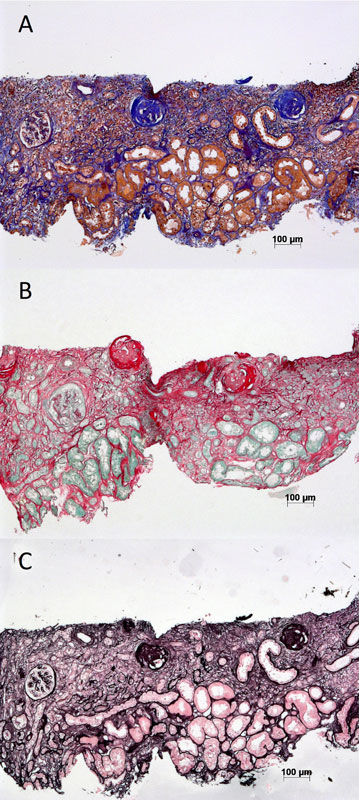
Figure 1 Examples of commonly used stains for histological assessment of renal interstitial fibrosis. (A) Trichrome. (B) Sirius red. (C) Silver methanamine.
DOI: https://doi.org/10.4414/smw.2017.14442
apparent diffusion coefficient
bone morphogenetic protein-7
chemokine (C-C motif) ligand 2
chronic kidney disease
estimated glomerular filtration rate
monocyte chemoattractant protein-1
malondialdehyde
microribonucleic acid
matrix metalloproteinase
magnetic resonance imaging
procollagen type III amino-terminal propeptide
retinol binding protein
transforming growth factor beta
tissue inhibitor of metalloproteinase
tumour necrosis factor-alpha
vitamin D binding protein
Chronic kidney disease (CKD) is defined as an alteration of kidney function and/or structure lasting for more than 3 months. It is a major public health issue since it affects 11% of the US and Swiss populations [1, 2]. CKD is also recognised as a condition that increases cardiovascular disease risk and is associated with a significant increase in mortality compared with the general population [3]. Fibrosis is the hallmark of CKD and the severity of CKD usually correlates with the magnitude of kidney cortical fibrosis.
Kidney fibrosis is characterised by increased synthesis and deposition of extracellular matrix components within the tubulointerstitial space (interstitial fibrosis) and glomeruli (glomerulosclerosis). Tubular atrophy often goes with interstitial fibrosis. Cortical interstitial fibrosis is better correlated with renal function loss than is glomerulosclerosis, and is a process common to a wide variety of kidney injuries [4]. The emergence of renal fibrosis is the consequence of maladaptive wound-healing processes after an injury [5]. Interstitial fibrosis remains the main factor contributing to kidney structural deterioration and loss of function. Therefore it is a crucial target for therapies since a wide variety of diseases converge into this single process [6]. The pathogenesis of fibrosis is very complex, and as yet not completely understood. It will not be detailed here but has been reviewed in depth elsewhere [7].
Evaluation of interstitial fibrosis is crucial to the assessment of prognosis and to guide therapy for most kidney diseases. However, how best to measure kidney fibrosis remains uncertain. Therefore, new approaches that allow a more accurate and reproducible assessment of renal interstitial fibrosis are required. Here, we review recent advances in the diagnosis and follow-up of renal fibrosis.
The histopathological findings associated with CKD are glomerulosclerosis, tubulointerstitial fibrosis and inflammation with loss of renal parenchyma characterised by tubular atrophy, capillary loss and podocyte depletion.
Renal interstitial fibrosis correlates with and predicts renal function loss in all experimental models of CKD [4]. In addition, the presence of interstitial inflammation in combination with kidney fibrosis is associated to an increased risk of kidney function decline in the long term [8–10]. Several studies have demonstrated that quantification of interstitial fibrosis and inflammation gives a more accurate prognosis of renal outcome in renal allografts [11] and native kidney diseases [12–14], including in lupus and immunoglobulin (Ig) A nephropathies.
Currently, several research protocols are aiming at reverting fibrosis, in both animals and human studies [15–20]. Although spontaneous regression of fibrosis has been documented in some renal pathologies [21], worsening of renal fibrosis is usually observed together with deterioration of kidney function [13]. The follow-up of fibrosis is therefore important for evaluation of the spontaneous evolution of CKD, and for the assessment of response to specific therapies, including emerging drugs [6, 18, 22].
Kidney fibrosis and inflammation are currently being evaluated by means of needle core biopsy followed by histopathological assessment. Percutaneous kidney biopsies are usually performed with ultrasonic guidance under local anaesthesia. After a renal biopsy, patients are observed on bed rest for a few hours to watch for possible complications (see below).
Kidney biopsy has several limitations. This procedure has become safer over recent years; however, complications still occur, including minor and major bleedings, pain, arteriovenous fistulas and perirenal soft tissue infections. These may lead to prolonged hospitalisation, loss of renal function and even death [23, 24]. Furthermore, the financial cost of the procedure is significant. In addition, the amount of tissue obtained from a needle core biopsy on some occasions may not be sufficient to assess the severity of a disease. Histological changes are usually scored semiquantitatively, with an element of subjectivity. Objective quantitative parameters such as Sirius red staining coupled with morphometric analysis are rarely used routinely [6]. The time needed for tissue preparation before examination may also be a limitation in some urgent circumstances, and serial kidney biopsies are difficult. Finally, one of the main intrinsic limitations to the assessment of fibrosis via biopsy is the sampling bias related to the technique.
Routine histological evaluation of a renal biopsy involves examination of the tissue under light, immunofluorescence, and electron microscopy. Conventional histological stains such as haematoxylin and eosin, periodic acid Schiff, silver methenamine and trichrome are routinely used (fig. 1). Fibrosis quantification by visual assessment of trichrome-stained slides is the standard practice in most pathology departments [25]. There are some limitations to this technique, since it has been reported to be only moderately reproducible [26]. Part of the poor reproducibility arises from subjective scores assigned by pathologists, whereas Masson trichrome may not be sensitive for mild fibrosis [26, 27]. Sirius red, viewed in both polarised and unpolarised light, may be used for fibrosis assessment [28].

Figure 1 Examples of commonly used stains for histological assessment of renal interstitial fibrosis. (A) Trichrome. (B) Sirius red. (C) Silver methanamine.
Quantification by use of morphometric techniques of staining such as trichrome, polarised and unpolarised Sirius red and immunohistochemistry (collagen III) has been developed with various results [29–32]. Correlation between specific stains and estimated glomerular filtration rate (eGFR) remains variable. More complex and objective techniques are being tested, with for example the use of a combination of different colourations [33]. Currently, the closest correlation to eGFR, best efficiency and best reproducibility are still with trichrome-stained and collagen III morphometry as shown by Farris et al., whereas unpolarised Sirius red also appears valuable [33]. A combination of whole section imaging and automated image analysis with Sirius red polarisation seems to be complementary to Masson trichrome [34].
Altogether, there is a lack of standardised assessment of interstitial fibrosis by pathologists, and morphometric methods are scarcely used routinely. Current assessment relies mainly on visual assessment of trichrome staining, a method that, although validated, is still subject to the interpretation of the pathologist involved. Given these limitations, although biopsy is currently the gold standard for the diagnosis of kidney fibrosis, new noninvasive techniques might help in the evaluation and follow-up of CKD patients.
There is no recognised radiological method to assess kidney interstitial fibrosis, unlike other organs such as the liver [35, 36].
Ultrasound has shown some promising results in providing a noninvasive means of measuring renal cortical stiffness resulting from pathological damage. Shear wave velocity imaging [37–42], transient elastography [43–46], real-time elastography [47], Doppler sonography [48, 49] and ultrasound corticomedullary strain [47, 48], among others, appear partially relevant in measuring renal cortical fibrosis. However, results are heterogeneous, with positive and negative studies. Furthermore, these methods are strongly dependent on external factors, such as blood pressure, kidney weight, body weight and the applied transducer force, not to mention also high intra- and interobserver variability.
The use of ultrasound in the assessment of renal interstitial fibrosis appears promising. However, its main limitation is the heterogeneity of renal parenchyma for elastography evaluation as well as the depth of native kidneys. This technique is therefore interesting but currently not as reliable as for the liver.
Magnetic resonance imaging (MRI), with use of various sequences including T1 mapping, blood oxygenation level dependent and diffusion-weighted imaging, is emerging as a promising non-invasive tool to assess kidney interstitial fibrosis.
T1 mapping creates a parametric map where each pixel represents the T1 spin-lattice relaxation time of the probed tissue. T1 relaxation time varies depending on the tissue physiopathology status, such as inflammation, oedema, fat or fibrosis. The vast majority of native T1 mapping applications are related to cardiac fibrosis, with increased T1 values recorded in diffuse myocardial fibrosis [50]. In the kidney, small animal studies have found increased T1 values in mice models of acute kidney injury [51] and of renal transplantation [52]. Similar associations were measured in human kidney: cortical T1 was negatively correlated with renal function in native and transplanted kidneys, suggesting that this sequence could be used to assess kidney interstitial fibrosis [53, 54]. Nevertheless, a strong correlation with fibrosis has not been confirmed by other studies, and T1 may rather be a marker of inflammation or vascularisation changes [55].
Renal blood oxygenation level dependent (BOLD) MRI (T2* sequence) allows probing renal tissue oxygenation in different pathophysiological conditions [56]. In mice, changes of renal T2* were associated with the severity of acute kidney injury and interstitial fibrosis [57]. In human, the effective transverse relaxation time T2* values of BOLD-MRI correlated with renal function, but not with histology in diabetic nephropathy, suggesting that other factors than kidney fibrosis, such as impairment of oxygenation, could contribute to image modifications in this MRI sequence [58].
Diffusion-weighted imaging (DWI) is another sequence investigated for quantification of interstitial fibrosis. DWI is sensitive to Brownian motion of water molecules within a given tissue. Currently DWI is quantified as the apparent diffusion coefficient (ADC). The emerging importance of ADC values for monitoring renal fibrosis was first validated in a small animal model of unilateral ureteral obstruction [59]. In CKD patients, a significant decrease of ADC was related to increased interstitial fibrosis obtained histopathologically in different studies [58, 60, 61]. Zhao et al. demonstrated that cortical and medullary ADC values correlated with interstitial fibrosis, as assessed with histology, in native kidney disease [61]. The major limitations of renal diffusion MRI in clinical practice are the absence of a standardised protocol, artefact linked to image acquisition [62] and large interindividual variation of ADC values due to intrinsic factors, such hydration status, and extrinsic factors such as magnetic field strength, MR sequence type, MRI manufacturer.
Currently, sequence optimisation is performed to overcome these limitations and decrease diffusion MRI variability. For example, we recently optimised diffusion MRI sequences for the kidney in healthy volunteers. We could demonstrate that a novel sequence (called RESOLVE) largely improved kidney image resolution in healthy volunteers [63]. This enhanced image resolution allowed better differentiation of the cortex from the medulla, and correction of absolute cortical ADC values to medullary values by deriving the difference between cortical and medullary ADC (delta ADC). This correction based on the delta ADC significantly decreased interindividual variability and improved discrimination for cortical fibrosis in patients [64]. Delta ADC correlated well with fibrosis in both experimental models of fibrosis in animals and kidney allograft recipients undergoing biopsies (R2 = 0.64, p <0.001) [64]. Extensive (>40%) fibrosis could be identified by negative delta ADC values (ADC values higher in the medulla than the cortex) (fig. 2). Although more work is needed to validate this index before application in the clinical setting, this study presents encouraging results for diffusion MRI as a novel tool for kidney fibrosis evaluation.
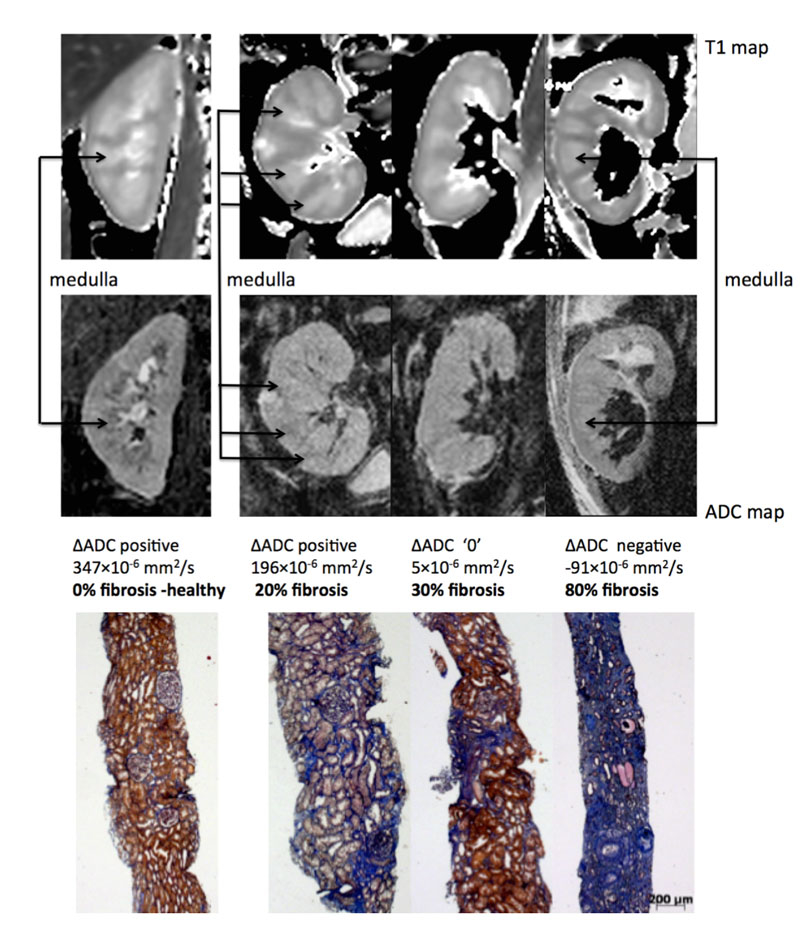
Figure 2 Representative biopsy and magnetic resonance images from patients. Morphological MOLLI T1 map used for the positioning of the regions of interest (top row) and apparent diffusion coefficient (ADC) maps (lower row) for 3 patients showing the different ΔADC cases – positive, zero and negative –with the corresponding fibrosis levels from histology (Masson trichrome staining) [59].
Altogether, MRI is a promising tool to evaluate kidney fibrosis. However, the methods remain to be confirmed by larger scale studies [65], because of the absence of consensus regarding MRI protocols, absence of reference values and the presence of artefacts, including respiratory motion and image distortion [62]. MRI, in particular with diffusion sequences, is one of the most promising tools to evaluate kidney fibrosis, but its use in the clinical setting still requires adaptation.
Several plasma or urinary biomarkers are currently being evaluated as indicators of fibrosis. The following list is not exhaustive, but includes some of the most promising markers to date. We have chosen to classify them according to their biological function.
Renal fibrosis is characterised by the accumulation of an excessive amount of extracellular matrix. Type III collagen is part of this extracellular matrix. It is synthesised as procollagen and the amino-terminal propeptide is cleaved during deposition. This procollagen type III amino-terminal propeptide (PIIINP) is then released from the extracellular matrix into the blood and urine. The urinary PIIINP/creatinine ratio was shown to be closely correlated with the extent of interstitial fibrosis in biopsies from CKD [66] and kidney allograft patients [67]. Circulating procollagen III correlated with interstitial fibrosis in 40 patients with subacute and chronic nephropathies [68]. Theses markers may be useful indicators of the extent of renal fibrosis. In the study by Ix et al., urine PIIINP was also associated with CKD progression and incident end-stage renal disease. These results suggest that higher urine and/or plasma PIIINP levels are associated (independently of eGFR) with interstitial fibrosis, CKD progression, risk of end-stage renal disease and death [69].
Periostin is an extracellular protein highly expressed during development and is overexpressed in various experimental types of CKD, where it appears to play an important profibrotic role [70]. In murine models, inhibition of periostin expression protected against the development of renal inflammation and fibrosis [71]. Tissue levels of periostin may correlate with kidney disease severity and might be used as an early marker of injury [72]. More recently, urinary periostin levels were shown to be elevated in chronic allograft nephropathy [73]. This protein may therefore emerge as a good predictor of fibrosis.
Transforming growth factor beta (TGF-beta) is a profibrotic cytokine central to the pathogenesis of kidney fibrosis [3, 74]. BMP-7 (bone morphogenetic protein-7) is a natural antagonist to TGF-beta1 with antifibrotic and anti-inflammatory properties. BMP-7 is being evaluated in mice for treatment of kidney fibrosis [75, 76]. None of these cytokines has been clearly demonstrated to correlate with the extent of kidney fibrosis. However, urinary TGF-beta1 increased in diabetic nephropathy and in membranous glomerulonephritis and was related to disease severity [77, 78]. Serum TGF-beta1 also increases in African-American subjects with end-stage kidney disease [79]. Finally, Wong and al. showed recently that the levels of circulating active TGF-beta1 or BMP-7 and their ratio were good predictors of renal function evolution in diabetic patients [80]. Adding these two biomarkers to the conventional predictors (urinary albumin-to-creatinine ratio, eGFR and clinical factors) provided a higher predictive value for predefined renal endpoints. Altogether, although more studies are needed and histological correlations are lacking, these two factors may be useful in assessing the risk of renal disease progression.
There is a link between macrophage infiltration and excessive extracellular matrix protein deposition. Chemokine (C-C motif) ligand 2 (CCL2), also called monocyte chemoattractant protein-1 (MCP1), is a receptor chemokine with chemoattractant properties for monocytes/macrophages and other inflammatory cells [81]. The majority of clinical studies have investigated its potential role as biomarker in renal allografts. Urinary CCL2 was tested in a multicentre cohort at 6 months after transplantation and was an independent predictor for the development of interstitial fibrosis at 24 months [82–84].
Renal cells produce matrix metalloproteinases (MMPs) which are secreted zinc-dependent endopeptidases that play a role in extracellular matrix remodelling. MMPs are important in the initiation of fibrogenesis in various organs, including the kidney. Moreover, MMPs are regulated by endogenous inhibitors known as tissue inhibitors of metalloproteinase (TIMPs), and dysregulation of the MMP/TIMP system is an additional contributor to the formation of interstitial fibrosis [85]. Observation shows that MMP-7 levels were elevated in serum and biopsies from patients with interstitial fibrosis [86]. Another study showed that proMMP-9 intragraft expression correlated with fibrotic change, whereas circulating proMMP2 and MMP9 did not differ between allograft patients with and without interstitial fibrosis [87]. MMP-2 expression decreased while TIMP-1 and serum creatinine increased with the rise of interstitial fibrosis grade in kidney transplant recipients [88]. However, in 40 allograft patients, urinary/serum MMPs/TIMPs did not clearly correlate with interstitial fibrosis [89]. Finally, in a recent study of 102 CKD patients, urinary MMP-7 levels were elevated as compared with control subjects and did correlate with kidney fibrosis [90].
Plasma levels of tumour necrosis factor alpha (TNF-alpha) increase during CKD. This biomarker is a proinflammatory mediator, which binds to soluble TNF receptors. The TNF receptor is a biomarker of inflammation in kidney disease. A high level of TNF receptor correlated with renal function decline in patients with type 1 and type 2 diabetes mellitus [91, 92]. In CKD, higher levels of TNF receptor were independently associated with faster rates of kidney function decline [93]. More recently, Sodona et al. have shown that, in IgA nephropathy, patients in the highest tertile of serum TNF receptor levels displayed more severe renal interstitial fibrosis than did those in the lowest or second tertiles [94].
Vitamin D binding protein (VDBP) transports vitamin D metabolites through the circulation. VDBP is filtered by the glomerulus and subsequently taken up by proximal tubular cells through receptor-mediated endocytosis. Since receptor-mediated uptake of proteins is energy-consuming, tubular injury may result in urinary VDBP (uVDBP) loss [95]. Mirkovic et al. investigated the value of uVDBP as a marker of tubulointerstitial inflammation and fibrosis in adriamycin rats, and of renal damage in humans. In rats, uVDBP was associated, independently of albuminuria, with macrophage accumulation and collagen III expression in the interstitium. In humans, uVDBP increased in albuminuric compared to normoalbuminuric subjects. Moreover, uVDBP was associated with tubular and inflammatory damage markers (kidney injury molecule-1, beta-2-microglobulin, cystatin C, MCP-1 and neutrophil gelatinase-associated lipocalin) independently of albuminuria [96].
Urinary excretion of retinol binding protein (RBP) is a sensitive marker of allograft fibrosis and can predict long-term graft loss independent of histology and urinary albumin. Indeed, the urinary protein profile correlated significantly with Banff scores (i, t, ci, ct) in 221 individuals 1 year after renal transplantation [97]. In 162 CKD patients, there was a significant correlation between the degree of interstitial fibrosis and the RBP/creatinine ratio. This correlation remained significant after adjustment for the estimated glomerular filtration rate, age, body mass index, alpha1-microglobulin, beta 2-microglobulin [98]. Thus, measurement of urinary VDBP and RBP may offer a potential for non-invasive evaluation of fibrosis.
MicroRNAs are a class of noncoding RNA that play a critical role in various cellular and physiological processes by modulating the degradation of messenger RNAs, therefore altering their expression levels [99]. MicroRNAs may play a key pathogenic role in kidney disease progression [100]. Some in vitro and in vivo models have shown a role of microRNA in the development and progression of diabetic nephropathy, as well as in other models of experimental CKD [101–103]. Urinary microRNAs may be considered noninvasive biomarkers for monitoring the progression of renal damage [104]. Muthukumar et al. have validated urinary microRNAs as diagnostic and prognostic biomarkers of acute cellular rejection, interstitial fibrosis and tubular atrophy [105]. Moreover, urine microRNA profiling was identified as a potential tool for monitoring graft function and anticipating progression to chronic allograft dysfunction in kidney transplant [106, 107]. MicroRNA-29c urinary levels correlated with glomerular filtration rate and tubulointerstitial fibrosis in 32 CKD patients and could predict the degree of tubulointerstitial fibrosis with area under the curve of 0.883 [108]. Furthermore, circulating microRNA-21 levels are associated with renal fibrosis in mice with unilateral ureteral obstruction and in transplant recipients [109].
Urinary miRNA may represent a promising novel noninvasive biomarker of renal fibrosis and a potential therapeutic approach to suppress renal fibrosis. Some limitations remain, because even if microRNAs appear stable in body fluids, a recent study suggested that circulating levels of some microRNAs are reduced in patients with severe kidney failure [110]. These very promising markers need to be better validated in clinical studies.
In summary, both plasma and urinary biomarkers to evaluate renal fibrosis are emerging. Several markers show great promise, among which urinary microRNAs and procollagens are probably the most studied and validated (table 1).
Table 1 Urinary and serum biomarkers of interstitial kidney fibrosis.
| Biomarker | Source | Key point | Ref | |
|---|---|---|---|---|
| Profibrotic and structural proteins | PIIINP | Blood Urine |
Positive association with fibrosis and CKD progression | Ghoul et al [62], Teppo et al [63], Soylemezuglu et al [64], Ix et al [65] |
| Periostin | Urine | Association with eGFR | Guerrot et al [66], Mael-Ainin et al [67] Sen et al [68] Satirapoj et al [69] | |
| TGF-beta BMP-7 |
Blood Urine |
Association with renal function. Prediction of function evolution | Basile et al [70] Wang et al [71] Wang et al [72] Sato et al [73] Honkanen et al [74] Suthanthiran et al [75] Wong et al [76] | |
| CCL2 | Urine | Predictor of fibrosis progression and eGFR decline in kidney allograft | Carr et al [77] Ho et al [78] Ho et al [79] Hirt-Minkowski et al [80] | |
| MMPs | Blood | Higher serum MMP7 with fibrosis | Tan et al [81] Rodder et al [82] Racca et al [83] Yan et al [84] Hirt-Minkowski [85] Zhou [86] | |
| TNF receptor | Blood | Higher TNF receptor associated to more extensive fibrosis and renal function decline | Niewczas et al [87] Gohda et al [88] Tonelli et al [89] Carlsson et al [90] | |
| Filtered proteins | VDBP | Urine | Correlation to collagen deposition in rats. Association to albuminuria and tubular damage markers in humans | Doorenbos et al [91] Mirkovic et al [92] |
| RBP | Urine | Higher urinary RBP is associated to fibrosis in CKD and kidney allografts | Amer et al [93] Pellet et al [94] | |
| miRNAs | miRNAs | Urine Blood |
High serum miR-21 and urinary miR-29 are associated to fibrosis but some are influence by renal function. Results to follow | Maluf et al [102] Anglicheau et al [103] Lv et al [104] Glowacki et al [105] Neal et al [106] |
BMP = bone morphogenetic protein; CCL2 = chemokine (C-C motif) ligand 2; CKD = chronic kidney disease; miRNAs = microribonucleic acids; MMPs = matrix metalloproteinases; PIIINP = procollagen type III amino-terminal propeptide; RBP = retinol binding protein; TGF = transforming growth factor; TIMPs = tissue inhibitors of metalloproteinases; TNF = tumour necrosis factor; VDBP = vitamin D binding protein
In recent years, several novel tools for the diagnosis of renal fibrosis have been identified. With advancing ultrasound and MRI techniques and emergence of urine and serum biomarkers, noninvasive monitoring of renal disease processes may become feasible in the near future (fig. 3). Early noninvasive markers identifying patients at highest risk of fibrosis would be useful to stratify patients for more or less intensive monitoring or therapy. Of importance, even if kidney biopsy remains the gold standard to diagnose kidney disease and to determine its severity, these tools could avoid unnecessary biopsies in the case of extensive fibrosis. However, because declining kidney function may modify biomarker expression, reproducibility and prospective validations remain major challenges for the future.
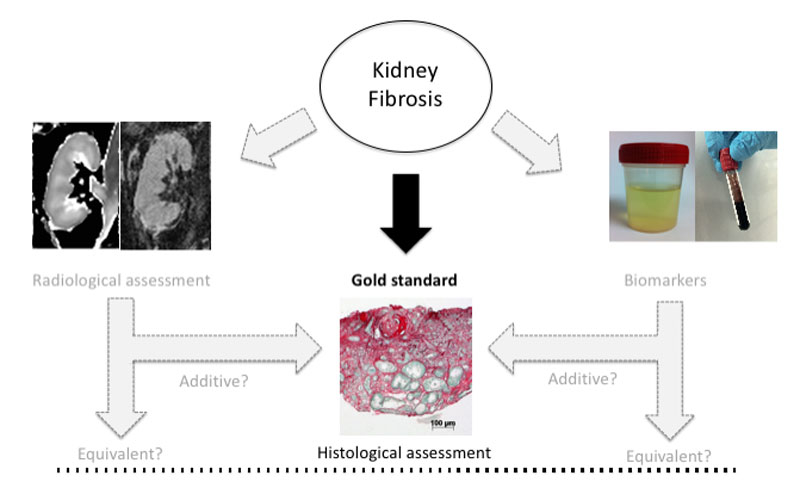
Figure 3 Kidney fibrosis is currently evaluated by histological assessment. Novel tools such as biomarkers or MRI are emerging. The place of these tools in the assessment process has yet to be determined as compared with the gold standard.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Coresh J , Astor BC , Greene T , Eknoyan G , Levey AS . Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. https://doi.org/10.1053/ajkd.2003.50007
2 Guessous I , Ponte B , Marques-Vidal P , Paccaud F , Gaspoz JM , Burnier M , et al. Clinical and biological determinants of kidney outcomes in a population-based cohort study. Kidney Blood Press Res. 2014;39(1):74–85. https://doi.org/10.1159/000355779
3 Fassett RG , Venuthurupalli SK , Gobe GC , Coombes JS , Cooper MA , Hoy WE . Biomarkers in chronic kidney disease: a review. Kidney Int. 2011;80(8):806–21. https://doi.org/10.1038/ki.2011.198
4 Schainuck LI , Striker GE , Cutler RE , Benditt EP . Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970;1(4):631–41. https://doi.org/10.1016/S0046-8177(70)80061-2
5 Liu Y . Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69(2):213–7. https://doi.org/10.1038/sj.ki.5000054
6 Boor P , Sebeková K , Ostendorf T , Floege J . Treatment targets in renal fibrosis. Nephrol Dial Transplant. 2007;22(12):3391–407. https://doi.org/10.1093/ndt/gfm393
7 Eddy AA . Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl (2011). 2014;4(1):2–8. https://doi.org/10.1038/kisup.2014.2
8 Cosio FG , Grande JP , Larson TS , Gloor JM , Velosa JA , Textor SC , et al. Kidney allograft fibrosis and atrophy early after living donor transplantation. Am J Transplant. 2005;5(5):1130–6. https://doi.org/10.1111/j.1600-6143.2005.00811.x
9 Park WD , Griffin MD , Cornell LD , Cosio FG , Stegall MD . Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol. 2010;21(11):1987–97. https://doi.org/10.1681/ASN.2010010049
10 Serón D , Moreso F , Ramón JM , Hueso M , Condom E , Fulladosa X , et al. Protocol renal allograft biopsies and the design of clinical trials aimed to prevent or treat chronic allograft nephropathy. Transplantation. 2000;69(9):1849–55. https://doi.org/10.1097/00007890-200005150-00019
11 Cosio FG , Grande JP , Wadei H , Larson TS , Griffin MD , Stegall MD . Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5(10):2464–72. https://doi.org/10.1111/j.1600-6143.2005.01050.x
12 Grimm PC , Nickerson P , Gough J , McKenna R , Jeffery J , Birk P , et al. Quantitation of allograft fibrosis and chronic allograft nephropathy. Pediatr Transplant. 1999;3(4):257–70. https://doi.org/10.1034/j.1399-3046.1999.00044.x
13 Risdon RA , Sloper JC , De Wardener HE . Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2(7564):363–6. https://doi.org/10.1016/S0140-6736(68)90589-8
14 Roberts IS , Cook HT , Troyanov S , Alpers CE , Amore A , Barratt J , et al.; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76(5):546–56. https://doi.org/10.1038/ki.2009.168
15 Adamczak M , Gross ML , Krtil J , Koch A , Tyralla K , Amann K , et al. Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats. J Am Soc Nephrol. 2003;14(11):2833–42. https://doi.org/10.1097/01.ASN.0000095248.91994.D3
16 Boffa JJ , Tharaux PL , Dussaule JC , Chatziantoniou C . Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension. 2001;37(2):490–6. https://doi.org/10.1161/01.HYP.37.2.490
17 Boffa JJ , Lu Y , Placier S , Stefanski A , Dussaule JC , Chatziantoniou C . Regression of renal vascular and glomerular fibrosis: role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14(5):1132–44. https://doi.org/10.1097/01.ASN.0000060574.38107.3B
18 Li Y , Wen X , Spataro BC , Hu K , Dai C , Liu Y . hepatocyte growth factor is a downstream effector that mediates the antifibrotic action of peroxisome proliferator-activated receptor-gamma agonists. J Am Soc Nephrol. 2006;17(1):54–65. https://doi.org/10.1681/ASN.2005030257
19 Wang S , Wilkes MC , Leof EB , Hirschberg R . Noncanonical TGF-beta pathways, mTORC1 and Abl, in renal interstitial fibrogenesis. Am J Physiol Renal Physiol. 2010;298(1):F142–9. https://doi.org/10.1152/ajprenal.00320.2009
20 Pontrelli P , Rossini M , Infante B , Stallone G , Schena A , Loverre A , et al. Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation. 2008;85(1):125–34. https://doi.org/10.1097/01.tp.0000296831.91303.9a
21 Hotta O , Furuta T , Chiba S , Tomioka S , Taguma Y . Regression of IgA nephropathy: a repeat biopsy study. Am J Kidney Dis. 2002;39(3):493–502. https://doi.org/10.1053/ajkd.2002.31399
22 Vilayur E , Harris DC . Emerging therapies for chronic kidney disease: what is their role? Nat Rev Nephrol. 2009;5(7):375–83. https://doi.org/10.1038/nrneph.2009.76
23 Whittier WL , Korbet SM . Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol. 2004;15(1):142–7. https://doi.org/10.1097/01.ASN.0000102472.37947.14
24 Parrish AE . Complications of percutaneous renal biopsy: a review of 37 years’ experience. Clin Nephrol. 1992;38(3):135–41.
25 Moreso F , Lopez M , Vallejos A , Giordani C , Riera L , Fulladosa X , et al. Serial protocol biopsies to quantify the progression of chronic transplant nephropathy in stable renal allografts. Am J Transplant. 2001;1(1):82–8. https://doi.org/10.1034/j.1600-6143.2001.010115.x
26 Marcussen N , Olsen TS , Benediktsson H , Racusen L , Solez K . Reproducibility of the Banff classification of renal allograft pathology. Inter- and intraobserver variation. Transplantation. 1995;60(10):1083–9. https://doi.org/10.1097/00007890-199511270-00004
27 Furness PN , Taub N ; Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60(5):1998–2012. https://doi.org/10.1046/j.1523-1755.2001.00030.x
28 Farris AB , Alpers CE . What is the best way to measure renal fibrosis?: A pathologist’s perspective. Kidney Int Suppl (2011). 2014;4(1):9–15. https://doi.org/10.1038/kisup.2014.3
29 Grimm PC , Nickerson P , Gough J , McKenna R , Stern E , Jeffery J , et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol. 2003;14(6):1662–8. https://doi.org/10.1097/01.ASN.0000066143.02832.5E
30 Sund S , Grimm P , Reisaeter AV , Hovig T . Computerized image analysis vs semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant. 2004;19(11):2838–45. https://doi.org/10.1093/ndt/gfh490
31 Servais A , Meas-Yedid V , Buchler M , Morelon E , Olivo-Marin JC , Lebranchu Y , et al. Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation. 2007;84(12):1595–601. https://doi.org/10.1097/01.tp.0000295749.50525.bd
32 Nicholson ML , Bailey E , Williams S , Harris KP , Furness PN . Computerized histomorphometric assessment of protocol renal transplant biopsy specimens for surrogate markers of chronic rejection. Transplantation. 1999;68(2):236–41. https://doi.org/10.1097/00007890-199907270-00013
33 Farris AB , Adams CD , Brousaides N , Della Pelle PA , Collins AB , Moradi E , et al. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22(1):176–86. https://doi.org/10.1681/ASN.2009091005
34 Street JM , Souza AC , Alvarez-Prats A , Horino T , Hu X , Yuen PS , et al. Automated quantification of renal fibrosis with Sirius Red and polarization contrast microscopy. Physiol Rep. 2014;2(7):e12088. https://doi.org/10.14814/phy2.12088
35 Bamber J , Cosgrove D , Dietrich CF , Fromageau J , Bojunga J , Calliada F , et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34(2):169–84. https://doi.org/10.1055/s-0033-1335205
36 Wilder J , Patel K . The clinical utility of FibroScan(®) as a noninvasive diagnostic test for liver disease. Med Devices (Auckl). 2014;7:107–14.
37 Gennisson JL , Grenier N , Combe C , Tanter M . Supersonic shear wave elastography of in vivo pig kidney: influence of blood pressure, urinary pressure and tissue anisotropy. Ultrasound Med Biol. 2012;38(9):1559–67. https://doi.org/10.1016/j.ultrasmedbio.2012.04.013
38 Grenier N , Poulain S , Lepreux S , Gennisson JL , Dallaudière B , Lebras Y , et al. Quantitative elastography of renal transplants using supersonic shear imaging: a pilot study. Eur Radiol. 2012;22(10):2138–46. https://doi.org/10.1007/s00330-012-2471-9
39 Syversveen T , Brabrand K , Midtvedt K , Strøm EH , Hartmann A , Jakobsen JA , et al. Assessment of renal allograft fibrosis by acoustic radiation force impulse quantification--a pilot study. Transpl Int. 2011;24(1):100–5. https://doi.org/10.1111/j.1432-2277.2010.01165.x
40 Syversveen T , Midtvedt K , Berstad AE , Brabrand K , Strøm EH , Abildgaard A . Tissue elasticity estimated by acoustic radiation force impulse quantification depends on the applied transducer force: an experimental study in kidney transplant patients. Eur Radiol. 2012;22(10):2130–7. https://doi.org/10.1007/s00330-012-2476-4
41 Wang L , Xia P , Lv K , Han J , Dai Q , Li XM , et al. Assessment of renal tissue elasticity by acoustic radiation force impulse quantification with histopathological correlation: preliminary experience in chronic kidney disease. Eur Radiol. 2014;24(7):1694–9. https://doi.org/10.1007/s00330-014-3162-5
42 Lee J , Oh YT , Joo DJ , Ma BG , Lee AL , Lee JG , et al. Acoustic Radiation Force Impulse Measurement in Renal Transplantation: A Prospective, Longitudinal Study With Protocol Biopsies. Medicine (Baltimore). 2015;94(39):e1590. https://doi.org/10.1097/MD.0000000000001590
43 Lukenda V , Mikolasevic I , Racki S , Jelic I , Stimac D , Orlic L . Transient elastography: a new noninvasive diagnostic tool for assessment of chronic allograft nephropathy. Int Urol Nephrol. 2014;46(7):1435–40. https://doi.org/10.1007/s11255-014-0697-y
44 Nakao T , Ushigome H , Nakamura T , Harada S , Koshino K , Suzuki T , et al. Evaluation of renal allograft fibrosis by transient elastography (Fibro Scan). Transplant Proc. 2015;47(3):640–3. https://doi.org/10.1016/j.transproceed.2014.12.034
45 Arndt R , Schmidt S , Loddenkemper C , Grünbaum M , Zidek W , van der Giet M , et al. Noninvasive evaluation of renal allograft fibrosis by transient elastography--a pilot study. Transpl Int. 2010;23(9):871–7.
46 Sommerer C , Scharf M , Seitz C , Millonig G , Seitz HK , Zeier M , et al. Assessment of renal allograft fibrosis by transient elastography. Transpl Int. 2013;26(5):545–51. https://doi.org/10.1111/tri.12073
47 Orlacchio A , Chegai F , Del Giudice C , Anselmo A , Iaria G , Palmieri G , et al. Kidney transplant: usefulness of real-time elastography (RTE) in the diagnosis of graft interstitial fibrosis. Ultrasound Med Biol. 2014;40(11):2564–72. https://doi.org/10.1016/j.ultrasmedbio.2014.06.002
48 Ozkan F , Yavuz YC , Inci MF , Altunoluk B , Ozcan N , Yuksel M , et al. Interobserver variability of ultrasound elastography in transplant kidneys: correlations with clinical-Doppler parameters. Ultrasound Med Biol. 2013;39(1):4–9. https://doi.org/10.1016/j.ultrasmedbio.2012.09.013
49 Gao J , Rubin JM , Xiang DY , He W , Auh YH , Wang J , et al. Doppler parameters in renal transplant dysfunction: correlations with histopathologic changes. J Ultrasound Med. 2011;30(2):169–75. https://doi.org/10.7863/jum.2011.30.2.169
50 Perea RJ , Ortiz-Perez JT , Sole M , Cibeira MT , de Caralt TM , Prat-Gonzalez S , et al. T1 mapping: characterisation of myocardial interstitial space. Insights Imaging. 2015;6(2):189–202. https://doi.org/10.1007/s13244-014-0366-9
51 Hueper K , Gutberlet M , Rong S , Hartung D , Mengel M , Lu X , et al. Acute kidney injury: arterial spin labeling to monitor renal perfusion impairment in mice-comparison with histopathologic results and renal function. Radiology. 2014;270(1):117–24. https://doi.org/10.1148/radiol.13130367
52 Hueper K , Hensen B , Gutberlet M , Chen R , Hartung D , Barrmeyer A , et al. Kidney Transplantation: Multiparametric Functional Magnetic Resonance Imaging for Assessment of Renal Allograft Pathophysiology in Mice. Invest Radiol. 2016;51(1):58–65. https://doi.org/10.1097/RLI.0000000000000205
53 Lee VS , Kaur M , Bokacheva L , Chen Q , Rusinek H , Thakur R , et al. What causes diminished corticomedullary differentiation in renal insufficiency? J Magn Reson Imaging. 2007;25(4):790–5. https://doi.org/10.1002/jmri.20878
54 Huang Y , Sadowski EA , Artz NS , Seo S , Djamali A , Grist TM , et al. Measurement and comparison of T1 relaxation times in native and transplanted kidney cortex and medulla. J Magn Reson Imaging. 2011;33(5):1241–7. https://doi.org/10.1002/jmri.22543
55 Hueper K , Gueler F , Bräsen JH , Gutberlet M , Jang MS , Lehner F , et al. Functional MRI detects perfusion impairment in renal allografts with delayed graft function. Am J Physiol Renal Physiol. 2015;308(12):F1444–51. https://doi.org/10.1152/ajprenal.00064.2015
56 Niendorf T , Pohlmann A , Arakelyan K , Flemming B , Cantow K , Hentschel J , et al. How bold is blood oxygenation level-dependent (BOLD) magnetic resonance imaging of the kidney? Opportunities, challenges and future directions. Acta Physiol (Oxf). 2015;213(1):19–38. https://doi.org/10.1111/apha.12393
57 Hueper K , Rong S , Gutberlet M , Hartung D , Mengel M , Lu X , et al. T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol. 2013;48(12):834–42. https://doi.org/10.1097/RLI.0b013e31829d0414
58 Inoue T , Kozawa E , Okada H , Inukai K , Watanabe S , Kikuta T , et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22(8):1429–34. https://doi.org/10.1681/ASN.2010111143
59 Togao O , Doi S , Kuro-o M , Masaki T , Yorioka N , Takahashi M . Assessment of renal fibrosis with diffusion-weighted MR imaging: study with murine model of unilateral ureteral obstruction. Radiology. 2010;255(3):772–80. https://doi.org/10.1148/radiol.10091735
60 Li Q , Li J , Zhang L , Chen Y , Zhang M , Yan F . Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: A preliminary clinical study. Eur J Radiol. 2014;83(5):756–62. https://doi.org/10.1016/j.ejrad.2014.01.024
61 Zhao J , Wang ZJ , Liu M , Zhu J , Zhang X , Zhang T , et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol. 2014;69(11):1117–22. https://doi.org/10.1016/j.crad.2014.06.011
62 Le Bihan D , Poupon C , Amadon A , Lethimonnier F . Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006;24(3):478–88. https://doi.org/10.1002/jmri.20683
63 Friedli I , Crowe LA , Viallon M , Porter DA , Martin PY , de Seigneux S , et al. Improvement of renal diffusion-weighted magnetic resonance imaging with readout-segmented echo-planar imaging at 3T. Magn Reson Imaging. 2015;33(6):701–8. https://doi.org/10.1016/j.mri.2015.02.023
64 Friedli I , Crowe LA , Berchtold L , Moll S , Hadaya K , de Perrot T , et al. New Magnetic Resonance Imaging Index for Renal Fibrosis Assessment: A Comparison between Diffusion-Weighted Imaging and T1 Mapping with Histological Validation. Sci Rep. 2016;6(1):30088. https://doi.org/10.1038/srep30088
65 Zhang JL , Sigmund EE , Chandarana H , Rusinek H , Chen Q , Vivier PH , et al. Variability of renal apparent diffusion coefficients: limitations of the monoexponential model for diffusion quantification. Radiology. 2010;254(3):783–92. https://doi.org/10.1148/radiol.09090891
66 Ghoul BE , Squalli T , Servais A , Elie C , Meas-Yedid V , Trivint C , et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010;5(2):205–10. https://doi.org/10.2215/CJN.06610909
67 Teppo AM , Törnroth T , Honkanen E , Grönhagen-Riska C . Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003;75(12):2113–9. https://doi.org/10.1097/01.TP.0000066809.60389.48
68 Soylemezoglu O , Wild G , Dalley AJ , MacNeil S , Milford-Ward A , Brown CB , et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant. 1997;12(9):1883–9. https://doi.org/10.1093/ndt/12.9.1883
69 Ix JH , Biggs ML , Mukamal K , Djousse L , Siscovick D , Tracy R , et al. Urine Collagen Fragments and CKD Progression-The Cardiovascular Health Study. J Am Soc Nephrol. 2015;26(10):2494–503. https://doi.org/10.1681/ASN.2014070696
70 Guerrot D , Dussaule JC , Mael-Ainin M , Xu-Dubois YC , Rondeau E , Chatziantoniou C , et al. Identification of periostin as a critical marker of progression/reversal of hypertensive nephropathy. PLoS One. 2012;7(3):e31974. https://doi.org/10.1371/journal.pone.0031974
71 Mael-Ainin M , Abed A , Conway SJ , Dussaule JC , Chatziantoniou C . Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J Am Soc Nephrol. 2014;25(8):1724–36. https://doi.org/10.1681/ASN.2013060664
72 Sen K , Lindenmeyer MT , Gaspert A , Eichinger F , Neusser MA , Kretzler M , et al. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol. 2011;179(4):1756–67. https://doi.org/10.1016/j.ajpath.2011.06.002
73 Satirapoj B , Witoon R , Ruangkanchanasetr P , Wantanasiri P , Charoenpitakchai M , Choovichian P . Urine periostin as a biomarker of renal injury in chronic allograft nephropathy. Transplant Proc. 2014;46(1):135–40. https://doi.org/10.1016/j.transproceed.2013.07.069
74 Basile DP . The transforming growth factor beta system in kidney disease and repair: recent progress and future directions. Curr Opin Nephrol Hypertens. 1999;8(1):21–30. https://doi.org/10.1097/00041552-199901000-00005
75 Wang S , Chen Q , Simon TC , Strebeck F , Chaudhary L , Morrissey J , et al. Bone morphogenic protein-7 (BMP-7), a novel therapy for diabetic nephropathy. Kidney Int. 2003;63(6):2037–49. https://doi.org/10.1046/j.1523-1755.2003.00035.x
76 Wang S , de Caestecker M , Kopp J , Mitu G , Lapage J , Hirschberg R . Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol. 2006;17(9):2504–12. https://doi.org/10.1681/ASN.2006030278
77 Sato H , Iwano M , Akai Y , Kurioka H , Kubo A , Yamaguchi T , et al. Increased excretion of urinary transforming growth factor beta 1 in patients with diabetic nephropathy. Am J Nephrol. 1998;18(6):490–4. https://doi.org/10.1159/000013415
78 Honkanen E , Teppo AM , Törnroth T , Groop PH , Grönhagen-Riska C . Urinary transforming growth factor-beta 1 in membranous glomerulonephritis. Nephrol Dial Transplant. 1997;12(12):2562–8. https://doi.org/10.1093/ndt/12.12.2562
79 Suthanthiran M , Gerber LM , Schwartz JE , Sharma VK , Medeiros M , Marion R , et al. Circulating transforming growth factor-beta1 levels and the risk for kidney disease in African Americans. Kidney Int. 2009;76(1):72–80. https://doi.org/10.1038/ki.2009.66
80 Wong MG , Perkovic V , Woodward M , Chalmers J , Li Q , Hillis GS , et al. Circulating bone morphogenetic protein-7 and transforming growth factor-β1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int. 2013;83(2):278–84. https://doi.org/10.1038/ki.2012.383
81 Carr MW , Roth SJ , Luther E , Rose SS , Springer TA . Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91(9):3652–6. https://doi.org/10.1073/pnas.91.9.3652
82 Ho J , Rush DN , Gibson IW , Karpinski M , Storsley L , Bestland J , et al. Early urinary CCL2 is associated with the later development of interstitial fibrosis and tubular atrophy in renal allografts. Transplantation. 2010;90(4):394–400. https://doi.org/10.1097/TP.0b013e3181e6424d
83 Ho J , Wiebe C , Gibson IW , Hombach-Klonisch S , Gao A , Rigatto C , et al. Elevated urinary CCL2: Cr at 6 months is associated with renal allograft interstitial fibrosis and inflammation at 24 months. Transplantation. 2014;98(1):39–46. https://doi.org/10.1097/01.TP.0000442776.40295.73
84 Hirt-Minkowski P , Rush DN , Gao A , Hopfer H , Wiebe C , Nickerson PW , et al. Six-Month Urinary CCL2 and CXCL10 Levels Predict Long-term Renal Allograft Outcome. Transplantation. 2016;100(9):1988–96. https://doi.org/10.1097/TP.0000000000001304
85 Tan RJ , Liu Y . Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302(11):F1351–61. https://doi.org/10.1152/ajprenal.00037.2012
86 Rödder S , Scherer A , Raulf F , Berthier CC , Hertig A , Couzi L , et al. Renal allografts with IF/TA display distinct expression profiles of metzincins and related genes. Am J Transplant. 2009;9(3):517–26. https://doi.org/10.1111/j.1600-6143.2008.02512.x
87 Racca MA , Novoa PA , Rodríguez I , Della Vedova AB , Pellizas CG , Demarchi M , et al. Renal dysfunction and intragraft proMMP9 activity in renal transplant recipients with interstitial fibrosis and tubular atrophy. Transpl Int. 2015;28(1):71–8. https://doi.org/10.1111/tri.12445
88 Yan Q , Sui W , Wang B , Zou H , Zou G , Luo H . Expression of MMP-2 and TIMP-1 in renal tissue of patients with chronic active antibody-mediated renal graft rejection. Diagn Pathol. 2012;7(1):141. https://doi.org/10.1186/1746-1596-7-141
89 Hirt-Minkowski P , Marti HP , Hönger G , Grandgirard D , Leib SL , Amico P , et al. Correlation of serum and urinary matrix metalloproteases/tissue inhibitors of metalloproteases with subclinical allograft fibrosis in renal transplantation. Transpl Immunol. 2014;30(1):1–6. https://doi.org/10.1016/j.trim.2013.11.004
90 Zhou D , Tian Y , Sun L , Zhou L , Xiao L , Tan RJ , et al. Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis. J Am Soc Nephrol. 2017;28(2):598–611.
91 Niewczas MA , Gohda T , Skupien J , Smiles AM , Walker WH , Rosetti F , et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507–15. https://doi.org/10.1681/ASN.2011060627
92 Gohda T , Niewczas MA , Ficociello LH , Walker WH , Skupien J , Rosetti F , et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23(3):516–24. https://doi.org/10.1681/ASN.2011060628
93 Tonelli M , Sacks F , Pfeffer M , Jhangri GS , Curhan G ; Cholesterol and Recurrent Events (CARE) Trial Investigators. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68(1):237–45. https://doi.org/10.1111/j.1523-1755.2005.00398.x
94 Carlsson AC , Larsson TE , Helmersson-Karlqvist J , Larsson A , Lind L , Ärnlöv J . Soluble TNF receptors and kidney dysfunction in the elderly. J Am Soc Nephrol. 2014;25(6):1313–20. https://doi.org/10.1681/ASN.2013080860
95 Doorenbos CR , van den Born J , Navis G , de Borst MH . Possible renoprotection by vitamin D in chronic renal disease: beyond mineral metabolism. Nat Rev Nephrol. 2009;5(12):691–700. https://doi.org/10.1038/nrneph.2009.185
96 Mirković K , Doorenbos CR , Dam WA , Lambers Heerspink HJ , Slagman MC , Nauta FL , et al. Urinary vitamin D binding protein: a potential novel marker of renal interstitial inflammation and fibrosis. PLoS One. 2013;8(2):e55887. https://doi.org/10.1371/journal.pone.0055887
97 Amer H , Lieske JC , Rule AD , Kremers WK , Larson TS , Franco Palacios CR , et al. Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival. Am J Transplant. 2013;13(3):676–84. https://doi.org/10.1111/ajt.12044
98 Pallet N , Chauvet S , Chassé JF , Vincent M , Avillach P , Levi C , et al. Urinary retinol binding protein is a marker of the extent of interstitial kidney fibrosis. PLoS One. 2014;9(1):e84708. https://doi.org/10.1371/journal.pone.0084708
99 Bartel DP . MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. https://doi.org/10.1016/j.cell.2009.01.002
100 Bhatt K , Kato M , Natarajan R . Mini-review: emerging roles of microRNAs in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2016;310(2):F109–18.
101 Schena FP , Serino G , Sallustio F . MicroRNAs in kidney diseases: new promising biomarkers for diagnosis and monitoring. Nephrol Dial Transplant. 2014;29(4):755–63. https://doi.org/10.1093/ndt/gft223
102 Putta S , Lanting L , Sun G , Lawson G , Kato M , Natarajan R . Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol. 2012;23(3):458–69. https://doi.org/10.1681/ASN.2011050485
103 Krupa A , Jenkins R , Luo DD , Lewis A , Phillips A , Fraser D . Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol. 2010;21(3):438–47. https://doi.org/10.1681/ASN.2009050530
104 Scian MJ , Maluf DG , David KG , Archer KJ , Suh JL , Wolen AR , et al. MicroRNA profiles in allograft tissues and paired urines associate with chronic allograft dysfunction with IF/TA. Am J Transplant. 2011;11(10):2110–22. https://doi.org/10.1111/j.1600-6143.2011.03666.x
105 Muthukumar T , Lee JR , Dadhania DM , Ding R , Sharma VK , Schwartz JE , et al. Allograft rejection and tubulointerstitial fibrosis in human kidney allografts: interrogation by urinary cell mRNA profiling. Transplant Rev (Orlando). 2014;28(3):145–54. https://doi.org/10.1016/j.trre.2014.05.003
106 Maluf DG , Dumur CI , Suh JL , Scian MJ , King AL , Cathro H , et al. The urine microRNA profile may help monitor post-transplant renal graft function. Kidney Int. 2014;85(2):439–49. https://doi.org/10.1038/ki.2013.338
107 Anglicheau D , Muthukumar T , Hummel A , Ding R , Sharma VK , Dadhania D , et al. Discovery and validation of a molecular signature for the noninvasive diagnosis of human renal allograft fibrosis. Transplantation. 2012;93(11):1136–46. https://doi.org/10.1097/TP.0b013e31824ef181
108 Lv LL , Cao YH , Ni HF , Xu M , Liu D , Liu H , et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol. 2013;305(8):F1220–7. https://doi.org/10.1152/ajprenal.00148.2013
109 Glowacki F , Savary G , Gnemmi V , Buob D , Van der Hauwaert C , Lo-Guidice JM , et al. Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One. 2013;8(2):e58014. https://doi.org/10.1371/journal.pone.0058014
110 Neal CS , Michael MZ , Pimlott LK , Yong TY , Li JY , Gleadle JM . Circulating microRNA expression is reduced in chronic kidney disease. Nephrol Dial Transplant. 2011;26(11):3794–802. https://doi.org/10.1093/ndt/gfr485
LB wrote and revised the manuscript; SdS wrote and revised the manuscript, JPV revised and approved final manuscript, SM revised and approved final manuscript, IF wrote and revised the manuscript, PYM read, edited and approved final manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.