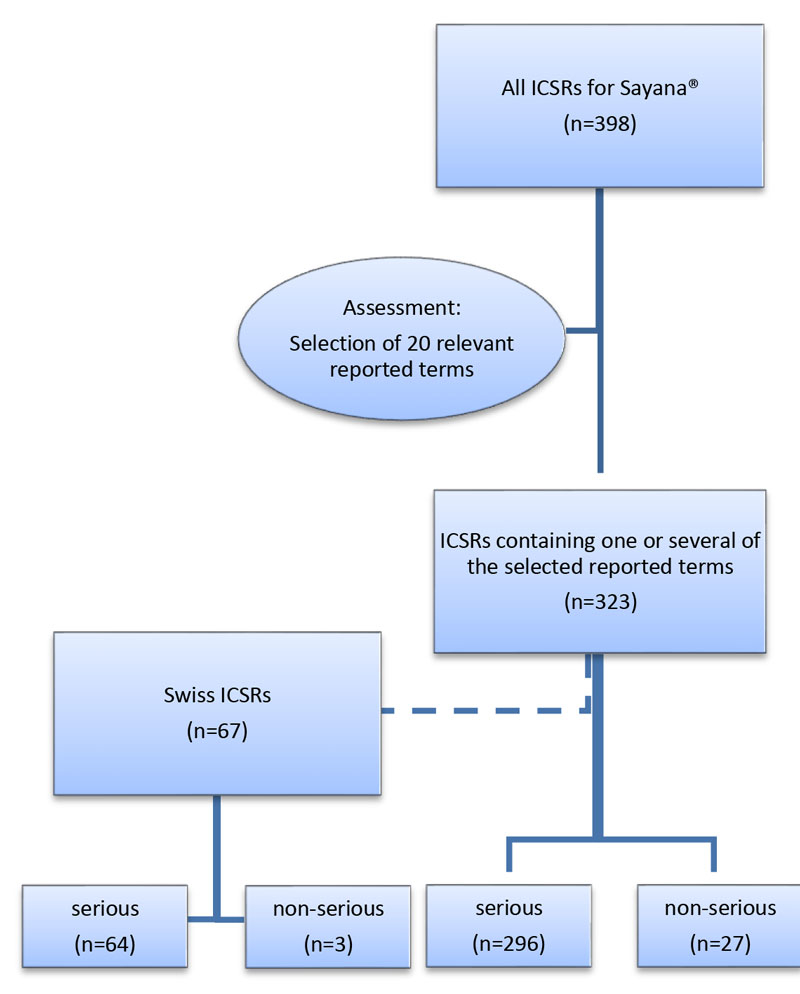
Figure 1 Case selection process: all individual case safety reports (ICRSs) up to 1 January 2016.
DOI: https://doi.org/10.4414/smw.2017.14432
adverse drug reaction
depot medroxprogesterone acetate
intramuscularly injected depot medroxprogesterone acetate
subcutaneously injected depot medroxprogesterone acetate
individual case safety report
Regional Pharmacovigilance Centre
World Health Organization
Depot medroxyprogesterone acetate (DMPA) injections have been effectively used for hormonal contraception for many years. The active ingredient inhibits the hypothalamus-hypophysis-gonad axis and therefore prevents ovulation and follicular maturation [1, 2]. It is considered a safe method of parenteral contraception [3].
In the past, DMPA was given as an intramuscular injection and administered by health professionals only. The introduction of Sayana® to the Swiss market on 5 April 2012 [4] provided a micronised DMPA formulation allowing subcutaneous injection (DMPA-SC). After administration, its contraceptive effect lasts for 3 months. It is dispensed in a prefilled syringe with a DMPA concentration of 104 mg in 0.65 ml, allowing self-administration by the patient into the subcutis of the thigh or abdominal wall.
In clinical trials, injection site reactions were reported as frequent adverse drug reactions (ADRs) [5, 6]. Within 10 months, the Regional Pharmacovigilance Centre (RPVC) Zurich received 11 reports of severe and persistent injection site reactions, after administration of subcutaneous Sayana®. Therefore, we analysed data on international and Swiss spontaneous pharmacovigilance reports in the World Health Organization (WHO) pharmacovigilance database Vigibase™.
This retrospective descriptive study was based on selected individual case safety reports (ICSRs) from the WHO global database VigiBase™ and a subgroup analysis of Swiss ICSRs. For more detailed data, we analysed the case series of 11 reports of ADRs issued to the RPVC of Zurich in 2014 and 2015.
VigiBase™ was established in 1968 within the WHO Programme for International Drug Monitoring and is run by the Uppsala Monitoring Centre in Sweden [7]. In January 2016 it contained more than 12.3 million ICSRs from 125 participating countries. An ICSR is an anonymised report for a single individual who suffered from one or more adverse events that may be linked to the use of one or more drugs. Since VigiBase™ is a register database, the international ICSRs contain neither narrative nor clinical or laboratory data. All ADRs are coded according to the reported term as well as to the WHO Adverse Reaction Terminology (WHO-ART) and the Medical Dictionary for Regulatory Activities (MedDRA). In order to receive information most closely related to the actual adverse event, original reported terms were used for our analysis.
We received the coded data elements of all international Sayana®-associated ICSRs from the WHO global database VigiBase™ as a Microsoft Excel file via VigiLyze™. The case selection process is illustrated in figure 1. All reported terms were discussed independently by two pharmacists and one clinical pharmacologist, to determine if they could be used to describe a persistent injection site reaction, comparable to those reported to the RPVC Zurich. Twenty reported terms were regarded as relevant and the corresponding ICSRs were selected for further study. From the selected ICSRs, a subgroup containing only ICSRs from Switzerland was formed. Both data sets were analysed for demographic data such as age at ADR onset and gender, completeness, reported ADR, outcome, concomitant drugs, seriousness and seriousness criteria. The adverse reactions were classified as “severe” owing to the persistence of the condition of lipodystrophy and tissue damage.

Figure 1 Case selection process: all individual case safety reports (ICRSs) up to 1 January 2016.
In Switzerland, ADRs are reported by both healthcare professionals and consumers to one of six RPVCs. These centres perform a causality assessment according to WHO/CIOMS (Council for International Organizations of Medical Sciences) criteria and forward the reports to the Swissmedic National Pharmacovigilance Centre. To categorise the causality between drug exposure and ADR as “certain”, the following criteria must be fulfilled: plausible time relationship to drug intake, plausible response to withdrawal, no alternative explanation by disease or other drugs and a positive rechallenge. A rechallenge is considered positive if the patients show the same ADR after exposure to the suspected drug for a second time. Adverse reactions filed by the pharmaceutical industry are reported directly to Swissmedic. The National Pharmacovigilance Centre collaborates with the International Centre for Drug Safety run by the WHO in Uppsala, Sweden [8].
Between June 2014 and April 2015, the RPVC Zurich received 11 reports of injection site lipodystrophy after subcutaneous Sayana® injection. In order to obtain complete case reports, as well as supplementary information and up-to-date follow-up information, the reporting physicians were contacted by the investigators. Data were analysed for age, indication and first-line therapy, previous contraception, the person using the prefilled syringe, the number of applications until the ADR onset, number of applications in total, the localisation and extent of persistent local reactions, rechallenge, latency time, treatment of ADR, outcome and causality assessments.
We performed descriptive analysis using Microsoft Office Excel (2010) and SPSS for Windows software (version 22). For variables with normally distributed numeric values, the arithmetic mean and standard deviation were calculated. For variables without normally distributed values, median and range were determined.
In the global database from the WHO VigiBase™ we identified 398 ICSRs for Sayana® up to 1 January 2016. A total of 620 ADRs were reported and 174 different reported terms were used. After excluding all cases not likely to be related to a persistent local reaction, 20 reported terms corresponding to 323 (81.2%) ICSRs remained for analysis.
All selected reported terms are listed together with their frequency and the outcome of the reaction in table 1. A total of 355 reported terms were used. The most frequently reported term was “injection site reaction” (n = 249, 70.1%) without further specification, followed by “injection site atrophy” (n = 29, 8.2%) and “injection site fat necrosis” (n = 15, 4.2%). The majority of the reactions (n = 193, 54.4%) did not recover; for 134 reactions (37.7%) the outcome was not reported. Of the 355 reported terms, only 1 (0.3%) was described as “recovered”.
Table 1 Reported terms and outcome of adverse drug reactions corresponding to 323 international individual case safety reports.
| Reported terms | Number | Outcome | ||||
|---|---|---|---|---|---|---|
| Not recovered | Recovered | Recovered with sequelae | Recovering | Unknown | ||
| Atrophy | 2 | 2 | 0 | 0 | 0 | 0 |
| Atrophy injection site | 7 | 3 | 0 | 0 | 1 | 3 |
| Atrophy skin | 1 | 1 | 0 | 0 | 0 | 0 |
| Dellen | 1 | 0 | 0 | 1 | 0 | 0 |
| Fat necrosis | 1 | 0 | 0 | 1 | 0 | 0 |
| Injection site atrophy | 29 | 18 | 0 | 5 | 0 | 6 |
| Injection site erosion | 1 | 1 | 0 | 0 | 0 | 0 |
| Injection site fat atrophy | 5 | 1 | 0 | 0 | 1 | 3 |
| Injection site fat necrosis | 15 | 10 | 0 | 1 | 1 | 3 |
| Injection site induration | 11 | 4 | 0 | 0 | 1 | 6 |
| Injection site lump | 2 | 1 | 0 | 1 | 0 | 0 |
| Injection site necrosis | 4 | 2 | 0 | 2 | 0 | 0 |
| Injection site reaction | 249 | 131 | 0 | 9 | 1 | 108 |
| Injection site reaction NOS | 2 | 2 | 0 | 0 | 0 | 0 |
| Injection site subcutaneous fat decreased | 1 | 1 | 0 | 0 | 0 | 0 |
| Lipoatrophy injection site | 10 | 7 | 0 | 0 | 0 | 3 |
| Lipodystrophy | 4 | 3 | 0 | 0 | 0 | 1 |
| Necrosis injection site | 2 | 0 | 0 | 2 | 0 | 0 |
| Partial lipodystrophy | 7 | 6 | 1 | 0 | 0 | 0 |
| Skin reaction localised | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 355 | 193 | 1 | 22 | 5 | 134 |
NOS = not otherwise specified
The selected 323 ICSRs were analysed for completeness, sex, age at onset, monotherapy and seriousness criteria (table 2). A total of 321 (99.4%) patients were female. The median age was 34 years (range 6–53 years). The average completeness score of the ICSRs was 0.35 (range 0.11–1). For most patients (n = 313, 96.9%), Sayana® was the only reported drug; the most frequently used concomitant drug was levothyroxine (n = 5, 1.5%). However, none of the concomitant drugs were administered subcutaneously. Overall, 91.6% (n = 296) of the cases related to local reactions were categorised as serious (only 8.1% were categorised as nonserious). The seriousness criteria were “caused/prolonged hospitalisation, other” (n = 2, 0.6%), “disabling/incapacitating” (n = 9, 2.8%) and “disabling/incapacitating, other” (n = 7, 2.2%). For the majority of the cases, reasons for the classification as serious was not further detailed (“other” [n = 278, 86.1%]).
Table 2 Characteristics of the individual case safety reports.
|
International
(n = 323) |
Switzerland
(n = 67) |
Regional
(n = 11) |
||||
|---|---|---|---|---|---|---|
| Characteristics | Frequency | Percent | Frequency | Percent | Frequency | Percent |
| Sex female | 321 | 99.4 | 66 | 98.5 | 11 | 100 |
| Sex unknown | 2 | 0.6 | 1 | 1.5 | 0 | 0 |
| Completeness score, median (range) | 0.35 (0.11–1) |
0.28 (0.11–1) |
0.90 (0.32–1) |
|||
| Age at onset (years), median (range) | 34 (6–53) |
34 (18–53) |
33 (24–50) |
|||
| Monotherapy Sayana® | 313 | 96.9 | 67 | 100 | 11 | 100 |
| Serious | 296 | 91.6 | 64 | 95.5 | 11 | 100 |
| Seriousness criteria: | ||||||
| Caused/prolonged hospitalisation, other | 2 | 0.6 | 0 | 0 | 0 | 0 |
| Disabling/incapacitating | 9 | 2.8 | 4 | 6 | 3 | 27.3 |
| Disabling/incapacitating, other | 7 | 2.2 | 3 | 4.5 | 2 | 18.2 |
| Other | 278 | 86.1 | 57 | 85.1 | 6 | 54.6 |
Figure 2 shows the countries of origin of the reports, stratified by the reporter’s qualification. Most reports were from Austria (n = 137, 42.4%), followed by Switzerland (n = 67, 20.7%) and Germany (n = 58, 18.0%). In total, 80.1% of the reports came from German-speaking countries. In the vast majority of ICSRs, physicians were involved in the reporting (88.2%), either as the only reporter or in combination with others. Only 7.1% were reported by other healthcare professionals and 2.8% by consumer/non-healthcare professionals.
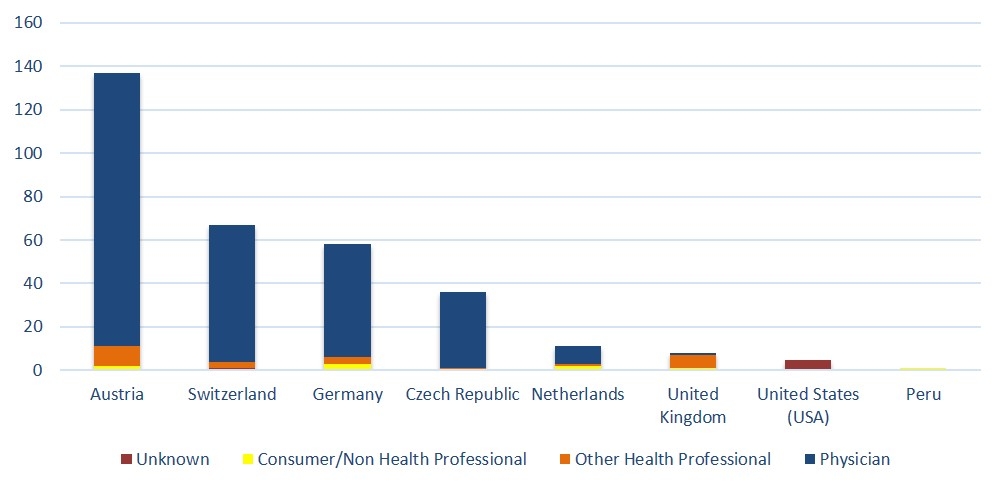
Figure 2 Number of individual safety reports stratified by country of origin and reporters’ qualifications.
In the selected 67 Swiss ICSRs, 77 ADRs were reported using 10 different terms including persistent local reactions such as lipodystrophy, atrophy or fat necrosis. The majority (n = 64, 95.5%) of the cases were categorised as serious. Only one patient recovered, 32 did not recover and for 40 ADRs the outcome was not reported. In these cases, the completeness score was lower than the international average (0.28, range 0.11–1). The median age was the same as in the international data (34 years, range 18–53 years). In all Swiss cases, Sayana® was administered as monotherapy.
The 11 cases were reported to the RPVC Zurich by six different physicians. The mean completeness score of regional ICSRs was higher at 0.90 (range 0.32–1.00). All patients were female and aged between 24 and 50 years (median 33 years). For 10 out of 11 patients, the indication for the use of Sayana® was contraception. Information on the patients’ weight was available for two cases, with both patients being normal weight. For all patients, the previous method of contraception could be evaluated: five had received another progestin-only depot contraceptive, which was injected intramuscularly every 3 months, another five patients took oral contraceptives and one used a hormonal contraceptive for vaginal administration. For three patients, Sayana® injections were administered by their physician, one patient performed the injections herself, and for seven patients the injections were given by medical practice assistants.
In 4 of the 11 cases (36.4%), the lipodystrophy was already observed after the first injection of Sayana®; another 5 patients had two injections before the ADR was noticed. The latency between the injection of Sayana® and the development of lipodystrophy at the injection site ranged from several days up to 5 months. For the majority of the patients (n = 8, 72.7%) the latency was 2–4 months. At least seven patients received an additional injection after the local ADR. For one patient the “dents” on the thighs were noticed, but not linked to the drug administration.
According to causality assessments by the RPVC, the causality was “certain” in six cases; for the other five cases the causality was “probable”. In seven cases, rechallenge was positive. The site of administration was changed from the thigh to the abdominal wall in three cases; for the other four patients the location was changed on the thighs. In two patients the change of the injection sites from the thigh to the abdominal wall led to a negative rechallenge. Two patients, who had no difficulties with previous injections, developed lipodystrophies after the second and the third application, respectively.
Longitudinal data with follow-up of up to 17 months after the initial report showed in 10 of the 11 cases no amelioration, but persistence of the local reaction. In one patient the “dent” was observable through her clothes. Another patient underwent plastic surgery for artificial infill of the affected area. The result of the injection of autologous fat was reported to be “satisfactory”.
In this retrospective study both international and Swiss pharmacovigilance data regarding severe injection site “dents” due to subcutaneous Sayana® administration were analysed. Additional qualitative aspects, such as latency time and follow-up information, were provided by exploring the 11 regional cases of persistent injection site fat tissue destruction.
“Injection site reaction” is a very nonspecific expression often used as an umbrella term for the encoding of local ADRs. It covers a wide range of reactions: both severe cases of fat tissue destruction and also mild, transient conditions such as skin redness, administration site pain, itchiness or swelling. The latter reactions are very well documented and frequently reported for locally administered drugs. Terms that may cover severe tissue destruction, such as atrophy or necrosis, have been extracted from the reported terms. Since Sayana® is the only subcutaneously administered DMPA preparation in Switzerland, we did not include other DMPA formulations used in other countries into our analyses. In 313 patients Sayana® was administered as monotherapy. All patients with comedications received Sayana® as the only subcutaneously administered drug. Hence, the injection site reactions cannot be imputed to the comedication.
Injection site reactions have been reported to be one of the most common reasons for discontinuation of therapy with DMPA-SC in the United States [9]. In two open-label phase III trials, Jain et al. assessed the safety of DMPA-SC (104 mg / 0.65 ml) for a period of 1 year. Nonallergic injection site reactions were reported in 1.6% of women taking part in the European/Asian trial and in 9.7% of the women in the American trial. The most common injection site reactions were described as injection site pain, granuloma or atrophy and were reported to be mild in intensity for most of the cases [10]. In a prospective case series, 11 of 50 patients (22%) experienced injection site reactions, with 3 patients (6%) developing a “dent” or “dimple” [6]. However, in these studies further information regarding reversibility or persistency of the reactions were not available. According to Prabhakaran et al., further observation regarding the lasting effects of the granulomas and atrophies was needed [6].
Out of our selected subset of 323 ICSRs, 296 ICSRs (91.6%) were classified as “serious”. Also, all 11 regional ICSRs were classified as serious owing to persistent localised atrophy, fat tissue necrosis or lipodystrophy. In 10 of the 11 regional cases the condition had been present for almost a year and therefore seems to be irreversible. In one patient, the affected area could even be seen through clothes. Another patient underwent plastic surgery to replace necrotic tissue. This action illustrates the severity of the situation for the affected patients. Consequently, this aspect was also emphasised by the primary reporters of these ADRs.
Since the mean latency time between exposure (subcutaneous injection) and detection of the dent was found to be between 2 and 4 months, in some patients the tissue atrophy was initially not linked to the drug injection. Therefore, some women received another injection after the event.
The reasons for the occurrence of lipodystrophy after Sayana® injections remain speculative. In none of the cases was a histological examination performed. For the intramuscular administration of Depo-Provera® only injection site swelling and skin colour changes at the administration site are labelled as ADRs in the Swiss product information [11]. Injection site reactions occurred in 8% of the participants who used DMPA-SC in contrast to only 0.4% for the DMPA-IM users in a randomized clinical study carried out by Kaunitz et al. [12]. Dorai et al. have shown in in-vitro studies that synthetic progestins such as medroxyprogesterone acetate show direct lipolytic activity on adipocytes [13]. Since adipocytes are abundant in the subcutaneous tissue, in contrast to the muscular tissue, a possible lipolytic effect cannot be ruled out [5]. Overweight or underweight as potential risk factors could not be analysed, since information regarding the patients’ body mass index was not available in most cases.
The intramuscular formulation of DMPA contains 150 mg / 1ml versus 104 mg / 0.65 ml for the subcutaneous preparation [11]. This results in an overall lower dose, but a higher concentration for the subcutaneous compared with the intramuscular preparation. Aside from having the same active ingredient, there are slight differences in the composition of the excipients in the intramuscular and subcutaneous formulations. In addition to macrogol 3350, polysorbate 80, sodium chloride and water for injection, the subcutaneous preparation contains a sodium-phosphate-buffer. The amino acid methionine and povidone are additional ingredients [2, 11]. Both formulations contain propylparahydroxybenzoate (E216) and methylparahydroxybenzoate (E218) as preserving agents, with a slightly higher concentration of the latter in the subcutaneous preparation (1.6 mg/ml vs 1.35 mg/ml for E218; 0.154 mg/ml vs 0.15 mg/ml for E216) [2, 11].
In a pilot study on self-administration of subcutaneous DMPA for contraception, 19 of 64 women (29.6%) were reported to have experienced difficulties in connection with the injection process [5]. All patients had received injection training before they performed the self-administration. Atrophy, induration and scarring were described in five patients. These conditions occurred most commonly after the second injection. Follow-up until the end of the study showed that one reaction had resolved, two were reported to be almost resolved, another two as ongoing. One case was lost to follow-up [5]. In the study of Prabhakaran et al. 12 of 50 patients (24%) experienced plunger resistance during their initial self-injection and in 17% of all injections device or plunger issues were reported [6]. This might indicate that, even with previous training, the administration process may be challenging for some women. In the regional case series, only one patient performed a self-injection and 10 received the injection either from a medical practice assistant or from their physician. Therefore, self-administration by laypeople might play only a minor role as a risk factor for these irreversible injection site reactions. Since most administrations were performed by medical professionals, insufficient disinfection of the administration site or contamination of the needle are possible, but unlikely. No injection site infection or quality issues regarding the product have been reported for the regional cases. The Swiss product information of Sayana® now labels injection site reactions, including persistent atrophy or lipodystrophy such as the occurrence of dents, nodules or dimples, as common adverse reactions [2]. Although injection site atrophy and the development of a “dent” or “dimple” has been observed in trials with subcutaneous DMPA [5, 6], to our knowledge, no post-marketing cases have been published so far.
A commonly described limitation in pharmacovigilance is the underreporting of ADRs in a spontaneous reporting system, which amounts to up to 94% [14]. The intensity of injection site reactions can vary between mild and severe and the size of the affected area may differ. Milder cases may not have been reported. As a result of the long latency time, the association of the event with the previous injection might not be obvious. Serious ADRs must be reported by law. However, a persistent dent at the site of injection might be subjectively assessed by the attending physician. Since “injection site reaction” is a very nonspecific term and no validation of the coding was possible, ICSRs that are not related to lipodystrophy, atrophy, fat tissue necrosis may have been included in our analysis. However, 91.6% of theses ICSRs were reported as serious.
Completeness of documentation of the ICSRs was not homogenous. ICSRs included in the WHO database had less information, as reflected by completeness scores. In addition, follow-up information was often lacking. Therefore, regional cases, with extensive information, causality assessment and longitudinal follow-up information were included to improve quality. In the WHO database, a causal relationship between drug and ADR could not be formally assessed, and coding or recoding could not be verified.
Administration site reactions during subcutaneous DMPA treatment occur frequently. The evaluation of the ICSRs in the WHO databank and regional cases demonstrate severe tissue damage at the injection site. These reactions include local lipodystrophy and persistent atrophy occurring several weeks to months after subcutaneous injection. These ADRs were recently integrated in the Swiss product information of Sayana®. Since these adverse effects can be a substantial burden for the young patients, physicians should be aware of them and include this information into their risk-benefit assessment for individual patients. Patients should also be informed and advised about these recently labelled and potentially irreversible adverse drug reactions at the injection site.
We would like to thank all physicians who reported the cases to the regional pharmacovigilance centre and provided supplementary information for their contributions.
The data for this study were obtained both from the WHO Collaborating Centre for International Drug Monitoring, Uppsala, Sweden and the Swiss health authority, Swissmedic, Bern, Switzerland. Owing to different reporting policies worldwide, data derived from spontaneous reporting is inhomogeneous, and there may be underreporting and reporting bias. The information contained in this study is therefore inhomogeneous with respect to origin and also to likelihood that Sayana® caused the adverse reactions. All conclusions drawn from these data do not necessarily represent the opinion of the World Health Organization.
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Royer PA , Jones KP . Progestins for contraception: modern delivery systems and novel formulations. Clin Obstet Gynecol. 2014;57(4):644–58. https://doi.org/10.1097/GRF.0000000000000072
2Product Information Sayana©, Pfizer AG, available from www.swissmedicinfo.ch, [updated Oct. 2014, accessed 2015-09-16].
3 Dragoman MV , Gaffield ME . The safety of subcutaneously administered depot medroxyprogesterone acetate (104mg/0.65mL): A systematic review. Contraception. 2016;94(3):202–15. https://doi.org/10.1016/j.contraception.2016.02.003
4 Swissmedic. Arzneimittel Statistik. Swissmedic Journal. 2012;4:347.
5 Cameron ST , Glasier A , Johnstone A . Pilot study of home self-administration of subcutaneous depo-medroxyprogesterone acetate for contraception. Contraception. 2012;85(5):458–64. https://doi.org/10.1016/j.contraception.2011.10.002
6 Prabhakaran S , Sweet A . Self-administration of subcutaneous depot medroxyprogesterone acetate for contraception: feasibility and acceptability. Contraception. 2012;85(5):453–7. https://doi.org/10.1016/j.contraception.2011.09.015
7Uppsala Monitoring Centre. VigiLyze. https://vigilyze.who-umc.org/. (accessed 2016-01-01).
8Swissmedic [Internet]. Market Surveillance, Pharmacovigilance. Available from https://www.swissmedic.ch/marktueberwachung/00135/00160/index.html?lang=en (accessed 2016-05-29).
9Micromedex® (Healthcare Series), (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com/ (accessed 2015-09-16).
10 Jain J , Jakimiuk AJ , Bode FR , Ross D , Kaunitz AM . Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70(4):269–75. https://doi.org/10.1016/j.contraception.2004.06.011
11Product Information Depo-Provera© 150, Pfizer AG [Internet], available from www.swissmedicinfo.ch, [updated Oct. 2014, accessed 2016-05-29].
12 Kaunitz AM , Darney PD , Ross D , Wolter KD , Speroff L . Subcutaneous DMPA vs. intramuscular DMPA: a 2-year randomized study of contraceptive efficacy and bone mineral density. Contraception. 2009;80(1):7–17. https://doi.org/10.1016/j.contraception.2009.02.005
13 Dorai V , Hazard MC , Paris J , Delansorne R . Lipolytic activity of progesterone and synthetic progestins on rat parametrial adipocytes in vitro. J Pharmacol Exp Ther. 1991;258(2):620–5.
14 Hazell L , Shakir SA . Under-reporting of adverse drug reactions : a systematic review. Drug Saf. 2006;29(5):385–96. https://doi.org/10.2165/00002018-200629050-00003
Annika Jödicke and Hendrike Dahmke contributed equally. All authors had access to the data and a role in writing the manuscript.
No financial support and no other potential conflict of interest relevant to this article was reported.