
Figure 1 Overview of SDB-specific pathophysiological mechanisms linking SDB with cerebro- and cardiovascular morbidity – a bidirectional crosstalk
DOI: https://doi.org/10.4414/smw.2017.14436
Over the past several decades, the prevalence of sleep-disordered breathing (SDB) has been continuously rising, and SDB, especially obstructive sleep apnoea (OSA), has become a common major health concern in industrialised countries [1–3]. Several factors are likely to have contributed to this increase, including the growing obesity epidemic in our societies, demographic changes with an aging population suffering from more comorbidities, and a raising awareness of SDB as a widespread disease. All forms of SDB may disturb the natural architecture of sleep, leading to excessive daytime sleepiness, fatigue, decreased alertness during the daytime and impaired cognitive functioning. Even more importantly, SDB, especially OSA, is a major risk factor for cerebro- and cardiovascular morbidity and mortality.
This association between SDB and cerebro- and cardiovascular diseases has recently been recognised by both clinicians and researchers. The growing evidence suggesting a causal link between SDB and cerebro- and cardiovascular morbidity has led to recent guidelines pertaining to SDB in the management of acute stroke [4], congestive heart failure [5] and arterial hypertension [6]. However, SDB remains underdiagnosed and undertreated, mainly because the clinical symptoms of SDB are nonspecific and, in many cases, unrecognised even by the affected patient [7].
We summarise available epidemiological data and focus on the main pathophysiological mechanisms linking SDB to cerebro- and cardiovascular complications. We also provide an overview of current diagnostic approaches.
Basically, any alteration of respiration during sleep that goes beyond the physiological adaption during the transition from wakefulness to sleep may be considered as sleep disordered breathing [7–10]. Based on the underlying pathophysiological mechanisms, sleep-related breathing disorders are defined and categorised, according to the third edition of the international classification of sleep disorders [8], into four main groups:
OSA is characterised by repetitive episodes of complete (apnoea) or partial (hypopnoea) collapse of the upper airways during sleep, with maintained respiratory drive and respiratory effort. It results from various causes of upper airway collapse such as an anatomically narrow upper airway due to obesity, and bony or soft tissue structures. Upper airway resistance or obstruction is generally exacerbated by muscle relaxation during sleep. By definition, OSA may be diagnosed if more than five obstructive respiratory events occur per hour of sleep, even if clinically asymptomatic [8]. Therefore, from a clinical perspective, it is important to distinguish OSA from obstructive sleep apnoea syndrome (OSAS), the latter requiring the presence of clinical daytime and/or sleep-related symptoms, such as excessive sleepiness, in addition to obstructive respiratory events (OSAS = OSA + clinical symptoms). This discrimination has clinical implications because treatment of asymptomatic OSA is usually recommended only if the apnoea-hypopnoea-index (AHI, mean number of apnoeas and hypopnoeas per hour of sleep) is >15/h, or in the presence of relevant cardiovascular comorbidities, whereas in symptomatic patients (OSAS) initiation of therapy should be considered in all cases [8, 11].
In central sleep apnoea (CSA) the upper airway remains patent. CSA is characterised by a reduction or a cessation of the airflow due to absent or reduced respiratory effort related to an impairment of the central respiratory regulation and/or respiratory muscle alteration. According to the third edition of the International Classification of Sleep Disorders (ICSD-3), the polysomnographic diagnostic criteria of CSA include three signs: the number of central apnoeas and/or hypopnoeas is >5/h of sleep, the total number of central events exceeds 50% of the total number of apnoeas/hypopnoeas, and the respiratory pattern shows Cheyne-Stokes respiration [8]. In adults, CSA includes several subgroups of disorders, namely primary CSA, central sleep apnoea with and without Cheyne-Stokes respiration, and CSA due to medication or substances [12]. CSA may also develop at high altitude (CSA due to high altitude periodic breathing), an adaptive reaction associated with high-altitude hypocapnic alkalosis that is completely reversible when the individual returns to sea level. CSA may also occur secondary to neurological disorders such as ischaemic stroke or cerebral haemorrhage where the respiratory centre in the brain stem is affected. Treatment-emergent CSA (formerly known as complex sleep apnoea) is another separate pattern of CSA that may develop in patients treated with positive airway pressure for OSA [8].
Sleep-related hypoventilation disorders are characterised by an abnormal nocturnal increase in the arterial partial pressure of carbon dioxide (PaCO2) due to decreased or impaired ventilation at night, either an increase of at least 10 mm Hg above awake values to 50 mm Hg for at least 10 minutes, or an increase to above 55 mm Hg for at least 10 minutes [8].
The category of sleep-related hypoxaemia disorder was introduced to distinguish cases with sustained periods of significant hypoxaemia during sleep in the absence of other predefined SDB or hypoventilation.
Different types of SDB can overlap in the same patient. Also, the features of both obstructive and central events can be found within the same respiratory episodes, when initially the respiratory effort is absent with the subsequent resumption. In these cases, mixed SDB is often diagnosed [13, 14].
The most comprehensive data on SDB epidemiology are available for OSA. The first large-scale population-based study from the USA, published in 1993, showed that 9% of females and 24% of males in a middle-aged population present with an AHI >5/h, and 2 and 4%, respectively, suffer from symptomatic OSAS [1]. These data were revised by Peppard et al. in 2013, and a significant increase in SDB prevalence during the previous two decades was established. According to Peppard et al., 26% of the general middle-aged population is affected by OSA and a rise, ranging from 14 to 55% depending on gender and age of the subgroups, was observed [3]. This increase in OSA prevalence has also been reported from European countries. Based on data from a Swiss cohort including 2121 randomly selected subjects (mean age 57, range 40–85 years) from the city of Lausanne, the SDB rates are estimated to be 60% in females and 83% in males (based on the presence of an AHI of ≥5/h), and 23 and 49%, respectively (based on a more stringent definition with an AHI ≥15/h) [2]. As described earlier, elderly vs younger subjects and males vs females showed higher rates.
Recently, studies demonstrated that the prevalence of relevant SDB is much higher in individuals with known cerebro- and cardiovascular events [15–18] and reaches values up to 50 to 90% in specific cohorts [7, 19–21]. This was confirmed in pooled populations in later meta-analyses [20]. This might imply a direct association between SDB and cerebro- and cardiovascular morbidity. However, proving that SDB can independently cause cerebro- and cardiovascular diseases remains a difficult issue, because most cerebro- and cardiovascular diseases share common risk factors with SDB, such as obesity, male sex, age, smoking, or metabolic disorders [22–25]. Further, more severe SDB is associated with a higher prevalence of cardiovascular diseases, indicating a dose-dependent association also present after a multivariate adjustment for major cardiovascular risk factors [15]. Interestingly, the type and distribution of SDB may vary in different diseases (table 1) with OSA (AHI ≥10/h) being commonly seen in systemic hypertension [11, 68, 69], coronary artery disease [34, 70], heart rhythm disorders [21, 41], pulmonary hypertension [54] and stroke [7, 15, 20, 49, 71]. In contrast, CSA is predominantly seen in patients with congestive heart failure, especially with left-ventricular systolic dysfunction (in up to 30–50%), typically with CSR that is characterised by cyclic fluctuations in breathing in a waxing-waning (crescendo-decrescendo) mode, and in stroke patients (up to 25–30% in acute stroke) [7, 38, 71–73].
Table 1 The prevalence of SDB in various cardiovascular diseases and co-morbidities and adjusted odds/hazard ratios for the presence of these diseases in patients with SDB, mainly OSA.
| Pathology | Prevalence of SDB | Odds/hazard ratio | Ref. |
|---|---|---|---|
| Arterial hypertension | AHI ≥5/h: 58–74% AHI ≥ 15/h: 10–30% |
(1.33–1.96) | [2, 26–31] |
| Resistant arterial hypertension | AHI ≥ 5/h: 88% AHI ≥10/h: 60–83% AHI ≥30/h: 26–32% |
[32, 33] | |
| Coronary artery disease (including acute myocardial infarction, post-revascularisation patients) | AHI ≥5/h: 83% AHI ≥10/h: 30–64% AHI ≥15/h: 64% |
(1.27–3.1)* | [15, 16, 34–37] |
| Congestive heart failure | AHI ≥10/h: 72% AHI ≥15/h: 60–64% AHI ≥20/h: 53% AHI ≥30/h: 36% |
(1.13–2.38)† | [13–16, 38–40] |
| Heart rhythm and conduction disorders | AHI ≥5/h: 60–66% AHI ≥10/h: 59% AHI ≥15/h: 14–47% AHI ≥30/h: 20–27% |
[41–44] | |
| Atrial fibrillation | AHI ≥5/h: 70–74% AHI ≥10/h: 49% AHI ≥15/h: 25–43% AHI ≥30/h: 13% |
(2.18–3.29)‡ | [21, 39, 45–48] |
| Stroke | AHI ≥5/h: 79–86% AHI ≥15/h: 35–40% AHI ≥30/h: 30% |
(1.76–1.97) | [7, 15, 34, 49–52] |
| Asymptomatic carotid stenosis | AHI ≥10/h: 69% | [53] | |
| Pulmonary hypertension | AHI ≥10/h: 60% AHI ≥15/h: 42% |
[54–56] | |
| Obesity | AHI ≥5/h: 8–78% AHI ≥15/h: 2–35% |
[57, 58] | |
| Diabetes mellitus | AHI ≥5/h: 60–63% AHI ≥15/h: 26–37% AHI ≥30/h: 10–12% |
(1.43–2.30)§ | [2, 22, 59–61] |
| Chronic kidney disease | AHI ≥5/h: 54% AHI ≥15/h: 32–39% AHI ≥30/h: 6% |
[62, 63] | |
| Haemodialysis | AHI ≥10/h: 89% AHI ≥15/h: 50% |
[64–67] |
AHI = apnoea/hypopnoea index; OSA = obstructive sleep apnoea; SDB = sleep-disordered breathing * Dose-dependent, the highest risk was observed in males aged <70 years † Dose-dependent; in men, the adjusted hazard ratio of incident heart failure was 1.13 per 10 AHI units increase, 1.58 for AHI ≥30/h vs AHI <5/h, no association in woman ‡ AHI >5/h; in multivariate analysis only the lowest oxygen desaturation was associated with atrial fibrillation (hazard ratio 3.29 per 1% decrease) § Adjusted odds ratio for AHI ≥15/h vs <5/h
A causal association between OSA and arterial hypertension was first suggested over 30 years ago, making it one of the most investigated and well-recognised relationships between OSA and a vascular comorbidity [74]. OSA is observed in up to 30 to 50% of all hypertensive individuals and in more than 80% of all patients suffering from drug-resistant arterial hypertension [11, 75]. The most commonly observed blood pressure features in OSA patients include elevated diastolic blood pressure, nocturnal hypertension and a non-dipping circadian blood pressure profile. Moreover, OSA (AHI ≥15/h) is recognised as one of the most prevalent causes of treatment-resistant hypertension; in a high-risk cohort, severe OSA was associated with a four-fold increase in the prevalence of resistant elevated blood pressure despite intensive antihypertensive treatment, even after adjustment for the major cardiovascular risk factors [68]. However, despite the growing evidence supporting this association, there are still some unanswered questions. The trials addressing the antihypertensive effects of continuous positive airway pressure (CPAP) used as sleep apnoea treatment are controversial. An antihypertensive effect of CPAP therapy for OSA has been demonstrated in a recent meta-analysis, but it was rather modest reaching only ˗2 to ˗3 mm Hg and was more profound for systolic and nocturnal blood pressure values [76, 77]. In a prospective observational study of 1889 participants followed for more than 11 years, Marin et al. demonstrated that CPAP prevents new-onset hypertension in treatment-compliant patients (hazard ratio 0.71, 95% confidence interval [CI ] 0.53–0.94]) compared with control subjects with AHI <5/h, and the protective effect was observed despite an increase in body mass index (BMI) [26]. However, this protective effect is not observed in asymptomatic (e.g., non-sleepy) OSA patients [78].
Current data demonstrate a strong association between SDB and ischaemic stroke, although the exact underlying mechanisms are still not completely elucidated. The prevalence of SDB (AHI ≥5/h) in stroke survivors significantly exceeds that in the general population and reaches 50 to 86% (AHI ≥30/h: 30%). Stroke localisation and lesion volume are discussed as potential influencing factors. However, no convincing evidence has until now been provided, and the influence of particular cerebral topographies are controversial [7, 49, 50, 79–81]. CSA with or without Cheyne-Stokes respiration is found in patients with lesions in the central autonomic network (e.g., medulla oblongata), suggesting a link to cardiorespiratory central control [7].
Some studies showed an association between SDB and nocturnal onset of cerebrovascular events, including the wake-up stroke that comprises up to 25 to 30% of all acute cerebrovascular events. Thus, wake-up stroke patients show more severe sleep apnoea than those with daytime stroke onset, and the frequency of moderate-to-severe SDB (AHI >15/h) is higher in wake-up stroke patients [82–84]. However, in the recently published SLEEP-TIGHT study, the frequencies of SDB in wake-up stroke and non-wake-up stroke patients were similar [84], and the causal relationship is still uncertain.
SDB tends to improve from the acute to the subacute phase of stroke, and this may be more the case for CSA than OSA [79, 85–87]. Various reasons for the improvement of SDB are discussed, including the amelioration of neurological deficits, a higher level of physical activity and less time spent in the supine position during sleep.
As mentioned above, comorbid cardiovascular diseases and metabolic dysregulations frequently seen in OSA patients may all promote stroke. However, OSA and CSA with or without Cheyne-Stokes respiration are frequently encountered in acute and chronic stroke patients (with OSA being the predominant type), even in the absence of other classical cardiovascular or metabolic risk factors [7, 49, 88]. Therefore, SDB itself may increase the risk of stroke independently of these factors. Some studies provided evidence of the dominant role of hypoxaemia (in particular when its duration exceeds 10% of sleep) in incident stroke in subjects with SDB [89]. The risk of stroke in SDB was confirmed to be three- to four-fold higher after adjustment for the major cardiovascular risk factors [90, 91]. Interestingly, some studies noted gender-specific differences, with a significantly higher impact of OSA on stroke incidence in men, but not in women.
There is growing evidence that SDB adversely affects early stroke outcome and is associated with a worse functional outcome in the acute and subacute phases [7, 80]. Presence of SDB is also an independent predictor of higher mortality rates after stroke, and mortality increases in proportion to the AHI values [51, 92]. A recently published systematic review focused on the effects of SDB after cerebrovascular events, and demonstrated that OSA is a risk factor for vascular event recurrence and all-cause mortality in post-stroke patients [93]. However, the effects of positive airway pressure (PAP) treatment on post-stroke outcomes are controversial [94–96]. At the same time, the prognostic cut-offs of SDB severity, and therefore the indication for treatment initiation after stroke, are not established and further investigations are required. An international multicentre study (SAS-CARE) addressing these issues recently completed patient recruitment, and first results will be published in 2017 [97]. A second prospective interventional randomised trial (eSATIS), evaluating early adaptive servoventilation treatment in acute stroke patients with severe SDB, was started in 2015 (ClinicalTrials.gov Identifier: NCT02554487) [98].
SDB has increasingly been recognised as a risk factor for cognitive impairment and dementia. Assumed mechanisms underlying this association include cerebral hypoperfusion, endothelial dysfunction, impaired cerebral vasomotor reactivity and neuroinflammation resulting in cerebral small vessel disease and subsequent white matter lesions, grey matter loss and neurodegenerative processes [99].
Because of the relationship between sleep disorders and stroke, as well as the need for multidisciplinary approaches in this field, a taskforce on “Sleep and Stroke” was initiated by four European societies (European Respiratory Society, European Stroke Organisation, European Academy of Neurology and European Sleep Research Society) in 2016. The taskforce is chaired by Professor C.L. Bassetti, Professor W. Randerath, and Dr V. Papavasileiou, and aims at developing position statements based on reviewed evidence.
As for other cerebro- and cardiovascular diseases, in patients with coronary artery disease the prevalence of OSA is higher than in the normal population [35, 70]. In 1999, Peker et al. reported a prevalence of OSA (defined as a respiratory disturbance index of >10/h) of 30% in patients admitted with an acute coronary syndrome, and identified an independent association between OSA and coronary artery disease in a multivariate model (odds ratio 3.1, 95% CI 1.2–8.3) [35]. Recent studies reported a wide range of SDB prevalence in patients with coronary artery disease, from 26 to 69% depending on the investigated population and the criteria to establish the diagnosis of OSA, such as values of AHI, scoring criteria [70, 100].
The interaction between SDB and coronary artery disease also manifests in higher mortality rates if both entities are concomitantly present in a patient. A decade ago, Gami et al. (2005) had already shown that SDB patients are more likely to die suddenly during the classical sleeping hours (from 10 p.m. to 6 a.m.), in contrast to the general population and subjects without sleep apnoea [45]. Almost 10 years later, the same group retrospectively evaluated a sample of US residents consisting of 10 701 adults, and demonstrated that OSA was a strong predictor of sudden cardiac death at night. Moreover, the magnitude of the risk was associated with several parameters characterising OSA severity, including AHI (≥20/h), mean nocturnal oxygen saturation (<93%) and lowest nocturnal oxygen saturation (<78%) [101]. Similarly, the risk of myocardial infarction at night (between midnight and 6 a.m.) is significantly higher in OSA patients than in non-OSA subjects, indirectly indicating a possible interrelation [102]. Supporting the deleterious link, large-scale prospective studies based on general population cohorts demonstrated higher all-cause mortality in untreated SDB patients, particularly in the most severe cases [103–105]. However, a more recent analysis of the Sleep Heart Health Study, with longitudinal data after an 8.7-year follow-up, did not demonstrate an association of OSA with incident coronary artery disease after adjusting for other risk factors. The risk of coronary artery disease was slightly increased in OSA patients younger than 70 years and in patients with severe OSA (AHI >30/h) [16]. The lack of a general association between SDB and coronary artery disease in the Sleep Heart Health Study may be explained in part by the cohort’s characteristics: predominantly elderly patients with a mean age of 62 years in whom risk factors other than OSA may play a more important role in an unfavourable prognosis, female prevalence, high frequency of asymptomatic forms (which may be disputable), etc.
Nevertheless, the body of literature addressing the role of OSA as an independent risk factor for coronary artery disease is constantly growing [106]. One supporting clue is the fact that OSA is independently associated with subclinical coronary atherosclerosis, measured as coronary calcification in computed tomography [107], and there is also a higher prevalence of noncalcified occlusive atherosclerotic plaques in OSA patients. In addition, data on the effects of PAP therapy are promising; it appears to reduce the risk of recurrent ischaemic events and the necessity of revascularisation procedures [108, 109].
Bradyarrhythmias, including sinus and atrioventricular block of different degrees, are found in 10 to 50% of OSA patients, depending on the population and diagnostic criteria applied [110]. On the other hand, the rate of SDB in patients with implanted pacemakers is up to 50% [111]. Moreover, CPAP therapy has a protective effect against bradyarrhythmias, as demonstrated in prospective studies [112, 113]. Therefore, sleep apnoea is currently considered to be one of the reversible causes of bradyarrhythmias, and a sleep study is recommended before pacemaker implantation [114, 115].
OSA prevalence was two-fold higher in patients with atrial fibrillation than in a general cohort of patients referred to the cardiology clinic (after adjustment for the main risk factors) [21]. In addition, OSA was associated with a higher risk of recurrent atrial fibrillation after radiofrequency ablation procedures [116], as well as atrial fibrillation onset in the postoperative period after coronary artery bypass grafting (odds ratio 1.89, 95% CI 1.24–2.80; p = 0.003) [117]. Although the available data suggest a strong relationship between atrial fibrillation and OSA, further studies are required to make definitive conclusions.
A variety of underlying SDB-specific pathophysiological mechanisms linking SDB with cerebro- and cardiovascular morbidity have been identified (fig. 1). Common key features in all types of SDB may contribute to future cerebro- and cardiovascular diseases: intermittent hypoxaemia, intermittent increases in carbon dioxide partial pressure and recurrent arousals. Moreover, unsuccessful respiratory efforts against obstructed upper airways in OSA also cause intrathoracic pressure swings, potentially affecting intrathoracic organs and blood flow towards the heart and the brain. These phenomena can initiate physiological and pathophysiological reactions that promote the development of cerebro- and cardiovascular sequelae.

Figure 1 Overview of SDB-specific pathophysiological mechanisms linking SDB with cerebro- and cardiovascular morbidity – a bidirectional crosstalk
Despite the afore summarised evidence of a causal relationship between SDB and cerebro- and cardiovascular events and comorbidities, one may assume that the higher cerebro- and cardiovascular risk in OSA patients is just a cumulative effect of shared risk factors, such as obesity, male gender, hypertension, hyperlipidaemia, etc. Intriguingly, however, the relationship between SDB and cerebro- and cardiovascular morbidities seems to be more complex and bidirectional. Specifically, cerebro- and cardiovascular diseases themselves might contribute to the development or aggravation of SDB. In stroke patients, there is a significant reduction in SDB prevalence and severity from the acute to the subacute stroke phase, indicating a direct impact of acute brain damage (and its complications) on SDB [7].
In recent years, a novel concept elucidating the development of SDB in congestive heart failure and other states that are associated with fluid retention (renal disease, hypoproteinaemia, treatment resistant hypertension, and others) has been introduced [130, 131]. In fact, this hypothesis adds some new insights to the traditional understanding and helps to put some pieces of the puzzle together. According to this theory, the recumbent position and the associated changes in gravity in patients with congestive heart failure (at night / during sleep) is associated with a fluid shift from the legs to the upper body, including lungs, neck and upper airway. This was confirmed in a series of high-level experiments with the application of lower body positive pressure in both healthy individuals and congestive heart failure patients. This intervention led to a rapid increase in neck circumference and increased collapsibility of the upper airways (precisely confirmed with magnetic resonance imaging and plethysmographic measurements), thus increasing the risk of OSA development. On the other hand, fluid shifts from the legs leads to an increase in venous return to the heart, thus increasing pulmonary capillary wedge pressure and pulmonary congestion. This stimulates pulmonary irritant receptors, and as a consequence, causes hyperventilation and a reduction in CO2 partial pressure below the apnoea threshold. The latter is crucial for ventilatory control during sleep. Against the background of impaired chemosensitivity that is commonly observed in congestive heart failure patients, even slight changes can cause significant fluctuations in ventilation. Changes in the severity of fluid retention and peripheral oedema can lead to the modulation of the degree of CSA and be responsible for the predominance of either OSA or CSA in patients with congestive heart failure. A bidirectional interaction between sleep apnoea and congestive heart failure is also suggested by the beneficial effects of cardiac resynchronisation therapy and heart transplantation on SDB, particularly with respect to central sleep apnoea [132–134].
Because of the higher prevalence of SDB in populations with cerebro- and cardiovascular diseases and their potential detrimental impacts, it is crucial to consider SDB as a potentially modifiable cardiovascular risk factor, especially given the availability of treatment options [11]. The recognition of a potential role of SDB and its association with cardio/cerebrovascular diseases is reflected in current guidelines for the management of specific diseases such as stroke, arterial hypertension and heart failure [4–6]. However, despite these guidelines and the growing evidence of a strong bidirectional relation between sleep apnoea and cerebro/cardiovascular diseases, the majority of cases still remain undiagnosed [1, 16, 70, 102]. Therefore, the routine implementation of diagnostic approaches and application of reliable and valid screening tools is important, although the results and further treatment strategies should be treated with caution since a general cardiovascular benefit has not yet been confirmed for all comorbidities in recent trials.
Exploration of the patient’s history for clinical signs and symptoms of SDB and typical physical risk factors such as obesity and relevant retrognathia should always be the first step (fig. 2). A more comprehensive SDB evaluation should follow in patients at risk. Patients should be asked more detailed questions regarding typical sleep-related and daytime signs and symptoms. These include snoring, witnessed apnoeas, dyspnoea/choking during sleep, repetitive awakenings, dry mouth and nocturnal sweating. Daytime manifestations include excessive daytime sleepiness, fatigue, morning (or nocturnal) headache, cognitive impairment and irritability, etc.
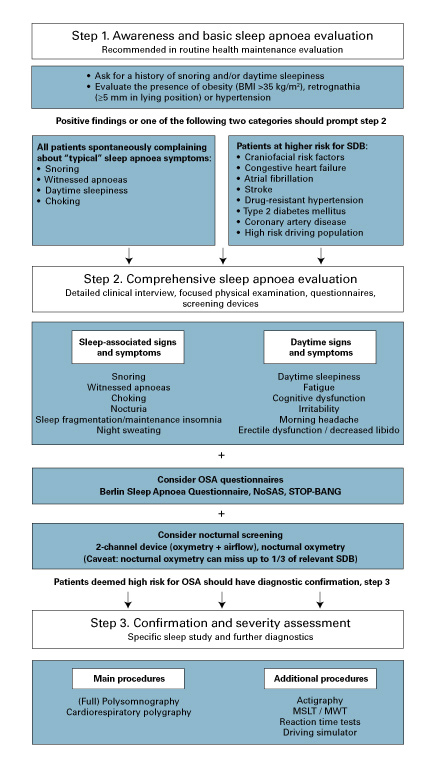
Figure 2 Suggestion for a simple three-step algorithm for the evaluation of sleep-disordered breathing (SDB).
BMI = body mass index; MSLT = multiple sleep latency test; MWT = maintenance of wakefulness text; OSA = obstructive sleep apnoea
The clinical manifestations of CSA may be less evident and are usually dominated by the underlying disease (e.g., heart failure). Fatigue, nonrestorative sleep, hyperventilation and disrupted sleep are frequently found [135].
Questionnaires evaluating the likelihood of clinically relevant SDB can be helpful, combined with a physical examination looking for features abetting SDB. For primary screening, there are various questionnaires that can be easily incorporated into routine clinical visits, such as the commonly used Berlin Sleep Apnea questionnaire, STOP-BANG questionnaire (and its modifications), and the recently introduced NoSAS-Score for OSA screening and Epworth sleepiness scale for daytime sleepiness assessment [136, 137]. However, recently published data have raised new issues regarding appropriate questionnaire screening tools in specific cohorts. For example, stroke patients usually demonstrate lower or normal values on the Epworth Sleepiness Scale and Berlin questionnaire as compared with non-stroke patients with SDB [7, 138]. The same is true for patients with atrial fibrillation or congestive heart failure [139, 140]. Such “masked” manifestation can lead to a significant underestimation of SDB burden in special conditions and potentially prevent timely treatment. For example, the HypnoLaus study has shown that the conventional clinical symptoms/signs are less predictive for the presence of SDB than the NoSAS score (a quantitative predictive scale for SDB probability evaluation that includes the following parameters: obesity, neck circumference, age, snoring and gender) [2, 136]. Thus, although disputed by some authors [137, 141], the common screening questionnaires seem to be inappropriate in some cohorts, and more differentiated diagnostic algorithms and individualised approaches are required [64].
Manifestations of SDB are heterogeneous in populations with differing comorbidities. Recently, a concept of different clinical phenotypes or “different clinical faces” of SDB/OSA has been suggested [142]. A collaborative Icelandic-American group identified three main clusters by grouping them according to the complaints/symptoms and comorbidities as follows: “disturbed sleep group”, “minimally symptomatic group” and the most prevalent “excessive daytime sleepiness group” consisting of 32.7%, 24.7%, and 42.6%, respectively, of the Icelandic cohort studied [142]. The probability of comorbid cardiovascular diseases differed between the subtypes, being lowest in the third cluster – the sleepiest one. Intriguingly, the probability of cerebro- and cardiovascular pathology was highest in the second group, which has minimal symptoms, and thus, according to current practice, is the last to be referred to sleep centres for specific SDB therapy [142, 143]. The heterogeneity of clinical manifestations might also be associated with different responses to therapy and/or adherence to treatment, potentially serving as a tool to choose treatment.
Therefore, objective diagnostic approaches should be applied at a low threshold for specific subgroups of patients, such as those with high estimated cerebro- or cardiovascular risks. In these cases, specialised instrumental examination may be considered the first step regardless of the presence of the clinical manifestations.
Based on American Academy of Sleep Medicine (AASM) criteria, four categories of diagnostic devices are distinguished (table 2), with attended observed video polysomnography as the “gold standard”. However, simple two-channel devices, including nocturnal oximetry and recording of the airflow, may be sufficient for screening and should be preferred to nocturnal oximetry only, which may miss at least one third of all relevant SDB. Cardiorespiratory polygraphy is usually referred to as a screening test, but it may be sufficient to establish the diagnosis in individuals with high clinical suspicion of SDB, as shown in recent studies reporting acceptable results for SDB identification by portable polygraphy in patients with cardiovascular comorbidities [144].
Table 2 Types of diagnostic tool (according to the American Academy of Sleep Medicine classification).
| Monitor | Type of the diagnostic tool | Parameters recorded | Benefits | Disadvantages |
|---|---|---|---|---|
| Type I | Attended, in-lab standard full (video) polysomnography |
≥7 channels including EEG, chin EMG, ECG, airflow, (chest, abdominal) respiratory efforts, oximetry, leg movements, position |
– “Gold standard” – Sleep structure assessment – Opportunity to perform interventions |
– Costly – Labour-intensive – Discomfort for the patient – Experienced and trained personnel (technician) – In-lab |
| Type II | Unattended full (video) polysomnography | ≥7 channels including EEG, chin EMG, heart rate or ECG, airflow, (chest, abdominal) respiratory efforts, oximetry, leg movements, position |
– Sleep structure assessment – Both in the lab and at home |
– Costly – Labour-intensive – Discomfort for the patient – No opportunity to perform interventions – Experienced and trained personnel (technician) |
|
|
|
|
– Inexpensive – Easy to perform – More comfortable – Portable (home) monitoring |
– Higher risk of false-positive and false-negative results |
|
|
|
|
– Inexpensive – Easy to perform (screening) – More comfortable – Portable, home monitoring |
– Higher risk of false-positive and false-negative results |
|
||||
One of the most relevant controversial issues is the choice of scoring criteria for sleep-associated respiratory events, independent of the diagnostic device used, since different recommended rules have been implemented in clinical practice and research during the last two decades. This may cause significant differences in the number of recorded respiratory events and, thus, the prevalence and severity of SDB. In a cohort of heart failure patients, a difference of 4.6 events per hour was established in AHI scoring according to AASM “recommended” and “alternative” rules, leading to a significant change in the detected SDB prevalence (29% vs 46%, p<0.001, based on an AHI ≥15/h) [145].
Currently, various alternative diagnostic tools are being developed, including portable, one-channel, non-contact devices (based on acoustic or bioradiolocation signals, etc.) [146, 147]. However, their utility in comorbid states has not yet been verified, although they appear to be promising screening devices and tools for long-term and repeated monitoring.
SDB is associated with cerebro- and cardiovascular diseases. Accumulating data provide new insights into the underlying mechanisms and need for novel management approaches. Undoubtedly, a wider implementation of screening tools is required, in particular in patients at high risk for cerebro- and/or cardiovascular diseases, as well as better application of preventive and therapeutic approaches. At present, some issues remain controversial, including the choice of diagnostic criteria and tools, the benefits of PAP therapy in some populations, the paradoxical effects of sleep apnoea in certain cohorts (e.g., preconditioning effects of sleep apnoea-associated intermittent hypoxaemia), etc.. These questions can be answered in multicentre trials and in large multidisciplinary collaborative research projects (e.g., the International Collaboration of Sleep Apnea Cardiovascular Trialists, INCOSACT, SAS-CARE, eSATIS [97, 98, 148] that could facilitate the promotion of research ideas, standardise procedures and regulations while advancing our scientific understanding on the role of SDB in cardiovascular morbidity and mortality.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Young T , Palta M , Dempsey J , Skatrud J , Weber S , Badr S . The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. https://doi.org/10.1056/NEJM199304293281704
2 Heinzer R , Vat S , Marques-Vidal P , Marti-Soler H , Andries D , Tobback N , et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–8. https://doi.org/10.1016/S2213-2600(15)00043-0
3 Peppard PE , Young T , Barnet JH , Palta M , Hagen EW , Hla KM . Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–14. https://doi.org/10.1093/aje/kws342
4 Kernan WN , Ovbiagele B , Black HR , Bravata DM , Chimowitz MI , Ezekowitz MD , et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. . Correction in: Stroke. 2015;46(2):e54. doi: 10.1161/STR.0000000000000059https://doi.org/10.1161/STR.0000000000000024
5 Yancy CW , Jessup M , Bozkurt B , Butler J , Casey DE, Jr , Drazner MH , et al.; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. https://doi.org/10.1016/j.jacc.2013.05.019
6 Rosendorff C , Lackland DT , Allison M , Aronow WS , Black HR , Blumenthal RS , et al.; American Heart Association, American College of Cardiology, and American Society of Hypertension. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American Heart Association, American College of Cardiology, and American Society of Hypertension. Circulation. 2015;131(19):e435–70. https://doi.org/10.1161/CIR.0000000000000207
7 Hermann DM , Bassetti CL . Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. 2016;87(13):1407–16. https://doi.org/10.1212/WNL.0000000000003037
8American Academy of Sleep Medicine. International Classification of Sleep Disorders. Vol 3d ed. Darien, IL: American Academy of Sleep Medicine; 2014.
9 Douglas NJ . Control of ventilation during sleep. Clin Chest Med. 1985;6(4):563–75.
10 Malik V , Smith D , Lee-Chiong T, Jr . Respiratory Physiology During Sleep. Sleep Med Clin. 2012;7(3):497–505. https://doi.org/10.1016/j.jsmc.2012.06.011
11 Parati G , Lombardi C , Hedner J , Bonsignore MR , Grote L , Tkacova R , et al.; EU COST Action B26 members. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J. 2013;41(3):523–38. https://doi.org/10.1183/09031936.00226711
12 Aurora RN , Chowdhuri S , Ramar K , Bista SR , Casey KR , Lamm CI , et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40. https://doi.org/10.5665/sleep.1580
13Greenberg H, Lakticova V, Scharf SM. Sleep Breathing Disorders. In: Principles and Practice of Sleep Medicine. 6th Ed. Ed. by M. Kryger et Al. Ch. 114. Vol; 2016:1110-1124.
14Eckert D, Butler J. Respiratory physiology: understanding the control of ventilation. In: Principles and Practice of Sleep Medicine. 6th Ed. Ed. by M. Kryger et Al. Ch. 16-17. Vol; 2016:167-173.
15 Shahar E , Whitney CW , Redline S , Lee ET , Newman AB , Nieto FJ , et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163(1):19–25. https://doi.org/10.1164/ajrccm.163.1.2001008
16 Gottlieb DJ , Yenokyan G , Newman AB , O’Connor GT , Punjabi NM , Quan SF , et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–60. https://doi.org/10.1161/CIRCULATIONAHA.109.901801
17 Gilat H , Vinker S , Buda I , Soudry E , Shani M , Bachar G . Obstructive sleep apnea and cardiovascular comorbidities: a large epidemiologic study. Medicine (Baltimore). 2014;93(9):e45. https://doi.org/10.1097/MD.0000000000000045
18 Bassetti C , Aldrich MS , Chervin RD , Quint D . Sleep apnea in patients with transient ischemic attack and stroke: a prospective study of 59 patients. Neurology. 1996;47(5):1167–73. https://doi.org/10.1212/WNL.47.5.1167
19 Hermann DM , Bassetti CL . Sleep-related breathing and sleep-wake disturbances in ischemic stroke. Neurology. 2009;73(16):1313–22. https://doi.org/10.1212/WNL.0b013e3181bd137c
20Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. JCSM. 2010;6(2):131-137. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2854698&tool=pmcentrez&rendertype=abstract.
21 Gami AS , Pressman G , Caples SM , Kanagala R , Gard JJ , Davison DE , et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–7. https://doi.org/10.1161/01.CIR.0000136587.68725.8E
22 Reichmuth KJ , Austin D , Skatrud JB , Young T . Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–5. https://doi.org/10.1164/rccm.200504-637OC
23 Tasali E , Mokhlesi B , Van Cauter E . Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133(2):496–506. https://doi.org/10.1378/chest.07-0828
24 Lam JCM , Mak JCW , Ip MSM . Obesity, obstructive sleep apnoea and metabolic syndrome. Respirology. 2012;17(2):223–36. https://doi.org/10.1111/j.1440-1843.2011.02081.x
25 Xu H , Guan J , Yi H , Zou J , Meng L , Tang X , et al.; Shanghai Sleep Health Study Research Group. Elevated low-density lipoprotein cholesterol is independently associated with obstructive sleep apnea: evidence from a large-scale cross-sectional study. Sleep Breath. 2016;20(2):627–34. https://doi.org/10.1007/s11325-015-1262-3
26 Marin JM , Agusti A , Villar I , Forner M , Nieto D , Carrizo SJ , et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–76. https://doi.org/10.1001/jama.2012.3418
27 Young T , Peppard P , Palta M , Hla KM , Finn L , Morgan B , et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–52. https://doi.org/10.1001/archinte.1997.00440360178019
28 Broström A , Sunnergren O , Årestedt K , Johansson P , Ulander M , Riegel B , et al. Factors associated with undiagnosed obstructive sleep apnoea in hypertensive primary care patients. Scand J Prim Health Care. 2012;30(2):107–13. https://doi.org/10.3109/02813432.2012.675563
29 Demede M , Pandey A , Zizi F , Bachmann R , Donat M , McFarlane SI , et al. Resistant hypertension and obstructive sleep apnea in the primary-care setting. Int J Hypertens. 2011;2011:340929. https://doi.org/10.4061/2011/340929
30 Di Guardo A , Profeta G , Crisafulli C , Sidoti G , Zammataro M , Paolini I , et al. Obstructive sleep apnoea in patients with obesity and hypertension. Br J Gen Pract. 2010;60(574):325–8. https://doi.org/10.3399/bjgp10X484174
31 Börgel J , Sanner BM , Keskin F , Bittlinsky A , Bartels NK , Büchner N , et al. Obstructive sleep apnea and blood pressure. Interaction between the blood pressure-lowering effects of positive airway pressure therapy and antihypertensive drugs. Am J Hypertens. 2004;17(12 Pt 1):1081–7. https://doi.org/10.1016/j.amjhyper.2004.06.026
32 Min HJ , Cho YJ , Kim CH , Kim DH , Kim HY , Choi JI , et al. Clinical Features of Obstructive Sleep Apnea That Determine Its High Prevalence in Resistant Hypertension. Yonsei Med J. 2015;56(5):1258–65. https://doi.org/10.3349/ymj.2015.56.5.1258
33 Pedrosa RP , Drager LF , Gonzaga CC , Sousa MG , de Paula LK , Amaro AC , et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–7. https://doi.org/10.1161/HYPERTENSIONAHA.111.179788
34 Mooe T , Franklin KA , Holmström K , Rabben T , Wiklund U . Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1910–3. https://doi.org/10.1164/ajrccm.164.10.2101072
35 Peker Y , Kraiczi H , Hedner J , Löth S , Johansson A , Bende M . An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14(1):179–84. https://doi.org/10.1034/j.1399-3003.1999.14a30.x
36 Aronson D , Nakhleh M , Zeidan-Shwiri T , Mutlak M , Lavie P , Lavie L . Clinical implications of sleep disordered breathing in acute myocardial infarction. PLoS One. 2014;9(2):e88878. https://doi.org/10.1371/journal.pone.0088878
37 Glantz H , Thunström E , Herlitz J , Cederin B , Nasic S , Ejdebäck J , et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10(4):350–6. https://doi.org/10.1513/AnnalsATS.201211-106OC
38 Herrscher TE , Akre H , Øverland B , Sandvik L , Westheim AS . High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17(5):420–5. https://doi.org/10.1016/j.cardfail.2011.01.013
39 Lanfranchi PA , Somers VK , Braghiroli A , Corra U , Eleuteri E , Giannuzzi P . Central sleep apnea in left ventricular dysfunction: prevalence and implications for arrhythmic risk. Circulation. 2003;107(5):727–32. https://doi.org/10.1161/01.CIR.0000049641.11675.EE
40 Sin DD , Fitzgerald F , Parker JD , Newton G , Floras JS , Bradley TD . Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–6. https://doi.org/10.1164/ajrccm.160.4.9903020
41 Guilleminault C , Connolly SJ , Winkle RA . Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52(5):490–4. https://doi.org/10.1016/0002-9149(83)90013-9
42 Grimm W , Apelt S , Timmesfeld N , Koehler U . Sleep-disordered breathing in patients with implantable cardioverter-defibrillator. Europace. 2013;15(4):515–22. https://doi.org/10.1093/europace/eus356
43 Koshino Y , Satoh M , Katayose Y , Yasuda K , Tanigawa T , Takeyasu N , et al. Association of sleep-disordered breathing and ventricular arrhythmias in patients without heart failure. Am J Cardiol. 2008;101(6):882–6. https://doi.org/10.1016/j.amjcard.2007.10.056
44 Garrigue S , Pépin J-L , Defaye P , Murgatroyd F , Poezevara Y , Clémenty J , et al. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European Multicenter Polysomnographic Study. Circulation. 2007;115(13):1703–9. https://doi.org/10.1161/CIRCULATIONAHA.106.659706
45 Gami AS , Howard DE , Olson EJ , Somers VK . Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–14. https://doi.org/10.1056/NEJMoa041832
46 Gami AS , Hodge DO , Herges RM , Olson EJ , Nykodym J , Kara T , et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–71. https://doi.org/10.1016/j.jacc.2006.08.060
47 Bitter T , Langer C , Vogt J , Lange M , Horstkotte D , Oldenburg O . Sleep-disordered breathing in patients with atrial fibrillation and normal systolic left ventricular function. Dtsch Arztebl Int. 2009;106(10):164–70. doi:.https://doi.org/10.3238/arztebl.2009.0164
48 Altmann DR , Ullmer E , Rickli H , Maeder MT , Sticherling C , Schaer BA , et al. Clinical impact of screening for sleep related breathing disorders in atrial fibrillation. Int J Cardiol. 2012;154(3):256–8. https://doi.org/10.1016/j.ijcard.2010.09.034
49 Stahl SM , Yaggi HK , Taylor S , Qin L , Ivan CS , Austin C , et al. Infarct location and sleep apnea: evaluating the potential association in acute ischemic stroke. Sleep Med. 2015;16(10):1198–203. https://doi.org/10.1016/j.sleep.2015.07.003
50 Ifergane G , Ovanyan A , Toledano R , Goldbart A , Abu-Salame I , Tal A , et al. Obstructive Sleep Apnea in Acute Stroke: A Role for Systemic Inflammation. Stroke. 2016;47(5):1207–12. https://doi.org/10.1161/STROKEAHA.115.011749
51 Sahlin C , Sandberg O , Gustafson Y , Bucht G , Carlberg B , Stenlund H , et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168(3):297–301. https://doi.org/10.1001/archinternmed.2007.70
52 Yaggi HK , Concato J , Kernan WN , Lichtman JH , Brass LM , Mohsenin V . Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–41. https://doi.org/10.1056/NEJMoa043104
53 Ehrhardt J , Schwab M , Finn S , Guenther A , Schultze T , Witte OW , et al. Sleep apnea and asymptomatic carotid stenosis: a complex interaction. Chest. 2015;147(4):1029–36. https://doi.org/10.1378/chest.14-1655
54 Laks L , Lehrhaft B , Grunstein RR , Sullivan CE . Pulmonary hypertension in obstructive sleep apnoea. Eur Respir J. 1995;8(4):537–41. doi:.https://doi.org/10.1183/09031936.95.08040537
55 Kessler R , Chaouat A , Weitzenblum E , Oswald M , Ehrhart M , Apprill M , et al. Pulmonary hypertension in the obstructive sleep apnoea syndrome: prevalence, causes and therapeutic consequences. Eur Respir J. 1996;9(4):787–94. https://doi.org/10.1183/09031936.96.09040787
56 Minic M , Granton JT , Ryan CM . Sleep disordered breathing in group 1 pulmonary arterial hypertension. J Clin Sleep Med. 2014;10(3):277–83. doi:.https://doi.org/10.5664/jcsm.3528
57 Lopez PP , Stefan B , Schulman CI , Byers PM . Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74(9):834–8.
58 Young T , Peppard PE , Taheri S . Excess weight and sleep-disordered breathing. J Appl Physiol (1985). 2005;99(4):1592–9. https://doi.org/10.1152/japplphysiol.00587.2005
59 Botros N , Concato J , Mohsenin V , Selim B , Doctor K , Yaggi HK . Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122(12):1122–7. https://doi.org/10.1016/j.amjmed.2009.04.026
60 Schober AK , Neurath MF , Harsch IA . Prevalence of sleep apnoea in diabetic patients. Clin Respir J. 2011;5(3):165–72. https://doi.org/10.1111/j.1752-699X.2010.00216.x
61 Zhang P , Zhang R , Zhao F , Heeley E , Chai-Coetzer CL , Liu J , et al. The prevalence and characteristics of obstructive sleep apnea in hospitalized patients with type 2 diabetes in China. J Sleep Res. 2016;25(1):39–46. https://doi.org/10.1111/jsr.12334
62 Markou N , Kanakaki M , Myrianthefs P , Hadjiyanakos D , Vlassopoulos D , Damianos A , et al. Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung. 2006;184(1):43–9. https://doi.org/10.1007/s00408-005-2563-2
63 Nicholl DDM , Ahmed SB , Loewen AHS , Hemmelgarn BR , Sola DY , Beecroft JM , et al. Clinical presentation of obstructive sleep apnea in patients with chronic kidney disease. J Clin Sleep Med. 2012;8(4):381–7. doi:.https://doi.org/10.5664/jcsm.2028
64 Forni Ogna V , Ogna A , Pruijm M , et al. Prevalence and Diagnostic Approach to Sleep Apnea in Hemodialysis Patients: A Population Study. BioMed Res Intern. 2015.2015:1–9. doi:.https://doi.org/10.1155/2015/103686
65 Merlino G , Piani A , Dolso P , Adorati M , Cancelli I , Valente M , et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21(1):184–90. https://doi.org/10.1093/ndt/gfi144
66 Unruh ML , Sanders MH , Redline S , Piraino BM , Umans JG , Hammond TC , et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol. 2006;17(12):3503–9. https://doi.org/10.1681/ASN.2006060659
67 Dharia SM , Unruh ML , Brown LK . Central Sleep Apnea in Kidney Disease. Semin Nephrol. 2015;35(4):335–46. https://doi.org/10.1016/j.semnephrol.2015.06.005
68 Walia HK , Li H , Rueschman M , Bhatt DL , Patel SR , Quan SF , et al. Association of severe obstructive sleep apnea and elevated blood pressure despite antihypertensive medication use. J Clin Sleep Med. 2014;10(8):835–43. doi:.https://doi.org/10.5664/jcsm.3946
69 Logan AG , Perlikowski SM , Mente A , Tisler A , Tkacova R , Niroumand M , et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–7. https://doi.org/10.1097/00004872-200112000-00022
70 Konecny T , Kuniyoshi FHS , Orban M , Pressman GS , Kara T , Gami A , et al. Under-diagnosis of sleep apnea in patients after acute myocardial infarction. J Am Coll Cardiol. 2010;56(9):742–3. https://doi.org/10.1016/j.jacc.2010.04.032
71 Bassetti CL , Milanova M , Gugger M . Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37(4):967–72. https://doi.org/10.1161/01.STR.0000208215.49243.c3
72 Siccoli MM , Valko PO , Hermann DM , Bassetti CL . Central periodic breathing during sleep in 74 patients with acute ischemic stroke - neurogenic and cardiogenic factors. J Neurol. 2008;255(11):1687–92. https://doi.org/10.1007/s00415-008-0981-9
73 Javaheri S . Central sleep apnea in congestive heart failure: prevalence, mechanisms, impact, and therapeutic options. Semin Respir Crit Care Med. 2005;26(1):44–55. https://doi.org/10.1055/s-2005-864206
74 Tilkian AG , Guilleminault C , Schroeder JS , Lehrman KL , Simmons FB , Dement WC . Hemodynamics in sleep-induced apnea. Studies during wakefulness and sleep. Ann Intern Med. 1976;85(6):714–9. https://doi.org/10.7326/0003-4819-85-6-714
75 Lévy P , McNicholas WT . Sleep apnoea and hypertension: time for recommendations. Eur Respir J. 2013;41(3):505–6. https://doi.org/10.1183/09031936.00007213
76 Fava C , Dorigoni S , Dalle Vedove F , Danese E , Montagnana M , Guidi GC , et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea: a systematic review and meta-analysis. Chest. 2014;145(4):762–71. https://doi.org/10.1378/chest.13-1115
77 Martínez-García MA , Capote F , Campos-Rodríguez F , Lloberes P , Díaz de Atauri MJ , Somoza M , et al.; Spanish Sleep Network. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–15. https://doi.org/10.1001/jama.2013.281250
78 Barbé F , Durán-Cantolla J , Sánchez-de-la-Torre M , Martínez-Alonso M , Carmona C , Barceló A , et al.; Spanish Sleep And Breathing Network. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307(20):2161–8. https://doi.org/10.1001/jama.2012.4366
79 Parra O , Arboix A , Bechich S , García-Eroles L , Montserrat JM , López JA , et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161(2 Pt 1):375–80. https://doi.org/10.1164/ajrccm.161.2.9903139
80 Camilo MR , Schnitman SV , Sander HH , Eckeli AL , Fernandes RM , Leite JP , et al. Sleep-disordered breathing among acute ischemic stroke patients in Brazil. Sleep Med. 2016;19:8–12. https://doi.org/10.1016/j.sleep.2015.11.008
81 Schipper MH , Jellema K , Rijsman RM . Occurrence of Obstructive Sleep Apnea Syndrome in Patients with Transient Ischemic Attack. J Stroke Cerebrovasc Dis. 2016;25(5):1249–53. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.01.046
82 Hsieh SW , Lai CL , Liu CK , Hsieh CF , Hsu CY . Obstructive sleep apnea linked to wake-up strokes. J Neurol. 2012;259(7):1433–9. https://doi.org/10.1007/s00415-011-6370-9
83 Šiarnik P , Kollár B , Čarnická Z , Šurda P , Klobučníková K , Sýkora M , et al. Association of Sleep Disordered Breathing with Wake-Up Acute Ischemic Stroke: A Full Polysomnographic Study. J Clin Sleep Med. 2016;12(4):549–54. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26612509. https://doi.org/10.5664/jcsm.5688
84 Koo BB , Bravata DM , Tobias LA , Mackey JS , Miech EJ , Matthias MS , et al. Observational study of obstructive sleep apnea in wake-up stroke: The SLEEP TIGHT study. Cerebrovasc Dis. 2016;41(5-6):233–41. https://doi.org/10.1159/000440736
85 Bassetti CL , Hermann DM . Sleep and stroke. Handb Clin Neurol. 2011;99:1051–72. https://doi.org/10.1016/B978-0-444-52007-4.00021-7
86 Iranzo A , Santamaría J , Berenguer J , Sánchez M , Chamorro A . Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;58(6):911–6. https://doi.org/10.1212/WNL.58.6.911
87 Harbison J , Ford GA , James OFW , Gibson GJ . Sleep-disordered breathing following acute stroke. QJM. 2002;95(11):741–7. https://doi.org/10.1093/qjmed/95.11.741
88 Bonnin-Vilaplana M , Arboix A , Parra O , García-Eroles L , Montserrat JM , Massons J . Cheyne-stokes respiration in patients with first-ever lacunar stroke. Sleep Disord. 2012;2012:257890. https://doi.org/10.1155/2012/257890
89 Stone KL , Blackwell TL , Ancoli-Israel S , Barrett-Connor E , Bauer DC , Cauley JA , et al.; Osteoporotic Fractures in Men (MrOS) Study Research Group. Sleep Disordered Breathing and Risk of Stroke in Older Community-Dwelling Men. Sleep. 2016;39(3):531–40. https://doi.org/10.5665/sleep.5520
90 Redline S , Yenokyan G , Gottlieb DJ , Shahar E , O’Connor GT , Resnick HE , et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–77. https://doi.org/10.1164/rccm.200911-1746OC
91 Loke YK , Brown JWL , Kwok CS , Niruban A , Myint PK . Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–8. https://doi.org/10.1161/CIRCOUTCOMES.111.964783
92 Parra O , Arboix A , Montserrat JM , Quintó L , Bechich S , García-Eroles L . Sleep-related breathing disorders: impact on mortality of cerebrovascular disease. Eur Respir J. 2004;24(2):267–72. https://doi.org/10.1183/09031936.04.00061503
93 Birkbak J , Clark AJ , Rod NH . The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: a systematic review. J Clin Sleep Med. 2014;10(1):103–8. doi:.https://doi.org/10.5664/jcsm.3376
94 Parra O , Sánchez-Armengol A , Bonnin M , Arboix A , Campos-Rodríguez F , Pérez-Ronchel J , et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J. 2011;37(5):1128–36. https://doi.org/10.1183/09031936.00034410
95 Parra O , Sánchez-Armengol Á , Capote F , Bonnin M , Arboix A , Campos-Rodríguez F , et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24(1):47–53. https://doi.org/10.1111/jsr.12181
96 Bravata DM , Concato J , Fried T , Ranjbar N , Sadarangani T , McClain V , et al. Continuous positive airway pressure: evaluation of a novel therapy for patients with acute ischemic stroke. Sleep. 2011;34(9):1271–7. doi:.https://doi.org/10.5665/SLEEP.1254
97 Cereda CW , Petrini L , Azzola A , Ciccone A , Fischer U , Gallino A , et al. Sleep-disordered breathing in acute ischemic stroke and transient ischemic attack: effects on short- and long-term outcome and efficacy of treatment with continuous positive airways pressure--rationale and design of the SAS CARE study. Int J Stroke. 2012;7(7):597–603. https://doi.org/10.1111/j.1747-4949.2012.00836.x
98 Bassetti CL , Ferini-Strambi L , Brown S , Adamantidis A , Benedetti F , Bruni O , et al. Neurology and psychiatry: waking up to opportunities of sleep.: State of the art and clinical/research priorities for the next decade. Eur J Neurol. 2015;22(10):1337–54. https://doi.org/10.1111/ene.12781
99 Rosenzweig I , Glasser M , Polsek D , Leschziner GD , Williams SCR , Morrell MJ . Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015;3(5):404–14. https://doi.org/10.1016/S2213-2600(15)00090-9
100 Lee C-H , Khoo S-M , Chan MY , Wong HB , Low AF , Phua QH , et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7(6):616–21. doi:.https://doi.org/10.5664/jcsm.1464
101 Gami AS , Olson EJ , Shen WK , Wright RS , Ballman KV , Hodge DO , et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013;62(7):610–6. https://doi.org/10.1016/j.jacc.2013.04.080
102 Kuniyoshi FHS , Garcia-Touchard A , Gami AS , Romero-Corral A , van der Walt C , Pusalavidyasagar S , et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343–6. https://doi.org/10.1016/j.jacc.2008.04.027
103 Marshall NS , Wong KKH , Cullen SRJ , Knuiman MW , Grunstein RR . Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–62. doi:.https://doi.org/10.5664/jcsm.3600
104 Marin JM , Carrizo SJ , Vicente E , Agusti AGN . Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53. https://doi.org/10.1016/S0140-6736(05)74229-X
105 Marshall NS , Wong KKH , Liu PY , Cullen SRJ , Knuiman MW , Grunstein RR . Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–85.
106 Monahan K , Redline S . Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26(6):541–7. https://doi.org/10.1097/HCO.0b013e32834b806a
107 Sorajja D , Gami AS , Somers VK , Behrenbeck TR , Garcia-Touchard A , Lopez-Jimenez F . Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133(4):927–33. https://doi.org/10.1378/chest.07-2544
108 Milleron O , Pillière R , Foucher A , de Roquefeuil F , Aegerter P , Jondeau G , et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur Heart J. 2004;25(9):728–34. https://doi.org/10.1016/j.ehj.2004.02.008
109 Garcia-Rio F , Alonso-Fernández A , Armada E , Mediano O , Lores V , Rojo B , et al. CPAP effect on recurrent episodes in patients with sleep apnea and myocardial infarction. Int J Cardiol. 2013;168(2):1328–35. https://doi.org/10.1016/j.ijcard.2012.12.015
110 Simantirakis EN , Schiza SI , Marketou ME , Chrysostomakis SI , Chlouverakis GI , Klapsinos NC , et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J. 2004;25(12):1070–6. https://doi.org/10.1016/j.ehj.2004.04.017
111 Fietze I , Röttig J , Quispe-Bravo S , Riedel F , Witte J , Baumann G , et al. Sleep apnea syndrome in patients with cardiac pacemaker. Respiration. 2000;67(3):268–71. https://doi.org/10.1159/000029509
112 Becker HF , Koehler U , Stammnitz A , Peter JH . Heart block in patients with sleep apnoea. Thorax. 1998;53(Suppl 3):S29–32. https://doi.org/10.1136/thx.53.2008.S29
113 Koehler U , Fus E , Grimm W , Pankow W , Schäfer H , Stammnitz A , et al. Heart block in patients with obstructive sleep apnoea: pathogenetic factors and effects of treatment. Eur Respir J. 1998;11(2):434–9. https://doi.org/10.1183/09031936.98.11020434
114 Brignole M , Auricchio A , Baron-Esquivias G , Bordachar P , Boriani G , Breithardt OA , et al., Document Reviewers. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34(29):2281–329. https://doi.org/10.1093/eurheartj/eht150
115 Epstein AE , Dimarco JP , Ellenbogen KA , et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:350–408. https://doi.org/10.1161/CIRCUALTIONAHA.108.189742
116 Kanagala R , Murali NS , Friedman PA , Ammash NM , Gersh BJ , Ballman KV , et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589–94. doi:.https://doi.org/10.1161/01.CIR.0000068337.25994.21
117 Qaddoura A , Kabali C , Drew D , van Oosten EM , Michael KA , Redfearn DP , et al. Obstructive sleep apnea as a predictor of atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. Can J Cardiol. 2014;30(12):1516–22. https://doi.org/10.1016/j.cjca.2014.10.014
118 Netzer N , Werner P , Jochums I , Lehmann M , Strohl KP . Blood flow of the middle cerebral artery with sleep-disordered breathing: correlation with obstructive hypopneas. Stroke. 1998;29(1):87–93. https://doi.org/10.1161/01.STR.29.1.87
119 Pizza F , Biallas M , Kallweit U , Wolf M , Bassetti CL . Cerebral hemodynamic changes in stroke during sleep-disordered breathing. Stroke. 2012;43(7):1951–3. https://doi.org/10.1161/STROKEAHA.112.656298
120 Coloma Navarro R , Jiménez Caballero PE , Vega G , Ayo-Martín O , Segura Martín T . Cerebral hemodynamics is altered in patients with sleep apnea/hypopnea syndrome. Springerplus. 2016;5(1):51. https://doi.org/10.1186/s40064-016-1691-x
121 Narkiewicz K , van de Borne PJH , Cooley RL , Dyken ME , Somers VK . Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–6. https://doi.org/10.1161/01.CIR.98.8.772
122 Narkiewicz K , Kato M , Phillips BG , Pesek CA , Davison DE , Somers VK . Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100(23):2332–5. https://doi.org/10.1161/01.CIR.100.23.2332
123 Seif F , Patel SR , Walia H , Rueschman M , Bhatt DL , Gottlieb DJ , et al. Association between obstructive sleep apnea severity and endothelial dysfunction in an increased background of cardiovascular burden. J Sleep Res. 2013;22(4):443–51. https://doi.org/10.1111/jsr.12026
124 Cereda CW , Tamisier R , Manconi M , Andreotti J , Frangi J , Pifferini V , et al. Endothelial dysfunction and arterial stiffness in ischemic stroke: the role of sleep-disordered breathing. Stroke. 2013;44(4):1175–8. https://doi.org/10.1161/STROKEAHA.111.000112
125 von Känel R , Natarajan L , Ancoli-Israel S , Mills PJ , Loredo JS , Dimsdale JE . Day/Night rhythm of hemostatic factors in obstructive sleep apnea. Sleep. 2010;33(3):371–7. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2831432&tool=pmcentrez&rendertype=abstract.
126 Steiner S , Jax T , Evers S , Hennersdorf M , Schwalen A , Strauer BE . Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology. 2005;104(2):92–6. https://doi.org/10.1159/000086729
127 Shamsuzzaman A , Amin RS , Calvin AD , Davison D , Somers VK . Severity of obstructive sleep apnea is associated with elevated plasma fibrinogen in otherwise healthy patients. Sleep Breath. 2014;18(4):761–6. https://doi.org/10.1007/s11325-014-0938-4
128 D’Silva L , Wilczynska M , Lewis K , Lawrence M , Hawkins K , Williams R , et al. Altered clot microstructure detected in obstructive sleep apnoea hypopnoea syndrome. Sleep Sci. 2016;9(1):14–9. https://doi.org/10.1016/j.slsci.2016.02.175
129 Steffanina A , Proietti L , Antonaglia C , Palange P , Angelici E , Canipari R . The Plasminogen System and Transforming Growth Factor-β in Subjects With Obstructive Sleep Apnea Syndrome: Effects of CPAP Treatment. Respir Care. 2015;60(11):1643–51. https://doi.org/10.4187/respcare.03571
130 White LH , Bradley TD . Logan a G. Pathogenesis of obstructive sleep apnoea in hypertensive patients: role of fluid retention and nocturnal rostral fluid shift. J Hum Hypertens. 2015;29(6):342–50. doi:.https://doi.org/10.1038/jhh.2014.94
131 Kasai T , Motwani SS , Elias RM , Gabriel JM , Taranto Montemurro L , Yanagisawa N , et al. Influence of rostral fluid shift on upper airway size and mucosal water content. J Clin Sleep Med. 2014;10(10):1069–74. doi:.https://doi.org/10.5664/jcsm.4102
132 Lüthje L , Renner B , Kessels R , Vollmann D , Raupach T , Gerritse B , et al. Cardiac resynchronization therapy and atrial overdrive pacing for the treatment of central sleep apnoea. Eur J Heart Fail. 2009;11(3):273–80. https://doi.org/10.1093/eurjhf/hfn042
133 Sinha AM , Skobel EC , Breithardt OA , Norra C , Markus KU , Breuer C , et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44(1):68–71. https://doi.org/10.1016/j.jacc.2004.03.040
134 Lamba J , Simpson CS , Redfearn DP , Michael KA , Fitzpatrick M , Baranchuk A . Cardiac resynchronization therapy for the treatment of sleep apnoea: a meta-analysis. Europace. 2011;13(8):1174–9. https://doi.org/10.1093/europace/eur128
135Mihaicuta S, Grote L. Sleep-related breathing disorders. In: Sleep Medicine Textbook. Ed. by C. Bassetti, Z. Dogas, P. Peigneux. 2014:233-250.
136 Marti-Soler H , Hirotsu C , Marques-Vidal P , Vollenweider P , Waeber G , Preisig M , et al. The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742–8. https://doi.org/10.1016/S2213-2600(16)30075-3
137 Boulos MI , Wan A , Im J , Elias S , Frankul F , Atalla M , et al. Identifying obstructive sleep apnea after stroke/TIA: evaluating four simple screening tools. Sleep Med. 2016;21(21):133–9. https://doi.org/10.1016/j.sleep.2015.12.013
138 Brill AK , Rösti R , Hefti JP , Bassetti C , Gugger M , Ott SR . Adaptive servo-ventilation as treatment of persistent central sleep apnea in post-acute ischemic stroke patients. Sleep Med. 2014;15(11):1309–13. https://doi.org/10.1016/j.sleep.2014.06.013
139 Albuquerque FN , Calvin AD , Sert Kuniyoshi FH , Konecny T , Lopez-Jimenez F , Pressman GS , et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141(4):967–73. https://doi.org/10.1378/chest.11-0975
140 Redeker NS , Muench U , Zucker MJ , Walsleben J , Gilbert M , Freudenberger R , et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33(4):551–60. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2849795&tool=pmcentrez&rendertype=abstract.
141 Katzan IL , Thompson NR , Uchino K , Foldvary-Schaefer N . A screening tool for obstructive sleep apnea in cerebrovascular patients. Sleep Med. 2016;21:70–6. https://doi.org/10.1016/j.sleep.2016.02.001
142 Ye L , Pien GW , Ratcliffe SJ , Björnsdottir E , Arnardottir ES , Pack AI , et al. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44(6):1600–7. https://doi.org/10.1183/09031936.00032314
143 Taranto Montemurro L , Floras JS , Millar PJ , Kasai T , Gabriel JM , Spaak J , et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest. 2012;142(5):1222–8. https://doi.org/10.1378/chest.11-2963
144 Boulos MI , Elias S , Wan A , Im J , Frankul F , Atalla M , et al. Unattended Hospital and Home Sleep Apnea Testing Following Cerebrovascular Events. J Stroke Cerebrovasc Dis. 2017;26(1):143–9. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.09.001
145 Ward NR , Roldao V , Cowie MR , Rosen SD , McDonagh TA , Simonds AK , et al. The effect of respiratory scoring on the diagnosis and classification of sleep disordered breathing in chronic heart failure. Sleep. 2013;36(9):1341–8. doi:.https://doi.org/10.5665/sleep.2960
146 Alekhin M , Anishchenko L , Zhuravlev A , Ivashov SI , Korostovtseva LS , Sviryaev YV , et al. Estimation of Information Value of Diagnostic Data Obtained by Bioradiolocation Pneumography in Non-contact Screening of Sleep Apnea Syndrome. Biomed Eng (NY). 2013;47(2):96–9. https://doi.org/10.1007/s10527-013-9343-8
147 Ryan CM , Wilton K , Bradley TD , Alshaer H . In-hospital diagnosis of sleep apnea in stroke patients using a portable acoustic device. Sleep Breath. 2016 Dec 2 [Epub ahead of print] https://doi.org/10.1007/s11325-016-1438-5
148 Gottlieb DJ , Craig SE , Lorenzi-Filho G , Heeley E , Redline S , McEvoy RD , et al. Sleep Apnea Cardiovascular Clinical Trials-Current Status and Steps Forward: The International Collaboration of Sleep Apnea Cardiovascular Trialists. Sleep. 2013;36(7):975–80. https://doi.org/10.5665/sleep.2790
Sebastian R. Ott and Lyudmila Korostovtseva contributed equally to the manuscript
No financial support and no other potential conflict of interest relevant to this article was reported.