Treatment challenges in type 1 diabetes after roux-en-Y gastric bypass
DOI: https://doi.org/10.4414/smw.2017.14420
Lucie
Favrea, François
Pralonga, Michel
Suterbc, François R.
Jornayvaza
aDepartment of Endocrinology, Diabetology and Metabolism, Lausanne University Hospital (CHUV),
bDepartment of Visceral Surgery, Lausanne University Hospital (CHUV),
cDepartment of Surgery, Riviera-Chablais Hospital, Rennaz,
Treatment challenges in type 1 diabetes after roux-en-Y gastric bypass
Summary
Bariatric surgery is an effective treatment of type 2 diabetes in obese patients. The obesity epidemic does not spare patients with type 1 diabetes mellitus (T1DM), but there is no consensus regarding the role of surgery in the management of obese T1DM patients. Published data consistently report significant weight loss after surgery in obese T1DM patients, but long-term glycaemic control remains difficult to achieve. Here we present our experience with a challenging patient and a review of the literature.
Our patient successfully underwent a roux-en-Y gastric bypass (RYGB) when she was 28 years old. Five years after surgery, she was diagnosed with latent autoimmune diabetes of adults and insulin therapy was initiated. Insulin therapy proved very difficult to adjust, with frequent episodes of postprandial hyperglycaemia. These difficulties could only be overcome by the initiation of a subcutaneous insulin infusion using a sensor-augmented insulin pump with automated suspension. This change allowed better glycaemic control.
Despite considerable weight loss with a concomitant decrease in insulin requirement, glycaemic control remained difficult after surgery. Due to their different impacts on glucose kinetics, the type of surgical operation should be part of the assessment. These patients might benefit from sensor-augmented insulin pump therapy with automated insulin suspension after bariatric surgery. The decision for surgical intervention in these patients should be carefully weighed against the difficulties in achieving adequate glycaemic control.
Introduction
The global epidemic of obesity that has developed over recent decades does not spare patients with type 1 diabetes mellitus (T1DM). In these patients, insulin resistance can accompany the deficient insulin secretion, and the term “double diabetes” has been suggested to describe this novel physiopathological entity. Moreover, a substantial fraction of patients suffering from type 2 diabetes (T2DM) also experience a progressive decline in beta-cell function of autoimmune origin [1]. Compared with lean individuals with type 1 diabetes, obese patients are at even higher risk of micro- and macro-vascular complications. They have a worse cardiovascular prognosis, justifying the requirement for tighter glycaemic control [2]. These patients represent a therapeutic challenge, because they require higher doses of insulin to achieve a good glycaemic control. This may result in further weight gain due to the anabolic effects of insulin, thus creating a vicious circle.
A growing number of patients with T1DM are thus theoretical candidates for metabolic surgery, but only a small number of such patients have been reported in the literature. According to recent reviews [3, 4], the vast majority of T1DM patients undergoing bariatric surgery experienced significant weight loss with ensuing decreases in their total insulin requirements. Nevertheless, long-term glycaemic control remained variable and somewhat unpredictable. An increase in the occurrence of hypoglycaemic episodes has also been reported [5]. Consequently, there are presently no definitive recommendations regarding the use and indications of bariatric surgery in obese T1DM patients. The aim of this article was to illustrate the challenges encountered in a patient who developed latent autoimmune diabetes of adults (LADA) several years after benefitting from a roux-en-Y gastric bypass (RYGB).
Case report
A 28-year-old woman weighing 141 kg and with a height of 167 cm (body mass index [BMI] 50.6 kg/m2) was referred for bariatric surgery. Metformin had been started 6 months before the intervention because of T2DM (HbA1c 7.8%, fasting plasma glucose 10 mmol/l). She underwent a RYGB. Metformin treatment was stopped postoperatively and 9 months later her HbA1c was down to 5.7% without treatment. Eighteen months after surgery, she had lost 57 kg, corresponding to 80% of excess weight loss, and thereafter her weight stabilised at 84 kg (BMI 30 kg/m2). At that time HbA1c was not measured, but fasting plasma glucose was 4.7 mmol/l. Five years after surgery, she experienced a further weight loss of 10 kg within 6 months. Laboratory analyses revealed a new elevation of HbA1c levels to 8.6%, and the diagnosis of LADA was made on the basis of a strongly positive test for autoantibodies against glutamic acid decarboxylase (GAD) (1 390 800 IU/ml, N <10).
The patient was diagnosed with T2DM shortly before surgery, but had normal blood glucose values for 5 years afterwards. A very high anti-GAD antibody titre led to the diagnosis of autoimmune diabetes of the LADA type when she relapsed. Later in the course of the disease, C-peptide was undetectable with a glycaemia of 12 mmol/l. Insulin therapy was initiated, with basal and prandial insulin injections, and she participated in an intensive insulin therapy education programme. In the weeks following the introduction of insulin, she began to experience dysphagia after eating, possibly related to the stress induced by the treatment. As a result, her food intake became limited, and she began to suffer from frequent hypoglycaemic episodes after prandial insulin (documented symptomatic hypoglycaemia almost daily, one severe episode of hypoglycaemia). In order to avoid these episodes, she was advised to delay the administration of prandial insulin until 15 minutes after the start of her meals. On this regimen, blood glucose control became highly erratic, with recurrent episodes of postprandial hyperglycaemia (>22.2 mmol/l). Continuous glucose monitoring confirmed the frequent occurrence of high postprandial glucose peaks (fig. 1). After a couple of weeks, the patient felt more at ease with the injections of insulin, the stress experienced at the beginning of therapy decreased and food ingestion returned to normal. Insulin boluses were therefore moved back to the start of the meal. This resulted in a lowering of postprandial peaks of glycaemia. Her overall diabetes control, however, was still insufficient. Insulin boluses were therefore moved back to 15 minutes before the start of the meal, which resulted in a better glycaemic control: HbA1c decreased from 8.9 to 7.4% within 10 weeks of this change (fig. 2). Unfortunately, the patient remained afraid of hypoglycaemic episodes and could not keep to this insulin regimen. She stopped her meticulous glycaemic control and her HbA1c gradually rose again. Her usual dietary intake of carbohydrates was between 30 and 60 g per meal. She was advised to limit refined carbohydrates in her diet in favour of low glycaemic index foods, but this was insufficient to correct her HbA1c.

Figure 1 Continuous monitoring of blood glucose (mmol/l) with multiple-dose insulin injections and prandial insulin administered 15 minutes after the start of meals.
Solid lines represent the record of six individuals and consecutive days. The dotted line is the average of the six recorded days. The shaded zone represents the target glucose value.
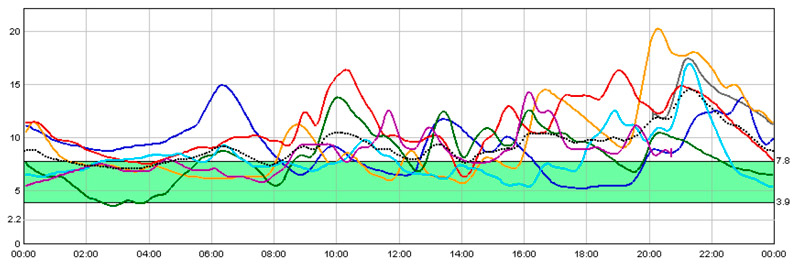
Figure 2 Continuous monitoring of blood glucose (mmol/l) with multiple-dose insulin injections and prandial insulin administered 15 minutes before the start of meals.
Solid lines represent the record of six individuals and consecutive days. The dotted line is the average of the six recorded days. The shaded zone represents the target glucose value.
As a result of the difficulties in optimally controlling blood glucose without hypoglycaemia, we decided to start continuous subcutaneous insulin infusion using a sensor-augmented insulin pump with automated insulin suspension (Minimed® 640G, Medtronic) in order to avoid hypoglycaemia. After the usual adaptations in the basal rate and boluses, overall glycaemic control improved, with an HbA1c of 7.6% without hypoglycaemic episodes (fig. 3). Her current total insulin dose is 60 IU/d (0.6 IU/kg/d) divided into 36 IU/d of basal insulin and 24 IU/d of bolus insulin. Her carbohydrate-to-insulin ratio is between 10 and 15 g/IU. Combination boluses are administrated 15 minutes before each meal. As can be seen in figure 4, during a day of fast the basal rate was not the main problem for treatment adjustment. The most difficult thing to handle was postprandial glycaemia.

Figure 3 Continuous glucose monitoring with continuous subcutaneous insulin infusion via a sensor-augmented insulin pump with automated insulin suspension. Insulin boluses administered 15 minutes before the start of the meals.
Solid lines represent the record of six individuals and consecutive days. The dotted line is the average of the six recorded days. The shaded zone represents the target glucose value.

Figure 4 Continuous glucose monitoring during a day of fasting and pump therapy.
Plus sign = calibration; inverted triangle = ALERT
Discussion
The patient described here illustrates the challenges faced by T1DM patients after RYGB surgery. Indeed, although weight outcomes after bariatric surgery are similar in patients with type 1 and type 2 diabetes [6, 7], glycaemic control may not necessarily improve in T1DM. This discrepancy may at least partially result from an increased variability in postprandial blood glucose concentrations after RYGB, combining marked hyperglycaemic peaks very early after meal ingestion with subsequent rapid decreases in glycaemia. Such variability can become problematic for adequate glycaemic control when patients who remain insulin dependent use multiple injections.
This evolution in the postprandial glycaemic profile after bariatric surgery is the consequence of the faster delivery of sugars to the jejunum. Jimenez et al. [8] reported the glucose response to a standardised mixed meal challenge after either RYGB or sleeve gastrectomy in 31 subjects who had remission of T2DM. After RYGB they found rapid increases in plasma glucose, with mean glucose peaks of 12 mmol/l occurring 33 minutes after the start of a meal. These peaks were followed by a pronounced fall in glycaemia (6.7 mmol/l) occurring 60 minutes thereafter. In the sleeve gastrectomy group, the mixed meal produced a lower glucose peak (8.9 mmol/l) and, importantly, a longer time to glucose peak (47 minutes) with a smaller subsequent glucose decline (3.5 mmol/l) in the 60 minutes after the peak. The large excursions in glucose concentrations reported after RYGB result in a dissociation between the rate of glucose absorption and the kinetics of insulin secretion. This dissociation may account for the difficulties encountered in the treatment of T1DM after this procedure. When considering glucose absorption, sleeve gastrectomy could be considered a more physiological and predictable intervention in terms of glucose absorption and thus may constitute a better solution for glucose control in T1DM. However, this hypothesis, also advocated by some authors [9], is awaiting definitive demonstration.
Published data regarding glycaemic control in patients with T1DM after obesity surgery are somewhat conflicting, possibly because of wide variations between diabetes therapies. Czupryniak et al. [10] were the first to report significant reductions in either insulin need or HbA1c after gastric bypass in three obese patients with poorly controlled T1DM before surgery. These data, however, are difficult to interpret, since C-peptide levels were not reported and treatment modalities were not indicated. In contrast, Chuang and al. [5] reported that HbA1c significantly worsened and hypoglycaemic episodes became more frequent after RYGB in two adolescents with T1DM. This occurred despite significant weight loss and improvements in cardiovascular risk factors such as dyslipidaemia or obstructive sleep apnoea. As in our patient, these two adolescents took insulin boluses postprandially because of the occurrence of gastrointestinal symptoms. Under such a regimen, a postprandial glucose peak cannot be avoided, because of the modification in glucose absorption described above. As a result, insulin is still acting when glucose levels are decreasing, creating a risk for hypoglycaemia.
In a larger study [11] reporting the evolution of ten patients undergoing various surgical procedures (RYGB, sleeve gastrectomy and adjustable gastric banding), nine achieved a significant excess weight loss (averaging 60%), but none could attain optimal glucose control. Their average HbA1c went from 10% before to 8.9% after the surgery. However, since neither the modality of diabetes treatment nor the surgical procedure were precisely reported, it is impossible to draw meaningful conclusions from these data. In another study [12], including 13 patients with T1DM who underwent RYGB or sleeve gastrectomy, the median postoperative decrease in HbA1c was 0.7% at 12 months, with great heterogeneity between individuals and an increase in minor hypoglycaemia. This heterogeneity in glucose control may be explained by the fact that two different surgical operations were performed in this study. In addition, data at 1 year could be biased because food intake is sometimes still irregular at this point. Consistent increases in HbA1c levels have been reported at 5 years [13]. A recent study by Vilarrasa et al. [14] of 32 patients with T1DM who underwent various surgical procedures (RYGB, sleeve gastrectomy or duodenal switch) confirmed the benefits of surgery on insulin requirement. This study also demonstrated that despite an initial decrease in HbA1c observed during the first year after surgery, the metabolic control was not sustained in the longer term.
In T1DM, short-acting insulin boluses before meals are associated with better glycaemic profiles than postprandial administration, and this could also be true after RYGB because of the faster glucose absorption. This hypothesis is in accordance with our present observation, where the best glycaemic control in the period preceding introduction of the sensor-augmented insulin pump was achieved with preprandial insulin boluses. However, this multiple daily injection regimen was also accompanied by an increased occurrence of hypoglycaemia.
In this respect, sensor-augmented insulin pump therapy with automated insulin suspension may constitute the treatment of choice for T1DM patients undergoing obesity surgery. In a report of three patients undergoing RYGB [15], two patients on an insulin pump (without sensor) remained stable after the surgery whereas the third patient, who used multiple daily injections, experienced a worsening of HbA1c levels. Nevertheless, achieving adequate glycaemic control remains challenging in these patients. In that study, insulin requirements were significantly decreased in all three patients after weight loss, but diabetes control remained inadequate in all three of them. A similar observation was made by Blanco et al. [6] in two T1DM patients who were started on an insulin pump because of insufficient glycaemic control with multiple daily injections.
There is currently no recommendation regarding the use of bariatric surgery in obese patients with T1DM. However, the bulk of existing data suggests that glycaemic control in these patients is at least not improved, and possibly rendered more difficult, by the intervention, although total daily insulin requirement is decreased. In some cases, a pancreatic transplant was eventually needed [16]. This suggests that a careful preoperative evaluation for specific types of diabetes mellitus is mandatory before proceeding with bariatric surgery. Failure to do so may have very deleterious consequences for patients misdiagnosed preoperatively with T2DM: a worsening of their HbA1c levels after surgery can lead to reclassification of their diabetes as LADA [7, 17].
In conclusion, the marked and sustained weight loss consistently observed after bariatric surgery is not sufficient to expect improved glycaemic control in T1DM. In the absence of residual beta-cell function, reaching optimal glycaemic control remains challenging because of the complex kinetics of postprandial glucose absorption after RYGB. For T1DM patients after bariatric surgery, we suggest that multiple daily injections of insulin should be replaced by sensor-augmented insulin pump therapy with automated insulin suspension to avoid postprandial hypoglycaemia. Moreover, preprandial insulin boluses should be carefully timed to limit glucose peaks and reduce the risk of postprandial hypoglycaemia. Nevertheless, more work is needed in order to better delineate both the most appropriate type of intervention as well as the best treatment regimen of type 1 diabetes after bariatric surgery.
Lucie Favre, MD, Department of Endocrinology, Diabetology and Metabolism, Lausanne University Hospital (CHUV), Chemin Mont-Paisible 18, CH-1011-Lausanne, lucie.favre[at]chuv.ch
References
1
Brooks-Worrell
BM
,
Boyko
EJ
,
Palmer
JP
. Impact of islet autoimmunity on the progressive β-cell functional decline in type 2 diabetes. Diabetes Care. 2014;37(12):3286–93. https://doi.org/10.2337/dc14-0961
2
Chillarón
JJ
,
Flores Le-Roux
JA
,
Benaiges
D
,
Pedro-Botet
J
. Type 1 diabetes, metabolic syndrome and cardiovascular risk. Metabolism. 2014;63(2):181–7. https://doi.org/10.1016/j.metabol.2013.10.002
3
Ashrafian
H
,
Harling
L
,
Toma
T
,
Athanasiou
C
,
Nikiteas
N
,
Efthimiou
E
, et al.
Type 1 Diabetes Mellitus and Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes Surg. 2016;26(8):1697–704. https://doi.org/10.1007/s11695-015-1999-6
4
Kirwan
JP
,
Aminian
A
,
Kashyap
SR
,
Burguera
B
,
Brethauer
SA
,
Schauer
PR
. Bariatric Surgery in Obese Patients With Type 1 Diabetes. Diabetes Care. 2016;39(6):941–8. https://doi.org/10.2337/dc15-2732
5
Chuang
J
,
Zeller
MH
,
Inge
T
,
Crimmins
N
. Bariatric surgery for severe obesity in two adolescents with type 1 diabetes. Pediatrics. 2013;132(4):e1031–4. https://doi.org/10.1542/peds.2012-3640
6
Blanco
J
,
Jiménez
A
,
Casamitjana
R
,
Flores
L
,
Lacy
A
,
Conget
I
, et al.
Relevance of beta-cell function for improved glycemic control after gastric bypass surgery. Surg Obes Relat Dis. 2014;10(1):9–13, quiz 189–90. https://doi.org/10.1016/j.soard.2013.07.020
7
Maraka
S
,
Kudva
YC
,
Kellogg
TA
,
Collazo-Clavell
ML
,
Mundi
MS
. Bariatric surgery and diabetes: Implications of type 1 versus insulin-requiring type 2. Obesity (Silver Spring). 2015;23(3):552–7. https://doi.org/10.1002/oby.20992
8
Jiménez
A
,
Ceriello
A
,
Casamitjana
R
,
Flores
L
,
Viaplana-Masclans
J
,
Vidal
J
. Remission of type 2 diabetes after Roux-en-Y gastric bypass or sleeve gastrectomy is associated with a distinct glycemic profile. Ann Surg. 2015;261(2):316–22. https://doi.org/10.1097/SLA.0000000000000586
9
Lannoo
M
,
Dillemans
B
,
Van Nieuwenhove
Y
,
Fieuws
S
,
Mathieu
C
,
Gillard
P
, et al.
Bariatric surgery induces weight loss but does not improve glycemic control in patients with type 1 diabetes. Diabetes Care. 2014;37(8):e173–4. https://doi.org/10.2337/dc14-0583
10
Czupryniak
L
,
Wiszniewski
M
,
Szymański
D
,
Pawłowski
M
,
Loba
J
,
Strzelczyk
J
. Long-term results of gastric bypass surgery in morbidly obese type 1 diabetes patients. Obes Surg. 2010;20(4):506–8. https://doi.org/10.1007/s11695-010-0074-6
11
Brethauer
SA
,
Aminian
A
,
Rosenthal
RJ
,
Kirwan
JP
,
Kashyap
SR
,
Schauer
PR
. Bariatric surgery improves the metabolic profile of morbidly obese patients with type 1 diabetes. Diabetes Care. 2014;37(3):e51–2. https://doi.org/10.2337/dc13-1736
12
Faucher
P
,
Poitou
C
,
Carette
C
,
Tezenas du Montcel
S
,
Barsamian
C
,
Touati
E
, et al.
Bariatric Surgery in Obese Patients with Type 1 Diabetes: Effects on Weight Loss and Metabolic Control. Obes Surg. 2016;26(10):2370–8. https://doi.org/10.1007/s11695-016-2106-3
13
Middelbeek
RJ
,
James-Todd
T
,
Cavallerano
JD
,
Schlossman
DK
,
Patti
ME
,
Brown
FM
. Gastric Bypass Surgery in Severely Obese Women With Type 1 Diabetes: Anthropometric and Cardiometabolic Effects at 1 and 5 Years Postsurgery. Diabetes Care. 2015;38(7):e104–5. https://doi.org/10.2337/dc15-0396
14
Vilarrasa
N
,
Rubio
MA
,
Miñambres
I
,
Flores
L
,
Caixàs
A
,
Ciudin
A
, et al.
Long-Term Outcomes in Patients with Morbid Obesity and Type 1 Diabetes Undergoing Bariatric Surgery. Obes Surg. 2016. https://doi.org/10.1007/s11695-016-2390-y
15
Mendez
CE
,
Tanenberg
RJ
,
Pories
W
. Outcomes of Roux-en-Y gastric bypass surgery for severely obese patients with type 1 diabetes: a case series report. Diabetes Metab Syndr Obes. 2010;3:281–3. https://doi.org/10.2147/DMSO.S9981
16
Porubsky
M
,
Powelson
JA
,
Selzer
DJ
,
Mujtaba
MA
,
Taber
T
,
Carnes
KL
, et al.
Pancreas transplantation after bariatric surgery. Clin Transplant. 2012;26(1):E1–6. https://doi.org/10.1111/j.1399-0012.2011.01559.x
17
Manning
SB
,
Pucci
A
,
Batterham
RL
,
Finer
N
. Latent autoimmune diabetes in adults presenting as diabetes “recurrence” after bariatric surgery: a case report. Diabetes Care. 2013;36(8):e120. https://doi.org/10.2337/dc13-0810



