Iron homeostasis in inflammation: a single centre prospective observational study in medical inpatients
DOI: https://doi.org/10.4414/smw.2017.14431
Carl
Chrobaka, Jan A.
Sidlera, Alix
O'Mearab, Sabine
Schädelinc, Balthasar L.
Huga
aDepartment of Internal Medicine, University Hospital Basel, Basel,
bOncology and Haematology, Spital Limmattal, Schlieren,
cClinical Trial Unit, University Hospital Basel,
Iron homeostasis in inflammation: a single centre prospective observational study in medical inpatients
Summary
AIMS OF THE STUDY: We aimed to assess a potential association of iron status with mortality and morbidity of inpatients with systemic inflammation.
METHODS
This was a single centre prospective observational study. From April 2014 to October 2014, all consecutive medical inpatients aged ≥18 years with a C-reactive protein value >5 mg/l on hospital admission were eligible for the study. We excluded pregnant women and patients with terminal renal insufficiency or past allogeneic stem cell transplantation. For all patients, a complete set of serum iron parameters was obtained on hospital admission. In the final analysis, the in-hospital all-cause mortality and several morbidity measures (length of stay, number of secondary diagnoses and Charlson Comorbidity Index) were compared between four distinct iron status groups: patients having iron deficiency anaemia, iron deficiency without anaemia, anaemia without iron deficiency, and normal iron status. Iron deficiency was quantifies as the serum transferrin receptor / ferritin index, with a cut-off level of 1.5.
RESULTS
A total of 438 patients were included in the final analysis. Patients with iron deficiency had a higher in-hospital mortality than patients with iron deficiency anaemia, anaemia without iron deficiency, or normal iron status (6% vs 1%, 5%, and 1%, respectively; p = 0.042). Patients with iron deficiency anaemia had a higher number of secondary diagnoses (mean 8.4; standard deviation 4.2) and a higher Charlson Comorbidity Index (mean 1.8; standard deviation 1.9) than patients with iron deficiency, anaemia without iron deficiency, or normal iron status (p <0.001 and p <0.001, respectively). The median length of stay did not differ significantly between the iron status groups (p = 0.080).
CONCLUSIONS
In our study population, iron status was significantly associated with mortality and morbidity. Further studies are required to assess the pathophysiological and clinical effects of an altered iron metabolism and iron substitution therapies in inflammation. (ClinicalTrial.gov, NCT02155114).
Introduction
Iron deficiency with or without concomitant anaemia is frequently observed in medical patients, particularly in the elderly, and can be due to various causes such as malnutrition, malabsorption, and subacute or chronic blood loss [1]. In a retrospective analysis, 62% of the medical inpatients at our institution had anaemia [2]; of patients with available iron panel test results, 14% had iron deficiency, half of whom had signs of inflammation defined as C-reactive protein (CRP) >5 mg/l.
Patients with an inflammatory process may present with decreased, normal or increased total body iron stores. Decreased iron stores in inflammatory diseases and infections can either represent true pre-existing iron deficiency or functional iron deficiency (anaemia of inflammation) [3, 4]. Inflammatory states increase serum ferritin levels as well as the release of inflammatory cytokines and may ultimately lead to hypoferraemia through an increase in hepcidin with consecutive iron sequestration in macrophages and duodenal enterocytes [5]. Furthermore, inflammation reduces erythropoietin and bone marrow activity through inflammatory cytokines, including tumour necrosis factor α, interleukin 1 and hepcidin [6].
Research regarding the clinical significance of different iron states in inflammatory diseases is conflicting, and guidelines for iron substitution are sparse and limited to patients with chronic kidney disease and malaria infection [7]. Several studies showed that the growth of Plasmodium species [8], viruses such as hepatitis C and human immunodeficiency virus [9], and bacteria [10] strongly depend on iron. Distinct species use different approaches to acquire iron from their hosts or environment, for instance by siderophores scavenging transferrin and other iron carrying proteins [11, 12]. The concept of “nutritional immunity”, i.e. the absence of iron restricting microbial growth during infections, is well studied in vitro and in mouse models [10, 12, 13]. Bacteria commonly encountered in clinical practice, such as Escherichia coli, Yersinia enterocolitica, Salmonella typhimurium and Staphylococcus epidermidis, show increased growth in human serum after oral iron supplementation [12, 14]. Furthermore, latent infections such as tuberculosis may reactivate after iron supplementation [15].
In contrast, in systemic inflammation not related to infections, patients seem to benefit from iron substitution – at least those with chronic kidney disease, inflammatory bowel disease and chronic heart failure [16–19]. Patients with chronic kidney disease, especially if dialysis dependent, exemplify a proinflammatory state and have been extensively studied in terms of iron and erythropoietin substitution [18, 20]. The formerly common use of erythropoietin in chronic kidney disease has been partly abandoned in favour of periodical intravenous iron infusions [21]. Patients with ferritin levels as high as 1200 μg/l can still qualify for iron substitution as a result of functional iron deficiency, although in the absence of inflammation such levels would be interpreted as iron overload [4, 21].
Ferritin has been used to estimate iron stores in the absence of inflammation, whereas its predictive value is limited in inflammation as it is an acute phase reactant [1]. The serum transferrin receptor (sTfR) is typically raised in iron deficiency anaemia, but low in anaemia of inflammation unless both conditions are present. New diagnostic tools like the serum transferrin receptor / ferritin (TfR/F) index are used to more accurately estimate iron storages during inflammation and have been extensively evaluated [22–25].
We hypothesised that in patients with systemic inflammation the iron status and presence of anaemia may considerably affect mortality and morbidity. We therefore aimed to assess a potential association of the iron status with mortality and morbidity using the TfR/F index as parameter for the iron status.
Materials and methods
Study design and setting
We conducted a prospective observational study at the Department of Internal Medicine of the University Hospital Basel – a 712-bed tertiary referral centre in Switzerland treating approximately 34 000 inpatients and 180 000 outpatients per year. Specialties comprise the whole spectrum of internal medicine, including renal and bone marrow transplantation.
The Ethics Committee of Northwestern and Central Switzerland approved the study (EKNZ 2014-053). The study was registered on ClinicalTrial.gov (NCT02155114).
Patient selection
From 4 April 2014 to 3 October 2014, all consecutive medical inpatients aged ≥18 years with a CRP level >5 mg/l on hospital admission were eligible for the study. We excluded pregnant women and patients with terminal renal insufficiency or past allogeneic stem cell transplantation.
Outcomes
The primary outcome measure was in-hospital all-cause mortality. Secondary outcome measures in terms of morbidity were the following: (i) the length of hospital stay (LOS), (ii) the number of secondary diagnoses, and (iii) the Charlson Comorbidity Index.
Data acquisition
For all patients included in the study, a complete set of serum iron parameters (ferritin, free serum iron, sTfR, transferrin and transferrin saturation) was obtained within the first 72 hours after hospital admission.
Relevant demographic, clinical and routine laboratory data were collected retrospectively. Clinical data were obtained from the Oracle database of the in-house clinical information system (ISMed, ProtecData AG, Boswil, Switzerland). Routine laboratory data were extracted from our laboratory information system (Dorner Health IT Solutions, Müllheim, Germany). Demographic data, International Classification of Diseases 10th revision (ICD-10) diagnoses, LOS and data about in-hospital mortality were retrieved from the SAP ERP system (Enterprise-Resource-Planning, SAP SE, Germany). All data collected had been anonymised.
ICD-10 diagnoses were coded by professional coders according to the German modification of ICD-10. Each patient had one main diagnosis and up to 33 secondary diagnoses. Only the first ten secondary diagnoses were included in our analysis because of low numbers in the residual diagnosis groups.
Definitions
On the basis of their iron status and presence of anaemia on hospital admission, we defined four distinct groups of patients: (i) iron deficiency anaemia (haemoglobin ≤130 g/l for men or ≤120 g/l for women and TfR/F index >1.5), (ii) iron deficiency without anaemia (haemoglobin >130 g/l for men or >120 g/l for women and TfR/F index ≤1.5), (iii) anaemia without iron deficiency (haemoglobin ≤130 g/l for men or ≤120 g/l for women and TfR/F index ≤1.5), and (iv) a normal iron status (haemoglobin >130 g/l for men or >120 g/l for women and TfR/F index ≤1.5). The TfR/F index was calculated as proposed by Punnonen et al, by using the TfR/log10 ferritin formula [25]. The Charlson Comorbidity Index was calculated from the adapted ICD-10 version according to Sundararajan et al. [26]. The first 72 hours after admission were defined as time of hospital admission. A patient with a CRP concentration >5 mg/l on hospital admission was defined as having a systemic inflammation.
Statistical analysis
The primary and secondary outcome measures were compared between the four predefined iron status groups: patients with iron deficiency anaemia, iron deficiency without anaemia, anaemia without iron deficiency, or normal iron status. Group comparisons for discrete variables were made with Fisher’s exact test or chi-square test as appropriate; for continuous variables the Kruskal-Wallis rank sum test was used. Data were analysed using “R” (version 3.2.1).
Results
Selection of patients
Within the 6-month study period, 1448 patients were eligible for the study (fig. 1). Of these patients, 438 were included in the final analysis after excluding 1010 patients for the following reasons: (i) not able to give informed consent (n = 143), (ii) did not understand the informed consent because of a language barrier (n = 200), (iii) did not give informed consent (n = 156), (iv) left hospital before screening (n = 62), (v) died before screening (n = 2), (vi) outliers with ferritin values >5000 µg/l (n = 4), and (vii) exclusion criteria (n = 443).
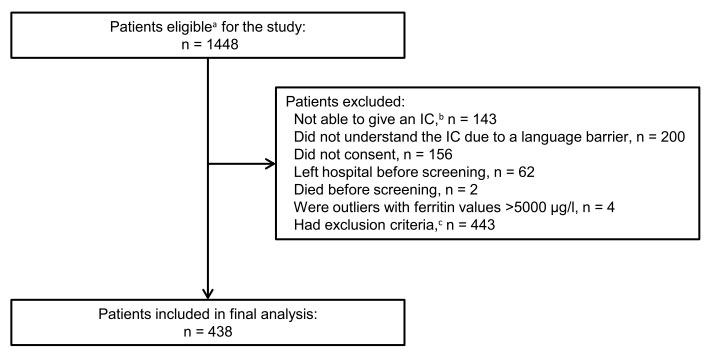
Figure 1 Selection of patients for final analysis.
IC = informed consent
a Patients age ≥18 years and hospitalised ≥24 hours with a concomitant C-reactive protein >5 mg/l on hospital admission. b Due to cognitive impairment, dementia, delirium, or aphasia. c Exclusion criteria: terminal renal insufficiency, pregnancy, and past allogeneic stem cell transplantation.
The four patients excluded because of exceptionally high ferritin values >5000 µg/l had severe lobar pneumonia, haemophagocytic syndrome, multiple organ dysfunction syndrome in sepsis, and cryptogenic acute liver failure, respectively.
Demographic and laboratory patient characteristics
Baseline characteristics were stratified by iron status and presence of anaemia on hospital admission (table 1); the largest groups were patients with iron deficiency anaemia (33%; 143/438) and anaemia without iron deficiency (32%; 142/438). Overall, the mean age was 65.9 years (standard deviation [SD] 15.5), 41% of patients (179/438) were female and the mean CRP value on hospital admission was 77.6 mg/l (SD 77.3).
Table 1 Demographic and laboratory data stratified by iron status and presence of anaemia on hospital admission (number of patients = 438).
|
Iron status / anaemia
|
|
Variables
|
ID anaemia
(n = 143) |
ID, no anaemia
(n = 53) |
No ID, anaemia
(n = 142) |
Normal*
(n = 100) |
| Age (years), mean (SD) |
67.3 (14.2) |
65.1 (17.9) |
67.2 (15.4) |
62.3 (15.7) |
| Female gender, n (%) |
64 (45) |
19 (36) |
56 (39) |
40 (40) |
|
Laboratory characteristics†, mean (SD)
|
| Haemoglobin (g/l) |
103.0 (15.9) |
137.5 (10.1) |
105.2 (15.2) |
140.1 (10.1) |
| Ferritin (µg/l) |
222.7 (258.4) |
221.5 (221.2) |
638.5 (688.4) |
501.7 (512.4) |
| Free iron (µmol/l) |
6.7 (6.7) |
7.3 (3.8) |
9.1 (6.9) |
11.5 (8.5) |
| sTfR (mg/l) |
6.4 (4.3) |
5.3 (3.4) |
2.6 (0.9) |
2.5 (0.7) |
| Transferrin (g/l) |
2.1 (0.5) |
2.3 (0.4) |
1.6 (0.4) |
2 (0.4) |
| Transferrin saturation (%) |
13.1 (11.7) |
12.7 (6.3) |
23.6 (19.4) |
22.5 (15.9) |
| CRP (mg/l) |
67.1 (64.7) |
67.7 (73.3) |
98.2 (87.9) |
68.8 (74.9) |
| Leucocyte count (G/l) |
8.2 (4.2) |
10.4 (5.6) |
8.8 (5.3) |
9.4 (3.9) |
| Procalcitonin‡ (ng/ml) |
1.4 (3.5) |
1.1 (1.9) |
1.0 (1.0) |
0.2 (0.2) |
The main hospitalisation diagnoses are presented in supplementary table S1 (see appendix). In all iron status groups, diseases of the respiratory tract and/or circulatory system were predominant.
In-hospital mortality
The in-hospital all-cause mortality was stratified by iron status (table 2); overall, 3% of patients died (12/437; missing data for one patient). Patients with an isolated iron deficiency had a higher in-hospital all-cause mortality than patients with iron deficiency anaemia, anaemia without iron deficiency, or a normal iron status (6% vs 1%, 5%, and 1%, respectively; p = 0.042).
Table 2 In-hospital all-cause mortality stratified by iron status and presence of anaemia on hospital admission (number of patients = 437).
|
Iron status / anaemia
|
|
In-hospital mortality* †
|
ID anaemia
(n = 143) |
ID, no anaemia
(n = 53) |
No ID, anaemia
(n = 141) |
Normal‡
(n = 100) |
Total
(n = 437) |
| Death, n (%) |
1 (1) |
3 (6) |
7 (5) |
1 (1) |
12 (3) |
| Survival, n (%) |
142 (99) |
50 (94) |
134 (95) |
99 (99) |
425 (97) |
Overall, 17% of patients (72/436; missing data for two patients) received iron substitution therapy during the hospitalisation period, with packed red blood cells, or intravenous or oral iron (table 3). Iron substitution was commonest in patients with iron deficiency anaemia (28%; 40/143) or anaemia without iron deficiency (20%; 29/142).
Table 3 Iron substitution stratified by iron status and presence of anaemia on hospital admission (number of patients = 436).
|
Iron status / anaemia
|
|
Iron substitution*†
|
ID anaemia
(n = 143) |
ID, no anaemia
(n = 51) |
No ID, anaemia
(n = 142) |
Normal‡
(n = 100) |
Total
(n = 436) |
| Yes, n (%) |
40 (28) |
1 (2) |
29 (20) |
2 (2) |
72 (17) |
| No, n (%) |
103 (72) |
50 (98) |
113 (80) |
98 (98) |
364 (83) |
Patients receiving any iron substitution therapy had a higher in-hospital all-cause mortality than patients not given iron (8 vs 2%, p = 0.006) (table 4).
Table 4 In-hospital all-cause mortality stratified by iron substitution (number of patients = 435).
|
Iron substitution*
|
|
In-hospital mortality†‡
|
Yes
(n = 71) |
No
(n = 364) |
Total
(n = 435) |
| Death, n (%) |
6 (8) |
6 (2) |
12 (3) |
| Survival, n (%) |
65 (92) |
358 (98) |
423 (97) |
Length of hospital stay, number of secondary diagnoses and Charlson Comorbidity Index
The length of stay and the number of secondary diagnoses differed significantly between the iron status groups (p <0.001 and p <0.001, respectively; table 5). Patients with iron deficiency anaemia had the highest number of secondary diagnoses (mean 8.4, SD 4.2) and the highest Charlson Comorbidity Index (mean 1.8, SD 1.9) as compared with patients with iron deficiency, anaemia without iron deficiency, or normal iron status. The median length of stay did not differ significantly between the four iron status groups (p = 0.080).
Table 5 Length of hospital stay, number of secondary diagnoses and Charlson Comorbidity Index stratified by iron status and presence of anaemia on hospital admission (number of patients = 438).
|
Iron status / anaemia
|
|
Variables*
|
ID anaemia
(n = 143) |
ID, no anaemia
(n = 53) |
No ID, anaemia
(n = 142) |
Normal†
(n = 100) |
Total
(n = 438) |
| LOS (days) mean (SD) |
12.6 (10.1) |
11.2 (8.2) |
11.0 (8.5) |
10.0 (8.2) |
11.3 (9.0) |
| Secondary diagnoses, mean n (SD) |
8.4 (4.2) |
7.2 (5.5) |
6.4 (3.8) |
4.8 (3.3) |
6.8 (4.3) |
| Charlson Comorbidity Index, mean (SD) |
1.8 (1.9) |
1.4 (1.9) |
1.6 (2.0) |
0.8 (1.3) |
1.5 (1.9) |
Discussion
To our knowledge, this is the first study that has prospectively assessed the iron status of medical inpatients with systemic inflammation. In our study population, we observed a high rate of iron deficiency and anaemia, with the admission iron status being significantly associated with mortality and morbidity.
For stratification of the iron status, we used the TfR/F index, which has been shown to be an accurate measure in the diagnosis of iron deficiency [25]. Conventional laboratory tests of iron status (e.g., serum iron, transferrin, transferrin saturation, ferritin) are widely used but are heavily influenced by acute phase reactants, which decreases their capacity to accurately discriminate between iron deficiency anaemia and anaemia of chronic disease [25]. The definition of systemic inflammation was based on the well-established serum biomarker CRP, which has repeatedly been used to detect and quantify systemic inflammation in epidemiological studies [27].
Regarding the iron status, the most common findings in our study population were iron deficiency anaemia and anaemia without iron deficiency in 33% and 32% of patients, respectively; furthermore, only 23% of patients had normal iron status on hospital admission. These results are in line with a previous study from 2013 [2], in which we observed a high prevalence of iron deficiency and anaemia in medical inpatients with and without inflammation. Functional iron deficiency is a challenge in clinical routine due to the lack of treatment guidelines for the majority of diseases [28]. In patients without systemic inflammation, typical causes for iron deficiency anaemia are blood loss, increased iron requirement or limited iron supply/uptake, which can be addressed specifically, whereas in patients with systemic inflammation, iron homeostasis and iron availability is heavily influenced by acute phase reactants. Recent years brought new insights into the regulation of iron homeostasis, and the identification of hepcidin led to a broader understanding of the mechanisms of iron homeostasis, particularly in inflammation [5]. Hepcidin has been shown to be the master regulator of iron homeostasis, which is closely connected to erythropoietin, bone marrow activity and inflammation [29]. Further, iron sensing via serum receptors allows regulation of hepcidin serum levels according to iron availability [30]. In chronic kidney disease [21], chronic heart failure [31] and inflammatory bowel disease [19, 32], iron substitution therapy seems to be beneficial for iron deficient and anaemic patients. In patients with other inflammatory diseases (e.g. rheumatic diseases), iron substitution could also be effective, but some patients display an iron refractory iron deficiency [33].
In our study, mortality differed significantly between the iron status groups. This observation might not be causal, as we did not adjust for the underlying comorbidities, severity of illness and other important cofactors; furthermore, we cannot exclude bias and confounding. Nevertheless, various prospective observational studies have concluded that iron status and, specifically, elevated ferritin and transferrin saturation are independently associated with increased mortality in the general population [34–39]. The iron status may be described as a biological marker of physiological and pathogenic processes reflecting, to some extent, the presence and severity of a variety of disease states, which ultimately lead to premature death [39].
Seventeen percent of patients received iron substitution therapy with packed red blood cells and/or intravenous/oral iron: These patients had a higher in-hospital all-cause mortality than patients not given iron (8% vs 2%). We hypothesise that these results can mainly be explained by the fact that patients receiving iron substitution, and particularly packed red blood cells, are per se at higher risk for dying independent of the iron substitution therapy (due to severe trauma, malignancies, etc.). The effects of iron substitution in systemic inflammation or infection have been mainly studied in mouse models [10, 12, 13], and human studies are scarce. Patient with iron overload, and particularly with African iron overload, a rare genetic disorder, seem to be at risk for worse outcome and reactivation of infections like tuberculosis [40–43]. Studies on the effect of iron substitution in humans in the context of infection either included small populations or showed considerable methodological challenges [12, 18, 44]. “Nutritional immunity”, the absence of iron in food supplements leading to decreased infection rates, seems a concept worth considering [10, 13]. Withholding iron from bacteria and certain viruses seems to be an important evolutionary factor that is associated with a survival benefit in some patients with infections.
In our study, patients with a pathological iron status had a higher number of secondary diagnoses and a higher Charlson Comorbidity Index than patients with a normal iron status, whereas the median length of stay did not differ significantly between the iron status groups. As for mortality, the primary outcome measure, we speculate that these results can be partly explained by the fact that the iron status is a marker for the presence and severity of various diseases and comorbidities which need to be diagnosed and treated adequately. With the increasing number of predictive routine markers, such information should be used to develop and validate risk models, which support decisions in daily clinical routine [45].
Our study has limitations. First, we had to exclude around 70% of eligible patients, which limits the generalisability of our results; particularly, we had to exclude patients who were unable to read or understand our patient information. Second, our follow-up was restricted to the actual hospitalisation period. Third, we could not adjust for important patient characteristics in our analyses (e.g., comorbidity, severity-of-illness, treatments), which could have affected the observed statistical associations. However, we believe that the results show the general importance of iron deficiency and other iron states in inflammation. Fourth, we observed an imbalance of main diagnoses, which might have distorted some of the results in the direction of the most prominent main diagnoses of pulmonary and cardiovascular diseases.
In conclusion, in our study population, iron status was significantly associated with mortality and morbidity. Further studies are required to assess the pathophysiological and clinical effects of an altered iron metabolism and iron substitution therapies in inflammation.
Appendix: Supplementary table
Table S1 ICD-10 main diagnosis groups stratified by iron status and presence of anemia on hospital admission (number of patients = 430).
|
|
Iron status / anaemia
|
|
ICD-10 chapter
|
Main diagnosis group*
|
ID anemia
(n = 141) |
ID, no anemia
(n = 52) |
No ID, anemia
(n = 137) |
Normal†
(n = 100) |
Total
(n = 430) |
|
|
n (%) |
n (%) |
n (%) |
n (%) |
n (%) |
| X |
Diseases of the respiratory system (J00‒J99) |
30 (21) |
17 (33) |
25 (18) |
27 (27) |
99 (23) |
| IX |
Diseases of the circulatory system (I00‒I99) |
31 (22) |
13 (25) |
23 (17) |
17 (17) |
84 (20) |
| II |
Neoplasms (C00‒D48) |
22 (16) |
4 (8) |
22 (16) |
13 (13) |
61 (14) |
| XI |
Diseases of the digestive system (K00‒K93) |
14 (10) |
1 (2) |
18 (13) |
6 (6) |
39 (9) |
| I |
Certain infectious and parasitic diseases (A00‒B99) |
10 (7) |
7 (13) |
10 (7) |
7 (7) |
34 (8) |
| XIII |
Diseases of the musculoskeletal system and connective tissue (M00–M99) |
14 (10) |
5 (10) |
7 (5) |
6 (6) |
32 (7) |
| XVIII |
Symptoms, signs and abnormal clinical and laboratory findings, not elsewhere classified (R00‒R99) |
3 (2) |
3 (6) |
2 (1) |
4 (4) |
12 (3) |
| XIV |
Diseases of the genitourinary system (N00‒N99) |
6 (4) |
1 (2) |
15 (11) |
9 (9) |
31 (7) |
| IV |
Endocrine, nutritional and metabolic diseases (E00‒E90) |
3 (2) |
1 (2) |
4 (3) |
1 (1) |
9 (2) |
| VI |
Diseases of the nervous system (G00‒G99) |
3 (2) |
0 (0) |
4 (3) |
2 (2) |
9 (2) |
| Other |
Other ICD-10 diagnoses |
5 (4) |
0 (0) |
7 (5) |
8 (8) |
20 (5) |
Acknowledgement
We are grateful to Prof. J.A. Schifferli for his kind support. We thank Dr R. Padiyath for his help regarding retrieval of coded diagnoses as well as T. Gaida for the administrative data.
Preliminary data were presented as posters during the Clinical Research Day of the University Hospital Basel on January 28, 2016, and during the International Forum on Quality and Safety in Healthcare in Gothenburg on April 12 to 15, 2016.
Authors’ contribution
CC and JAS: Contributed equally
Balthasar L. Hug, MD, MBA, MPH, Department of Internal Medicine, University Hospital Basel, Petersgraben 4, CH-4031 Basel, b.hug[at]unibas.ch
References
1
Lopez
A
,
Cacoub
P
,
Macdougall
IC
,
Peyrin-Biroulet
L
. Iron deficiency anaemia. Lancet. 2016;387(10021):907–16.https://doi.org/10.1016/S0140-6736(15)60865-0
2
Hug
BL
,
Tichelli
A
,
Benkert
P
,
Stirnimann
G
,
Schifferli
JA
. Diagnosis and treatment of iron deficiency in medical inpatients at a Swiss tertiary university referral hospital: a retrospective observational cohort study of clinical practice. Swiss Med Wkly. 2013;143:w13847.
3
Nemeth
E
,
Ganz
T
. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28(4):671–81, vi.https://doi.org/10.1016/j.hoc.2014.04.005
4
Thomas
DW
,
Hinchliffe
RF
,
Briggs
C
,
Macdougall
IC
,
Littlewood
T
,
Cavill
I
; British Committee for Standards in Haematology. Guideline for the laboratory diagnosis of functional iron deficiency. Br J Haematol. 2013;161(5):639–48.https://doi.org/10.1111/bjh.12311
5
Ganz
T
,
Nemeth
E
. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15(8):500–10.https://doi.org/10.1038/nri3863
6
Weiss
G
. Anemia of chronic disorders: New diagnostic tools and new treatment strategies. Semin Hematol. 2015;52(4):313–20.https://doi.org/10.1053/j.seminhematol.2015.07.004
7
Kliger
AS
,
Foley
RN
,
Goldfarb
DS
,
Goldstein
SL
,
Johansen
K
,
Singh
A
, et al.
KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis. 2013;62(5):849–59.https://doi.org/10.1053/j.ajkd.2013.06.008
8
Prentice
AM
,
Verhoef
H
,
Cerami
C
. Iron fortification and malaria risk in children. JAMA. 2013;310(9):914–5.https://doi.org/10.1001/jama.2013.6771
9
Drakesmith
H
,
Prentice
A
. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–52.https://doi.org/10.1038/nrmicro1930
10
Hood
MI
,
Skaar
EP
. Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol. 2012;10(8):525–37.https://doi.org/10.1038/nrmicro2836
11
Chu
BC
,
Garcia-Herrero
A
,
Johanson
TH
,
Krewulak
KD
,
Lau
CK
,
Peacock
RS
, et al.
Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals. 2010;23(4):601–11.https://doi.org/10.1007/s10534-010-9361-x
12
Weinberg
ED
. Iron availability and infection. Biochim Biophys Acta. 2009;1790(7):600–5.https://doi.org/10.1016/j.bbagen.2008.07.002
13
Michels
K
,
Nemeth
E
,
Ganz
T
,
Mehrad
B
. Hepcidin and host defense against infectious diseases. PLoS Pathog. 2015;11(8):e1004998.https://doi.org/10.1371/journal.ppat.1004998
14
Cross
JH
,
Bradbury
RS
,
Fulford
AJ
,
Jallow
AT
,
Wegmüller
R
,
Prentice
AM
, et al.
Oral iron acutely elevates bacterial growth in human serum. Sci Rep. 2015;5:16670.https://doi.org/10.1038/srep16670
15
Murray
MJ
,
Murray
AB
,
Murray
MB
,
Murray
CJ
. The adverse effect of iron repletion on the course of certain infections. BMJ. 1978;2(6145):1113–5.https://doi.org/10.1136/bmj.2.6145.1113
16
Moore
RA
,
Gaskell
H
,
Rose
P
,
Allan
J
. Meta-analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord. 2011;11(1):4.https://doi.org/10.1186/1471-2326-11-4
17
Anker
SD
,
Comin Colet
J
,
Filippatos
G
,
Willenheimer
R
,
Dickstein
K
,
Drexler
H
, et al.; FAIR-HF Trial Investigators. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361(25):2436–48.https://doi.org/10.1056/NEJMoa0908355
18
Feldman
HI
,
Santanna
J
,
Guo
W
,
Furst
H
,
Franklin
E
,
Joffe
M
, et al.
Iron administration and clinical outcomes in hemodialysis patients. J Am Soc Nephrol. 2002;13(3):734–44.
19
Gisbert
JP
,
Bermejo
F
,
Pajares
R
,
Pérez-Calle
J-L
,
Rodríguez
M
,
Algaba
A
, et al.
Oral and intravenous iron treatment in inflammatory bowel disease: hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15(10):1485–91.https://doi.org/10.1002/ibd.20925
20
Oberg
BP
,
McMenamin
E
,
Lucas
FL
,
McMonagle
E
,
Morrow
J
,
Ikizler
TA
, et al.
Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65(3):1009–16.https://doi.org/10.1111/j.1523-1755.2004.00465.x
21
Improving Global Outcomes (KDIGO) Anemia Working Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. Available from: http://www.kdigo.org/clinical_practice_guidelines/pdf/KDIGO-Anemia
22
Skikne
BS
,
Punnonen
K
,
Caldron
PH
,
Bennett
MT
,
Rehu
M
,
Gasior
GH
, et al.
Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol. 2011;86(11):923–7.https://doi.org/10.1002/ajh.22108
23
Marković
M
,
Majkić-Singh
N
,
Subota
V
. Usefulness of soluble transferrin receptor and ferritin in iron deficiency and chronic disease. Scand J Clin Lab Invest. 2005;65(7):571–6.https://doi.org/10.1080/00365510500206542
24
Rimon
E
,
Levy
S
,
Sapir
A
,
Gelzer
G
,
Peled
R
,
Ergas
D
, et al.
Diagnosis of iron deficiency anemia in the elderly by transferrin receptor-ferritin index. Arch Intern Med. 2002;162(4):445–9.https://doi.org/10.1001/archinte.162.4.445
25
Punnonen
K
,
Irjala
K
,
Rajamäki
A
. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89(3):1052–7.
26
Sundararajan
V
,
Henderson
T
,
Perry
C
,
Muggivan
A
,
Quan
H
,
Ghali
WA
. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004;57(12):1288–94.https://doi.org/10.1016/j.jclinepi.2004.03.012
27
Tsounis
D
,
Bouras
G
,
Giannopoulos
G
,
Papadimitriou
C
,
Alexopoulos
D
,
Deftereos
S
. Inflammation markers in essential hypertension. Med Chem. 2014;10(7):672–81.https://doi.org/10.2174/1573406410666140318111328
28
Fraenkel
PG
. Understanding anemia of chronic disease. Hematology (Am Soc Hematol Educ Program). 2015;2015(1):14–8.https://doi.org/10.1182/asheducation-2015.1.14
29
Dallalio
G
,
Law
E
,
Means
RT, Jr
. Hepcidin inhibits in vitro erythroid colony formation at reduced erythropoietin concentrations. Blood. 2006;107(7):2702–4.https://doi.org/10.1182/blood-2005-07-2854
30
Ganz
T
,
Nemeth
E
. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–43.https://doi.org/10.1016/j.bbamcr.2012.01.014
31
Enjuanes
C
,
Klip
IT
,
Bruguera
J
,
Cladellas
M
,
Ponikowski
P
,
Banasiak
W
, et al.
Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174(2):268–75.https://doi.org/10.1016/j.ijcard.2014.03.169
32
Gasche
C
,
Berstad
A
,
Befrits
R
,
Beglinger
C
,
Dignass
A
,
Erichsen
K
, et al.
Guidelines on the diagnosis and management of iron deficiency and anemia in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13(12):1545–53.https://doi.org/10.1002/ibd.20285
33
Weiss
G
,
Schett
G
. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9(4):205–15.https://doi.org/10.1038/nrrheum.2012.183
34
Ellervik
C
,
Tybjærg-Hansen
A
,
Nordestgaard
BG
. Total mortality by transferrin saturation levels: two general population studies and a metaanalysis. Clin Chem. 2011;57(3):459–66.https://doi.org/10.1373/clinchem.2010.156802
35
Kim
KS
,
Son
HG
,
Hong
NS
,
Lee
DH
. Associations of serum ferritin and transferrin % saturation with all-cause, cancer, and cardiovascular disease mortality: Third National Health and Nutrition Examination Survey follow-up study. J Prev Med Public Health. 2012;45(3):196–203.https://doi.org/10.3961/jpmph.2012.45.3.196
36
Mainous
AG, 3rd
,
Wells
B
,
Carek
PJ
,
Gill
JM
,
Geesey
ME
. The mortality risk of elevated serum transferrin saturation and consumption of dietary iron. Ann Fam Med. 2004;2(2):139–44.https://doi.org/10.1370/afm.82
37
Stack
AG
,
Mutwali
AI
,
Nguyen
HT
,
Cronin
CJ
,
Casserly
LF
,
Ferguson
J
. Transferrin saturation ratio and risk of total and cardiovascular mortality in the general population. QJM. 2014;107(8):623–33.https://doi.org/10.1093/qjmed/hcu045
38
Wells
BJ
,
Mainous
AG, 3rd
,
King
DE
,
Gill
JM
,
Carek
PJ
,
Geesey
ME
. The combined effect of transferrin saturation and low density lipoprotein on mortality. Fam Med. 2004;36(5):324–9.
39
Ellervik
C
,
Mandrup-Poulsen
T
,
Tybjærg-Hansen
A
,
Nordestgaard
BG
. Total and cause-specific mortality by elevated transferrin saturation and hemochromatosis genotype in individuals with diabetes: two general population studies. Diabetes Care. 2014;37(2):444–52.https://doi.org/10.2337/dc13-1198
40
Boelaert
JR
,
Vandecasteele
SJ
,
Appelberg
R
,
Gordeuk
VR
. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195(12):1745–53.https://doi.org/10.1086/518040
41
Lounis
N
,
Truffot-Pernot
C
,
Grosset
J
,
Gordeuk
VR
,
Boelaert
JR
. Iron and Mycobacterium tuberculosis infection. J Clin Virol. 2001;20(3):123–6.https://doi.org/10.1016/S1386-6532(00)00136-0
42
Weinberg
ED
. Iron loading and disease surveillance. Emerg Infect Dis. 1999;5(3):346–52.https://doi.org/10.3201/eid0503.990305
43
Gangaidzo
IT
,
Moyo
VM
,
Mvundura
E
,
Aggrey
G
,
Murphree
NL
,
Khumalo
H
, et al.
Association of pulmonary tuberculosis with increased dietary iron. J Infect Dis. 2001;184(7):936–9.https://doi.org/10.1086/323203
44
Barry
DM
,
Reeve
AW
. Increased incidence of gram-negative neonatal sepsis with intramuscular iron administration. Pediatrics. 1977;60(6):908–12.
45
Beeler
PE
,
Bates
DW
,
Hug
BL
. Clinical decision support systems. Swiss Med Wkly. 2014;144:w14073.
