“Real world” experience in cardiac resynchronisation therapy at a Swiss tertiary care centre: update 2016
DOI: https://doi.org/10.4414/smw.2017.14425
Stephan
Winnika, Christian
Elsenera, Burkhardt
Seifertb, Christoph
Starckc, Agnes
Strauba, Ardan M.
Sagunera, Alexander
Breitensteina, Nazmi
Krasniqia, Markus J.
Wilhelmd, Laurent
Haegelia, Firat
Durua, Stefano
Benussid, Francesco
Maisanod, Thomas F.
Lüschera, Johannes
Holzmeistera, David
Hürlimanna, Frank
Ruschitzkaa, Jan
Steffela
aUniversity Heart Centre Zurich, Department of Cardiology, Zurich,
bEpidemiology, Biostatistics and Prevention Institute, University of Zurich,
cDeutsches Herzzentrum Berlin, Charité Berlin,
dUniversity Heart Centre Zurich, Department of Cardiovascular Surgery, Zurich,
“Real world” experience in cardiac resynchronisation therapy at a Swiss tertiary care centre: update 2016
Summary
BACKGROUND
Based on a reduction in morbidity and mortality, cardiac resynchronisation therapy (CRT) has evolved as a standard therapy for patients with advanced heart failure.
OBJECTIVE
To provide insight into patient demographics, safety, echocardiographic remodelling and long-term follow-up of patients treated with CRT in a “real-world” setting at a Swiss tertiary care centre.
METHODS
Patients implanted with a CRT device at the University Heart Centre Zurich between 2000 and 2015 were consecutively enrolled. Initial clinical and echocardiographic therapy response as well as long-term follow-up for mortality (defined as all-cause death, heart transplantation or ventricular assist device implantation) and hospitalisation for heart failure were assessed.
RESULTS
A total of 418 patients with a median age of 66 years at the time of CRT implantation (78% male) were enrolled. Serious peri-interventional complications (from the time of implantation up to 14 days thereafter) were rare and included systemic infections in 2.4%, pneumothorax in 3.3% and haematoma requiring revision in 2.2% of cases. Overall, the Kaplan-Meier estimate for 5-year freedom from the composite endpoint (hospitalisation for heart failure or mortality) was 55.8%; the Kaplan-Meier estimate for 5-year freedom from mortality was 64.1%. CRT was associated with a significant symptomatic improvement and left ventricular reverse remodelling.
Overall, 3.9% of patients did not respond to cardiac resynchronisation therapy (decline in left ventricular ejection fraction [LVEF] >5%), whereas 35.1% experienced neither a continued decline nor a relevant improvement of LVEF (±5%). In the remaining 61% of patients we observed an improvement in LVEF of more than 5%. Forty percent and 31% of patients were super responders, defined as an absolute LVEF improvement of ≥10% and by a relative reduction of left ventricular end-diastolic volume index by 20% or more. Super-response to CRT was associated with a significant benefit in terms of survival and rehospitalisation rates.
CONCLUSION
Our data are consistent with large multicentre trials and indicate that CRT is similarly effective in a real-world setting in Switzerland.
Introduction
On the basis of accumulating evidence demonstrating its safety and effectiveness, cardiac resynchronisation therapy (CRT) has emerged as a standard of care in patients with heart failure and severely reduced left ventricular ejection fraction (HFrEF) [1–5]. During recent years, however, selection criteria for CRT have changed substantially. Initially, the benefit of CRT was demonstrated for highly symptomatic patients (New York Heart Association [NYHA] class III and ambulatory IV) with a left ventricular ejection fraction (LVEF) ≤35% [1–3]; growing evidence has since shown a reduction in morbidity and mortality also in oligosymptomatic patients (NYHA II) [4, 5]. Hence, current guidelines for the diagnosis and treatment of acute and chronic heart failure recommend CRT as standard therapy in symptomatic patients with an LVEF ≤35% despite optimal medical therapy and a QRS complex ≥130 ms [6]. In contrast, patients with a narrow QRS complex (<120 ms and <130 ms) do not seem to benefit from CRT and may, in some cases, even derive harm [7, 8].
Whether the positive results observed in the above-mentioned large, randomised, controlled trials also translate to patients selected in daily clinical practice in Switzerland is less clear [9]. Indeed, a considerable number of patients do not respond to CRT [9, 10]. Therefore, observational research in a “real-world” setting, i.e. registries and surveys, is important to assess the safety and efficacy of evolving therapies in daily clinical practice [9, 11, 12]. Consecutive enrolment into such registries largely avoids the often criticised selection bias of interventional trials. In the current study we aimed to provide an update to our previously published results [9, 13], not only with respect to patient demographics, but also regarding periprocedural safety, echocardiographic response and long-term outcome.
Methods
Study population and follow-up
From November 2000 to July 2015 all patients implanted with a CRT device at the University Heart Centre Zurich and who provided informed consent were consecutively enrolled. Patients were followed up for clinical and echocardiographic improvement (left ventricular remodelling), as well as for hospitalisation for heart failure and mortality (defined as death from any cause, heart transplantation or ventricular assist device [VAD] implantation). Clinical and echocardiographic follow-up was performed in-house or by the patients’ private cardiologist. Long-term follow-up involved a telephone interview and/or chart review, either in-house or in cooperation with the family physician. Definition of clinical and echocardiographic therapy response as well as definition of outcomes (all-cause death, heart transplantation or ventricular assist device implantation and hospitalisation for heart failure) was defined post hoc. The study was approved by the local ethics committee (KEK-ZH-NR: 2011-0304). All enrolled patients provided informed consent.
CRT implantation
The vast majority of transvenous leads were implanted under local anaesthesia with mild conscious sedation. Devices and leads of the vendors Biotronik, Guidant / Boston Scientific, Medtronic, St. Jude Medical, and Sorin / LivaNova were implanted. After intubation of the coronary sinus, a coronary sinus venogram was obtained during transient balloon occlusion of the coronary sinus in order to visualise vessel anatomy. Target veins were either lateral or posterolateral coronary veins, to achieve optimal separation of right and left ventricular pacing. Left ventricular leads were advanced into the target vein in an over-the-wire technique. When transvenous coronary sinus lead placement was not possible because of either anatomical or technical obstacles, an epicardial lead was placed via a separate approach. Documented peri-interventional complications included any complication between the time of device implantation and 14 days thereafter. Decision to implant a CRT-pacemaker (CRT-P) or CRT-defibrillator (CRT-D) was left to the clinical judgment of the treating physician, based on a patient-centred approach taking into account individual risk stratification, physician experience, cost-effectiveness and patient expectations.
Echocardiographic super-response was defined post-hoc as either an absolute improvement of LVEF by ≥10% or a relative reduction of the left ventricular end-diastolic volume index (LVEDVI) by 20% or more.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges (IQRs), if not indicated otherwise. Categorical and ordinal variables are presented as patient number per total number and percentage. Pre- and postoperative values of continuous and ordinal variables were compared using Wilcoxon signed rank tests. All p-values are two-sided. Survival curves for time-to-event variables were constructed using Kaplan-Meier estimates based on all available data. Survival curves of different patient groups were compared using log-rank tests. Significance was accepted for p <0.05. All statistical analyses were performed in IBM SPSS Statistics, version 22.
Results
Baseline characteristics
Between November 2000 and July 2015, 418 patients were implanted with a CRT device. Median age at the time of implantation was 66 years. The leading cause of cardiomyopathy was ischaemic (n = 175, 41.9%), followed by dilative cardiomyopathy (n = 157, 37.6%). Median time from diagnosis of heart failure (HF) to CRT implantation was 14 months. Baseline characteristics were rather constant across the different time periods of device implantation (table 1 and supplementary table S1 in appendix).
Table 1 Patient characteristics at baseline (the time of cardiac resynchronisation therapy implantation).
| Age at implantation – years |
66 (58–73) |
| Male sex – no. / total no. (%) |
325/418 (77.8) |
| Height – m |
1.72 (1.65–1.77) |
| Weight – kg |
78 (68–88) |
| BMI – kg/m2
|
26.6 (23.6–30.3) |
| Body surface area – m2
|
1.93 (1.79–2.07) |
| Ischaemic cardiomyopathy – no. / total no. (%) |
175/418 (41.9) |
| Blood pressure while sitting – mm Hg |
|
| Systolic |
114 (102–128) |
| Diastolic |
70 (60–77) |
| Heart rate – min-1
|
72 (63–81) |
| Creatinine – μmol/l |
106 (88–140) |
| proBNP – ng/ml |
2138 (981–4581) |
| Na+ – mmol/l |
139 (137–141) |
| K+ – mmol/l |
4.2 (3.9–4.6) |
| NYHA class – no. / total no. (%) |
|
| I |
24/414 (5.8) |
| II |
108/414 (26.1) |
| III |
250/414 (60.4) |
| ambulatory IV |
27/414 (6.5) |
| Systemic arterial hypertension – no. / total no. (%) |
222/418 (53.1) |
| Prior stroke – no. / total no. (%) |
35/417 (8.4) |
| Diabetes – no. / total no. (%) |
105/418 (25.1) |
| Coronary artery disease – no. / total no. (%) |
209/417 (50.0) |
| Chronic obstructive lung disease – no. / total no. (%) |
37/418 (8.9) |
| Time of diagnosis to CRT implantation – months |
14.1 (2.1–73.1) |
| Left ventricular ejection fraction – %, n = 412 |
26 (20–32) |
| Sinus rhythm – no. / total no. (%) |
293/417 (70.3) |
| QRS duration – ms |
151 (130–170) |
| QRS ≤120 ms – no. / total no. (%) |
57/342 (16.7) |
| QRS >120 ms to ≤150 ms – no. / total no. (%) |
111/342 (32.5) |
| QRS >150 ms – no. / total no. (%) |
174/342 (50.9) |
| Left bundle-branch block – no. / total no. (%) |
247/344 (71.8) |
At the time of CRT implantation, the majority of patients were on state-of-the-art medical heart failure therapy with angiotensin converting-enzyme (ACE) inhibitors or angiotensin receptor blockers (90.7%), beta blockers (80.1%), loop diuretics (74.6%), and aldosterone antagonists (51.7%) (table S2). Most patients were symptomatic, with dyspnoea NYHA class II (n = 108, 26.1%) or class III (n = 250, 60.4%) (table 1). Median LVEF was 26% (table 1, table S3). A baseline electrocardiogram revealed sinus rhythm in 70.4% of patients (n = 293), atrial fibrillation in 11% (n = 46), and a paced rhythm in 18.7% (n = 78). Excluding paced patients, 16.7% (n = 57) had a QRS width <120 ms. The majority of patients (71.8%, n = 247) had left bundle-branch block; only a minority of 5.5% (n = 19) presented with a right bundle-branch block (table 1, table S4).
Implantation procedure
Initial left ventricular lead placement was successful in 88% of patients (n = 366). In the remaining 52 patients (12.4%), left ventricular lead placement was successful during a second attempt. Overall, 11.5% (n = 48) received an epicardial left ventricular lead via a surgical approach. Of these 48 surgical left ventricular lead placements, 16 were primary surgical approaches, whereas 32 were performed after an initial, unsuccessful transvenous attempt (table S5A). The median duration of the associated hospital stay was 2 days (table S5A). Complications, which were documented from the time of device implantation until 14 days thereafter, occurred in a limited number of cases (table S5B). The most common complications were left ventricular lead dislocation (5.5%, n = 23), infection (local 2.2%, n = 9; systemic, necessitating device extraction 2.4%, n = 10), pneumothorax (3.3%, n = 14), and haematoma necessitating revision (2.2%, n = 9). There were no peri-interventional deaths. In 10.6% of cases (n = 44) post-interventional diaphragmatic capture occurred. Reprogramming was sufficient to abolish diaphragmatic capture in the majority of cases (6.2%, n = 26); left ventricular lead repositioning was successfully performed in 5 patients (1.2%). In 11 patients (2.6%) with only intermittent diaphragmatic capture, no intervention was necessary, and in the remaining 2 patients (0.5%) left ventricular pacing had to be discontinued (table S5B).
Postoperative course and echocardiographic follow-up
At clinical follow-up after a median time of 5.9 months from CRT implantation, which was available for 305 patients (73%), 63.5% of patients reported symptomatic improvement of at least one point on the NYHA scale. Overall, the proportion of patients in lower NYHA categories increased significantly compared with the NYHA distribution before CRT implantation (fig. 1).
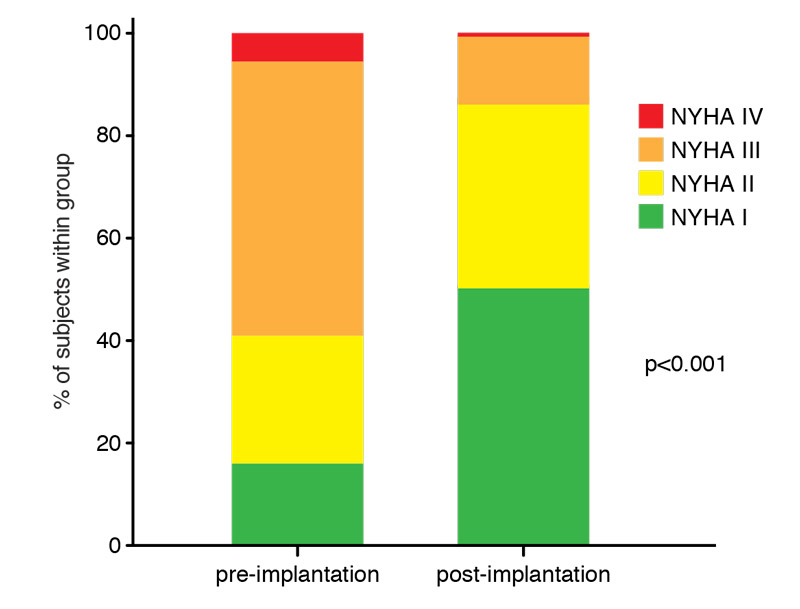
Figure 1 Clinical effects of cardiac resynchronisation therapy (CRT). Distribution of New York Heart Association (NYHA) class before and after CRT implantation (data available in 305 patients, 73%). Median follow-up time of 6 months. Wilcoxon signed rank test.
Echocardiographic follow-up was available for 369 patients (88.3%). A median absolute increase in LVEF of 7% from 26% to 33% (fig. 2A) was observed across the entire study group. Only 3.9% of patients experienced a continued decline in LVEF (>5%) and, thus, did not respond to CRT. In 35.1% of patients we observed neither a continued decline nor a relevant improvement of LVEF (±5%). In the remaining 61% of patients we observed an improvement of the LVEF of more than 5%. Based on an absolute improvement of LVEF of ≥10%, 40% of patients (n = 146/365) were considered super-responders. Based on a relative reduction of the LVEDVI by 20% or more, 31% of patients (n = 50/288) were super-responders. Fifty-one percent of patients demonstrated a relevant decline (reduction of >10%), whereas 38% had no relevant change in LVEDVI (±10%) and 11% experienced an increase in LVEDVI by 10% or more. Over the entire population median LVEDVI, left ventricular endsystolic volume index (LVESVI), and left atrial endsystolic diameter (LAESD) were significantly reduced (fig. 2A). Mitral regurgitation also improved following CRT implantation (fig. 2B). Interestingly, we observed a reduction of the systolic right ventricular over right atrial pressure from a median of 30 mm Hg to a median pressure of 26 mm Hg (fig. 2A). No difference was found in right ventricular fractional area change and tricuspid annular movement (fig. 2A).
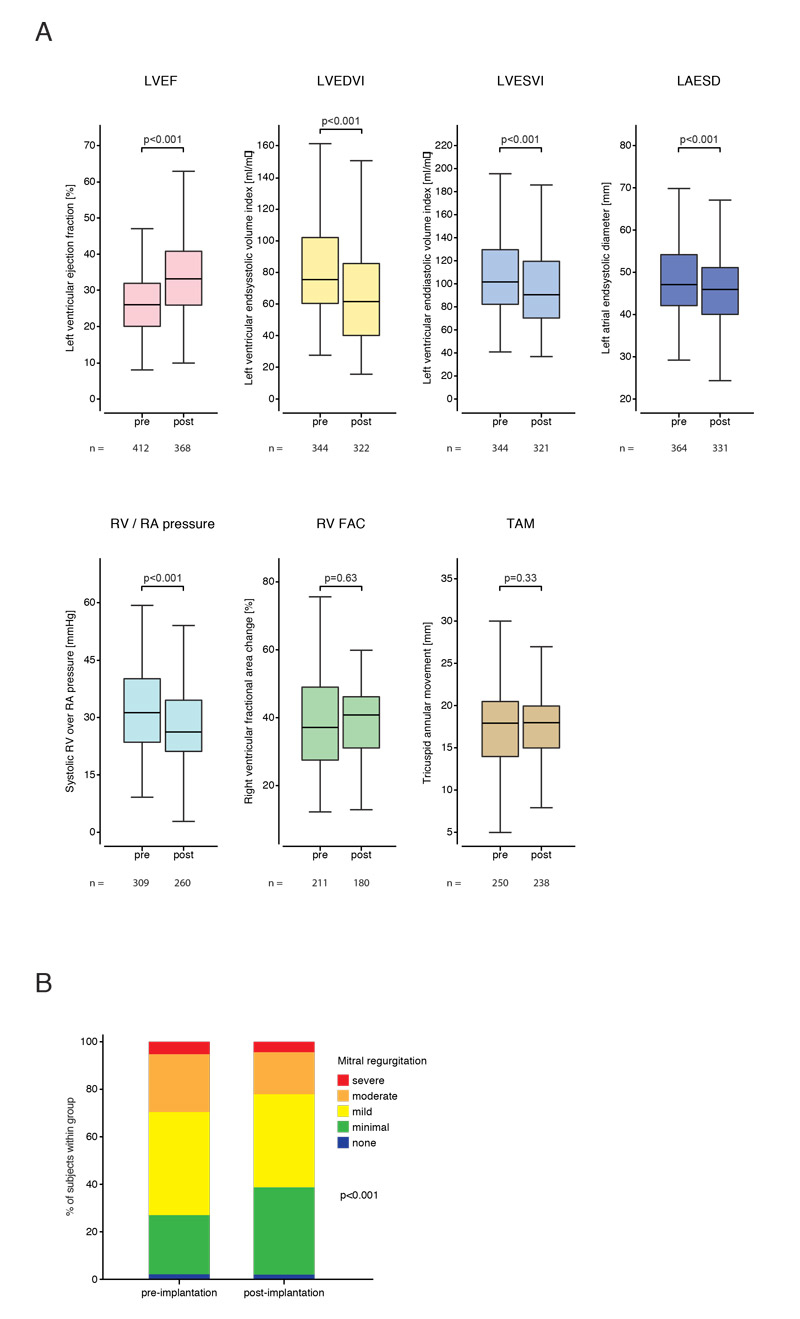
Figure 2 Effects of cardiac resynchronisation therapy (CRT) on echocardiographic reverse remodelling. Comparisons pre- vs post-CRT implantation of echocardiographic parameters of cardiac remodelling. (A) Comparisons of LVEF (left ventricular ejection fraction), LVEDVI (left ventricular end-diastolic volume index), LVESVI (left ventricular endsystolic volume index), LAESD (left atrial endsystolic diameter), RV/RA (systolic right ventricular over right atrial pressure), RV RAC (right ventricular fractional area change), TAM (tricuspid annular movement). (B) Comparison of mitral regurgitation. Wilcoxon singed rank tests. Box plots show interquartile ranges, whiskers indicate minimum and maximum values. Median follow-up time of 6 months. Follow-up echocardiographic data were available for 369 patients (88.3%).
Excluding patients with right ventricular pacing at baseline, we observed consistently higher proportions of positive echocardiographic response in those patients with a left bundle-branch block morphology compared with those without (table S6).
Long-term clinical follow-up
Patients were followed-up for rehospitalisation for heart failure, heart transplantation, implantation of a left ventricular assist device (LVAD) and death for a median time of 3.6 years (IQR 1.9–5.7 years) after CRT implantation. The Kaplan-Meier estimate for freedom from the composite endpoint of hospitalisation for heart failure and mortality (all-cause death, heart transplantation or ventricular assist device [VAD] implantation) at 5 years after CRT implantation was 55.8%. The Kaplan-Meier estimate for freedom from mortality (all-cause death, heart transplantation or VAD implantation) at 5 years after CRT implantation was 64.1% (fig. 3, table 2).
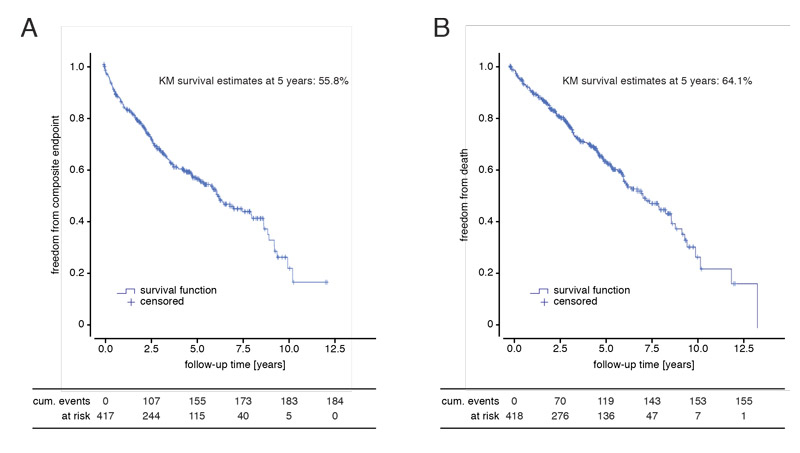
Figure 3 Follow-up for survival and rehospitalisation for congestive heart failure. Kaplan-Meier survival estimates for (A) the composite endpoint of hospitalisation for heart failure or all-cause mortality (defined as death, heart transplantation or ventricular assist device implantation) and (B) the endpoint of mortality alone. Follow-up began at the time point of cardiac resynchronisation therapy implantation. Median follow-up time was 3.6 years (interquartile range 1.9–5.7).
Table 2 Endpoints during long-term follow-up.
| Death – no. / total no. (%) |
140/418 (33.5) |
| Heart transplantation – no. / total no. (%) |
16/418 (3.8) |
| VAD implantation – no. / total no. (%) |
14/418 (3.3) |
| First hospitalisation for heart failure – no. / total no. (%) |
101/399 (25.3) |
Both groups of super responders – patients with an absolute LVEF improvement of ≥10%, and patients with a relative LVEDVI reduction of ≥20% – had a significantly improved survival and freedom from hospitalisation for heart failure compared with the corresponding control groups (fig. 4, table 2). Accordingly, 5-year estimates both for freedom from the composite endpoint of mortality or hospitalization for heart failure, as well as for mortality alone were higher in either super-responder group compared to the respective remaining patient population (fig. 4, table 2).
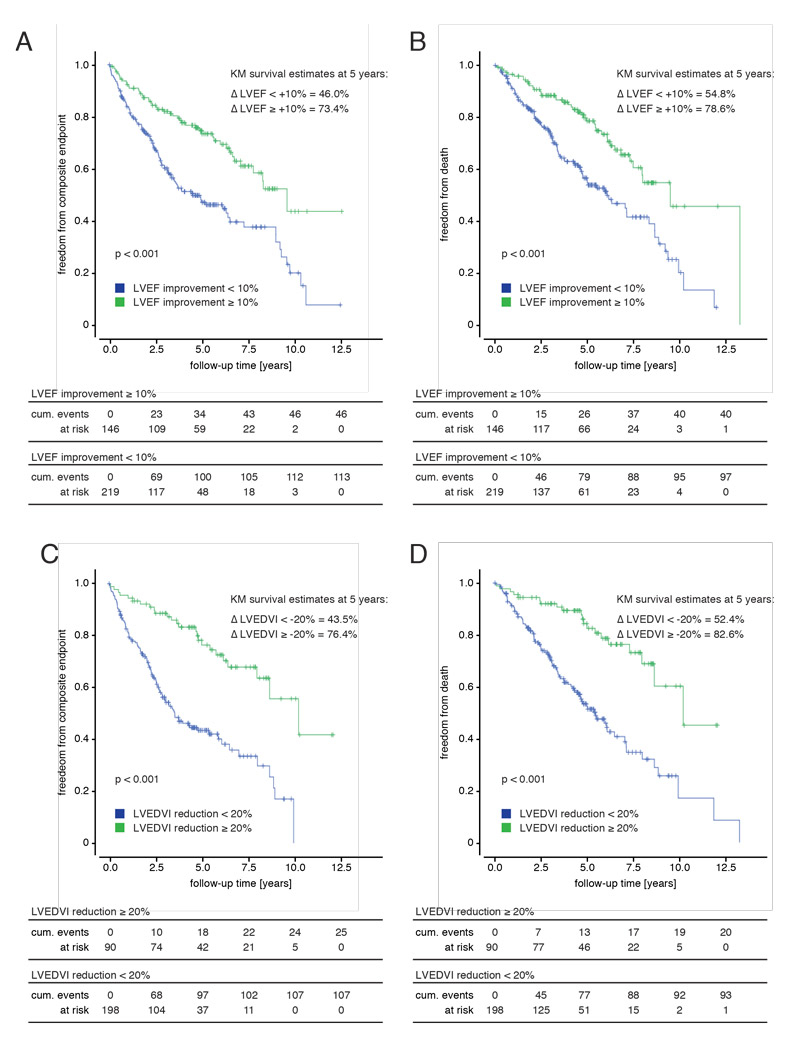
Figure 4 Subgroup analyses for super-responders to cardiac resynchronisation therapy (CRT). Kaplan-Meier survival estimates for the composite endpoint of hospitalisation for heart failure and mortality (defined as death, heart transplantation or ventricular assist device implantation) as well as for mortality alone. (A, B) Comparison of the subgroups of super-responders by left ventricular ejection fraction (LVEF) (absolute LVEF increase of 10% or greater) with non-super-responders. (C, D) Comparison of super-responders by left ventricular end-diastolic volume index (LVEDVI) (reduction of 20% or greater) with non-super-responders. Groups were compared with log rank tests. Follow-up began at the time point of CRT implantation. Median follow-up time was 3.6 years (interquartile range 1.9–5.7).
Discussion
The present real-world experience of patients with CRT supports the beneficial effects of CRT observed in large randomised trials. CRT is associated with a marked symptomatic improvement and left ventricular reverse remodelling in patients with advanced heart failure. A substantial number of patients were super-responders, which was associated with a significant survival benefit compared with the remaining patient population. Only a few patients did not profit from CRT and experienced a continued decline in left ventricular function.
CRT has evolved as an integral therapeutic modality for patients with advanced heart failure and reduced ejection fraction. However, there is often uncertainty as to whether patient populations from large clinical trials truly represent the patients encountered in daily clinical practice. Therefore, in order to evaluate the benefit of comparatively novel therapies such as CRT, it is prudent to analyse their safety and effectiveness under real-world conditions.
Constant technical advancement of the material (guiding catheters, leads) has made the implantation of left ventricular leads easier than in the early years of CRT. In our current cohort, left ventricular lead placement was successful during the initial session in 87.5% of cases. Importantly, transvenous left ventricular lead placement was performed only in presence of an appropriate target vessel (lateral or posterolateral vein). Pro forma implantation, such as into an anterior cardiac vein, which has been shown to be of little benefit in cardiac resynchronisation [14, 15], was avoided. In such cases an epicardial lead was surgically implanted via mini-thoracotomy, if surgical risk was deemed acceptable.
Independent of its beneficial effect on morbidity and mortality, CRT implantation is an invasive procedure and, therefore, is associated with a certain risk of periprocedural complications. Overall, complications occurred in a limited number of cases and were comparable with other registries, such as the European CRT Survey [12]. Of note, the European registry reported complications only until hospital discharge, whereas our registry included complications up to 14 days after CRT implantation. Importantly, the necessity of left ventricular lead repositioning due to phrenic nerve capture or insufficient left ventricular capture has become increasingly rare since the use of quadripolar leads began in 2011.
The current registry included 418 CRT implantations at our centre. Consecutive enrolment reduced any possible bias to a minimum. The demographics of our cohort are very similar to those of the large clinical trials and also closely resemble the baseline characteristics of the patient cohort in the European CRT Survey (which included 2438 patients enrolled at 141 centres in 13 countries) [12]. Interestingly, patient demographics remained largely unchanged compared with the beginning of data collection in 2010 [16], and represent a typical heart failure population.
Before the EchoCRT trial, a number of small studies suggested that patients with echocardiographic dyssynchrony and a narrow QRS complex (<120 ms) may profit from CRT [17–20]. However, EchoCRT demonstrated the opposite: CRT may even increase mortality in patients with a narrow QRS complex, irrespective of echocardiographically evident mechanical dyssynchrony [7]. The reasons for this finding are the subject of current research and ongoing analyses. The substantial number of patients with a QRS >120 ms in our overall cohort (85%) largely reflects the practice of CRT implantation prior to EchoCRT. Since then, only a few patients with narrow QRS received CRT for specific indications, such as an expected high percentage of right ventricular pacing in the presence of an atrioventricular block [21] or upgrades from chronic right ventricular pacing [22] as these patient subgroups may benefit more from biventricular pacing than from right ventricular pacing only.
Our data further indicate that in this real-world cohort of consecutively included patients, CRT results in substantial reverse left ventricular remodelling and marked clinical improvement. In the absence of a uniform definition of super-response, two frequently employed cut-offs were used, yielding very similar results. Up to 40% of patients turned out to be super-responders, who in turn displayed a survival benefit. In the majority of the remaining patients, further deterioration of left ventricular function could be avoided, which in itself may be considered a form of response to CRT [23]. CRT had probably no effect on the natural course of declining left ventricular function in only a minority of patients (4 to 11%). Importantly, however, overall Kaplan-Meier survival estimates constitute a 5-year freedom from the composite endpoint of all-cause death, VAD implantation, heart transplantation or hospitalisation for heart failure of 55.8%, and a 5-year survival free of heart transplantation or LVAD implantation of 64.1% – both of which are at least as high as those in the available large clinical trials or registries [11, 12, 24, 25].
Limitations
The current study has to be interpreted in the light of the following limitations, most of which are inherent to any single-centre real-world observational study. All patients were recruited at a single centre, which may introduce a selection, as well as a referral bias. Clinical and echocardiographic follow-up is incomplete, which is owing to frequent patient referral from out-patient care-givers, limiting at least in part accessibility to external patient data. Importantly, however, long-term follow-up for hard endpoints including survival and hospitalisation for worsening heart failure are very complete. As a result of the consecutive enrolment of patients until July 2015, follow-up time ranges from <6 months (7.7% of patients) to 13.2 years. Median follow-up time was 3.6 years. Moreover, echocardiographic response to CRT based on LVEF is inherently limited by intra-observer variability, which is reported to range from 6 to 10%. Therefore, we defined any change in LVEF of ±5% as “no relevant change”. Echocardiographic super-response lacks a universal definition. We therefore applied two different definitions of different sensitivity: an absolute LVEF increase of >10% and, more sensitive, a relative LVEDVI reduction of >20%. Importantly, both definitions were associated with a significantly better outcomes for survival and rehospitalisation due to worsening heart failure.
Implications and perspectives
CRT remains an important part of current heart failure therapy. Based on the results of landmark clinical trials, patient selection for CRT is continuously optimised, resulting in a further refinement of patients selected for this therapy. Our real-world analysis from a large Swiss tertiary centre confirmed the findings of pivotal clinical trials and show that, in a real-world setting of everyday clinical practice, CRT is safe and effective. In order for patients to derive the maximum benefit from this important therapy, implantation at centres with sufficient volume and experience in the implantation procedure and the dedicated follow-up of these patients is critical [11, 26].
Appendix: Supplementary tables
Table S1 Patient characteristics at baseline stratified according to the time period of cardiac resynchronisation therapy device implantation.
|
Patient group (no. / % total)
|
2000–2005
(35/8.4)
|
2006-2010
(204/48.8)
|
2011-2015
(179/42.8)
|
| Age at implantation – years |
64 (57–72) |
66 (58–72) |
68 (60–74) |
| Male sex – no. / total no. (%) |
35/35 (n.a.) |
152/204 (74.5) |
138/179 (77.1) |
| Height – m |
1.74 (1.7–1.78) |
1.72 (1.65–1.77) |
1.72 (1.64–1.77) |
| Weight – kg |
85 (74–96) |
79 (70–88) |
76 (66–88) |
| BMI – kg/m2
|
26.9 (24.2–32.3) |
26.9 (24.1–30.3) |
25.7 (22.7–29.4) |
| Body surface area – m2
|
2.02 (1.88–2.16) |
1.95 (1.81–2.07) |
1.90 (1.75–2.04) |
| Ischaemic cardiomyopathy – no. / total no. (%) |
17/35 (48.6%) |
94/204 (46.1) |
64/179 (35.8) |
| Blood pressure while sitting – mm Hg |
|
|
|
| Systolic |
111 (110–126) |
111 (104–125) |
118 (100–132) |
| Diastolic |
70 (60–75) |
70 (62–78) |
68 (58–76) |
| Heart rate – min-1
|
72 (68–80) |
72 (64–80) |
70 (62–81) |
| Creatinine – μmol/l |
126 (105–145) |
107 (88–139) |
101 (86–140) |
| proBNP – ng/ml |
1808 (1030–3485) |
2138 (988–4704) |
2308 (944–4207) |
| Na+ – mmol/l |
137 (136–140) |
140 (138–141) |
139 (137–140) |
| K+ – mmol/l |
4.3 (3.9–4.7) |
4.2 (3.8–4.5) |
4.2 (3.9–4.7) |
| NYHA classification – no. / total no. (%) |
|
|
|
| I |
1/35 (2.9) |
11/202 (5.5) |
17/177 (9.6) |
| II |
11/35 (31.4) |
36/202 (17.8) |
61/177 (34.5) |
| III |
22/35 (62.9) |
140/202 (69.3) |
88/177 (49.7) |
| ambulatory IV |
1/35 (2.8) |
15/202 (7.4) |
11/177 (6.2) |
| Systemic arterial hypertension – no. / total no. (%) |
19/35 (54.3) |
97/204 (47.6) |
109/179 (60.9) |
| Prior stroke – no. / total no. (%) |
1/35 (2.9) |
18/203 (8.9) |
16/179 (8.9) |
| Diabetes – no. / total no. (%) |
9/35 (25.7) |
51/204 (25.0) |
45/179 (25.1) |
| Coronary artery disease – no. / total no. (%) |
17/35 (48.6) |
96/203 (47.3) |
96/179 (53.6) |
| Chronic obstructive lung disease – no. / total no. (%) |
3/35 (8.6%) |
19/204 (9.3) |
15/179 (8.4) |
| Time of diagnosis to CRT implantation – months |
8.9 (0.7–50.8) |
6.5 (1.7–46.3) |
30.6 (4.7–108.7) |
| Left ventricular ejection fraction – %, total n = 412 |
20 (17–28) |
17 (20–31) |
28 (21–33) |
| Sinus rhythm – no. / total no. (%) |
15/34 (44.1) |
145/204 (71.1) |
133/179 (74.3) |
| QRS duration – ms |
170 (137–193) |
155 (130–180) |
157 (134–176) |
| Left bundle-branch block – no. / total no. (%) |
17/35 (48.6%) |
121/204 (59.3) |
117/179 (65.4) |
Table S2 Cardiac medication at the time of cardiac resynchronisation therapy device implantation.
|
No. / total no. (%)
|
| ACEI/ARB |
379/417 (90.9) |
| Beta blockers |
335/417 (80.3) |
| Aldosterone antagonists |
216/416 (51.9) |
| Loop diuretics |
312/408 (76.5) |
| Thiazide diuretics |
72/404 (17.8) |
| Nitrates |
50/415 (12.0) |
| Digitalis |
54/414 (13.0) |
| Amiodarone |
75/414 (18.1) |
| Aspirin |
193/417 (46.3) |
| ADP antagonists |
55/417 (13.2) |
| Oral anticoagulation |
200/417 (48) |
| Lipid lowering therapy |
247/416 (59.4) |
| Calcium antagonists |
24/414 (5.8) |
Table S3 Echocardiographic characteristics at the time of cardiac resynchronisation therapy device implantation.
| Left ventricular ejection fraction – % (n = 412) |
26 (20–32) |
| Left ventricular end-diastolic volume – ml/m2 (n = 344) |
100.9 (82.0–128.8) |
| Left ventricular endsystolic volume – ml/m2 (n = 344) |
73.4 (57.9–100.8) |
| Left ventricular end-diastolic diameter – mm (n = 374) |
65 (60–72) |
| Left ventricular endsystolic diameter – mm (n = 360) |
55 (48–63) |
| Left ventricular fractional shortening – % (n = 358) |
16 (11–21) |
| Right ventricular diastolic area – cm2 (n = 201) |
19 (15–25) |
| Right ventricular fractional area change – % (n = 211) |
38 (37–48) |
| Tricuspid Annulus Movement – mm (n = 250) |
17 (13–20) |
| Systolic RV over RA pressure – mm Hg (n = 309) |
30 (23–40) |
| Mitral insufficiency– no. / total no. (%) |
|
| None |
10/389 (2.6) |
| Grade 1 |
101/389 (26) |
| Grade 2 |
154/389 (39.6) |
| Grade 3 |
101/389 (26) |
| Grade 4 |
23/389 (5.9) |
| Diastolic dysfunction – no. / total no. (%) |
|
| None |
35/198 (17.7) |
| Grade 1 (abnormal relaxation) |
82/198 (41.4) |
| Grade 2 (pseudonormal relaxation pattern) |
23/198 (11.6) |
| Grade 3 (reversible restrictive relaxation pattern) |
34/198 (17.2) |
| Grade 4 (irreversible restrictive relaxation pattern) |
24/198 (12.1) |
Table S4 Electrocardiographic characteristics at the time of cardiac resynchronisation therapy device implantation.
| Rhythm |
|
| Sinus rhythm – no. / total no. (%) |
293/417 (70.3) |
| Atrial fibrillation – no. / total no. (%) |
46/417 (11) |
| Right ventricle paced – no. / total no. (%) |
78/417 (18.7) |
| Atrioventricular conduction |
|
| PQ – ms |
189 (165–214) |
| AVB I° – no. / total no. (%) |
95/410 (23.2) |
| AVB II° Mobitz 1 – no. / total no. (%) |
3/410 (0.7) |
| AVB II° Mobitz 2 – no. / total no. (%) |
7/410 (1.7) |
| AVB III° – no. / total no. (%) |
25/410 (6.0) |
| Intraventricular conduction |
|
| QRS – ms |
151 (130–170) |
| QRS ≤120 ms – no. / total no. (%) |
57/342 (16.7) |
| QRS >120 ms to ≤150 ms – no. / total no. (%) |
111/342 (32.5) |
| QRS >150 ms – no. / total no. (%) |
174/342 (50.9) |
| No BBB – no. / total no. (%) |
45/344 (13.1) |
| Incomplete RBBB – no. / total no. (%) |
3/344 (0.9) |
| RBBB – no. / total no. (%) |
19/344 (5.5) |
| Incomplete LBBB – no. / total no. (%) |
7/344 (2.0) |
| LBBB – no. / total no. (%) |
247/344 (71.8) |
| Unspecific intraventricular block – no. / total no. (%) |
21/344 (6.1) |
Table S5
A: Perioperative characteristics and devices.
| Time of surgery – min |
100 (72–130) |
| Time of fluoroscopy – min |
15.5 (11.5–23.7) |
| CRT-D – no. / total no. (%) |
366/418 (87.6) |
|
De novo implantation – no. / total no. (%) |
265/418 (63.4) |
| PM upgrade – no. / total no. (%) |
124/418 (29.7) |
| ICD upgrade – no. / total no. (%) |
29/418 (6.9) |
| Successful LV lead placement in initial attempt (transvenous or epicardial) – no. / total no. (%) |
366/418 (87.6) |
| Successful LV lead placement in second attempt (transvenous or epicardial) – no. / total no. (%) |
52/418 (12.4) |
| Epicardial LV lead placement – no. / total no. (%) |
48/418 (11.5) |
| Device vendor |
|
| Biotronik – no. / total no. (%) |
153/418 (36.6) |
| Boston Scientific – no. / total no. (%) |
30/418 (7.2) |
| Medtronic – no. / total no. (%) |
139/418 (33.3) |
| Sorin – no. / total no. (%) |
4/419 (1.0) |
| St. Jude Medical – no. / total no. (%) |
92/418 (22.0) |
Table S5B Peri- and postoperative complications.
|
No. / total no. (%)
|
| Coronary sinus dissection |
5/417 (1.2) |
| Left ventricular lead dislocation |
23/417 (5.5) |
| Diaphragmatic capture |
44/417 (10.6) |
| Intermittent, no intervention |
11/417 (2.6) |
| Successful reprogramming |
26/417 (6.2) |
| Successful lead repositioning |
5/417 (1.2) |
| Termination of left ventricular pacing |
2/417 (0.5) |
| Infection |
19/418 (4.5) |
| Conservative management |
9/418 (2.2) |
| Device/lead explantation |
10/418 (2.4) |
| Coronary sinus perforation |
2/418 (0.5) |
| Pneumothorax |
14/416 (3.4) |
| Haematoma, conservative management |
17/416 (4.1) |
| Haematoma, operative management |
9/416 (2.2) |
Table S6 Echocardiographic therapy response stratified by bundle branch block morphology.
|
Rhythm at baseline
|
No. / total no. (%)
|
|
No LBBB
|
LBBB
|
| LVEF improvement >5% |
43/83 (51.8) |
139/222 (62.6) |
| LVEF improvement ≥10% |
29/85 (34.1) |
90/223 (40.4) |
| EDVI reduction ≥10% |
27/67 (40.3) |
94/176 (53.4) |
| EDVI reduction ≥20% |
15/67 (22.4) |
60/176 (34.1) |
Jan Steffel, MD, FESC, FHRS, Co-head, Invasive Electrophysiology and Cardiac Devices, University Heart Center Zurich, Department of Cardiology, Raemistr. 100, CH-8091 Zurich, j.steffel[at]gmx.ch
References
1
Abraham
WT
,
Fisher
WG
,
Smith
AL
,
Delurgio
DB
,
Leon
AR
,
Loh
E
, et al.; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–53. https://doi.org/10.1056/NEJMoa013168
2
Bristow
MR
,
Saxon
LA
,
Boehmer
J
,
Krueger
S
,
Kass
DA
,
De Marco
T
, et al.; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140–50. https://doi.org/10.1056/NEJMoa032423
3
Cleland
JG
,
Daubert
JC
,
Erdmann
E
,
Freemantle
N
,
Gras
D
,
Kappenberger
L
, et al.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539–49. https://doi.org/10.1056/NEJMoa050496
4
Goldenberg
I
,
Kutyifa
V
,
Klein
HU
,
Cannom
DS
,
Brown
MW
,
Dan
A
, et al.
Survival with cardiac-resynchronization therapy in mild heart failure. N Engl J Med. 2014;370(18):1694–701. https://doi.org/10.1056/NEJMoa1401426
5
Zareba
W
,
Klein
H
,
Cygankiewicz
I
,
Hall
WJ
,
McNitt
S
,
Brown
M
, et al.; MADIT-CRT Investigators. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;123(10):1061–72. https://doi.org/10.1161/CIRCULATIONAHA.110.960898
6
Ponikowski
P
,
Voors
AA
,
Anker
SD
,
Bueno
H
,
Cleland
JG
,
Coats
AJ
, et al.; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128
7
Ruschitzka
F
,
Abraham
WT
,
Singh
JP
,
Bax
JJ
,
Borer
JS
,
Brugada
J
, et al.; EchoCRT Study Group. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med. 2013;369(15):1395–405. https://doi.org/10.1056/NEJMoa1306687
8
Steffel
J
,
Robertson
M
,
Singh
JP
,
Abraham
WT
,
Bax
JJ
,
Borer
JS
, et al.
The effect of QRS duration on cardiac resynchronization therapy in patients with a narrow QRS complex: a subgroup analysis of the EchoCRT trial. Eur Heart J. 2015;36(30):1983–9. https://doi.org/10.1093/eurheartj/ehv242
9
Steffel
J
,
Hürlimann
D
. Current practice of cardiac resynchronization therapy (CRT) in the real world: insights from the European CRT survey. Eur Heart J. 2009;30(20):2433–5. https://doi.org/10.1093/eurheartj/ehp366
10
Kashani
A
,
Barold
SS
. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46(12):2183–92. https://doi.org/10.1016/j.jacc.2005.01.071
11
Bogale
N
,
Priori
S
,
Gitt
A
,
Alings
M
,
Linde
C
,
Dickstein
K
, et al.; Scientific Committee, National coordinators, and investigators. The European cardiac resynchronization therapy survey: patient selection and implantation practice vary according to centre volume. Europace. 2011;13(10):1445–53. https://doi.org/10.1093/europace/eur173
12
Dickstein
K
,
Bogale
N
,
Priori
S
,
Auricchio
A
,
Cleland
JG
,
Gitt
A
, et al.; Scientific Committee; National Coordinators. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30(20):2450–60. https://doi.org/10.1093/eurheartj/ehp359
13
Steffel
J
,
Milosevic
G
,
Hürlimann
A
,
Krasniqi
N
,
Namdar
M
,
Ruschitzka
F
, et al.
Characteristics and long-term outcome of echocardiographic super-responders to cardiac resynchronisation therapy: ‘real world’ experience from a single tertiary care centre. Heart. 2011;97(20):1668–74. https://doi.org/10.1136/heartjnl-2011-300222
14
Auricchio
A
. Cardiac resynchronization therapy: does varying the pacing site or combination of sites improve cardiac function?
Nat Clin Pract Cardiovasc Med. 2005;2(6):288–9. https://doi.org/10.1038/ncpcardio0222
15
Butter
C
,
Auricchio
A
,
Stellbrink
C
,
Fleck
E
,
Ding
J
,
Yu
Y
, et al.; Pacing Therapy for Chronic Heart Failure II Study Group. Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation. 2001;104(25):3026–9. https://doi.org/10.1161/hc5001.102229
16
Hürlimann
DSJ
,
Milosevic
G
,
Krasniqi
N
,
Gruner
C
,
Oswlad
F
,
Rahn
M
, et al.
Cardiac resynchronization therapiy - “real world” experience from a swiss tertiary center. Cardiovasc Med. 2010;13:334–41.10.4414/cvm.2010.01543
17
Achilli
A
,
Sassara
M
,
Ficili
S
,
Pontillo
D
,
Achilli
P
,
Alessi
C
, et al.
Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42(12):2117–24. https://doi.org/10.1016/j.jacc.2003.08.024
18
Bleeker
GB
,
Holman
ER
,
Steendijk
P
,
Boersma
E
,
van der Wall
EE
,
Schalij
MJ
, et al.
Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006;48(11):2243–50. https://doi.org/10.1016/j.jacc.2006.07.067
19
Holzmeister
J
,
Hürlimann
D
,
Steffel
J
,
Ruschitzka
F
. Cardiac resynchronization therapy in patients with a narrow QRS. Curr Heart Fail Rep. 2009;6(1):49–56. https://doi.org/10.1007/s11897-009-0009-5
20
Yu
CM
,
Chan
YS
,
Zhang
Q
,
Yip
GW
,
Chan
CK
,
Kum
LC
, et al.
Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48(11):2251–7. https://doi.org/10.1016/j.jacc.2006.07.054
21
Curtis
AB
,
Worley
SJ
,
Adamson
PB
,
Chung
ES
,
Niazi
I
,
Sherfesee
L
, et al., Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368(17):1585–93. https://doi.org/10.1056/NEJMoa1210356
22
Fröhlich
G
,
Steffel
J
,
Hürlimann
D
,
Enseleit
F
,
Lüscher
TF
,
Ruschitzka
F
, et al.
Upgrading to resynchronization therapy after chronic right ventricular pacing improves left ventricular remodelling. Eur Heart J. 2010;31(12):1477–85. https://doi.org/10.1093/eurheartj/ehq065
23
Steffel
J
,
Ruschitzka
F
. Superresponse to cardiac resynchronization therapy. Circulation. 2014;130(1):87–90. https://doi.org/10.1161/CIRCULATIONAHA.113.006124
24
Moss
AJ
,
Hall
WJ
,
Cannom
DS
,
Klein
H
,
Brown
MW
,
Daubert
JP
, et al.; MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361(14):1329–38. https://doi.org/10.1056/NEJMoa0906431
25
Tang
AS
,
Wells
GA
,
Talajic
M
,
Arnold
MO
,
Sheldon
R
,
Connolly
S
, et al.; Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363(25):2385–95. https://doi.org/10.1056/NEJMoa1009540
26
Hernández Madrid
A
,
Matía Francés
R
,
Moro
C
,
Zamorano
J
. Cardiac resynchronization therapy: do patient selection and implant practice vary depending on the volume a center handles?
Pacing Clin Electrophysiol. 2013;36(7):863–71. https://doi.org/10.1111/pace.12135



