
Figure 1 Social outcomes of teenage and young adult cancer survivors in comparison to healthy controls. * Proportions and numbers for controls weighted according to age, sex, and migration background of survivors.
DOI: https://doi.org/10.4414/smw.2017.14419
Teenage and young adult (TYA) cancer patients face the cancer diagnosis at the age of 16–24 years [1]. This is a challenging period of psychosocial development [2–4] characterised by completing education, starting a professional career, gaining social independence and establishing romantic relationships [2–6]. A diagnosis of cancer places TYAs at high risk for disruptions or delays in this developmental process [4, 7–9]. Studies in the US including adolescent and young adult cancer survivors found that more than 50% of full-time workers/students encountered educational or work-related problems following the diagnosis [10], and a higher risk of divorce compared with healthy controls [11]. International comparisons of social outcomes after cancer during adolescence and young adulthood are hindered by large disparities in the respective schooling and social security systems. However, there are very limited data outside the US [12], and the majority of psychosocial oncology research has focused on paediatric or older adult populations [13].
In addition to interrupted developmental processes, TYA cancer survivors are at high risk for long-term late effects attributable to cancer and its treatment [14, 15]. Late effects may further interfere with long-term psychosocial adjustment [15]. In Switzerland, long-term survivors of childhood cancer (CCSs) encountered problems during schooling and completed their professional education with some delay [16]. Other studies on Swiss CCSs found that, long after treatment has ended, survivors were less likely than their peers to be employed and to have a life partner [17, 18]. However, TYA cancer patients and survivors may be faced with different developmental needs compared with childhood cancer survivors, and there are still substantial knowledge gaps about the impact of cancer during adolescence and young adulthood in the Swiss and European context.
Understanding the long-term social impact of cancer on TYAs and identifying survivors at risk for adverse social outcomes is critical to developing country-specific support strategies to promote healthy psychosocial development [3, 13, 19]. A recent systematic review on social well-being in adolescent and young adults with cancer concluded that more research is needed in this specific population, including more heterogeneous cancer samples and comparison groups to develop appropriate supportive care [12]. We therefore aimed to (1) determine differences in long-term social outcomes (educational achievement, employment status, marital status, and life partnership) between TYA cancer survivors and healthy controls, and (2) identify sociodemographic and cancer-related factors associated with adverse social outcomes.
In our cross-sectional survey, we included TYA cancer survivors who resided in the Canton of Zurich, Switzerland, at time of diagnosis and who were registered in the Cancer Registry Zurich and Zug. Eligible survivors were aged 16–25 years at diagnosis, diagnosed between 1990 and 2005, and survived ≥5 years. The survey of TYA cancer survivors was performed in the context of a larger project that included survivors of childhood cancer. Therefore, we only included survivors diagnosed with leukaemia, lymphoma, central nervous system (CNS) tumours, neuroblastoma, renal, hepatic and bone tumours, soft tissue sarcoma, and germ cell tumours because these are the most common cancer diagnoses among children. Addresses at diagnosis were available from the Cancer Registry Zurich and Zug, and were updated by web search and by contacting community registries. Between 2010 and 2012, we sent a cover letter, information about the purpose of the study, informed consent form, questionnaire and a prepaid return envelope to all eligible survivors with a valid current address. After 4 weeks, we sent a reminder with another copy of the questionnaire to all nonresponders. Ethical approval was provided by the Cantonal Ethics Committee of Zurich (REF Nr. EK: 2010-0228/2). Informed consent was provided by all study participants.
Control data were obtained from the Swiss Health Survey (SHS) 2012. The SHS is a computer-assisted telephone survey of a random sample of Swiss residents aged ≥15 years, conducted every 5 years by the Swiss Federal Statistical Office. It provides data on participants’ current health, health behaviour, use of health services and sociodemographic information [20, 21]. A random sample was obtained by stratified selection in two stages. Firstly, households were randomly selected from each canton; secondly, in each household one person was randomly selected for the telephone interview. In total, 21 597 interviews were performed (response rate 53%) [21]. For our study, we included only participants aged 20–50 years resident in the Canton of Zurich.
The questionnaire focused on follow-up care attendance and preferences for the organisation of follow-up care in Switzerland, quality of life, psychological distress and various socioeconomic characteristics [22].
The outcomes of interest were educational achievement, employment status, marital status and (for survivors only) life partnership. We used the same questions as in the SHS for assessing educational achievement and divided educational achievement into four categories: basic education (nine years of compulsory schooling only), vocational training / apprenticeship (including grammar school, teachers’ college), upper secondary (higher technical and professional training, university of applied sciences) and university education [16]. Survivors were asked in the questionnaire about their current labour market situation (employed, unemployed, not in the workforce, in education, receiving a disability pension). Controls were asked whether they were in full-time employment, part-time employment or unemployed. We dichotomised the employment status of survivors and controls into employed (including full-time or part-time employment) and not employed (including unemployed, not in the workforce, in education and receiving a disability pension). Civil status was recorded as single, married, widowed and divorced for survivors, or single, married, widowed, divorced and separated for controls. Participants who indicated that they were married were coded as being married. We asked survivors whether they had a life partner (yes/no). Survivors who indicated yes were categorised as having a life partner. Information regarding life partners was not available for controls.
For survivors and controls we assessed age at the time of the study, sex, and migration background (if they were not Swiss citizens or had moved to Switzerland after birth). Age at the time of the study was divided into 20–29 years, 30–39 years and ≥40 years [22] in order to identify whether a particular age group of TYAs is at risk for adverse social outcomes.
For TYA cancer survivors, we extracted the following clinical data from the Cancer Registry Zurich and Zug: diagnosis, treatment, age at diagnosis (16–20 years, 21–25 years), and time since diagnosis (5–10 years, 11–15 years, ≥16 years). Diagnosis was coded according to the International Classification of Childhood Cancer, Third Edition (ICCC-3) [23]. For the analysis, diagnosis was categorised into leukaemia/lymphoma, CNS tumours, and other tumours. This grouping was chosen in order to investigate whether survivors of CNS tumours were at risk for adverse social outcomes, as previously shown in Swiss childhood cancer survivors [16, 18]. We looked separately at leukaemia/lymphoma survivors because of the relatively long-lasting treatment, which might more strongly interfere with social achievements. Treatment was coded hierarchically as surgery only, chemotherapy (may have had surgery) and radiotherapy (may have had surgery and/or chemotherapy). In the questionnaire we asked survivors whether they had ever experienced a cancer relapse (yes/no) and whether they suffer from physical or psychological late effects attributable to cancer and/or its treatment (yes/no).
All analyses were performed using Stata version 14.1 (StataCorp LP, College Station, TX). We used descriptive statistics, chi-square tests or Fisher’s exact test and t-tests to compare participating with nonparticipating survivors, and participating survivors with controls. Controls were more often female, older, and more often had a migration background (see table 1). To account for these differences, we standardised controls for age (categorical), sex and migration background according to the marginal distribution in survivors. We used multivariable logistic regression with being a control as outcome to calculate appropriate weights for this standardisation [24]. The weight for survivors was set to one. All analyses were performed with weighted controls using the survey command in Stata. Stata’s survey command fits models for survey data by adjusting the results of a command for previously defined settings such as the weights for controls [25]. For aim 1, we used a combined dataset of survivors and controls to compare educational achievement, employment and marital status using descriptive statistics and chi-square tests. To address aim 2, we fitted univariable and multivariable logistic regression models to determine associations between being a survivor and sociodemographic factors, with educational achievement, employment status and marital status in the combined dataset. Educational achievement was dichotomised into basic education and higher education (vocational training/apprenticeship, upper secondary education, university education) for all regression analyses. In the regression analyses, we included variables for the respective outcome according to the model proposed in figure S1 (appendix 1). We used interaction tests to determine whether associations differed between survivors and controls. All variables associated with the respective outcome at p <0.10 in univariable regression were included in multivariable analyses. For survivors only, we investigated associations between cancer-related variables and educational achievement, employment status, marital status and life partnership in univariable exact logistic regression. Exact logistic regression is recommended when analysing small samples and subgroups as it produces more accurate estimates than the standard maximum-likelihood based logistic regression [26]. All p-values <0.05 were considered statistically significant.
Of 469 eligible TYA cancer survivors, 389 (82.9%) could be contacted. Of those, 160 (41.1%) returned the questionnaire. Among TYA cancer survivors, participants and nonparticipants were similar in regard to sociodemographic and cancer-related characteristics (table 1). The mean age of survivors was 33.5 years with a mean time since diagnosis of 11.9 years. Most survivors were diagnosed with lymphoma, followed by germ cell tumours. The SHS sample consisted of 999 eligible controls.
Table 1 Characteristics of TYA cancer survivors and controls.
| TYA cancer survivors (n = 469) | Controls (n = 999) | ||||||
|---|---|---|---|---|---|---|---|
|
Non-
participants (n = 309) |
Participants
(n = 160) |
Weighteda | Not weighted | ||||
| n (%b) | n (%b) | p-valuec | n (%b) | p-valued | n (%b) | p-valuee | |
| Basic sociodemographic variables | |||||||
| Sex | 0.110 | n.a.a | 0.006 | ||||
| Male | 210 (68.6) | 98 (61.3) | 617 (61.8) | 496 (49.7) | |||
| Female | 96 (31.4) | 62 (38.8) | 382 (38.2) | 503 (50.4) | |||
| Age at time of study | 0.569 | n.a.a | <0.001 | ||||
| ≥40 years | 55 (17.8) | 32 (20.0) | 199 (20.0) | 424 (42.4) | |||
| 30–39 years | 180 (58.3) | 85 (53.1) | 528 (52.9) | 365 (36.5) | |||
| 20–29 years | 74 (24.8) | 43 (26.9) | 272 (27.2 | 210 (21.0) | |||
| Migration background | n.a.f | n.a.a | <0.001 | ||||
| No | – | 135 (84.9) | 849 (85.0) | 581 (58.2) | |||
| Yes | – | 24 (15.1) | 150 (15.0) | 418 (41.8) | |||
| Outcome variables | |||||||
| Educational achievement | n.a.f | 0.012 | 0.001 | ||||
| Basic education | – | 13 (8.2) | 48 (4.8) | 88 (8.8) | |||
| Vocational training / apprenticeship | – | 74 (46.5) | 471 (47.2) | 444 (44.6) | |||
| Upper secondary education | – | 53 (33.3) | 266 (26.7) | 218 (21.9) | |||
| University education | – | 19 (11.9) | 212 (21.3) | 246 (24.7) | |||
| Employment status | n.a.f | 0.515 | 0.282 | ||||
| No | – | 14 (8.8) | 105 (10.5) | 117 (11.7) | |||
| Yes | – | 145 (91.2) | 894 (89.5) | 882 (88.3) | |||
| Marital status | n.a.f | 0.357 | 0.003 | ||||
| No | – | 101 (63.5) | 594 (59.5) | 507 (50.8) | |||
| Yes | – | 58 (36.5) | 404 (40.5) | 491 (49.2) | |||
| Life partnership | n.a.f | n.a.f | n.a.f | ||||
| No | – | 37 (23.1) | – | – | |||
| Yes | – | 123 (76.9) | – | – | |||
| Cancer-related variables | |||||||
| Diagnosis (ICCC-3) | 0.131g | n.a.f | n.a.f | ||||
| Leukaemia | 28 (9.1) | 13 (8.1) | – | – | |||
| Lymphoma | 91 (29.5) | 60 (37.5) | – | – | |||
| CNS tumour | 36 (11.7) | 15 (9.4) | – | – | |||
| Neuroblastoma | 2 (0.7) | 2 (1.3) | – | – | |||
| Renal tumour | 1 (0.3) | 3 (1.9) | – | – | |||
| Hepatic tumour | 2 (0.7) | 0 (0.0) | – | – | |||
| Bone tumour | 15 (4.9) | 6 (3.8) | – | – | |||
| Soft tissue sarcoma | 17 (5.5) | 15 (9.4) | – | – | |||
| Germ cell tumour | 117 (37.9) | 46 (28.8) | – | – | |||
| Treatment | 0.509 | n.a.f | n.a.f | ||||
| Surgery | 108 (47.0) | 57 (44.5) | – | – | |||
| Chemotherapy | 63 (27.4) | 31 (24.2) | – | – | |||
| Radiotherapy | 59 (25.7) | 40 (31.3) | – | – | |||
| Age at diagnosis | 0.373 | n.a.f | n.a.f | ||||
| 21–25 years | 187 (60.5) | 90 (56.3) | – | – | |||
| 16–20 years | 122 (39.5) | 70 (43.8) | – | – | |||
| Time since diagnosis | 0.976 | n.a.f | |||||
| ≥16 years | 99 (32.0) | 50 (31.3) | – | – | |||
| 11–15 years | 99 (32.0) | 51 (31.9) | – | – | |||
| 5–10 years | 111 (35.9) | 59 (36.9) | – | – | |||
| Self-reported relapse | n.a.f | n.a.f | |||||
| No | – | 136 (85.0) | – | – | |||
| Yes | – | 24 (15.0) | – | – | |||
| Self-reported late effects | n.a.f | n.a.f | |||||
| No | – | 112 (71.8) | – | – | |||
| Yes | – | 44 (28.2) | – | – | |||
|
Mean
(SD) |
Mean
(SD) |
p-valuec | Mean (SD) | p-valued |
Mean
(SD) |
p-valuee | |
| Age at study | 33.7 (5.6) | 33.5 (5.9) | 0.768 | 34.0 (9.9) | n.a.a | 36.9 (7.9) | <0.001 |
| Age at diagnosis | 21.2 (2.9) | 21.1 (2.9) | 0.614 | – | – | n.a.e | |
| Time since diagnosis | 12.0 (4.8) | 11.9 (4.7) | 0.788 | – | – | n.a.e | |
CNS = central nervous system; ICCC-3 = International Classification of Childhood Cancer, Third Edition; n.a. = not available/applicable; SD = standard deviation; TYA = teenage and young adult Bold type indicates a p-value lower than 0.05. a Standardised on age, sex, and migration background according to TYA cancer survivors. b Percentages are based upon available data for each variable. c p-value calculated from chi-square statistics or t-tests comparing participating TYA cancer survivors with nonparticipants. d p-value calculated from chi-square statistics or t-tests comparing participating TYA cancer survivors and weighted controls. e p-value calculated from chi-square statistics or t-tests comparing participating TYA cancer survivors and non-weighted controls. f Information was not available for nonparticipating TYA cancer survivors and controls. g p-value calculated from Fisher’s exact test comparing participating TYA cancer survivors to nonparticipants.
Educational achievement of survivors differed significantly from that of controls (p = 0.012). More survivors than controls reported basic education (fig. 1; n = 13, 8.2% vs n = 48, 4.8%) or upper secondary education (n = 53, 33.3% vs n = 266, 26.7%). Fewer survivors reported having achieved a university education (n = 19, 11.9% vs n = 212, 21.3%). We found no significant differences for employment (p = 0.515) and marital status (p = 0.357). The majority of survivors (n = 145, 91.2%) and controls (n = 894, 89.5%) were employed. Most survivors (n = 101, 63.5%) and controls (n = 594, 59.5%) were not married. The majority of survivors reported having a life partner (n = 123, 76.9%).

Figure 1 Social outcomes of teenage and young adult cancer survivors in comparison to healthy controls. * Proportions and numbers for controls weighted according to age, sex, and migration background of survivors.
In univariable regression of the combined dataset including TYA cancer survivors and controls we found that survivors and controls with a migration background were more likely to have only basic education (table 2), which remained significant in multivariable regression (table 3; odds ratio [OR] 10.23, 95% confidence interval [CI] 4.64–22.55). We found a marginally significant association with having only basic education and being a TYA cancer survivor (OR 1.93, 95% CI 0.95–3.91). Being unemployed was significantly associated with female sex (OR 2.52, 95% CI 1.36–4.68), having only basic education (OR 2.78, 95% CI 1.01–7.65) and being married (OR 0.53, 95% CI 0.29–0.98) in multivariable analysis. Not being married was associated with younger age at the time of the study (OR for 20–29 years 6.77, 95% CI 3.68–12.46) and having a migration background (OR 0.48, 95% CI 0.30–0.79). We observed a significant interaction for sex and marital status: female survivors were less likely to be married than female controls (ORinteraction 2.69, 95% CI 1.18–6.12). Other associations were similar in both populations (all p-values for interaction >0.05).
Table 2 Factors associated with educational achievement, employment status, and marital status from univariable logistic regression models (combined dataset including TYA cancer survivors and controls).
|
Educational achievement
(having basic education) |
Employment status
(being unemployed) |
Marital status
(not being married) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | p-valued |
p-value
inter- actione |
ORb | 95% CI | p-valued |
p-value
inter- actione |
ORc | 95% CI | p-valued |
p-value
inter- actione |
|
| Population | 0.088 | – | 0.516 | - | 0.357 | - | ||||||
| Controlsf | 1.00 | 1.00 | 1.00 | |||||||||
| Survivors | 1.77 | 0.92–3.40 | 0.82 | 0.45–1.50 | 1.19 | 0.83–1.70 | ||||||
| Basic sociodemographic variables | ||||||||||||
| Sex | 0.175 | 0.500 | 0.001 | 0.650 | 0.301 | 0.032 | ||||||
| Male | 1.00 | 1.00 | 1.00 | |||||||||
| Female | 1.68 | 0.79–3.54 | 2.65 | 1.47–4.78 | 1.21 | 0.84–1.74 | ||||||
| Age at time of study | 0.792 | 0.858 | 0.221 | 0.589 | <0.001 | 0.175 | ||||||
| ≥40 years | 1.00 | 1.00 | 1.00 | |||||||||
| 30–39 years | 1.41 | 0.51–3.94 | 1.40 | 0.62–3.17 | 1.41 | 0.91–2.20 | ||||||
| 20–29 | 1.21 | 0.39–3.78 | 2.04 | 0.88–4.74 | 6.13 | 3.36–11.20 | ||||||
| Migration background | <0.001 | 0.284 | 0.282 | 0.712 | 0.006 | 0.974 | ||||||
| No | 1.00 | 1.00 | 1.00 | |||||||||
| Yes | 9.94 | 4.59–21.54 | 1.47 | 0.73–2.96 | 0.52 | 0.33–0.82 | ||||||
| Other sociodemographic variables g | ||||||||||||
| Educational achievement | – | – | 0.007 | 0.434 | 0.168 | 0.627 | ||||||
| Higher education | n.a. | 1.00 | 1.00 | |||||||||
| Basic education | – | – | 3.32 | 1.38–7.99 | 0.59 | 0.28–1.25 | ||||||
| Marital status | – | – | 0.059 | 0.798 | – | – | ||||||
| Married | n.a. | 1.00 | n.a. | |||||||||
| Not married | – | – | 0.58 | 0.32–1.02 | – | – | ||||||
CI = confidence interval; n.a. = not applicable; OR = odds ratio; TYA = teenage and young adult. Bold type indicates a p-value lower than 0.05. a OR for having basic education. b OR for being unemployed. c OR for not being married. d p-value calculated from Wald tests. e p-value for interaction between study population (TYA cancer survivors and controls) and the respective variable. f Standardised on age, sex, and migration background according to TYA cancer survivors. g Other sociodemographic variables were included in the respective models according to figure S1.
Table 3 Factors associated with educational achievement, employment status and marital status from multivariable logistic regression modelsa (combined dataset including TYA cancer survivors and controls).
|
Educational achievement
(having basic education) |
Employment status
(being unemployed) |
Marital status
(not being married) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORb | 95% CI | p-valuee |
p-value inter-
actionf |
ORc | 95% CI | p-valuee |
p-value
inter- actionf |
ORd | 95% CI | p-valuee |
p-value inter-
actionf |
||
| Population | 0.068 | – | – | – | 0.446 | – | |||||||
| Controlsg | 1.00 | n.a. | 1.00 | ||||||||||
| Survivors | 1.93 | 0.95–3.91 | – | – | 0.83 | 0.51–1.34 | |||||||
| Basic sociodemographic variables | |||||||||||||
| Sex | – | – | 0.004 | – | 0.092 | 0.018 | |||||||
| Male | n.a. | 1.00 | 1.00 | ||||||||||
| Female | – | – | 2.52 | 1.36–4.68 | 0.75 | 0.54–1.05 | |||||||
| Age at time of study | – | – | – | – | <0.001 | – | |||||||
| ≥40 years | n.a. | n.a. | 1.00 | ||||||||||
| 30–39 years | 1.50 | 0.96–2.36 | |||||||||||
| 20–29 years | – | – | – | – | 6.77 | 3.68–12.46 | |||||||
| Migration background | <0.001 | – | – | – | 0.004 | – | |||||||
| No | 1.00 | n.a. | 1.00 | ||||||||||
| Yes | 10.23 | 4.64–22.55 | – | – | 0.48 | 0.30–0.79 | |||||||
| Other sociodemographic variables | |||||||||||||
| Educational achievement | – | – | 0.048 | – | – | – | |||||||
| Higher education | n.a. | 1.00 | n.a. | ||||||||||
| Basic education | – | – | 2.78 | 1.01–7.65 | – | – | |||||||
| Marital status | – | – | 0.042 | – | – | – | |||||||
| Married | n.a. | 1.00 | n.a. | ||||||||||
| Not married | – | – | 0.53 | 0.29–0.98 | – | – | |||||||
CI = confidence interval; n.a. = not applicable; OR = odds ratio; TYA = teenage and young adult. Bold type indicates a p-value lower than 0.05. aAll variables significantly (p<0.10) associated with the respective outcome in univariable regression were included in the multivariable model. b OR for having basic education. c OR for being unemployed. d OR for not being married. e p-value calculated from Wald tests. f p-value for interaction between study population (TYA cancer survivors and controls) and the respective variable. g Standardised on age, sex, and migration background according to TYA cancer survivors.
We found few cancer-related variables to be associated with social outcomes (table 4). No cancer-related factors were associated with having only basic education. Unemployment was associated with younger age at diagnosis (OR 5.29, 95% CI 1.32–30.79) and self-reported late effects (OR 4.70, 95% CI 1.26–19.49). Survivors with younger age at diagnosis were more likely not to be married (OR 2.65, 95% CI 1.28–5.66) and not to have a life partner (OR 2.28, 95% CI 1.02–5.24).
Table 4 Cancer-related variables associated with educational achievement, employment status, marital status and life partnership in TYA cancer survivors from univariable exact logistic regression models.
| Educational achievement (having basic education) |
Employment status
(being unemployed) |
Marital status
(not being married) |
Life partnership
(not having a life partner) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | p-value | ORb | 95% CI | p-value | ORc | 95% CI | p-value | ORd | 95% CI | p-value | |
| Diagnosis (ICCC-3) | 0.909 | 0.602 | 0.614 | 0.115 | ||||||||
| Leukaemia/ lymphoma |
1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| CNS tumour | 0.68 | 0.01–6.00 | 2.24 | 0.19–15.79 | 0.58 | 0.16–2.10 | n.e. | – | ||||
| Other tumourse | 0.72 | 0.17–2.77 | 1.46 | 0.38–6.15 | 0.83 | 0.40–1.75 | 1.97 | 0.86–4.62 | ||||
| Treatment | 0.827 | 0.459 | 0.110 | 0.282 | ||||||||
| Surgery | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Chemotherapy | 1.39 | 0.19–8.84 | 1.04 | 0.20–4.52 | 1.07 | 0.40–2.92 | 1.12 | 0.39–3.15 | ||||
| Radiotherapy | 0.69 | 0.06–5.08 | 0.37 | 0.04–2.10 | 2.60 | 0.95–7.74 | 0.50 | 0.16–1.47 | ||||
| Age at diagnosis | 0.395 | 0.010 | 0.005 | 0.037 | ||||||||
| 21–25 years | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 16–20 years | 0.56 | 0.12–2.10 | 5.29 | 1.32–30.79 | 2.65 | 1.28–5.66 | 2.28 | 1.02–5.24 | ||||
| Time since diagnosis | 0.669 | 0.690 | 0.108 | 0.487 | ||||||||
| ≥16 years | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| 11–15 years | 0.71 | 0.10–4.42 | 1.49 | 0.33–7.70 | 1.48 | 0.62–3.57 | 0.68 | 0.23–1.99 | ||||
| 5–10 years | 1.27 | 0.28–6.52 | 0.82 | 0.14–4.65 | 2.36 | 0.99–5.75 | 1.18 | 0.46–3.10 | ||||
| Self-reported relapse | 0.693 | 1.000 | 0.495 | 0.804 | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 0.45 | 0.01–3.31 | 0.98 | 0.10–4.93 | 1.47 | 0.53–4.50 | 0.86 | 0.23–2.63 | ||||
| Self-reported late effects | 1.000 | 0.009 | 0.715 | 0.833 | ||||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Yes | 0.83 | 0.14–3.55 | 4.70 | 1.26–19.49 | 1.18 | 0.54–2.65 | 1.16 | 0.46–2.78 | ||||
CI = confidence interval; CNS = central nervous system; ICCC-3 = International Classification of Childhood Cancer - Third Edition; n.e. = not estimated (no variation in outcome); OR = odds ratio; TYA = teenage and young adult. Bold type indicates a p-value lower than 0.05. a OR for having basic education b OR for being unemployed. c OR for not being married. d OR for not having a life partner. e Other tumours include germ cell tumour, neuroblastoma, renal tumour, bone tumour and soft tissue sarcoma.
This study compared social outcomes between TYA cancer survivors and healthy controls in Switzerland. We found similar employment and marriage rates for long-term TYA cancer survivors and healthy controls. However, our findings indicate that survivors completed applied higher education rather than a university education. Younger age at diagnosis and self-reported late effects were identified as the main cancer-related factors associated with unemployment and not being married.
We found that more TYA cancer survivors (33%) than healthy controls (27%) reported upper secondary education as their highest educational achievement. However, fewer survivors (12%) than controls (21%) reported gaining a university degree. In Switzerland, after nine years of compulsory schooling, a majority of pupils continues with a structured vocational training. This may be complemented by an upper secondary education later on, and thereafter by a university degree. However, the more typical pathway to a university degree is via grammar school [27]. Our findings suggest that survivors may favour a step by step approach to higher education, rather than aiming at a university degree as a long-term goal. This is in line with a study showing that Swiss childhood cancer survivors aimed rather for short-term educational goals involving more intermediate steps, but with a final educational achievement comparable to the general population [16].
TYA cancer survivors in our study may have encountered additional barriers to pursuing a university education. TYAs are faced with the cancer diagnosis during a period of identity formation [3] involving decisions on future educational achievement. A cancer diagnosis during this critical period may alter survivors’ priorities in life. A US study showed that the cancer diagnosis had a negative impact on education-related plans in one third of survivors diagnosed at age 15-20 years [2]. Other barriers may include time away from school during cancer treatment [2, 14, 28]. Future research should focus on the reasons for and satisfaction with the observed educational pathways in order to develop specific educational services for TYAs if needed. We identified no cancer-related characteristics associated with survivors’ educational achievement, and associations with sociodemographic variables were similar to those of controls. National estimates in Switzerland have indicated increased risks for poor educational outcomes among migrants [27]. These findings were confirmed in the present study for both survivors and controls. A structured integration of these TYAs into the Swiss educational system and society may help to improve their long-term educational achievement.
In our study, the employment status of survivors was comparable to that of the controls. This is in contrast to a study in the US, which showed lower return to work and employment rates in TYA cancer survivors compared with national averages [10, 11, 29]. However, our findings are similar to other European countries, where unemployment among childhood cancer survivors was comparable to that in the general population [30]. A possible explanation may be the generally higher employment rates in Switzerland than in the US. Alternatively, it was hypothesised that employers in the US may have different views regarding long-term cancer survivors compared with Europe [30]. Work-related discrimination may be higher in the US and employers may be more reluctant to hire cancer survivors as a result of fears of lower productivity [30].
In our study, female sex, having only basic education and marriage were associated with unemployment in survivors and controls. Similarly, US TYA cancer survivors with a lower level of education were less likely to return to work/school after diagnosis [10]. Specific educational support for survivors and controls at risk for low education may help them to stay in the workforce in the future. US studies found that female TYA cancer survivors had higher beliefs that cancer had a negative impact on plans for work [10]. Female survivors of childhood cancer were also shown to be particularly vulnerable for unemployment and lower-skill occupations [31, 32]. Potential explanations may be that women may suffer from higher work-related discrimination than men and have other priorities in life such as taking care of children. Future studies should address the reasons for this gender-related vulnerability. The association between marriage and unemployment may be explained by personal, as well as socially expected, priorities in Switzerland, such as leaving employment to care for children. Although not statistically significant, our findings indicated that survivors and controls aged 20 to 29 years were more likely to be unemployed. Some of them may still be in education and thus not have a full-time employment.
Among TYA cancer survivors, we found that those with younger age at diagnosis and self-reported late effects were particularly at risk for unemployment. A cancer diagnosis at age 16 to 20 years may more strongly interfere with vocational training that typically starts at this age in Switzerland. Such a disruption may affect the survivors’ employment opportunities in the long term. Late effects such as cognitive impairments, cardiac toxicity or second malignancies [4, 14, 15] may, in contrast, interfere with the ability to maintain employment in the long term. Although the generalisability of our findings is limited because the subgroup of unemployed survivors was small, we suggest that work-related problems among survivors should be discussed in the context of psychosocial follow-up care.
We found that similar proportions of survivors and controls were married. Among survivors, 37% were married and 77% had a life partner. Our estimates are similar to results from a study in Germany [5]. In contrast, a study in the US found that young adult cancer survivors were less likely than controls to be currently married [11]. We were not able to compare life partnership between survivors and controls as this information was not available for controls. Life partnership may be a better proxy for relationship status since marital status strongly depends on religious or sociocultural beliefs. In addition, cohabitation and other partnership arrangements are increasingly common in Switzerland [18]. Female survivors were less likely to be married than female controls in our study. One reason might be higher concerns of female survivors regarding fertility impairment, pregnancy complications or family planning [33, 34]. We also found that survivors who were diagnosed at a younger age were less likely to be married or in a life partnership. However, this group was also younger at the time of study. TYAs with cancer may also face difficulties in disclosing a cancer history and its likely consequences to potential partners because of fear of rejection, which may preclude engagement in romantic relationships [35]. This may be of particular concern if cancer is diagnosed in teenage years when identity formation is still in progress [35]. Fertility concerns and disclosure of the cancer history should be addressed in future studies to inform the development of supportive services for Swiss TYAs diagnosed with cancer.
A limitation is the relatively small sample size of TYA cancer survivors, which reduced the accuracy of effect estimates in subgroup analyses, particularly in regard to cancer-related characteristics. Another limitation is the response rate of 41.1%. However, our response rate was comparable to other studies in the field [36]. The nonresponder analysis in table 1 showed that participants in our study adequately reflected the entire population of TYA cancer survivors. TYAs are a mobile and difficult-to-contact age group generally showing lower response rates than other age groups [36, 37]. Those aged between 15 and 25 years were described as particularly difficult to contact as a result of personal changes related to educational progress, starting professional careers or marriage [36]. The restriction of our study population of TYA cancer survivors to selected diagnostic groups further limits the generalisability of our findings to survivors of other frequent malignancies such as carcinomas. No in-depth questions on social outcomes (e.g., duration of education, type of and satisfaction with employment, quality of partner relationship) or other potentially confounding variables, such as personal income or the number of children, were available. These aspects need to be investigated in future studies. In addition, the cross-sectional study design did not allow investigation of social outcomes along the cancer trajectory since no data on social outcomes prior to the study were available.
A major strength of the study is the population-based sample of TYA cancer survivors in a large region of Switzerland. Clinical information was obtained from the population-based Cancer Registry Zurich and Zug. We used similar inclusion criteria and outcome measures for survivors and controls, and both surveys were performed in the same time period. We further maximised comparability by weighting controls according to survivors.
In conclusion, employment and marriage rates of TYA cancer survivors were comparable to those of healthy controls. However, our findings indicate that cancer during adolescence and young adulthood interfered with long-term educational achievement, with more survivors achieving an applied higher education than a university degree when compared with controls. Future studies including larger samples of TYA cancer survivors are needed to validate our findings and to explore the reasons for and satisfaction with the observed educational pathways.
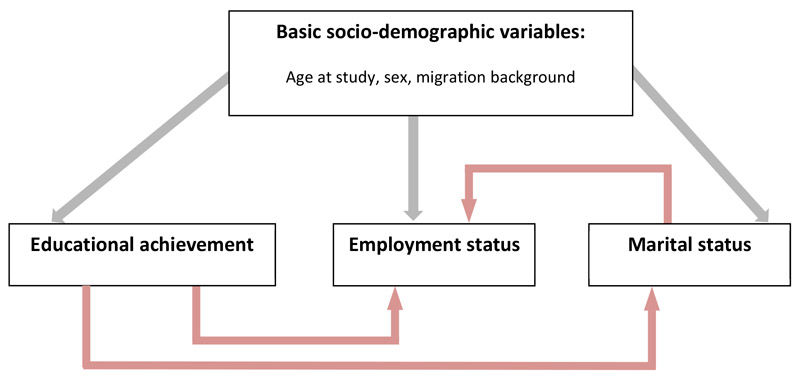
Figure S1 Causal pathway regression models in the combined dataset of teenage and young adult cancer survivors and controls.
We thank all TYA cancer survivors for participating in our survey, the Cancer Registry Zurich and Zug for its support, and the Swiss Federal Statistical Office for providing data for the Swiss Health Survey 2012.
This study was funded by the Swiss National Science Foundation (Ambizione grant PZ00P3_121682/1 and PZ00P3-141722 to GM; Grant 100019_153268 / 1). All authors declare that they have no competing interests that may be relevant to the submitted work.
No potential conflict of interest relevant to this article was reported.
1 Ahmad SS , Reinius MA , Hatcher HM , Ajithkumar TV . Anticancer chemotherapy in teenagers and young adults: managing long term side effects. BMJ. 2016;354:i4567.https://doi.org/10.1136/bmj.i4567
2 Bellizzi KM , Smith A , Schmidt S , Keegan TH , Zebrack B , Lynch CF , et al.; Adolescent and Young Adult Health Outcomes and Patient Experience (AYA HOPE) Study Collaborative Group. Positive and negative psychosocial impact of being diagnosed with cancer as an adolescent or young adult. Cancer. 2012;118(20):5155–62.https://doi.org/10.1002/cncr.27512
3 Zebrack BJ . Psychological, social, and behavioral issues for young adults with cancer. Cancer. 2011;117(10, Suppl):2289–94.https://doi.org/10.1002/cncr.26056
4 Patterson P , McDonald FE , Zebrack B , Medlow S . Emerging issues among adolescent and young adult cancer survivors. Semin Oncol Nurs. 2015;31(1):53–9.https://doi.org/10.1016/j.soncn.2014.11.006
5 Geue K , Schmidt R , Sender A , Sauter S , Friedrich M . Sexuality and romantic relationships in young adult cancer survivors: satisfaction and supportive care needs. Psychooncology. 2015;24(11):1368–76.https://doi.org/10.1002/pon.3805
6 Olsson M , Jarfelt M , Pergert P , Enskär K . Experiences of teenagers and young adults treated for cancer in Sweden. Eur J Oncol Nurs. 2015;19(5):575–81.https://doi.org/10.1016/j.ejon.2015.03.003
7 Seitz DCM , Besier T , Debatin KM , Grabow D , Dieluweit U , Hinz A , et al. Posttraumatic stress, depression and anxiety among adult long-term survivors of cancer in adolescence. Eur J Cancer. 2010;46(9):1596–606.https://doi.org/10.1016/j.ejca.2010.03.001
8 Zebrack B . Information and service needs for young adult cancer survivors. Support Care Cancer. 2009;17(4):349–57.https://doi.org/10.1007/s00520-008-0469-2
9 Zebrack B , Bleyer A , Albritton K , Medearis S , Tang J . Assessing the health care needs of adolescent and young adult cancer patients and survivors. Cancer. 2006;107(12):2915–23.https://doi.org/10.1002/cncr.22338
10 Parsons HM , Harlan LC , Lynch CF , Hamilton AS , Wu XC , Kato I , et al. Impact of cancer on work and education among adolescent and young adult cancer survivors. J Clin Oncol. 2012;30(19):2393–400.https://doi.org/10.1200/JCO.2011.39.6333
11 Kirchhoff AC , Yi J , Wright J , Warner EL , Smith KR . Marriage and divorce among young adult cancer survivors. J Cancer Surviv. 2012;6(4):441–50.https://doi.org/10.1007/s11764-012-0238-6
12 Warner EL , Kent EE , Trevino KM , Parsons HM , Zebrack BJ , Kirchhoff AC . Social well-being among adolescents and young adults with cancer: A systematic review. Cancer. 2016;122(7):1029–37.https://doi.org/10.1002/cncr.29866
13 Pearce S . Policy and practice in teenage and young adult cancer care in England: looking to the future. Eur J Oncol Nurs. 2009;13(3):149–53.https://doi.org/10.1016/j.ejon.2009.05.003
14 Soliman H , Agresta SV . Current issues in adolescent and young adult cancer survivorship. Cancer Contr. 2008;15(1):55–62.
15 Woodward E , Jessop M , Glaser A , Stark D . Late effects in survivors of teenage and young adult cancer: does age matter? Ann Oncol. 2011;22(12):2561–8.https://doi.org/10.1093/annonc/mdr044
16 Kuehni CE , Strippoli MP , Rueegg CS , Rebholz CE , Bergstraesser E , Grotzer M , et al.; Swiss Pediatric Oncology Group (SPOG). Educational achievement in Swiss childhood cancer survivors compared with the general population. Cancer. 2012;118(5):1439–49.https://doi.org/10.1002/cncr.26418
17 Wengenroth L , Sommer G , Schindler M , Spycher BD , von der Weid NX , Stutz-Grunder E , et al.; Swiss Paediatric Oncology Group (SPOG). Income in Adult Survivors of Childhood Cancer. PLoS One. 2016;11(5):e0155546.https://doi.org/10.1371/journal.pone.0155546
18 Wengenroth L , Rueegg CS , Michel G , Essig S , Ammann RA , Bergstraesser E , et al.; Swiss Paediatric Oncology Group (SPOG). Life partnerships in childhood cancer survivors, their siblings, and the general population. Pediatr Blood Cancer. 2014;61(3):538–45.https://doi.org/10.1002/pbc.24821
19 D’Agostino NM , Edelstein K . Psychosocial challenges and resource needs of young adult cancer survivors: implications for program development. J Psychosoc Oncol. 2013;31(6):585–600.https://doi.org/10.1080/07347332.2013.835018
20Bundesamt für Statistik. Schweizerische Gesundheitsbefragung 2012 - Übersicht [Swiss Health Survey 2012 - Overview]. Neuchâtel: 2013.
21Bundesamt für Statistik. Die Schweizerische Gesundheitsbefragung 2012 in Kürze - Konzept, Methode, Durchführung [Swiss Health Survey 2012 - Concept, methods, implementation]. Neuchâtel: Schweizerische Eidgenossenschaft; 2013.
22 Christen S , Vetsch J , Mader L , Dehler S , Korol D , Kuehni C , et al. Preferences for the organization of long-term follow-up in adolescent and young adult cancer survivors. Support Care Cancer. 2016;24(8):3425–36.https://doi.org/10.1007/s00520-016-3157-7
23Steliarova-Foucher E, Stiller C, Lacour B, Kaatsch P. International Classification of Childhood Cancer, third edition. Cancer 2005;103: 1457-67.
24 Mader L , Rueegg CS , Vetsch J , Rischewski J , Ansari M , Kuehni CE , et al.; Swiss Paediatric Oncology Group (SPOG). Employment Situation of Parents of Long-Term Childhood Cancer Survivors. PLoS One. 2016;11(3):e0151966.https://doi.org/10.1371/journal.pone.0151966
25StataCorp. 2013. Stata Survey Data Reference Manual - Release 13. College Station, TX: Stata Press.
26Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed2013. 500 S. p.
27Bundesamt für Statistik. Bildung und Wissenschaft: Panorama [Education and Science: Panorama]. Neuchâtel: Schweizerische Eidgenossenschaft; 2015.
28 Grinyer A . The biographical impact of teenage and adolescent cancer. Chronic Illn. 2007;3(4):265–77.https://doi.org/10.1177/1742395307085335
29 Tai E , Buchanan N , Townsend J , Fairley T , Moore A , Richardson LC . Health status of adolescent and young adult cancer survivors. Cancer. 2012;118(19):4884–91.https://doi.org/10.1002/cncr.27445
30 de Boer AG , Verbeek JH , van Dijk FJ . Adult survivors of childhood cancer and unemployment: A metaanalysis. Cancer. 2006;107(1):1–11.https://doi.org/10.1002/cncr.21974
31 Kirchhoff AC , Krull KR , Ness KK , Park ER , Oeffinger KC , Hudson MM , et al. Occupational outcomes of adult childhood cancer survivors: A report from the childhood cancer survivor study. Cancer. 2011;117(13):3033–44.https://doi.org/10.1002/cncr.25867
32 Pang JWY , Friedman DL , Whitton JA , Stovall M , Mertens AC , Robison LL , et al. Employment status among adult survivors in the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2008;50(1):104–10.https://doi.org/10.1002/pbc.21226
33 Crawshaw M . Psychosocial oncofertility issues faced by adolescents and young adults over their lifetime: a review of the research. Hum Fertil (Camb). 2013;16(1):59–63.https://doi.org/10.3109/14647273.2012.733480
34 Kent EE , Parry C , Montoya MJ , Sender LS , Morris RA , Anton-Culver H . “You’re too young for this”: adolescent and young adults’ perspectives on cancer survivorship. J Psychosoc Oncol. 2012;30(2):260–79.https://doi.org/10.1080/07347332.2011.644396
35 Murphy D , Klosky JL , Reed DR , Termuhlen AM , Shannon SV , Quinn GP . The importance of assessing priorities of reproductive health concerns among adolescent and young adult patients with cancer. Cancer. 2015;121(15):2529–36.https://doi.org/10.1002/cncr.29466
36 Harlan LC , Lynch CF , Keegan TH , Hamilton AS , Wu XC , Kato I , et al.; AYA HOPE Study Collaborative Group. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. J Cancer Surviv. 2011;5(3):305–14.https://doi.org/10.1007/s11764-011-0173-y
37 Rosenberg AR , Bona K , Wharton CM , Bradford M , Shaffer ML , Wolfe J , et al. Adolescent and Young Adult Patient Engagement and Participation in Survey-Based Research: A Report From the “Resilience in Adolescents and Young Adults With Cancer” Study. Pediatr Blood Cancer. 2016;63(4):734–6.https://doi.org/10.1002/pbc.25843
This study was funded by the Swiss National Science Foundation (Ambizione grant PZ00P3_121682/1 and PZ00P3-141722 to GM; Grant 100019_153268 / 1). All authors declare that they have no competing interests that may be relevant to the submitted work.
No potential conflict of interest relevant to this article was reported.