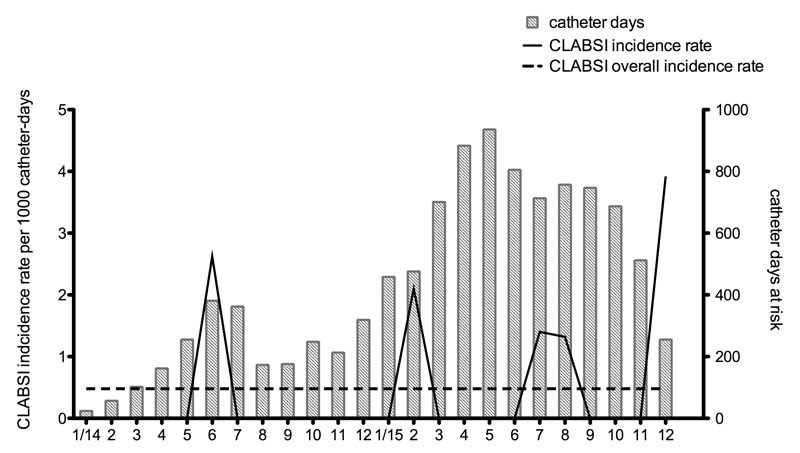
Figure 1 Incidence rate of central line-associated bloodstream infections (CLABSIs) per catheter-days per month.
DOI: https://doi.org/10.4414/smw.2017.14441
Peripherally inserted central catheters (PICCs) are intravenous access lines inserted from a peripheral vein of the arm (usually basilic or cephalic vein), whose tips terminate in the inferior third of the vena cava at the junction with the right atrium. Their main indications are for the (outpatient) administration of chemotherapy, antibiotics and parenteral nutrition, and they may be used for therapies lasting a few days up to 12 months [1–3]. Since their introduction in the late 1970s, the use of PICCs has steadily increased, especially in the oncology setting [4], owing to a less invasive insertion technique (i.e., not requiring implantation or tunnelling) and a low rate of periprocedural mechanical complications, as well as a safe and easy removal technique [5, 6].
In contrast to those benefits, the safety of PICCs has occasionally been questioned because of the significant rate of complications occurring during catheter use, such as catheter-associated infection and thrombosis.
The rate of infections varies according to patient characteristics, catheter-related factors and the different settings and standards of care [7–10]. This rate is reported as the number of bloodstream infections (according to a specific definition) per total number of catheter-days in place (commonly a denominator of 1000 catheter days is used as standard) [11, 12]. A complicating factor, however, is that definitions differ between studies. Mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) is a new category that takes into account the fact that cancer patients may suffer bloodstream infection from gut translocation while having a central catheter in place (as opposed to true central line-associated bloodstream infection, CLABSI). However, the role of MBI-LCBI in surveillance of PICC complications is unclear to date. For acute-care patients, both in and outside the intensive care unit (ICU) setting, the bloodstream infection rates are comparable to those associated with other central venous catheters [9, 10, 13, 14].
There is an increased risk of upper-extremity deep venous thrombosis associated with PICCs [15], for which reduced vein diameter with subsequent venous stasis and endothelial injury due to catheter displacement has been postulated as the main contributing factor. There is conflicting evidence for high-risk patients, and newer data indicate a downward trend in the number of thrombotic events [16–18]. A correlation between the number of lumens and PICC-associated bloodstream infection and thrombosis is also well established [8, 9, 19].
Other complications including phlebitis, occlusion and mechanical problems (catheter tear or dislodgment) are known to occur [6, 16] and, although not life-threatening, may require catheter removal. As described in prevention guidelines, targeted interventions including patient and staff education, and optimal PICC insertion practice and maintenance, as well as a multidisciplinary approach to implementation, currently seem the best strategy to achieve low rates of complications [3, 17, 20, 21].
Our goal was to introduce PICCs for medium- and long-term outpatient intravenous therapy in a tertiary care university hospital, with the support of a multidisciplinary team (including an interventional radiologist, oncologist, infection prevention expert, infectious disease physician and nurse practitioners experienced with venous access devices).
In our hospital, a previous attempt to introduce PICCs was aborted in 2012 after an unsystematic approach, seemingly accompanied by high rates of complications. We hypothesised that support from the infection prevention team in the form of structured surveillance for infectious and noninfectious complications could provide quality indicators to assure patient safety during the implementation phase of this vascular access new to our hospital.
We conducted a prospective surveillance study at our 950-bed academic hospital (Bern University Hospital, Bern, Switzerland), collecting data of all patients from the oncology and infectious diseases departments who underwent PICC insertion from 1 January 2014 on and had the catheter removed by 31 December 2015. Since our study was a quality assessment project, ethical approval was not required.
All patients requiring intravenous therapy for more than 7 days primarily in the outpatient setting, according to the decision of the treating oncologist or infectious diseases specialist, were eligible for inclusion. All patients were followed up during the PICC placement time irrespective of the management setting (outpatient, inpatient, intermittently hospitalised after insertion). The primary author (ELP) collected data by manual review of each patient’s electronic medical record, from the catheter booklet and by contacting both the hospital-based and home care nursing teams. The following patient data were collected: demographic characteristics; cancer type, organ and stage; nononcological diagnosis; body mass index (BMI) and Charlson comorbidity score [22]. Catheter information included date, site of insertion, number of lumen, clinical indication, infused drugs, total days in place and reason for removal. The date of catheter insertion was defined as day one. In our analysis, we included only major complications, which are defined and listed below. Educational sessions were offered to the hospital-based (infection prevention team and vascular access team) and community-based (home care nurse) nursing services under the lead of a nurse practitioner (M.F.).
Our primary goal was to measure the incidence rate of central catheter-associated infections expressed in events per 1000 catheter-days, defined according to the Centers for Disease Control and Prevention (CDC) / National Healthcare Safety Network (NHSN) definition [11, 12]. For the definition of central catheter-associated bloodstream infection, both laboratory-confirmed bloodstream infection (LCBI) and the recently added subcategory mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) were used. Exit-site infection was diagnosed according to the CDC/NHSN surveillance definitions for arterial or venous infection (VASC).
The occurrence of the following noninfectious complications was a secondary endpoint: catheter related-venous thrombosis, catheter tip migration, catheter occlusion and mechanical complications (catheter rupture, haemothorax, pneumothorax, cardiac tamponade or arrhythmia). For the diagnosis of venous thrombosis and tip migration, clinical symptoms had to be confirmed radiologically (ultrasound, venography or computed tomography). Catheter occlusion was defined as the inability to flush and/or withdraw blood. Other noninfectious complications were determined from the diagnoses made by the treating physician. Each complication that occurred during catheterisation was counted as a separate event.
The introduction of PICCs was planned in two phases: during phase one (January 2014 to June 2014) only cancer patients fulfilling the inclusion criteria were eligible for PICC insertion; during phase two (July 2014 to December 2015) the indication was extended to infectious diseases patients. A single lumen polyurethane 4F catheter (PRO-PICC® CT, medCOMP, Harleysville, PA, USA) was chosen to be the standard for all patients. Before catheter insertion, all patients gave their informed consent. All catheters were placed under maximum sterile barrier precautions by an interventional radiologist. The dedicated team consisted of three senior interventional radiologists, who gradually trained a team of junior physicians. For skin antisepsis, 2% chlorhexidine-alcohol solution was used. The selection of the insertion site and vein to cannulate was based on patient preference and vascular anatomy. Veins were cannulated under sonographic guidance; subsequent catheter insertion and tip localisation were evaluated under fluoroscopic guidance. The use of venography was restricted to selected cases. After insertion, the catheter was fixed using a suture-free device (STATLOCK® stabilisation device, BardMedical®, Louisville, CO, USA) and the exit site covered with a transparent dressing and sterile gauze (to obtain light compression in order to reduce the risk of initial bleeding at the insertion site). Within the next 24 hours, the preliminary dressing was replaced with a chlorhexidine gel pad dressing (TEGADERM CTH®, 3M, St. Paul, MN, USA) and thereafter changed every 7 days (or sooner if damp, loose or visibly soiled). Only trained personnel were allowed to manipulate the catheters. As a general rule, the catheter hub was always disinfected before handling. Catheter patency was tested before each use. The catheter was flushed by exerting intermittent pressure with a 0.9% saline solution before and after each use. Following use, the catheter was locked with a heparin solution (200 IU). Every patient received a PICC handbook to document any procedure done at home or in the hospital. The catheter care protocol and material used by the home nursing team differed from those used by the hospital nursing team. The main differences consisted of the following elements: suture-free device (GRIP-LOK®, TIDI products®, Neenah, WI, USA); use of a silver alginate pad instead of a chlorhexidine gel pad, catheter lock with citrate prefilled syringes (Dura Lock®, medComp®, Harleysville, PA, USA), and a different needleless device (NeutraClear®, ICUmedical®, San Clemente, CA, USA). Neither antibiotics nor antithrombotic prophylaxis was routinely administered.
Data were collected into an Excel electronic database (Microsoft Corporation, Seattle, WA). The study population was characterised by use of descriptive statistics. SPSS 21.0 (SPSS, Inc., an IBM Company, Chicago, IL) was used for all statistical analyses. Time to PICC removal was evaluated with a Kaplan–Meier curve to estimate the cumulative probability that the catheter would still be in place at any time since placement. Data were censored at the time of catheter removal.
Between 1 January 2014 and 31 December 2015, a total of 135 catheters where inserted into 124 patients. The median age of the patients was 60.5 years; the majority were female (n = 70, 56.5%). Oncological patients prevailed (n = 107, 86.3%); most of them were affected by solid tumours (n = 72, 67.3%). Further patient characteristics are listed in table 1. Overall, the indications for PICC insertion were chemotherapy (n = 97, 71.9%), antibiotic therapy (n = 24, 17.8%), total parenteral nutrition (n = 8, 5.9%), blood product transfusion (n = 4, 3.0%) and palliative care (n = 2, 1.5%).
Table 1 Characteristics of the 124 patients within the PICC pilot programme.
| Total, n (%) | 124 (100) |
| Female, n (%) | 70 (56.5) |
| Age, median (range) | 60.5 (15-85) |
| BMI, median (range) | 25.3 (16-52) |
| CCI, median (range) | 2 (0-11) |
| Underlying condition, n (%) | |
| Cancer | 107 (86.3) |
| Haematological malignancies | 34 (31.8) |
| Solid tumours | 73 (68.2) |
| Stage | |
| <III | 23 (31.5) |
| ≥III | 50 (68.5) |
| Noncancer | 17 (13.7) |
| Orthopaedic SSI | 9 (52.9) |
| Osteomyelitis | 2 (11.7) |
| Brain abscess | 2 (11.7) |
| Endocarditis | 1 (5.9) |
| Abdominal SSI | 1 (5.9) |
| Lower airways infection | 1 (5.9) |
| Total parenteral nutrition | 1 (5.9) |
| Primary indication for PICC, n (%) | |
| Chemotherapy | 97 (71.9) |
| Intravenous antibiotics | 24 (17.8) |
| Total parenteral nutrition | 8 (5.9) |
| Blood products transfusion | 4 (3.0) |
| Palliative care | 2 (1.5) |
BMI = body mass index; CCI = Charlson comorbidity score; PICC = peripherally inserted central catheter; SSI = surgical site infection
The 135 PICCs were in place for a total of 10 402 catheter-days, with a median dwell time of 62 days (range 2–450 days) and all were monitored until removal. Catheter dwell time was longer when the PICC indication was chemotherapy (median 80, range 2-450). Additional PICC characteristics are listed in table 2. We followed up patients in any healthcare setting, including ambulatory and in-hospital care. Of the 124 patients, 62 (50%) were hospitalised for at least 24 hours during the observation period. Fifty-five patients (44.4%) were managed in the outpatient setting only and seven were managed solely as inpatients (5.6%). None of the patients were hospitalised in an ICU.
Table 2 Catheter data for the 135 devices inserted during the peripherally inserted central catheter (PICC) pilot programme.
| PICC characteristic, n (%) | |
|---|---|
| Total | 135 (100) |
| Number of lumens | |
| 1 | 132 (97.8) |
| 2 | 2 (1.5) |
| 3 | 1 (0.7) |
| Arm | |
| Left | 101 (74.8) |
| Right | 34 (25.2) |
| Vein | |
| Basilic vein | 109 (80.7) |
| Brachial vein | 23 (17.0) |
| Cephalic vein | 1 (0.7) |
| Median vein | 1 (0.7) |
| Femoral vein | 1 (0.7) |
| Catheter-days, total | 10 402 |
| Catheter-days, median (range) | 62 (2–450) |
| Dwell time by indication (days), median (range) | |
| Chemotherapy | 80 (2–450) |
| Intravenous antibiotics | 31 (8–87) |
| Total parenteral nutrition | 71 (3–160) |
| Blood products transfusion | 29 (18–60) |
| Palliative care | 10 (8–11) |
| Reason for removal, n (%) | |
| End of treatment | 86 (63.7) |
| PICC-unrelated death | 18 (13.3) |
| Other | 9 (6.7) |
| PICC-associated infection | 7 (5.2) |
| PICC tip migration | 7 (5.2) |
| Suspected PICC-associated infection | 5 (3.7) |
| PICC-associated venous thrombosis | 2 (1.5) |
| PICC occlusion | 1 (0.7) |
Five CLABSIs occurred in five cancer patients, yielding an incidence rate of 0.48 per 1000 catheter-days. In figure 1, the overall incidence rates of CLABSI that occurred are displayed with total monthly catheter-days. One episode fulfilled the criteria for an MBI-LCBI (in a haemato-oncology patient with chemotherapy-induced neutropenia). Two CLABSIs where caused by coagulase-negative staphylococci and occurred 11 and 14 days after catheter insertion, respectively. Another two were caused by Enterobacter cloacae and occurred 114 and 119 days after insertion. The episode of MBI-LCBI was also caused by Enterobacter cloacae and occurred 29 days after catheter insertion. Two exit-site infections occurred, both identified by the presence of purulent exudate (no exit site swab was taken). All catheter-related infections resulted in PICC removal (n = 7; 5.2%). There were five (3.7%) additional cases of suspected catheter-related infection, for which the catheter was removed although the epidemiological definition was not fulfilled.

Figure 1 Incidence rate of central line-associated bloodstream infections (CLABSIs) per catheter-days per month.
Seven radiologically confirmed venous thromboses occurred in seven patients (six of them were cancer patients: four lymphomas, one testicular cancer and one ovarian cancer), accounting for a rate of 0.68 per 1000 catheter-days. All thromboses affected the upper extremity where the catheter was inserted, four in the deep venous system and three in the superficial venous system, and in two cases led to catheter removal because of an associated catheter dysfunction. Ten episodes of catheter tip migration occurred in ten patients, and resulted in PICC removal in seven cases.
The most frequent complication was catheter flow dysfunction (not related to the above-cited complications) and occurred in 22 separate episodes. In seven patients, it was due to device occlusion, which yields an incidence rate of 0.68 per 1000 catheter-days. In 18 of 22 cases the flow could be successfully re-established by use of an alteplase lock. In one case, the catheter had to be removed owing to irreversible occlusion, and in the three remaining cases the flow dysfunction spontaneously resolved without further intervention. Overall, at the end of the surveillance period, 46 complications had occurred, with a rate of 4.5 per 1000 catheter-days. Seventeen catheters (12.6%) had to be removed because of a complication. The catheter survival data according to the time of onset of complication for the four major complications that required catheter removal (infection, thrombosis, tip migration and occlusion) are shown in figure 2. Only one periprocedural complication occurred: significant bleeding at the insertion site in a patient with thrombopenia and concurrent medication with the antiplatelet agent clopidogrel.
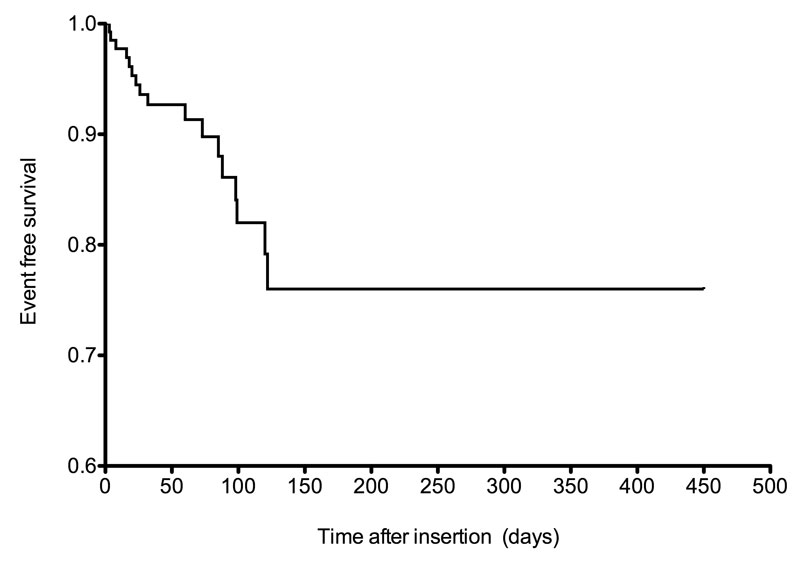
Figure 2 Kaplan-Meier curve of catheter survival based on the occurrence of complications that led to catheter removal.
PICCs are an established and safe alternative to central venous catheters inserted into the jugular or subclavian vein for medium- and long-term intravenous therapy [16, 23]; our surveillance data provide additional support in favour of their use. To assure maximum quality of care and patient safety, it is essential to collect and utilise quality indicators in order to assess complications [24]. The overall complication rate we encountered in this study was low, taking into account the heterogeneous patient population with a high proportion of oncological patients. Cancer patients, especially those with haematological malignancies, are notable for being at increased risk for catheter-related complications [6, 13, 15, 25]. Our CLABSI rate was comparable to the rate in a recent study that included a similar patient population and care setting, with a reported CLABSI rate of 0.95 per 1000 catheter days [26]. However, another recent study showed a low bloodstream infection rate when PICCs were used in the outpatient setting in a cancer patient population, with a catheter-related bloodstream infection (CRBSI) rate as low as 0.05 per 1000 catheter-days [16]. This low infection rate should be cautiously compared to ours since a different definition (CRBSI), which included additional microbiological information (quantitative culture and time to positivity), was used. In fact, it is well known that the CLABSI definition tends to overestimate the real number of catheter-related infections [27], and although CRBSI is more clinically accurate, CLABSI is more suitable for surveillance purposes [28]. A meta-analysis by Chopra et al. comparing PICC to other central venous access devices supported the fact that the former was associated with a lower risk of CLABSI in ambulatory settings. In our cohort, five catheters were removed for suspected infection, the decision for which was taken by the treating physician. The appropriate indication for catheter removal should be based on the clinical condition (e.g., sepsis, thrombophlebitis) and microbiology results (e.g., Staphylococcus aureus, Gram-negative bacilli, fungi) as highlighted in the 2009 guideline of the Infectious Disease Society of America [29].
The rate of thrombosis we encountered was higher than previously reported for central venous catheters in cancer patients, as shown by Lee et al. (0.3 per 1000 catheter days). Nevertheless, wide differences in similar patient populations, ranging from 0.05 to 0.95 per 1000 catheter days, were demonstrated in two recent studies [16, 26]. A possible explanation for our findings was the inclusion of patients with lymphoma and ovarian cancer, who are known to be at increased risk for thrombosis [30, 31] and who represented five out of the six cancer patients with a catheter-related thrombosis. Another potential risk factor could have been hospitalisation during follow-up [32]
The multidisciplinary nature of the study team, a characteristic shown to have an effect on complication rates in other centres as reported by Curto-Garcia et al. [17], ensured the needed broad support and sustained acceptance in our hospital, and, in our opinion, has to be considered as the central element in all institutions wishing to introduce new vascular devices and to include hospital-based and community-based healthcare personnel. In their surveillance study, the authors saw PICC-related complications decrease in patients with haematological malignancies receiving intensive chemotherapy after a multidisciplinary team had been charged with establishing evidence-based guidelines and education programmes. The team consisted of two haematologists, one medical microbiologist, three haematology nurses, one ICU nurse and one emergency department nurse.
The establishment of evidence-based guidelines for the introduction of the new vascular access highlights the importance of implementation science in promoting the integration of evidence-based findings into daily clinical practice. In fact, evidence-based data summaries for central venous catheter insertion and maintenance care have been confirmed to be an efficacious measure in preventing CLABSI, as recently documented in a meta-analysis by Ista et al. [33]. The efficacy of such interventions has primarily been demonstrated in the ICU setting [34], whereas there is currently weak evidence regarding catheters outside the ICU [35]. In our study, the infection prevention team provided an objective evaluation of how PICCs perform by monitoring essential quality indicators during the entire process, which were reviewed by the interdisciplinary team on a regular basis. Infectious complications represented only 15% of the adverse events seen in our population, indicating that a substantial effort should be directed at the prevention of noninfectious complications during the implementation of PICCs. More evidence for the prevention of the latter complication group needs to be accrued to establish guidelines supporting clinical decision making.
We noticed that the two CLABSI associated with Gram-positive bacteria occurred rather early after insertion, in contrast to a later presentation of Gram-negative bacteria-associated CLABSI. The single MBI-LCBSI also occurred relatively early (in the first month); a similar finding was previously reported by a study in a paediatric population [36]. In contrast, a retrospective study in adults showed that coagulase-negative staphylococci and Enterobacteriaceae were typical pathogen groups associated with CLABSI throughout catheter dwell time [37]. The event numbers in our cohort were too small to further elucidate this aspect.
Study limitations included the presence of a heterogeneous population in different management settings and various protocols of care that limited more specific analysis; however, these questions were out of the scope of our study. Interpretation of the reported infection rate should take into account that additional preventive measures were used throughout the implementation phase (such as chlorhexidine dressing and silver alginate). Despite the fact that chlorhexidine dressing and silver alginate are second-line measures to prevent CLABSI [24], we decided to introduce chlorhexidine gel pads as a tool to reduce the risk of infectious adverse events [38] that could have led to an early project failure. The single-centre design cannot guarantee the generalisability of the processes of care to other settings. We could also have missed certain complication events if a patient was hospitalised in another region of Switzerland (despite the regular checks of the patient’s catheter booklet). Since the vast majority (97.8%) of the PICCs were single lumen catheters we cannot extrapolate these encouraging results to multilumen PICCs.
The strength of our study includes the prospective multidisciplinary design and the use of clear epidemiological definitions for catheter-related infections and clear clinical definitions for noninfectious complications. Our findings could help facilities willing to introduce PICCs to decide in favour of early involvement of the infection prevention team during the implementation of a new device. The strategy presented here could also easily be extended to other intravascular devices. The efficacy of this approach has recently been highlighted in the study of Kim-Saechao et al. [39], where the implementation of a mandatory electronic communication tool overseen by multidisciplinary leadership, which included the infection control team, resulted in a decreased rate of PICC-related complications.
We introduced PICCs in an academic hospital by implementing a surveillance programme for complications. Both infectious and noninfectious complications were rare. Infection prevention specialists should be actively involved during the introduction of new intravascular devices in order to provide quality indicators and assure patient safety
We thank all nurses and doctors who participated in the programme.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 O’Grady NP , Alexander M , Burns LA , Dellinger EP , Garland J , Heard SO , et al.; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4, Suppl 1):S1–34. https://doi.org/10.1016/j.ajic.2011.01.003
2 Gallieni M , Pittiruti M , Biffi R . Vascular access in oncology patients. CA Cancer J Clin. 2008;58(6):323–46. https://doi.org/10.3322/CA.2008.0015
3 Chopra V , Flanders SA , Saint S , Woller SC , O’Grady NP , Safdar N , et al.; Michigan Appropriateness Guide for Intravenouse Catheters (MAGIC) Panel. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann Intern Med. 2015;163(6, Suppl):S1–40. https://doi.org/10.7326/M15-0744
4 Johansson E , Hammarskjöld F , Lundberg D , Arnlind MH . Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Oncol. 2013;52(5):886–92. https://doi.org/10.3109/0284186X.2013.773072
5 Walker G , Todd A . Nurse-led PICC insertion: is it cost effective? Br J Nurs. 2013;22(19):S9–15. https://doi.org/10.12968/bjon.2013.22.Sup19.S9
6 Walshe LJ , Malak SF , Eagan J , Sepkowitz KA . Complication rates among cancer patients with peripherally inserted central catheters. J Clin Oncol. 2002;20(15):3276–81. https://doi.org/10.1200/JCO.2002.11.135
7 Rhee Y , Heung M , Chen B , Chenoweth CE . Central line-associated bloodstream infections in non-ICU inpatient wards: a 2-year analysis. Infect Control Hosp Epidemiol. 2015;36(4):424–30. https://doi.org/10.1017/ice.2014.86
8 Chopra V , Ratz D , Kuhn L , Lopus T , Chenoweth C , Krein S . PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319–28. https://doi.org/10.1016/j.amjmed.2014.01.001
9 Pongruangporn M , Ajenjo MC , Russo AJ , McMullen KM , Robinson C , Williams RC , et al. Patient- and device-specific risk factors for peripherally inserted central venous catheter-related bloodstream infections. Infect Control Hosp Epidemiol. 2013;34(2):184–9. https://doi.org/10.1086/669083
10 Ajenjo MC , Morley JC , Russo AJ , McMullen KM , Robinson C , Williams RC , et al. Peripherally inserted central venous catheter-associated bloodstream infections in hospitalized adult patients. Infect Control Hosp Epidemiol. 2011;32(2):125–30. https://doi.org/10.1086/657942
11Centers for Disease Control (CDC)/Healthcare Infection Control Practices Advisory Committee (HICPAC), Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-central line-associated Bloodstream Infection). 2015; Available at: http://www.cdc.gov/nhsn/. Accessed 3 December 2015.
12Centers for Disease Control (CDC)/Healthcare Infection Control Practices Advisory Committee (HICPAC). CDC/NHSN Surveillance Definitions for Specific Types of Infections [Internet]. [cited 2015 Mar 26]. Available from: http://www.cdc.gov/nhsn/
13 Chopra V , O’Horo JC , Rogers MA , Maki DG , Safdar N . The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2013;34(9):908–18. https://doi.org/10.1086/671737
14 Safdar N , Maki DG . Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128(2):489–95. https://doi.org/10.1378/chest.128.2.489
15 Chopra V , Anand S , Hickner A , Buist M , Rogers MA , Saint S , et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311–25. https://doi.org/10.1016/S0140-6736(13)60592-9
16 Cotogni P , Barbero C , Garrino C , Degiorgis C , Mussa B , De Francesco A , et al. Peripherally inserted central catheters in non-hospitalized cancer patients: 5-year results of a prospective study. Support Care Cancer. 2015;23(2):403–9.
17 Curto-García N , García-Suárez J , Callejas Chavarria M , Gil Fernández JJ , Martín Guerrero Y , Magro Mazo E , et al. A team-based multidisciplinary approach to managing peripherally inserted central catheter complications in high-risk haematological patients: a prospective study. Support Care Cancer. 2016;24(1):93–101. https://doi.org/10.1007/s00520-015-2754-1
18 Nolan ME , Yadav H , Cawcutt KA , Cartin-Ceba R . Complication rates among peripherally inserted central venous catheters and centrally inserted central catheters in the medical intensive care unit. J Crit Care. 2016;31(1):238–42. https://doi.org/10.1016/j.jcrc.2015.09.024
19 O’Brien J , Paquet F , Lindsay R , Valenti D . Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864–8. https://doi.org/10.1016/j.jacr.2013.06.003
20 Yap Y-S , Karapetis C , Lerose S , Iyer S , Koczwara B . Reducing the risk of peripherally inserted central catheter line complications in the oncology setting. Eur J Cancer Care (Engl). 2006;15(4):342–7. https://doi.org/10.1111/j.1365-2354.2006.00664.x
21 Alexandrou E , Spencer TR , Frost SA , Mifflin N , Davidson PM , Hillman KM . Central venous catheter placement by advanced practice nurses demonstrates low procedural complication and infection rates--a report from 13 years of service*. Crit Care Med. 2014;42(3):536–43. https://doi.org/10.1097/CCM.0b013e3182a667f0
22 Charlson M , Szatrowski TP , Peterson J , Gold J . Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51. https://doi.org/10.1016/0895-4356(94)90129-5
23 Shrestha NK , Shrestha J , Everett A , Carroll D , Gordon SM , Butler RS , et al. Vascular access complications during outpatient parenteral antimicrobial therapy at home: a retrospective cohort study. J Antimicrob Chemother. 2016;71(2):506–12. https://doi.org/10.1093/jac/dkv344
24 Marschall J , Mermel LA , Fakih M , Hadaway L , Kallen A , O’Grady NP , et al.; Society for Healthcare Epidemiology of America. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(7):753–71. https://doi.org/10.1086/676533
25 Cheong K , Perry D , Karapetis C , Koczwara B . High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours. Intern Med J. 2004;34(5):234–8. https://doi.org/10.1111/j.1444-0903.2004.00447.x
26 Bertoglio S , Faccini B , Lalli L , Cafiero F , Bruzzi P . Peripherally inserted central catheters (PICCs) in cancer patients under chemotherapy: A prospective study on the incidence of complications and overall failures. J Surg Oncol. 2016;113(6):708–14. https://doi.org/10.1002/jso.24220
27 Freeman JT , Elinder-Camburn A , McClymont C , Anderson DJ , Bilkey M , Williamson DA , et al. Central line-associated bloodstream infections in adult hematology patients with febrile neutropenia: an evaluation of surveillance definitions using differential time to blood culture positivity. Infect Control Hosp Epidemiol. 2013;34(1):89–92. https://doi.org/10.1086/668431
28 Chaftari A-M , Jordan M , Hachem R , Al Hamal Z , Jiang Y , Yousif A , et al. A clinical practical approach to the surveillance definition of central line-associated bloodstream infection in cancer patients with mucosal barrier injury. Am J Infect Control. 2016;44(8):931–4. https://doi.org/10.1016/j.ajic.2016.03.011
29 Mermel LA , Allon M , Bouza E , Craven DE , Flynn P , O’Grady NP , et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(1):1–45. Corrected in Clin Infect Dis. 2010;50(7):1079 (Dosage error in article text)https://doi.org/10.1086/599376
30 Lee AYY , Levine MN , Butler G , Webb C , Costantini L , Gu C , et al. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol. 2006;24(9):1404–8. https://doi.org/10.1200/JCO.2005.03.5600
31 Zhang X , Huang J-J , Xia Y , Li C-F , Wang Y , Liu P-P , et al. High risk of deep vein thrombosis associated with peripherally inserted central catheters in lymphoma. Oncotarget. 2016;7(23):35404–11.
32 Ahn DH , Illum HB , Wang DH , Sharma A , Dowell JE . Upper extremity venous thrombosis in patients with cancer with peripherally inserted central venous catheters: a retrospective analysis of risk factors. J Oncol Pract. 2013;9(1):e8–12. https://doi.org/10.1200/JOP.2012.000595
33 Ista E , van der Hoven B , Kornelisse RF , van der Starre C , Vos MC , Boersma E , et al. Effectiveness of insertion and maintenance bundles to prevent central-line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(6):724–34. https://doi.org/10.1016/S1473-3099(15)00409-0
34 Pronovost P , Needham D , Berenholtz S , Sinopoli D , Chu H , Cosgrove S , et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–32. https://doi.org/10.1056/NEJMoa061115
35 O’Neil C , Ball K , Wood H , McMullen K , Kremer P , Jafarzadeh SR , et al. A Central Line Care Maintenance Bundle for the Prevention of Central Line-Associated Bloodstream Infection in Non-Intensive Care Unit Settings. Infect Control Hosp Epidemiol. 2016;37(6):692–8. https://doi.org/10.1017/ice.2016.32
36 Milstone AM , Reich NG , Advani S , Yuan G , Bryant K , Coffin SE , et al. Catheter dwell time and CLABSIs in neonates with PICCs: a multicenter cohort study. Pediatrics. 2013;132(6):e1609–15. https://doi.org/10.1542/peds.2013-1645
37 Chittick P , Azhar S , Movva K , Keller P , Boura JA , Band J . Early onset versus late onset peripherally inserted central venous catheter infections: an analysis of risk factors and microbiology. Infect Control Hosp Epidemiol. 2013;34(9):980–3. https://doi.org/10.1086/671726
38 Safdar N , O’Horo JC , Ghufran A , Bearden A , Didier ME , Chateau D , et al. Chlorhexidine-impregnated dressing for prevention of catheter-related bloodstream infection: a meta-analysis*. Crit Care Med. 2014;42(7):1703–13. https://doi.org/10.1097/CCM.0000000000000319
39Kim-Saechao SJ, Almario E, Rubin ZA. A novel infection prevention approach: Leveraging a mandatory electronic communication tool to decrease peripherally inserted central catheter infections, complications, and cost. Am J Infect Control [Internet]. 2016 May [cited 2016 May 22]; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0196655316002819
No financial support and no other potential conflict of interest relevant to this article was reported.