Cessation of extracorporeal photopheresis in chronic lung allograft dysfunction: effects on clinical outcome in adults
DOI: https://doi.org/10.4414/smw.2017.14429
Cécile
Robinsona, Lars C
Hubera, Christian
Murera, Macé M.
Schuurmansa, Malcolm
Kohlera, Günther F
Hofbauerb, Christian
Bendena
aDivision of Pulmonology, University Hospital Zurich,
bDepartment of Dermatology, University Hospital Zurich,
Cessation of extracorporeal photopheresis in chronic lung allograft dysfunction: effects on clinical outcome in adults
Summary
BACKGROUND
Extracorporeal photopheresis (ECP) has been reported to be safe and the ultimate treatment option in lung transplant recipients with chronic lung allograft dysfunction (CLAD), the main overall cause of mortality in lung transplant recipients. However, ECP is not reimbursed in selected health jurisdictions, and reimbursement by health insurance providers is a major issue. In Switzerland, ECP is not recognised by the health authorities as a therapy option for CLAD; thus by the end of 2014, ECP had to be stopped in the majority of adult lung transplant recipients cared for at the University Hospital Zurich because of lack of continuous funding.
OBJECTIVE
To describe the outcome of lung transplant recipients after forced cessation of ECP treatment.
METHOD
We retrospectively analysed outcome of 12 lung transplant recipients undergoing ECP for different phenotypes of CLAD (bronchiolitis obliterans syndrome, restrictive allograft syndrome) at our centre followed-up for 12 months after forced cessation of ECP.
RESULTS
Within the 12 months after treatment cessation, seven patients (58%) died with a median survival of 207 days (range 6–365 days). Lung function (FEV1, forced expiratory volume in 1 second) declined significantly 6 months after ECP cessation (p = 0.003).
CONCLUSION
Our data support the role of ECP as valuable treatment option in lung transplant recipients with CLAD.
Introduction
Chronic lung allograft dysfunction (CLAD) is the main cause of death in lung transplant recipients [1]. The mechanisms of CLAD are not completely understood. Different CLAD “phenotypes” have been described, such as bronchiolitis obliterans syndrome (BOS) and restrictive allograft syndrome (RAS) [2]. Bronchiolitis obliterans syndrome is the main cause of death in lung transplant recipients after the first post-transplant year; it is observed in approximately 50% of patients by 5 years after transplantation and >75% after 10 years [3]. Early diagnosis and initiation of treatment is considered to be of vital importance to slow progression, preserve lung function and maintain quality of life. The initial management of BOS consists of augmentation of immunosuppression and immunomodulation using macrolide antibiotics. The role of statins and leukotriene receptor antagonists for the prevention of CLAD progression is less clear [4].
Extracorporeal photopheresis (ECP) is considered as second-line treatment for lung transplant recipients developing CLAD whose condition deteriorates following augmentation of immunosuppression and use of macrolide antibiotics [2]. Recently published work suggested that ECP may be able to stabilise or slow lung function loss without an increased infection risk and also to improve survival in patients with BOS without causing significant adverse effects [2, 5, 6]. The exact mechanisms of ECP are elusive but it is thought to induce apoptosis of lymphocytes and to generate regulatory T cells, which modulate transplant immune rejection [7–9]. At our centre, ECP treatment has been performed since 1997 for various indications including CLAD [5].
From the autumn of 2014, financial reimbursement for ECP was progressively withdrawn by health insurance providers, despite repeated appeals in each individual case, and by the end of 2014 ECP had to be stopped for the vast majority of adult lung transplant recipients at our centre. We assessed clinical outcome of these patients over a period of 1 year after cessation of ECP treatment, including lung allograft function and patient survival.
Methods
Study design
We performed a retrospective patient data collection and analysis, using electronic charts. We included all lung transplant recipients who had received ECP treatment for BOS or RAS and in whom ECP treatment was stopped because of withdrawal of reimbursement by health insurance providers by the end of December 2014. Data from 12 months before cessation of ECP up to 12 months thereafter was collected. Patients who survived for 12 months after ECP was stopped, and patients who were granted reimbursement and continued ECP were followed up until 30 November 2016. Analysis includes baseline patient demographics, CLAD phenotype and lung function. Autopsy reports were reviewed where available.
The local Ethics Committee approved the study (KEK # 2016-00534).
Extracorporeal photopheresis
ECP was performed in collaboration with the Department of Dermatology, University Hospital of Zurich. Since 1997, a standardised protocol had been used in Zurich for ECP, with cycles (1 cycle = 2 days) every 4 weeks. This protocol was changed in 2013 for to be comparable to the protocol used by the Hannover and Vienna group: an induction phase was introduced, with ECP cycles every 2 weeks for 3 months and 4 weekly thereafter [2, 10]. As previously described, extracorporeal photopheresis was performed using the UVARXTS system (Therakos, Exton, PA) approved by the US Food and Drugs Administration. Briefly, leukapheresis in the UVAR system is followed by photoactivation with methoxsalen (8-methoxypsoralen) and UVA irradiation, and subsequent reinfusion of irradiated cells over 3 to 4 hours. During ECP, peripheral blood mononuclear cells are separated from the whole blood in a Latham centrifuge at 2700 g. Uvadex® methoxsalen solution (Therakos) is added directly into the buffy coat bag before extracorporeal irradiation with UVA (1–2 J/m2), reducing systemic methoxsalen toxicity. The number of blood mononuclear cells exposed to methoxsalen and UVA during each procedure is usually 2 to 5% of the total circulating white cells [5].
Spirometry was performed at each ECP and outpatient attendance according to ATS/ERS guidelines [11]. For each month the highest available FEV1 value (forced expired volume in 1 second) and FVC (forced vital capacity) were tabulated. All patients were diagnosed and graded for CLAD according to a recently published practice guideline consensus document [12].
Patients
On 31 August 2014, 15 lung transplant recipients were undergoing ECP treatment for BOS and RAS (fig. 1). From September to December 2014, ECP treatment had to be stopped in 12 patients, despite repeated appeal in each individual case. Demographic information for these patients is presented in table 1. Seven patients were female. The median age was 49 years (range 30–70 years). Underlying diagnosis leading to bilateral lung transplantation included COPD (n = 4), cystic fibrosis (n = 3), sarcoidosis (n = 2), idiopathic pulmonary fibrosis (n = 1), pulmonary arterial hypertension (n = 1) and diffuse interstitial lung disease (n = 1). The patients were on ECP treatment for BOS 2 (n = 3), BOS 3 (n = 7) and RAS (n = 2). Most patients (n = 9) were under dual immunosuppression with prednisone/ciclosporin or prednisone/tacrolimus, three patients had additional treatment with mycophenolate mofetil (n = 3).
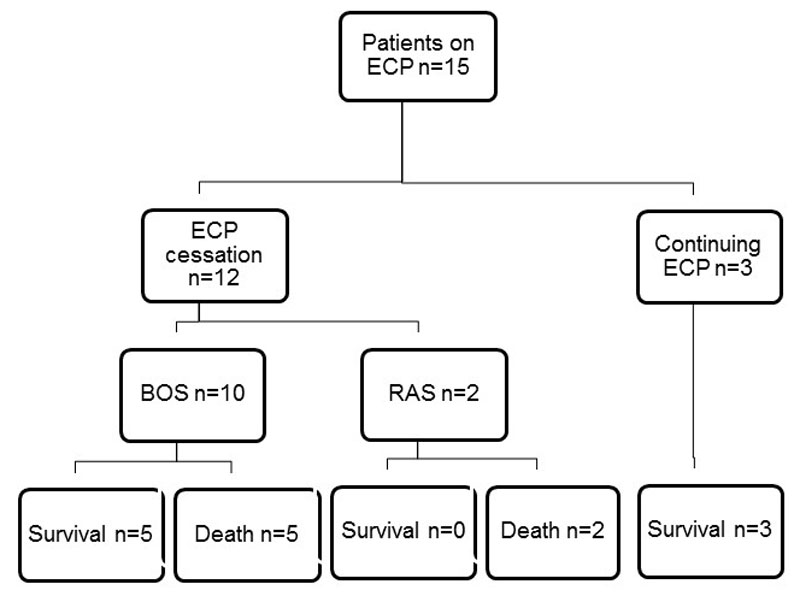
Figure 1 ECP follow-up and survival. On 31 August 2014, 15 patients were undergoing ECP treatment; 12 were stopped between September and December 2014, 3 patients continued and survived the follow-up period. Of the 12 patients stopping ECP, 10 were treated for BOS and 2 for RAS. 5 BOS patients and 2 RAS patients died (death rate 58%).
BOS = bronchiolitis obliterans syndrome; ECP = extracorporeal photopheresis; RAS = restrictive allograft syndrome
Table 1 Demographics for all patients (survival reported up to 12 months), for those who died within 12 months following ECP cessation and those who survived a minimum 12 months after ECP was stopped (survival reported until 30 November 2016).
|
|
Total
(n = 12)
|
Deaths
(n = 7)
|
Survivals
(n = 5)
|
| Sex |
Male (%) |
5 (42) |
2 |
3 |
| Female (%) |
7 (58) |
5 |
2 |
| Median age (range), years |
|
49 (30–70) |
50 (37–70) |
40 (30–59) |
| Underlying disease |
COPD (%) |
4 (34) |
4 |
0 |
| Cystic fibrosis (%) |
3 (26) |
0 |
3 |
| Sarcoidosis (%) |
2 (16) |
1 |
1 |
| IPF (%) |
1 (8) |
1 |
0 |
| PAH (%) |
1 (8) |
0 |
1 |
| Diffuse interstitial lung disease (%) |
1 (8) |
1 |
0 |
| Phenotype |
BOS (%) |
10 (84) |
5 |
5 |
| RAS (%) |
2 (16) |
2 |
0 |
| Median number of ECP cycles (range) |
|
44 (8–142) |
33 (8–129) |
72 (12–142) |
| Median time under ECP treatment (range) |
|
1001 (168–6054) |
898 (168–4568) |
2716 (260–5054) |
| Death (%) |
|
7 (58) |
|
3 (60) |
| Median survival time (range), days |
|
207 (6–365) |
156 (6–334) |
699 (525–675) |
| Cause of death |
Infection (%) |
3 (43) |
|
0 |
| Progression (%) |
3 (43) |
|
2 |
| Cancer (%) |
1 (14) |
|
0 |
| Other (%) |
|
|
1 |
Statistical analysis
Data are reported as medians with ranges. Continuous variables were compared using the Mann-Whitney-U-test. Two-sided t-tests and Wilcoxon signed rank tests were used to compare variables before and after ECP treatment cessation. Kaplan-Meier estimates of survival were calculated. For all analyses p-values <0.05 were considered statistically significant.
Results
From September to December 2014, ECP treatment had to be stopped in 12 patients. At the time of ECP cessation the 12 patients had received a median of 44 ECP cycles (8–142) with a median duration of ECP treatment of 1001 days (168–5054 days). Four patients started ECP treatment less than 12 months before cessation of ECP, three 8 months and one 10 months earlier. As shown in figures 1 and 2
, seven of the 12 patients (58%) died within 12 months after ending ECP treatment (58%). Median survival time after cessation of ECP was 207 days (range 6–365 days). Three patients died of CLAD progression, three as a result of infection, including one patient who died (from infection) 6 days after ECP cessation, and one (14%) of extra-pulmonary cancer. All patients who died from infection had concomitant CLAD progression. Patients did not receive any additional treatment (rabbit antithymocyte globulin, alemtuzimab, total body irradiation) potentially contributing to infection. Autopsy reports were available in four patients only, all of which confirmed the clinically suspected cause of death. Survival time did not significantly differ between different CLAD phenotypes, sex, age and underlying disease.
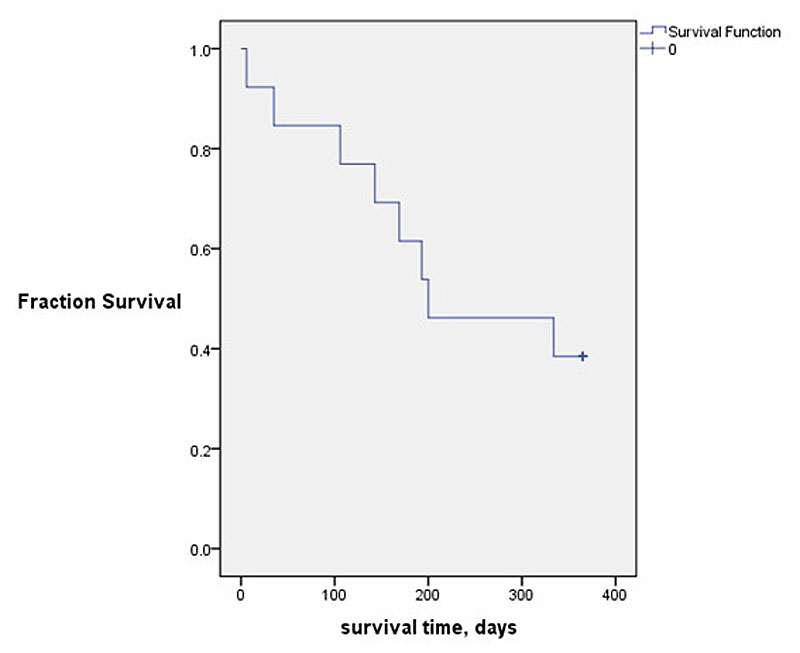
Figure 2 Kaplan-Meier plot of survival time. Seven of 12 patients (58%) died within the 12 months of follow-up. Median survival time was 207 days (range 6–365).
At baseline, median FEV1 was 920 ml (610–3870 ml) (table 2). Prior to ECP cessation, lung function was stable in all patients, with a median FEV1 loss of 13 ml/month (8–110 ml/month); lung function values were available in all patients for each month. After ECP cessation, FEV1 declined rapidly (median FEV1 of 800 ml, range 520–3720 ml; p = 0.003) within 6 months. The median FEV1 loss per month, measured at the end of the 12-month observation period (or last available value before death), was 17 ml (range 6–163 ml). There was no significant difference in monthly lung function loss before and after ECP cessation. As a result of their impaired condition, monthly lung function testing results were unavailable in some of patients (median FEV1 measurements 5 per 12 months, range 0–12).
Table 2 Clinical outcome indicators.
|
ECP
|
After ECP
|
p-value
|
| Median FEV1 (range), ml |
920 (610–3660) |
800 (520–3720) |
0.003 |
| Median FEV1 decline per month (range), ml |
13 (8–110) |
17 (6–163) |
NS |
| Mean lymphocyte count (range), ×109/l |
0.50 (0.32–0.65) |
1.03(0.42–1.54) |
0.002 |
Of the five patients who survived the 12 months following ECP cessation, two died from CLAD progression 585 and 675 days after ECP was stopped, one patient died from complications following repeat lung transplantation 525 days later. Within the 12 months following ECP cessation they showed a median FEV1 loss of 13 ml/month (range 0–980 ml/month).
For comparison, of the three CLAD patients who were granted reimbursement for continued ECP (one of them after a direct patient appeal to the insurance provider), two remained stable without any lung function decline until the 30 November 2016; one developed CLAD progression starting in the summer of 2016 and was still alive on 30 November 2016.
Immunosuppressive treatment was not changed after ECP cessation. Most patients (n = 9) were under dual immunosuppression with prednisone/ciclosporin or prednisone/tacrolimus; three patients had additional treatment with mycophenolate mofetil. Prednisone doses did not have to be augmented after ECP cessation.
Discussion
In this retrospective analysis, we investigated the effects of ECP cessation in a unique cohort of lung transplant recipients. Our data show a decline of lung function and a high mortality rate within 12 months after ECP cessation. This large study analysed clinical outcome in a cohort of lung transplant recipients after forced ECP cessation, emphasising the potential benefits of ECP for maintaining already impaired allograft function in patients with CLAD.
In 1995, ECP was first described as a treatment for severe acute lung allograft rejection following infection [13]. Later, ECP had also been administered, by our group and others, to patients with BOS, who showed stabilisation of lung function [5, 14, 15]. The only prospective study to date showed stabilisation of lung function and significantly longer survival of patients with CLAD under ECP treatment [10]. In all published data to date, ECP has shown to be a safe treatment without significant adverse effects. Our study analysed the effect of ECP cessation for non-medical reasons (reimbursement issues with Swiss healthcare providers) in lung transplant recipients with CLAD.
We observed a monthly FEV1 loss of 13 ml during ECP treatment, which is in line with other reports [2, 5]. Whereas no increase of monthly lung functional decline could be observed after ECP cessation, we found a decline in median FEV1 (after 6 months or last FEV1 value available). These findings imply that the calculated monthly FEV1 loss after ECP cessation is likely underestimated, probably because monthly lung function data could not be obtained from all patients,.
Following ECP cessation, a very high mortality rate of 58% was observed within 12 months in our lung transplant recipient cohort (median survival time 207 days, range 3–365 days), which is in strong contrast to the fact that most patients had received long-term ECP (median of 44 cycles, median duration of ECP 1001 days). Death was mainly due to infection and progression of CLAD, confirming the findings of other studies [2, 5, 10]. No difference in survival time between different CLAD phenotypes was revealed. The high mortality rate after ECP cessation raises the question of whether ECP withdrawal triggers an immunogenic response that, in turn, results in further CLAD progression, but this remains speculative at this time.
Of our total cohort, three CLAD patients were able to continue ECP after multiple appeals to the health insurance providers. Although the small group of patients is not suited to serve as a well-matched control group, it is of note that all three patients remained clinically stable and maintained their lung function while continuing ECP. All three patients are currently still alive with a median patient survival of 15 years (range 13–20). Overall survival of patients who received lung transplants at our centre in Zurich has recently been reported to be 84%, 71% and 67% at 1, 3 and 5 years, respectively [16]. Lung function remained stable in two patients with no decline until November 2016, and one patient recently developed CLAD progression.
Limitations of our retrospective study include the small sample size and limited lung function data after cessation of ECP as a result of the patients’ severely impaired condition. Moreover, the overall sample size was too small to distinguish the effects of ECP cessation on on outcome and survival in the different CLAD phenotypes. In conclusion, we document a high mortality rate of lung transplant recipients within 1 year after forced ECP cessation, emphasising the important role of ECP for preservation of lung function and prolonged survival in patients with CLAD.
Since 2016, ECP has newly been covered by health insurance (OKP-Leistungskatalog) for treatment of CLAD.
References
1
Verleden
GM
,
Vos
R
,
Verleden
SE
,
De Wever
W
,
De Vleeschauwer
SI
,
Willems-Widyastuti
A
, et al.
Survival determinants in lung transplant patients with chronic allograft dysfunction. Transplantation. 2011;92(6):703–8. https://doi.org/10.1097/TP.0b013e31822bf790
2
Greer
M
,
Dierich
M
,
De Wall
C
,
Suhling
H
,
Rademacher
J
,
Welte
T
, et al.
Phenotyping established chronic lung allograft dysfunction predicts extracorporeal photopheresis response in lung transplant patients. Am J Transplant. 2013;13(4):911–8. https://doi.org/10.1111/ajt.12155
3
Yusen
RD
,
Christie
JD
,
Edwards
LB
,
Kucheryavaya
AY
,
Benden
C
,
Dipchand
AI
, et al.; International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report--2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):965–78. https://doi.org/10.1016/j.healun.2013.08.007
4
Meyer
KC
,
Raghu
G
,
Verleden
GM
,
Corris
PA
,
Aurora
P
,
Wilson
KC
, et al.; ISHLT/ATS/ERS BOS Task Force Committee; ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44(6):1479–503. https://doi.org/10.1183/09031936.00107514
5
Benden
C
,
Speich
R
,
Hofbauer
GF
,
Irani
S
,
Eich-Wanger
C
,
Russi
EW
, et al.
Extracorporeal photopheresis after lung transplantation: a 10-year single-center experience. Transplantation. 2008;86(11):1625–7. https://doi.org/10.1097/TP.0b013e31818bc024
6
Morrell
MR
,
Despotis
GJ
,
Lublin
DM
,
Patterson
GA
,
Trulock
EP
,
Hachem
RR
. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2010;29(4):424–31. https://doi.org/10.1016/j.healun.2009.08.029
7
Meloni
F
,
Cascina
A
,
Miserere
S
,
Perotti
C
,
Vitulo
P
,
Fietta
AM
. Peripheral CD4(+)CD25(+) TREG cell counts and the response to extracorporeal photopheresis in lung transplant recipients. Transplant Proc. 2007;39(1):213–7. https://doi.org/10.1016/j.transproceed.2006.10.227
8
Maeda
A
,
Schwarz
A
,
Kernebeck
K
,
Gross
N
,
Aragane
Y
,
Peritt
D
, et al.
Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol. 2005;174(10):5968–76. https://doi.org/10.4049/jimmunol.174.10.5968
9
Lamioni
A
,
Parisi
F
,
Isacchi
G
,
Giorda
E
,
Di Cesare
S
,
Landolfo
A
, et al.
The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79(7):846–50. https://doi.org/10.1097/01.TP.0000157278.02848.C7
10
Jaksch
P
,
Scheed
A
,
Keplinger
M
,
Ernst
MB
,
Dani
T
,
Just
U
, et al.
A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2012;31(9):950–7. https://doi.org/10.1016/j.healun.2012.05.002
11
Miller
MR
,
Hankinson
J
,
Brusasco
V
,
Burgos
F
,
Casaburi
R
,
Coates
A
, et al.; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. https://doi.org/10.1183/09031936.05.00034805
12
Meyer
KC
,
Raghu
G
,
Verleden
GM
,
Corris
PA
,
Aurora
P
,
Wilson
KC
, et al.; ISHLT/ATS/ERS BOS Task Force Committee; ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44(6):1479–503. https://doi.org/10.1183/09031936.00107514
13
O’Hagan
AR
,
Stillwell
PC
,
Arroliga
A
,
Koo
A
. Photopheresis in the treatment of refractory bronchiolitis obliterans complicating lung transplantation. Chest. 1999;115(5):1459–62. https://doi.org/10.1378/chest.115.5.1459
14
Salerno
CT
,
Park
SJ
,
Kreykes
NS
,
Kulick
DM
,
Savik
K
,
Hertz
MI
, et al.
Adjuvant treatment of refractory lung transplant rejection with extracorporeal photopheresis. J Thorac Cardiovasc Surg. 1999;117(6):1063–9. https://doi.org/10.1016/S0022-5223(99)70241-2
15
Jaksch
P
,
Knobler
R
. ECP and solid organ transplantation. Transfus Apheresis Sci. 2014;50(3):358–62. https://doi.org/10.1016/j.transci.2014.04.006
16
Inci
I
,
Schuurmans
MM
,
Boehler
A
,
Weder
W
. Zurich University Hospital lung transplantation programme: update 2012. Swiss Med Wkly. 2013;143:w13836. https://doi.org/10.4414/smw.2013.13836

