Early biomarker response and patient preferences to oral and intramuscular vitamin B12 substitution in primary care: a randomised parallel-group trial
DOI: https://doi.org/10.4414/smw.2017.14421
Corina
Metaxasa, Deborah
Mathisb, Cyrill
Jegerc, Kurt E.
Hersbergera, Isabelle
Arneta, Philipp
Waltera d
aPharmaceutical Care Research Group, University of Basel,
bDivision of Clinical Chemistry and Biochemistry, University Children’s Hospital Zurich,
cRing Praxis Olten,
dInstitute of Laboratory Medicine, Solothurn Hospitals,
Early biomarker response and patient preferences to oral and intramuscular vitamin B12 substitution in primary care: a randomised parallel-group trial
Summary
BACKGROUND
Vitamin B12 (VB12) deficiency can be treated with oral high-dose substitution or intramuscular (IM) injection of VB12. Whenever alternative routes of administration exist, patient preferences should be considered when choosing the treatment. We aimed to assess outpatient preferences towards oral or IM VB12 substitution and confirm noninferiority of early biomarker response with oral treatment, in a typical primary care population.
METHODS
Prospective randomised nonblinded parallel-group trial. Patients were recruited by their general practitioner and randomly assigned to oral or IM treatment. Group O-oral was given 28 tablets of 1000 µg cyanocobalamin in a monthly punch card fitted with an electronic monitoring system. Group I-IM received four weekly injections of 1000 µg hydroxocobalamin. Blood samples were drawn before the first administration and after 1, 2 and 4 weeks of treatment, and analysed for VB12, holotranscobalamin (HoloTc), homocysteine (Hcy) and methylmalonic acid (MMA). For group O-oral, treatment adherence and percentage of days with ≥2 dosing events were calculated. Before and after 28 days of treatment, patients were asked to fill in a questionnaire about their preference for the therapy options and associated factors.
RESULTS
Between November 2013 and December 2015, 37 patients (age: 49.5 ± 18.5 years; women: 60.5%) were recruited for oral (19) or IM (18) treatment. Baseline values with 95% confidence intervals for serum VB12, HoloTc, Hcy and MMA were 158 pmol/l [145–172], 49.0 pmol/l [40.4–57.5], 14.8 µmol/l [12.0–17.7] and 304 nmol/l [219–390], respectively, in group O-oral and 164 pmol/l [154–174], 50.1 pmol/l [38.7–61.6], 13.0 µmol/l [11.0–15.1] and 321 nmol/l [215–427], respectively, in group I-IM (not significant). After 1 month of treatment, levels of VB12 and HoloTc showed a significant increase compared with baseline (group O-oral: VB12 354 pmol/l [298–410] and HoloTc 156 pmol/l [116–196]; group I-IM: VB12 2796 pmol/l [1277–4314] and HoloTc 1269 pmol/l [103–2435]). Hcy and MMA levels showed a significant decrease compared with baseline (group O-oral: Hcy 13.8 µmol/l [10.7–16.8] and MMA 168 nmol/l [134–202]; group I-IM: Hcy 8.5 µmol/l [7.1–9.8] and MMA 156 nmol/l [121–190]). HoloTc and MMA levels were normalised in all patients after 4 weeks of treatment, whereas normalisation of VB12 and Hcy was reached by all patients in group I-IM only. Response of VB12, HoloTc and Hcy was more pronounced in group I-IM (p <0.01) and the primary hypothesis that oral VB12 treatment would be noninferior to IM treatment was rejected. Average adherence to therapy was 99.6 ± 1.1% and days with ≥2 dosing events reached 5.6%. Before randomisation, preference was in favour of oral treatment (45.9%, n = 17) over IM administration (21.6%, n = 8). Twelve patients (32.4%) had no preference. Nine (24.3%) patients changed their preference after treatment. Patients who obtained their preferred route of administration maintained their preference in the case of oral treatment and changed their preference after IM treatment.
CONCLUSIONS
Differences in VB12 levels between groups were higher than expected. Therefore, noninferiority of oral treatment had to be rejected. However, normalisation of HoloTc and MMA was reached by all patients after a 1-month treatment period. The clinical benefit of the exaggerated biomarker response after IM treatment within a typical primary care population is questionable. Midterm biomarker effects and patient preferences should be considered when a therapeutic scheme is chosen. Initial rating in favour of either IM or oral therapy can change over time and justifies repeated re-evaluation of patient preferences. (ClinicalTrials.gov ID NCT01832129)
Background
Depending on the definition used, prevalence of vitamin B12 (VB12) deficiency is 8–16% and 5–40% in adults (26–64 years) [1] and the elderly [2], respectively. However, the true prevalence of VB12 deficiency in the general population is still uncertain, but it is known to rise with age, probably because of impaired absorption [3, 4].
Causes of VB12 deficiency can be divided into nutritional [5, 6], malabsorption syndromes and other gastrointestinal causes [5]. Pernicious anaemia typically presents with haematological signs and is associated with antibodies to intrinsic factor and/or gastric parietal cells, but accounts for only a small proportion of the observed cases of VB12 deficiency [7]. Furthermore, defective transport mechanisms due to genetic factors account for a very small proportion of cases [8]. Long-term treatment with acid-lowering agents [9, 10] and metformin [11] may also play a role in the development of VB12 deficiency.
Clinical symptoms of VB12 deficiency are numerous. Besides nonspecific symptoms such as tiredness and a loss of appetite, haematological manifestations (megaloblastic anaemia), neurological disorders (e.g., polyneuropathy, ataxia) and symptoms of a psychiatric nature (e.g., depression) are possible [8, 12]. Additionally, cardiovascular manifestations, which accompany hyperhomocysteinaemia, are described [13–16]. Because VB12 deficiency is a reversible cause of demyelinating nervous system disease and bone marrow failure, its early detection and treatment are important [12].
The indications for VB12 supplementation is VB12 deficiency of various causes (e.g., pernicious anaemia, gastrectomy, dietary deficiency) [12]. In addition, preventive treatment should be initiated in pure vegetarians, pregnant women on a Mediterranean diet, patients with gastric surgery and nitrous oxide exposure [17]. However, there are no official threshold concentrations for initiating treatment. Biochemically, VB12 deficiency is characterised by subnormal to borderline serum VB12 levels. Holotranscobalamin (HoloTc) is the bioactive form of VB12 and has been discussed controversially as a more specific and sensitive marker of VB12 deficiency [18–20]. Functional VB12 deficiency is characterised by an increase of methylmalonic acid (MMA) and/or homocysteine (Hcy). Functional testing is recommended when VB12 deficiency is highly expected, levels of serum VB12 are moderately low (148–221 pmol/l), in patients with unexplained macrocytosis or unexplained neurological issues, and when VB12 deficiency is highly suspected to be a treatable cause of dementia [17]. Further laboratory findings are haematological abnormalities such as macrocytosis, pancytopenia and hypersegmented neutrophils. Haematological changes can be found in the more severe cases, whereas biochemical findings go in parallel with less specific clinical manifestations of VB12 deficiency. However, no clear-cut limits exist for the prediction of symptoms [12]. Subclinical VB12 deficiency occurs and is found in up to 10–25% of the aged population. Treatment of these patients is common, even though the long-term benefits of such treatment are unclear [17].
The treatment of VB12 deficiency consists of VB12 supplementation, which can be either oral or by intramuscular (IM) injection. Patients with severe VB12 deficiency should receive injections of 1000 μg VB12 at least several times per week for 1 to 2 weeks, then weekly until clear improvement is shown, followed by monthly injections [12, 17]. Initial oral treatment with high dose VB12 can be considered in patients with mild malabsorption or dietary deficiency [12]. Given the unpredictable absorption of oral VB12, in severe cases the oral route should be used only after the serum VB12 level has been normalised with parenteral treatment or when the response to the treatment is monitored frequently with measurement of serum VB12 and MMA [17]. Measurements for monitoring response to treatment after VB12 substitution comprise VB12 itself, its active fraction HoloTc and either Hcy or MMA as a functional marker (in the case of a mild form without haematological manifestations), and potassium, iron status, lactate dehydrogenase and bilirubin (in cases of VB12-associated anaemia).
In Switzerland, VB12 supplementation is predominantly given as IM injections [21], which are usually painful. No high-dose VB12 oral monopreparation is currently available, despite evidence of its effectiveness [22–24]. Good response to oral supplementation has been observed even in the presence of gastrointestinal diseases that are commonly associated with VB12 deficiency. One study showed that VB12 deficiency could even be reversed in patients who had undergone gastrectomy [25]. However, evidence for the effectiveness of oral high-dose VB12 substitution from randomised trials comparing oral and IM administration is limited [26].
Oral treatment with VB12 may be superior to IM injections in terms of patient acceptance and cost-effectiveness [27]. Patient preferences in treatment-related decisions should be elicited and taken into account, because patients who felt less empowered with regard to treatment decisions reported lower rates of adherence [28]. Finally, a better understanding of patient preferences and values for making choices is fundamental to achieving shared decision-making and ultimately improving adherence.
We aimed to assess outpatient preferences for VB12 supplementation by the oral or IM route, and to confirm noninferiority of early biomarker response with oral treatment in a typical primary-care population with biochemically defined VB12 deficiency.
Material and methods
This prospective, randomised, nonblinded parallel-group trial was approved by the ethics committee of the cantons Aargau and Solothurn, Switzerland, and has been registered at ClinicalTrials.gov ID NCT01832129. The study was conducted in accordance with the Declaration of Helsinki and follows the International Conference on Harmonisation Good Clinical Practice (ICH-GCP) guidelines. The primary hypothesis was that oral VB12 treatment would be noninferior to IM treatment in terms of serum VB12 response after 1 month of treatment. Patients were expected to prefer oral treatment over IM injections before and after treatment.
Participants
Recruitment was initiated at three general practitioner (GP) practices in the area of Olten, Switzerland. Eligible patients had a VB12 serum concentration <200 pmol/l, an indication for VB12 supplementation according to their GP’s estimation, were ≥18 years old and were able to give written informed consent. Exclusion criteria were: the concurrent intake of vitamin preparations containing VB12, previously diagnosed dementia, known hereditary transcobolamin transportation defects, or lack of written and/or oral understanding of German, French, Italian or English.
Recruitment
A letter with the patient information leaflet and a written informed consent form was given to patients whose physician had ordered a laboratory test for the biochemical confirmation of VB12 deficiency. Patients were asked to bring the informed consent form to their next scheduled visit with their GP, during which the results of the laboratory test would be discussed. Eligible patients were asked by their GP to participate in the study. Patients who gave written informed consent were randomly assigned to group O-oral daily treatment or to group I-IM conventional weekly treatment. Blocks of four were generated from computer software. Each GP practice received two blocks (four O-oral group and four I-IM group) each packed in sealed and unlabelled envelopes. Once a patient had consented, the GP or his staff opened one envelope to reveal which group of the study the patient had been randomised to. Upon request, further blocks were available.
Interventions
Patients of group O-oral were instructed to ingest one tablet of 1000 µg cyanocobalamin daily (B12 “Ankermann”; Wörwag Pharma GmbH & Co, Böblingen, Germany) for 28 consecutive days supplied in a 7x4 punch card with electronic adherence monitoring. Polymedication electronic monitoring system (POEMS) technology [29] was used to assess adherence to the oral VB12 intake. POEMS consists of a film with imprinted electronic components that measure the electrical resistance and record the time of its changes when a loop is broken, i.e., when a cavity is emptied. A first punch card fitted with POEMS was handed out for 14 days. A second identical punch card was handed out for a further 2 weeks at the third visit 2 weeks later. Patients were instructed to return the punch cards for pill count and for the extraction of the electronic adherence data. Patients of the group I-IM received conventional supplementation with weekly injections of 1000 µg hydroxocobalamin (Vitarubin® Depot 1000 µg / 1ml; Streuli Pharma AG, Uznach, Switzerland, mixed with Lidocaine 1% 1 ml before injection). The treatment options were not blinded.
Adherence outcomes
For each patient in the group O-oral, we calculated two adherence rates: adherence with pill count, defined as the percentage of days with performed intakes divided by the days with prescribed intakes, and dosing irregularities, defined as the percentage of days with ≥2 dosing events from the POEMS data.
Biomarker assessment
Venous blood samples were drawn before the first administration (V0), and after 1 (V7), 2 (V14), and 4 weeks of treatment (V28). Blood samples were analysed by means of immunological assays on a Beckman Coulter DxC 860i (VB12), Roche cobas® 6000 (homocysteine, folic acid), and Abbott Architect i2000SR (holotranscobalamin). Methylmalonic acid was measured with liquid chromatography mass spectrometry (LC-MS/MS) on a Thermo Scientific UltiMate 3000 Rapid Separation LC coupled to an AB Sciex 5500 TripleQuad MS. Blood cell count was determined on a Beckman Coulter DxH 800. Normalisation of VB12-associated biomarkers was defined as a serum VB12 >258 pmol/l, HoloTc >37 pmol/l, Hcy <15 µmol/l and MMA <270 nmol/l. Folate deficiency was defined as a serum value <9.1 nmol/l. GPs were informed about biomarker levels after V28.
Patient preferences
Preferences were determined before block randomisation (V0) and after 4 weeks of treatment (V28), with use of a scenario-based approach. Patients were asked to select treatment by ticking tablets, syringes or no preference in a questionnaire, given that oral and IM substitution was equally effective. The questionnaire consisted of nine items focusing on factors influencing preference: pain, disgust, side effects, effectiveness, inconvenience, difficulties, time consumption, costs, and nonadherence to treatment schedule. Each item was to be answered twice for each therapy option (oral and IM). Answers could be given on a 10-point Likert scale.
Sample size
Sample size estimation was based on assumptions regarding outcomes after 4 weeks. Patients were expected to display baseline VB12 concentrations of 100–150 pmol/l. Based on published data, patients reach levels of approximately 600 pmol/l with an estimated standard deviation of 120 pmol/l after treatment. A difference of ≤100 pmol/l between levels after intramuscular or oral supplementation was deemed acceptable for noninferiority, on the presumption that this difference is clinically meaningless.
With the hypothesis that there is no difference between the groups, 50 patients were required to show with 90% confidence that the lower limit of a one-sided 95% confidence interval will be above the noninferiority limit of 100 pmol/l.
Statistical analysis
Values are given as mean ± standard deviation (SD), median with quartiles and percentages where appropriate. Frequencies were analysed using chi‐square tests or Fishers test. The Mann‐Whitney test was used to compare numerical variables between two groups and the Kruskal-Wallis test was used to compare between three groups. Spearman’s r was calculated to assess correlations between numerical variables and interpreted with the following criteria: 0–0.25 = little or no correlation; 0.26–0.50 = small correlation; 0.51–0.75 = moderate to good correlation, and >0.75 = very good to excellent correlation. A p-value ≤0.05 was considered significant.
Results
Between November 2013 and December 2015, 37 patients (age 49.5 ± 18.5 years; 60.5% women) were recruited for oral (n = 19) or IM (n = 18) treatment. No patient reported any harms or side effects during the study period. Recruitment was terminated after an anticipated analysis showed sufficient biomarker response for both treatment options and an enormous difference between groups in mean VB12 values at V28 (2442 pmol/l), leading to the rejection of the primary noninferiority hypothesis for oral treatment. Post-hoc sample size estimation was based on VB12 outcomes at V28. Patients in group O-oral and group I-IM had VB12 levels of approximately 350 pmol/l with a standard deviation of 120 pmol/l and 2700 pmol/l with a standard deviation of 2700 pmol/l, respectively. With the hypothesis that there is a difference between the groups, a total of 28 patients were required to ensure that the 90% confidence interval includes the true difference between groups with an α of 5%.
The baseline characteristics were equally distributed between the two treatment groups (table 1). The study population contained patients with the following diagnosis or risk factors associated with VB12 deficiency: pernicious anaemia (n = 2), metformin intake (n = 2), use of acid lowering drugs (n = 4), vegetarian or low dietary intake of VB12-containing food (n = 18), diagnosed alcohol abuse or daily intake of alcohol (n = 4), and gastric stapling (n = 1). No established risk factor for VB12 deficiency was identified in six patients. Distribution of risk factors was balanced between the groups (data not shown).
Table 1 Baseline characteristics in patients receiving oral or IM vitamin B12 substitution (n = 37).
|
Normal values
|
|
Group O-oral (n = 19)
|
Group I-IM (n = 18)
|
p-value
|
| Women (n) |
|
% (n) |
68.4% (n = 13) |
55.6% (n = 10) |
0.508 |
| Age (years) |
|
Mean ± SD |
47.3 ± 17.8 |
51.5 ± 19.6 |
0.543 |
| Body mass index (kg/m2) |
|
Mean ± SD |
27.3 ± 7.00 |
25.6 ± 4.20 |
0.715 |
| Vitamin B12 (pmol/l) |
>258 |
Mean ± SD
Median (quartiles) |
158 ± 27.4
164 (135/177) |
164 ± 20.1
161 (152/178) |
0.578 |
| Holotranscobalamin (pmol/l) |
>37 |
Mean ± SD
Median (quartiles) |
49.0 ± 17.7
49.9 (32.1/63.2) |
50.1 ± 23.0
43.1 (35.8/65.5) |
0.940 |
| Homocysteine (µmol/l) |
<15 |
Mean ± SD
Median (quartiles) |
14.8 ± 5.80
13.2 (10.9/16.7) |
13.0 ± 4.10
13.4 (9.6/16.7) |
0.408 |
| Methylmalonic acid (nmol/l) |
<270 |
Mean ± SD
Median (quartiles) |
304 ± 172
284 (160/379) |
321 ± 183
249 (183/332) |
0.757 |
| Haemoglobin (g/l) |
120–160 (women)
135–175 (men) |
Mean ± SD
Median (quartiles) |
138 ± 13.1
136 (127/145) |
136 ± 11.8
137 (127/141) |
0.822 |
| Mean corpuscular volume (fl) |
85–101 |
Mean ± SD
Median (quartiles) |
91.7 ± 6.40
91 (89/96) |
93.7 ± 6.10
93 (90/95) |
0.663 |
| Mean corpuscular haemoglobin (pg) |
28–33 |
Mean ± SD
Median (quartiles) |
30.7 ± 2.10
31 (30/32) |
31.2 ± 2.00
31 (30/32) |
0.799 |
| Folic acid (nmol/l) |
9.1–42.4 |
Mean ± SD
Median (quartiles) |
16.2 ± 5.80
16.6 (11.8/18.4) |
17.6 ± 5.80
16.7 (13.7/22.0) |
0.558 |
| Sodium (mmol/l) |
136–145 |
Mean ± SD
Median (quartiles) |
140 ± 1.9
140 (139/141) |
140 ± 1.7
141 (139/141) |
0.775 |
| Potassium (mmol/l) |
3.5–5.1 |
Mean ± SD
Median (quartiles) |
4.6 ± 1.0
4.2 (4.1/4.5) |
4.2 ± 0.3
4.2 (4.0/4.3) |
0.298 |
| Creatinine (µmol/l) |
49–90 (women)
64–104 (men) |
Mean ± SD
Median (quartiles) |
74 ± 14.1
72 (64/83) |
74 ± 12.7
73 (65.25/79.5) |
0.916 |
| Alanine aminotransferase (U/l) |
<55 |
Mean ± SD
Median (quartiles) |
8.0 ± 8.6
8.0 (0/11) |
6.6 ± 5.5
6.5 (0/12) |
0.964 |
| Aspartate aminotransferase (U/l) |
5–34 |
Mean ± SD
Median (quartiles) |
25.9 ± 5.9
24 (21/32) |
22.6 ± 3.9
22 (20/25) |
0.105 |
| Gamma-glutamyltransferase (U/l) |
9–46 |
Mean ± SD
Median (quartiles) |
39.2 ± 53.2
15 (10/46) |
19.5 ± 10.5
17.5 (10/23.75) |
0.916 |
A total of 38 electronic punch cards containing oral treatment were delivered, one of which was not returned (excluded from analysis) and 13 only partially detected the removals because of technical problems. From the 518 expected events, 356 were recorded (31.3% missed data). Days with ≥2 dosing events occurred in 5.6% of all recordings. Average treatment adherence by pill count was 99.6% ± 1.1%
Blood counts and levels of VB12, HoloTc, Hcy, MMA, folic acid, sodium, potassium, creatinine and liver enzymes did not differ at baseline (V0) between groups (table 1). Levels of VB12 and HoloTc were significantly increased at V7, V14 and V28 compared with baseline for both groups (p <0.01) (table 2). For group I-IM at each assessment point, VB12 and HoloTc response was significantly higher (p<0.01, fig. 1) and the level of Hcy was significantly more reduced (p <0.01) compared with group O-oral. Reduction of Hcy levels compared with baseline was significant at V7 for group I-IM and at V28 for both groups (group O-oral p <0.05; group I-IM p <0.01). MMA levels were significantly decreased compared with baseline at V7 for both groups (p <0.01) and did not differ between groups (fig. 1). Blood count and folic acid levels did not change significantly between V0 and V28 in both groups (data not shown).
Table 2 Serum concentrations of vitamin B12, holotranscobalamin, homocysteine and methylmalonic acid at baseline (V0) and after 7, 14 and 28 days of treatment (V7, V14 and V28).
|
Group
|
Mean concentration (95% confidence interval)
|
|
V0
|
V7
|
V14
|
V28
|
|
VB 12 (pmol/l)
|
O-oral |
158 (145–172) |
304 (250–357) |
319 (284–355) |
354 (298–410) |
| I-IM |
164 (154–174) |
1088 (934–1236) |
1897 (1219–2574) |
2796 (1277–4314) |
| p-value |
0.58 |
<0.001 |
<0.001 |
<0.001 |
|
HoloTc (pmol/l)
|
O-oral |
49.0 (40.4–57.5) |
148 (109–187) |
144 (115–173) |
156 (116–196) |
| I-IM |
50.1 (38.7–61.6) |
244 (200–288) |
495 (373–617) |
1269 (103–2435) |
| p-value |
0.94 |
<0.01 |
<0.001 |
<0.001 |
|
Hcy (µmol/l)
|
O-oral |
14.8 (12.0–17.7) |
14.6 (11.7–17.3) |
14.3 (11.4–17.3) |
13.8 (10.7–16.8) |
| I-IM |
13.0 (11.0–15.1) |
9.5 (8.2 – 11.0) |
9.0 (7.4–10.7) |
8.5 (7.1–9.8) |
| p-value |
0.41 |
<0.001 |
<0.001 |
<0.001 |
|
MMA (nmol/l)
|
O-oral |
304 (219–390) |
188 (148–227) |
187 (151–223) |
168 (134–202) |
| I-IM |
321 (215–427) |
172 (135–208) |
161 (127–197) |
156 (121–190) |
| p-value |
0.76 |
0.53 |
0.16 |
0.51 |
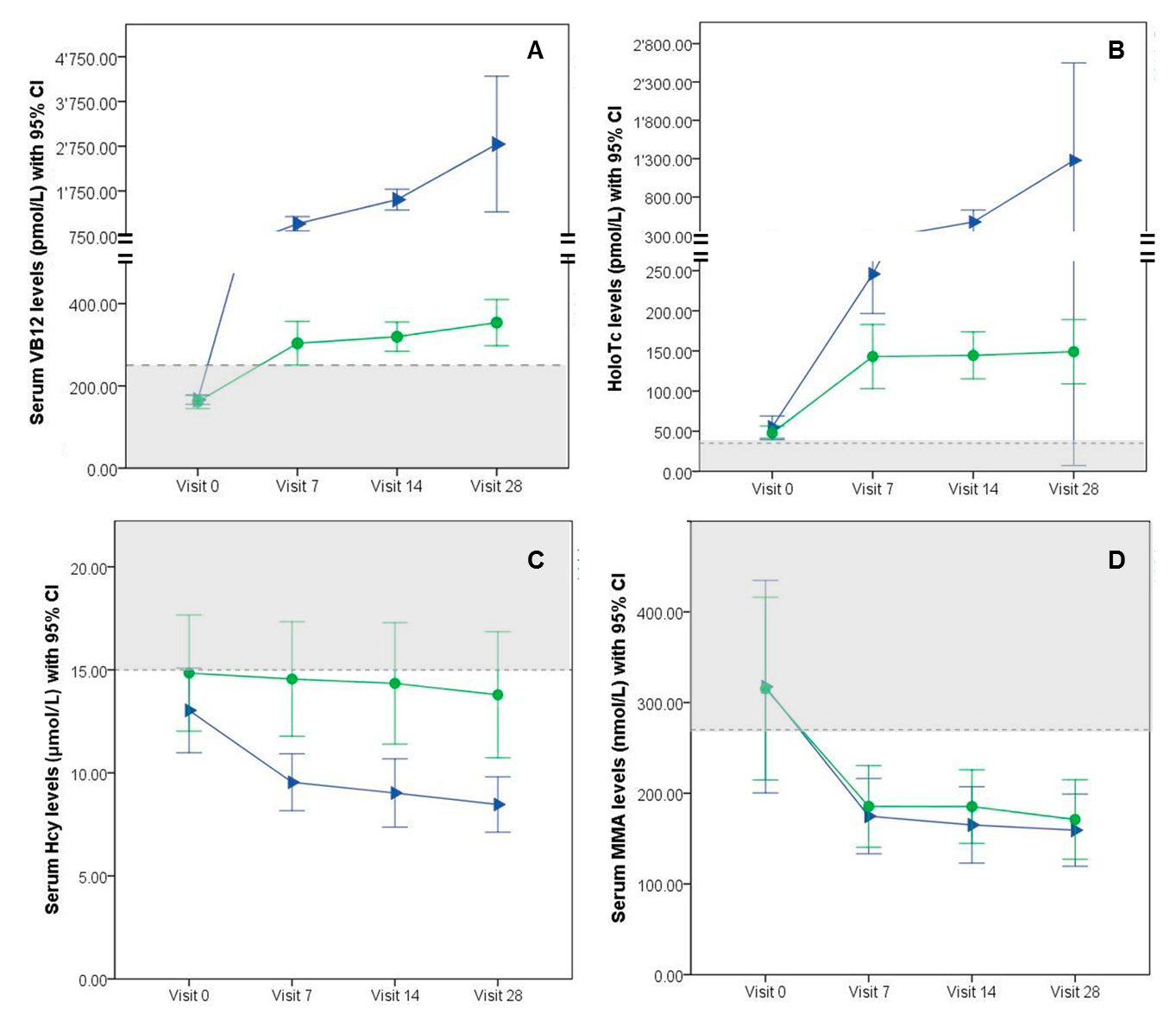
Figure 1 Biomarker levels (mean and 95 confidence intervals [CI]) at baseline (Visit 0), Visit 7, Visit 14 and Visit 28 for group O-oral treatment (●) and group I-IM treatment (►). Grey areas indicate subtherapeutic levels, dotted lines indicate threshold values for biomarker normalisation. (A) Mean serum vitamin B12 (VB12) levels, dotted line at 258 pmol/l; (B) Mean holotranscobalamin (HoloTc) levels, dotted line at 37 pmol/l; (C) Mean homocysteine (Hcy) levels, dotted line at 15 µmol/l; (D) Mean methylmalonic acid (MMA) levels, dotted line at 270 nmol/l.
After 28 days of treatment, in group O-oral normalised VB12 levels were reached by 16 (84.2%) patients, normal Hcy levels by 14 (73.9%) patients and normal HoloTc and MMA levels by 19 (100%) patients (fig. 2). After 28 days of treatment in group I-IM, all 18 patients (100%) had normal VB12, HoloTc, Hcy and MMA levels. Percentage of patients with a normalisation of all biomarkers at V28 was significantly higher in group I-IM compared with group O-oral (100% vs 63.2%, p <0.05).
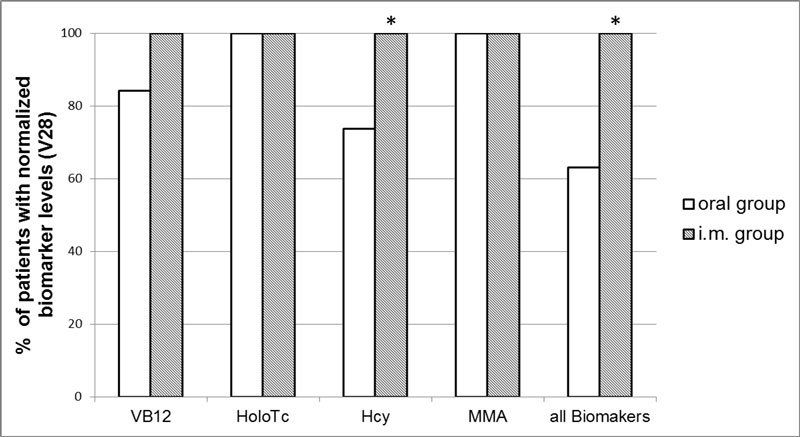
Figure 2 Proportion of patients with normalised VB12-associated biomarkers after 28 days of treatment administered by the oral (n = 19; white bar) or IM route (n = 18; grey bar).
* indicates significant difference (p< 0.05). Hcy = homocysteine; HoloTc = holotranscobalamin; MMA = methylmalonic acid; VB12 = vitamin B12
At V0, VB12 or HoloTc did not correlate with Hcy or MMA within the study population. Within group O-oral a non-significant small correlation between Hcy levels and VB12 was found at V7 (r = ˗0.369; p = 0.12), V14 (r = ˗0.388; p = 0.10) and V28 (r = ˗0.341; p = 0.15) and between Hcy and HoloTc at V14 (r = ˗0.397; p = 0.10) and V28 (r = ˗0.392; p = 0.10). Levels of VB12 or HoloTc did not correlate with MMA. Within group I-IM a moderate correlation was found for VB12 and Hcy levels at V7 (r = ˗0.725; p <0.001), V14 (r = ˗0.507; p <0.05) and a small nonsignificant correlation at V28 (r = ˗0.254; p = 0.38). Levels of HoloTc and Hcy did not correlate nor did levels of MMA correlate with VB12 or HoloTc levels. Correlation between Hcy and creatinine levels was moderate for the whole study population at V0 (r = 0.522; p <0.001) and for the group O-oral at V28 (r = 0.491; p <0.05). A high correlation was observed for group I-IM at V28 (r = 0.713, p <0.001).
Before randomisation, 17 patients preferred oral treatment (45.9%) and eight patients preferred IM treatment (21.6%). Twelve patients (32.4%) had no preference. Concerns were compared between patients grouped by preference. For the patients who preferred tablets, therapy with syringes would raise more concerns about the pain (p = 0.001), disgust (p = 0.004), side effects (p = 0.017), inconvenience (p = 0.001), difficulties (p = 0.001) and time consumption (p = 0.001). Patients preferring IM treatment indicated their concerns about forgetting to take the medicine regularly (addressed as nonadherence to treatment schedule) (p = 0.018), inconvenience (p = 0.024) or higher time consumption when taking tablets (p = 0.001) (table 3).
Table 3 Patients’ answers to nine items known to influence treatment preference, before randomisation. Answers are sorted according to the first question “Which treatment do you prefer?” tablets / syringes / no preference. Each item was to be answered twice for therapy with syringes and with tablets. The higher the score the higher the anticipated effect of the corresponding item on a Likert scale 1–10. Data are given as mean ±SD. A p-value <0.05 indicates a significant difference between the group with the highest values versus the group with the lowest values.
|
Item
|
|
Prefers tablets
(n = 17)
|
Prefers syringes
(n = 8)
|
No preference
(n = 12)
|
p-value between three groups
|
| Pain |
Syringes
Tablets |
5.7 ± 2.0
1.0 ± 0.0 |
3.1 ± 1.6
2.1 ± 2.2 |
2.6 ± 1.2
1.1 ± 0.3 |
0.001
0.101 |
| Disgust |
Syringes
Tablets |
5.9 ± 2.4
3.1 ± 1.7 |
3.1 ± 2.2
4.6 ± 2.3 |
3.2 ± 2.4
2.5 ± 1.4 |
0.004
0.078 |
| Side effects |
Syringes
Tablets |
4.2 ± 2.6
2.4 ± 1.3 |
2.1 ± 1.8
2.8 ± 2.5 |
2.3 ± 1.5
2.3 ± 1.5 |
0.017
0.950 |
| Effectiveness of the treatment |
Syringes
Tablets |
8.4 ± 1.5
7.8 ± 2.2 |
9.0 ± 1.1
6.9 ± 2.3 |
8.8 ± 1.3
8.0 ± 1.5 |
0.740
0.454 |
| Inconvenience |
Syringes
Tablets |
6.8 ± 1.8
2.7 ± 1.5 |
2.6 ± 1.4
4.9 ± 2.4 |
2.3 ± 1.2
2.3 ± 2.0 |
0.001
0.024
|
| Difficulties |
Syringes
Tablets |
4.7 ± 2.7
1.7 ± 1.7 |
1.8 ± 1.8
1.9 ± 1.6 |
1.4 ± 0.7
1.3 ± 0.6 |
0.001
0.808 |
| Time consumption |
Syringes
Tablets |
7.0 ± 2.6
1.2 ± 0.5 |
4.1 ± 2.8
2.6 ± 0.9 |
2.4 ± 1.0
1.3 ± 0.7 |
0.001
0.001
|
| Costs |
Syringes
Tablets |
4.5 ± 2.6
2.8 ± 1.7 |
3.9 ± 3.1
2.6 ± 1.8 |
3.5 ± 2.7
2.4 ± 2.7 |
0.503
0.504 |
| Nonadherence to treatment schedule |
Syringes
Tablets |
3.3 ± 2.6
2.4 ± 2.6 |
3.8 ± 2.8
4.3 ± 1.7 |
2.6 ± 2.6
2.7 ± 2.9 |
0.390
0.018
|
Nine patients (24.3%) changed their preference after treatment. Of the patients who were allocated to the nonpreferred administration group, 20% changed their mind regardless of whether they were exposed to oral or IM treatment: patients who received oral treatment changed their mind in favour of oral treatment (100%) and patients receiving IM treatment changed their mind in both direction at V28. Patients who preferred oral treatment and were assigned to group O-oral maintained their preference (100% congruence). Patients who preferred IM treatment and obtained parenteral treatment changed their preference at V 28 (0% congruence, see figure S1 in the appendix).
Discussion
In our study, levels of VB12 and HoloTc were significantly increased and levels of Hcy and MMA significantly decreased after 28 days of treatment with high-dose VB12 administered by either the oral or IM route. These findings are in line with other trials, of which two assessed the effect of oral high-dose VB12 substitution (1000 µg cyanocobalamin) vs placebo [24, 30], whereas three other randomised, controlled trials compared cyanocobalamin therapy administered orally (1000–2000 µg) or parenterally (1000 µg cyanocobalamin) [22, 23, 31].
In contrast to prior studies, we observed an exaggerated response after IM administration and therefore the hypothesis for noninferiority of oral in comparison to IM treatment had to be rejected. Because we used electronic punch cards and monitored an almost perfect intake of tablets (99.6% adherence), nonadherence can be ruled out as a contributor to the less pronounced response of VB12, HoloTc and Hcy in patients following oral administration. Thus, the enormous difference must have a chemical or physiological explanation. One reason might be the use of hydroxocobalamin, a physiological intermediate form that is more available to cells than other cobalamin forms [32]. In children with VB12 deficiency, one injection of 400 µg hydroxocobalamin resulted in improvement in motor function and cobalamin repletion [33]. Additionally, hydroxocobalamin is retained in plasma longer than equivalent doses of cyanocobalamin, which allows less frequent dosing. However, owing to its low stability, hydroxocobalamin is less suited for oral supplementation, whereas cyanocobalamin is best suited for oral supplementation as it is a more stable and inexpensive [34]. Surprisingly, sustainability of biomarker response after IM hydroxocobalamin administration has been poorly described. In one study among 8 patients with VB12 levels below 80 pg/ml (59.4 pmol/l), VB12 levels between 300 and 1100 pg/l (221–812 pmol/l) were obtained 10 days after the injection of 1000 µg hydroxocobalamin. The levels fell below 200 pg/l (148 pmol/l) between 4 and 10 weeks later [35]. In our study, wide interindividual variation in VB12 and HoloTc responses was observed within the IM group, which corresponds to the wide interindividual variations in hydroxocobalamin pharmacokinetics reported by others [36, 37].
To our knowledge, there are no reports of high levels of VB12 and HoloTc with daily oral VB12 substitution over a longer treatment period similar to those we observed after IM treatment. In one study with high-dose oral substitution of VB12 for 3 months, patients with initially low levels of VB12 (186 ± 56 pmol/l) reached higher VB12 levels (mean 477 pmol/l) than we observed after 28 days of treatment, albeit levels of HoloTc (183 pmol/l), Hcy (13.4 µmol/l) and MMA (0.23 µmol/ 230 nmol/l) were comparable to the levels we observed after 7 days (HoloTc and MMA) and after 28 days (Hcy) of oral treatment [30]. Continuation of treatment for up to 6 months did not result in additional significant changes in VB12, HoloTc, Hcy and MMA [30]. A further study observed a plateau in serum VB12 levels with a mean of 1164 pg/ml (858 pmol/l) after 3 months when patients received a loading dose of 1000 µg hydroxocobalamin and a subsequent 18 months of treatment with 1000 µg oral cyanocobalamin [38]. These findings suggest that continuous oral treatment with high dose VB12 reaches saturation in serum VB12 levels after 3 months of treatment. In our study, three patients did not have normal levels of VB12 after oral treatment. However, two out of those three patients had VB12 levels above the normal range at V14, which slightly decreased afterwards. In the absence of any rational explanations (e.g., patients did not stop treatment prematurely), results indicate that time to VB12 saturation after therapy and the level of saturation may vary between patients. The other patient responded slowly to oral substitution, probably owing to the underlying cause of VB12 deficiency, which was gastric stapling (VB12 levels at V0 and V28: 108 pmol/l and 182 pmol/l, respectively). Furthermore, the active part of vitamin B12, HoloTc, was normalised in all patients at V28. Pernicious anaemia could be another explanation for nonresponse in the O-oral group. However, because all patients had some kind of response or a physiological rationale for a slower response (i.e. gastric stapling), this explanation is very unlikely.
Five patients in the oral group did not reach normalised Hcy levels at V28. However, there is as yet no agreement on normal ranges for VB12-associated biomarkers [39]. Hcy lacks specificity, and response may be confounded by folic acid and vitamin B6 deficiency, as well as by renal insufficiency, liver insufficiency and genetic abnormalities. Additionally, Hcy levels are influenced by lifestyle factors, such as consumption of coffee, alcohol and tobacco [40]. The incomplete normalisation of Hcy levels in our cohort could be explained in two patients with folic acid deficiency or diagnosed renal insufficiency, respectively. Additionally, renal function might have affected normalisation in other patients as well, indicated by the correlation between Hcy and creatinine. Compared with IM treatment, decrease in Hcy levels was slower with oral treatment. Other studies have also found Hcy levels to respond slowly to oral treatment [24], with a trend to further decrease over a period of 18 months [38].
A negative concentration-effect relationship was observed for Hcy and VB12 in the group I-IM. A trend towards similar correlation between low Hcy levels and high VB12 levels was observed for group O-oral. A stronger concentration-effect relationship may be observed in patients with a more pronounced deficiency of VB12-associated biomarkers. Before drawing conclusions on concentration-effect relationship between VB12 and Hcy, results should be verified in a bigger sample of anaemic patients with consideration of further factors such as renal function and lifestyle.
Interestingly, the functional biomarkers MMA and Hcy did not respond consistently in either group. Patients in both groups reached normal MMA levels at V7. Hcy levels in the I-IM group were decreased at V7 too, whereas levels in O-oral-group were decreased at V28, and significantly higher at each assessment point compared with the I-IM group. The different enzyme systems converting Hcy and MMA in the human body might explain the observed difference in functional biomarker response between groups. In vitro studies showed that hydroxocobalamin induces the activation of one of these enzyme systems (methionine synthase) more strongly and quickly than cyanocobalamin [41].
In summary, supratherapeutic levels were observed for VB12 and HoloTc after the IM treatment with hydroxocobalamin, which might never be reached through oral substitution. Additionally, normalisation of all biomarkers was significantly higher in group I-IM compared with group O-oral (100% vs 63.2%, p<0.05). However, incomplete response in group O-oral was limited to VB12 and Hcy. Therefore, the benefit of such an exaggerated response after IM injection seems limited to a practical advantage in the form of fewer administrations, i.e. longer treatment intervals and in the case of symptomatic patients needing rapid normalisation of VB12-associated biomarkers. A large prospective randomised controlled trial comparing high-dose oral with intramuscular cyanocobalamin in elderly patients is currently underway (PMID: 22650964, NCTNCT 01476007). This study is expected to report on long-term oral and IM VB12 substitution (8, 26 and 52 weeks). However, no reports on short-term biomarker response or patient preferences are expected. Further studies are required to assess the effects of different cobalamin forms on biomarker response and on clinical outcomes. Accordingly, observed differences between cobalamin forms should be incorporated into guidelines for treatment of VB12 deficiency.
As expected, patients preferred oral treatment to IM treatment, before the assignment to treatment as well as after treatment completion. Our findings are in line with reports from two studies on patient preferences in relation to oral VB12 treatment [38, 42]. In a study in primary care, 83% of patients preferred oral to IM treatment [38]. In another study involving patients receiving VB12 as injection and willing to try oral administration, the majority were satisfied with the switch and wished to remain permanently on oral therapy. Important factors for switching to oral therapy were the disadvantage of injections and their association with many visits to their healthcare providers, higher costs and the convenience of oral treatment [42]. We also found time consumption and inconvenience of IM treatment as important factors in favour of oral treatment.
There was a slight change in patient preferences after receiving oral therapy (n = 2, 10.5%), whilst changes occurred only in favour of oral treatment. This may indicate that patients appreciate the route of administration more after experiencing oral treatment at first hand. After experiencing IM treatment, 11 patients (38.9%) changed their preference in various directions. This is interesting in view of the pretreatment attitudes towards important factors associated with patient preferences, regarding a therapy with syringes (n = 6) and regarding a therapy with tablets (n = 3). These findings suggest that patients may have more prejudice regarding syringes, which may explain the numerous and various changes after experiencing IM treatment.
Given the exaggerated response after IM treatment, the required frequency of injections with hydroxocobalamin in clinical practice may be lower in patients with mild VB12 deficiency, which may augment the preference for IM administration. Additional research with validated methods is needed to gain an insight into the patient preferences, especially when therapeutic options with comparable efficacy and safety are available.
Limitations of the study include the fact that we enrolled patients mostly without haematological symptoms and not necessarily abnormal functional biomarkers. Therefore, patients in our study were less likely to respond to VB12 substitution, which affects our ability to generalise our results to a symptomatic, anaemic, VB12-deficient population. Second, the questionnaire we used to assess patients’ preferences consisted of re-used questions from several assessment tools but was not validated completely as an entity. Nevertheless, the single questions can be judged valid for retrieving patient preferences. Additionally, we did not assess patient preferences for maintenance therapy and therefore cannot evaluate whether preferences would change in this long-term situation. However, it seems justified to re-evaluate treatment 1 month after initiation, irrespective of treatment schedule as received treatment is strongly suspected to influence attitudes and ultimately patient preferences. Third, we had a high proportion of technical issues leading to a loss of one third of the electronically gained adherence data; therefore, days with more dosing events might have occurred more frequently, as suggested by our calculations. However, this technical drawback had no impact on the calculation of adherence based on the conventional pill count method. Last, we stopped the study prematurely after an anticipated analysis and thus obtained a small sample size. However, the normalised levels reached in both groups did not justify continuing the study since the hypothesis was rejected with the small number of patients.
Conclusion
Differences in VB12, HoloTc and Hcy levels between groups were higher than expected. Therefore, the hypothesis of noninferiority of oral treatment had to be rejected. However, HoloTc and MMA were normalised in all patients and VB12 and Hcy in the majority of patients within group O-oral after 1 month of treatment. The clinical benefit of the exaggerated biomarker response after IM treatment within a typical primary care population is questionable. Midterm biomarker effects and patient preferences should be considered in the choice of therapeutic scheme. Initial rating in favour of either IM or oral therapy can change over time and justifies repeated re-evaluation of patient preferences. However, the majority of patients preferred oral treatment before and after the study, indicating the need for a high-dose oral VB12 preparation in Switzerland. Further research may help to evaluate which route of administration, oral vs IM, of long-term VB12 treatment, will be appropriate to yield a sustained biomarker response.
Appendix: Supplementary figure
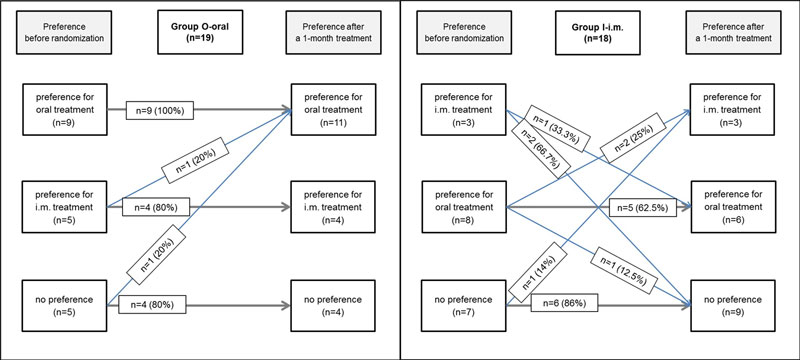
Figure S1 Patients preferences before and after 28 days of treatment administered by the oral (n = 19) or IM route (n = 18).
Acknowledgements
We thank the participating general practitioners and their staff for patient recruitment. The authors thank Jean Pierre Rothen for the initiation of the study, Noortje Vriiends, who contributed to the development of the questionnaire to assess patient preferences, and Ursula Zoli (Aarelab, Olten) and the laboratory technicians at the Institute of Laboratory Medicine in Olten for their practical contribution. We thank Benjamin Berger for proof-reading the manuscript and Michael Mittag for statistical support.
Corina Metaxas, MSc, Universität Basel, Pharmaceutical Care Research Group, Pharmazentrum, Klingelbergstrasse 50, CH-4056 Basel, corina.metaxas[at]unibas.ch
References
1
Tucker
KL
,
Rich
S
,
Rosenberg
I
,
Jacques
P
,
Dallal
G
,
Wilson
PW
, et al.
Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am J Clin Nutr. 2000;71(2):514–22.
2
Wong
CW
. Vitamin B12 deficiency in the elderly: is it worth screening?
Hong Kong Med J. 2015;21(2):155–64.
3
Andrès
E
,
Affenberger
S
,
Vinzio
S
,
Kurtz
JE
,
Noel
E
,
Kaltenbach
G
, et al.
Food-cobalamin malabsorption in elderly patients: clinical manifestations and treatment. Am J Med. 2005;118(10):1154–9. https://doi.org/10.1016/j.amjmed.2005.02.026
4
Andrès
E
,
Loukili
NH
,
Noel
E
,
Kaltenbach
G
,
Abdelgheni
MB
,
Perrin
AE
, et al.
Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171(3):251–9. https://doi.org/10.1503/cmaj.1031155
5
Oh
R
,
Brown
DL
. Vitamin B12 deficiency. Am Fam Physician. 2003;67(5):979–86.
6
Herrmann
W
,
Schorr
H
,
Obeid
R
,
Geisel
J
. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr. 2003;78(1):131–6.
7
Snow
CF
. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–98. https://doi.org/10.1001/archinte.159.12.1289
8
Dali-Youcef
N
,
Andrès
E
. An update on cobalamin deficiency in adults. QJM. 2009;102(1):17–28. https://doi.org/10.1093/qjmed/hcn138
9
Lam
JR
,
Schneider
JL
,
Zhao
W
,
Corley
DA
. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310(22):2435–42. https://doi.org/10.1001/jama.2013.280490
10
Dharmarajan
TS
,
Kanagala
MR
,
Murakonda
P
,
Lebelt
AS
,
Norkus
EP
. Do acid-lowering agents affect vitamin B12 status in older adults?
J Am Med Dir Assoc. 2008;9(3):162–7. https://doi.org/10.1016/j.jamda.2007.10.004
11
de Jager
J
,
Kooy
A
,
Lehert
P
,
Wulffelé
MG
,
van der Kolk
J
,
Bets
D
, et al.
Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B-12 deficiency: randomised placebo controlled trial. BMJ. 2010;340(may19 4):c2181. https://doi.org/10.1136/bmj.c2181
12
Stabler
SP
. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149–60. https://doi.org/10.1056/NEJMcp1113996
13
Bergen
C
,
Compher
C
. Total homocysteine concentration and associated cardiovascular and renal implications in adults. J Cardiovasc Nurs. 2006;21(1):40–6. https://doi.org/10.1097/00005082-200601000-00009
14
Pawlak
R
. Is vitamin B12 deficiency a risk factor for cardiovascular disease in vegetarians?
Am J Prev Med. 2015;48(6):e11–26. https://doi.org/10.1016/j.amepre.2015.02.009
15
Nygård
O
,
Nordrehaug
JE
,
Refsum
H
,
Ueland
PM
,
Farstad
M
,
Vollset
SE
. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230–7. https://doi.org/10.1056/NEJM199707243370403
16
Seshadri
S
,
Beiser
A
,
Selhub
J
,
Jacques
PF
,
Rosenberg
IH
,
D’Agostino
RB
, et al.
Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–83. https://doi.org/10.1056/NEJMoa011613
17Schrier SL. Diagnosis and treatment of vitamin B 12 and folic acid deficiency (last update September 2016). UpToDate [Internet]. Available from: http://www.uptodate.com/contents/diagnosis-and-treatment-of-vitamin-b12-and-folate-deficiency.
18
Hvas
AM
,
Nexo
E
. Holotranscobalamin--a first choice assay for diagnosing early vitamin B deficiency?
J Intern Med. 2005;257(3):289–98. https://doi.org/10.1111/j.1365-2796.2004.01437.x
19
Nexo
E
,
Hoffmann-Lücke
E
. Holotranscobalamin, a marker of vitamin B-12 status: analytical aspects and clinical utility. Am J Clin Nutr. 2011;94(1):359S–65S. https://doi.org/10.3945/ajcn.111.013458
20
Yetley
EA
,
Pfeiffer
CM
,
Phinney
KW
,
Bailey
RL
,
Blackmore
S
,
Bock
JL
, et al.
Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94(1):313S–21S. https://doi.org/10.3945/ajcn.111.013243
21
Kind
A
,
Marty
F
. Vitamin B12 alle drei Monate substituieren?
Prim Care. 2008;8(10):177–8
. [Article in German.].
22
Kuzminski
AM
,
Del Giacco
EJ
,
Allen
RH
,
Stabler
SP
,
Lindenbaum
J
. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92(4):1191–8.
23
Bolaman
Z
,
Kadikoylu
G
,
Yukselen
V
,
Yavasoglu
I
,
Barutca
S
,
Senturk
T
. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther. 2003;25(12):3124–34. https://doi.org/10.1016/S0149-2918(03)90096-8
24
Favrat
B
,
Vaucher
P
,
Herzig
L
,
Burnand
B
,
Ali
G
,
Boulat
O
, et al.
Oral vitamin B12 for patients suspected of subtle cobalamin deficiency: a multicentre pragmatic randomised controlled trial. BMC Fam Pract. 2011;12(1):2. https://doi.org/10.1186/1471-2296-12-2
25
Adachi
S
,
Kawamoto
T
,
Otsuka
M
,
Todoroki
T
,
Fukao
K
. Enteral vitamin B12 supplements reverse postgastrectomy B12 deficiency. Ann Surg. 2000;232(2):199–201. https://doi.org/10.1097/00000658-200008000-00008
26
Vidal-Alaball
J
,
Butler
CC
,
Cannings-John
R
,
Goringe
A
,
Hood
K
,
McCaddon
A
, et al.
Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2005;(3):CD004655.
27
van Walraven
C
,
Austin
P
,
Naylor
CD
. Vitamin B12 injections versus oral supplements. How much money could be saved by switching from injections to pills?
Can Fam Physician. 2001;47:79–86.
28
Webb
DG
,
Horne
R
,
Pinching
AJ
. Treatment-related empowerment: preliminary evaluation of a new measure in patients with advanced HIV disease. Int J STD AIDS. 2001;12(2):103–7. https://doi.org/10.1258/0956462011916875
29
Arnet
I
,
Walter
PN
,
Hersberger
KE
. Polymedication Electronic Monitoring System (POEMS) - a new technology for measuring adherence. Front Pharmacol. 2013;4:26. https://doi.org/10.3389/fphar.2013.00026
30
Eussen
SJ
,
de Groot
LC
,
Joosten
LW
,
Bloo
RJ
,
Clarke
R
,
Ueland
PM
, et al.
Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr. 2006;84(2):361–70.
31
Castelli
MC
,
Friedman
K
,
Sherry
J
,
Brazzillo
K
,
Genoble
L
,
Bhargava
P
, et al.
Comparing the efficacy and tolerability of a new daily oral vitamin B12 formulation and intermittent intramuscular vitamin B12 in normalizing low cobalamin levels: a randomized, open-label, parallel-group study. Clin Ther. 2011;33(3):358–371.e2. https://doi.org/10.1016/j.clinthera.2011.03.003
32
Hall
CA
,
Begley
JA
,
Green-Colligan
PD
. The availability of therapeutic hydroxocobalamin to cells. Blood. 1984;63(2):335–41.
33
Torsvik
I
,
Ueland
PM
,
Markestad
T
,
Bjørke-Monsen
AL
. Cobalamin supplementation improves motor development and regurgitations in infants: results from a randomized intervention study. Am J Clin Nutr. 2013;98(5):1233–40. https://doi.org/10.3945/ajcn.113.061549
34
Obeid
R
,
Fedosov
SN
,
Nexo
E
. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol Nutr Food Res. 2015;59(7):1364–72. https://doi.org/10.1002/mnfr.201500019
35
Marshall Chalmers
JN
,
Shinton
NK
. Comparison of hydroxocobalamin and cyanocobalamin in the treatment of pernicious anaemia. Lancet. 1965;286(7426):1305–8. https://doi.org/10.1016/S0140-6736(65)92336-6
36
Tillemans
MP
,
Donders
EM
,
Verweij
SL
,
Van der Hoeven
RT
,
Kalisvaart
KJ
. Effect of administration route on the pharmacokinetics of cobalamin in elderly patients: a randomized controlled trial. Curr Ther Res Clin Exp. 2014;76:21–5. https://doi.org/10.1016/j.curtheres.2014.01.001
37
Hertz
H
,
Kristensen
HP
,
Hoff-Jorgensen
E
. Studies on Vitamin B12 Retention. Comparison of Retention Following Intramuscular Injection of Cyanocobalamin and Hydroxocobalamin. Scand J Haematol. 1964;1(1):5–15. https://doi.org/10.1111/j.1600-0609.1964.tb00001.x
38
Nyholm
E
,
Turpin
P
,
Swain
D
,
Cunningham
B
,
Daly
S
,
Nightingale
P
, et al.
Oral vitamin B12 can change our practice. Postgrad Med J. 2003;79(930):218–20. https://doi.org/10.1136/pmj.79.930.218
39
Aparicio-Ugarriza
R
,
Palacios
G
,
Alder
M
,
González-Gross
M
. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med. 2015;53(8):1149–59. https://doi.org/10.1515/cclm-2014-0784
40
Hvas
AM
,
Nexo
E
. Diagnosis and treatment of vitamin B12 deficiency--an update. Haematologica. 2006;91(11):1506–12.
41
Pezacka
E
,
Green
R
,
Jacobsen
DW
. Glutathionylcobalamin as an intermediate in the formation of cobalamin coenzymes. Biochem Biophys Res Commun. 1990;169(2):443–50. https://doi.org/10.1016/0006-291X(90)90351-M
42
Kwong
JC
,
Carr
D
,
Dhalla
IA
,
Tom-Kun
D
,
Upshur
RE
. Oral vitamin B12 therapy in the primary care setting: a qualitative and quantitative study of patient perspectives. BMC Fam Pract. 2005;6(1):8. https://doi.org/10.1186/1471-2296-6-8


