Use it or lose it! Cognitive activity as a protective factor for cognitive decline associated with Alzheimer’s disease
DOI: https://doi.org/10.4414/smw.2017.14407
Panagiota
Mistridisa, Jutta
Matab, Stefan
Neuner-Jehlecd, Jean-Marie
Annonie, Andreas
Biedermannf, Irene
Bopp-Kistlerg, Dominique
Brandh, Andrea Brioschi
Guevarai, Hedi
Decrey-Wickj, Jean-François
Démoneti, Ulrich
Hemmeterk, Reto W
Kressiglm, Brian
Martinn o, Luca
Rampap, Egemen
Savaskanq, Andreas E.
Stuckr, Philipp
Tschopps, Dina
Zekryt, Andreas U.
Monschal
aMemory Clinic, University Centre for Medicine of Aging, Felix Platter Hospital, Basel,
bHealth Psychology, Department of Social Sciences, University of Mannheim,
cInstitute of Primary Care, University of Zurich and University Hospital Zurich,
dSwiss College of Primary Care Medicine, Fribourg,
eNeurology Unit, Department of Medicine, Faculty of Science, University of Fribourg,
fPublic Health Services, Berne,
gMemory Clinic, University Clinic of Geriatrics, Stadtspital Waid, Zurich,
hGeneral Practitioner, Internal Medicine and Geriatrics, Courtelary,
iLeenaards Memory Centre, University Hospital Lausanne,
jGeneral Practitioner, Corseaux,
kPsychiatric Services St Gallen-North, Centre of Education and Research (COEUR), St Gallen,
lUniversity of Basel,
mUniversity Centre for Medicine of Aging, Felix Platter Hospital, Basel,
nChief Medical Officer of the Canton of Basel-Country, Liestal,
oInstitute of Epidemiology, Biostatistics and Prevention EBPI, University of Zurich,
pUniversity Hospital of Old Age Psychiatry, University of Bern,
qUniversity Hospital of Psychiatry Zurich, Department of Geriatric Psychiatry, Zurich,
rDepartment of Geriatrics, Inselspital, Berne University Hospital, University of Berne,
sGeneral Practitioner, Basel,
tDepartment of Internal Medicine, Rehabilitation and Geriatrics, Geneva University Hospitals and University of Geneva,
Use it or lose it! Cognitive activity as a protective factor for cognitive decline associated with Alzheimer’s disease
Summary
Because of the worldwide aging of populations, Alzheimer’s disease and other dementias constitute a devastating experience for patients and families as well as a major social and economic burden for both healthcare systems and society. Multiple potentially modifiable cardiovascular and lifestyle risk factors have been associated with this disease. Thus, modifying these risk factors and identifying protective factors represent important strategies to prevent and delay disease onset and to decrease the social burden. Based on the cognitive reserve hypothesis, evidence from epidemiological studies shows that low education and cognitive inactivity constitute major risk factors for dementia. This indicates that a cognitively active lifestyle may protect against cognitive decline or delay the onset of dementia. We describe a newly developed preventive programme, based on this evidence, to stimulate and increase cognitive activity in older adults at risk for cognitive decline. This programme, called “BrainCoach”, includes the technique of “motivational interviewing” to foster behaviour change. If the planned feasibility study is successful, we propose to add BrainCoach as a module to the already existing “Health Coaching” programme, a Swiss preventive programme to address multiple risk factors in primary care.
Introduction
The increase of life expectancy over the last century is paralleled by an elevated number of individuals with dementia, placing an enormous social and economic burden on society and healthcare systems, in addition to the devastating consequences for patients and their families. According to the World Alzheimer Report 2015, an estimated 47 million people worldwide suffer from dementia [1]. Based on current simulation models, the number of cases is expected to at least double every 20 years, reaching almost 132 million cases by 2050. However, recent reports suggest that the age-specific incidence of dementia might be decreasing in persons with at least a high school diploma, possibly owing to an improvement in cardiovascular health [2, 3]. Nevertheless, and irrespective of how fast the growth in the number of people with dementia will be, the economic impact associated with dementia is huge and increasing. Worldwide, the current total costs have increased by 35% in the last five years and were estimated to be USD 818 billion in 2015, representing 1% of the global gross domestic product [1]. These costs include direct medical care costs (dementia treatment in primary and secondary care), direct social care costs (community care professionals and residential homes) and informal care costs (unpaid care by family and others) [1]. In Switzerland, based on current simulation models, the number of people with dementia was estimated to be 119 000 in 2015, and this number is predicted to increase up to 300 000 by 2050 [4]. Annually, there are 27 000 new cases of dementia. The annual costs amount to approximately CHF 7 billion, imposing an enormous economic burden on Swiss society [5].
Although several causes of dementia exist, we will focus this review on its most common cause, Alzheimer's disease. Alzheimer’s disease is clinically characterised by an insidious onset and a progressive deterioration of cognitive function, which typically starts with a decline in memory functions [6, 7]. Pathologically, Alzheimer’s disease is characterised by increased levels of extracellular amyloid-β, intracellular hyperphosphorylated tau proteins, and neuronal and synaptic loss in the neocortex [8, 9]. Despite decades of research, the pathological mechanisms underlying these disease-related changes still remain largely unclear. However, there is a broad consensus that Alzheimer’s disease has a complex multifactorial aetiology and is modulated by various risk factors [10–13]. While advancing age [14] and specific genetic predispositions (e.g., APOE-ε4 [15, 16]) are well-recognised unmodifiable main risk factors for sporadic Alzheimer’s disease, additional potentially modifiable factors have been linked to the development of the disease. Specifically, observational studies indicate that cardiovascular risk factors (e.g., hypertension, diabetes, cerebrovascular disease, obesity, metabolic syndrome), lifestyle factors (e.g., unhealthy diet, smoking, high alcohol intake, low cognitive, social and physical activity, chronic stress) and psychosocial factors (e.g., lack of a rich social network, depressive symptoms) have an impact on the risk for Alzheimer’s disease and other forms of dementia (for an overview see [12, 17–19]. Additionally, individual risk factors are often closely linked and thus co-occur during a person’s lifetime: A recent estimate by Norton et al. [11] implies that, taking into account the interdependency of these factors, about one third of Alzheimer’s disease cases worldwide may be attributable to seven potentially modifiable risk factors: diabetes, midlife hypertension, midlife obesity, depression, physical inactivity, smoking, and low educational attainment (fig. 1; see also [10]).
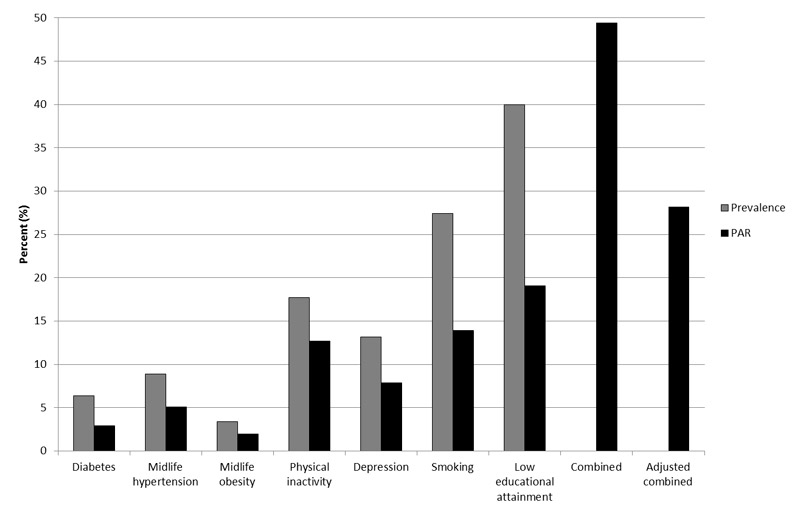
Figure 1 Prevalence and estimates for population-attributable risks for Alzheimer’s disease in 2010. Data source: Norton et al. [11].
PAR = population-attributable risk, the proportion of Alzheimer’s disease cases in the population that can be attributed to individual risk factors calculated by using the population prevalence and the relative risk of the risk factor. Combined = calculated prevalence and PAR for the seven individual risk factors combined, assuming independence of the risk factors. Adjusted combined = calculated prevalence and PAR for all seven risk factors combined with adjustment for non-independence of the risk factors.
Whereas current pharmacological and nonpharmacological treatments may decelerate disease progression, no curative or disease-modifying intervention exists to prevent the pathogenesis of dementia [20, 21]. Thus, there is a pressing need to identify preventive measures and strategies aiming to maintain brain health and delay cognitive decline. Importantly, because neurodegenerative disorders such as Alzheimer’s disease have a long “silent” phase with no or only very subtle symptoms [22], such preventive programmes should optimally be implemented at the earliest possible time when cognitive impairments are not yet manifest [23–26]. Figure 2 illustrates that preventive strategies require a lifespan perspective. Thus, children and adolescents need to be targeted with regard to education and, starting at midlife, optimal management of potential risk factors is essential [27].
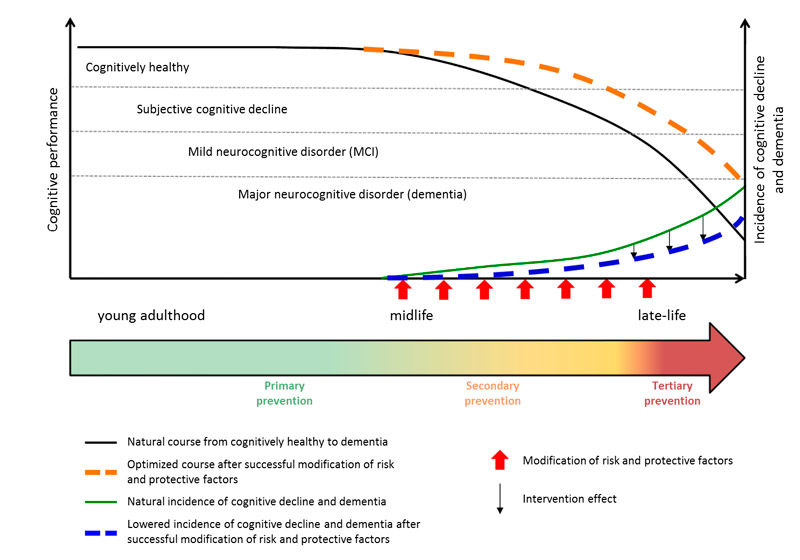
Figure 2 Hypothesised model of neurocognitive disorders [26] across lifespan without (black line) and with (orange dashed line) successful modification of risk and protective factors and the consequence on dementia incidence rates: natural course (green line) vs result of optimally timed preventive strategies through primary, secondary and tertiary prevention (blue dashed line). Figure adapted from [24] and [25], reproduced with permission.
Different modifiable factors to preserve cognitive health have been described. A healthy diet (e.g., Mediterranean) rich in antioxidants, vitamins (e.g., vitamins B12, E and D) and polyunsaturated fatty acids (e.g., omega-3 [28]) may lower the risk of cognitive decline and dementia by reducing, for example, the risk for cardiovascular diseases [29, 30]. However, although findings from different studies suggest that specific dietary patterns or nutritional components may represent promising interventions in delaying cognitive decline, evidence in this regard is still weak and further investigation on this topic is needed [31, 32]. Additionally, physical activity is also known to exert beneficial effects on brain health. Promising results from different observational and intervention studies, as well as systematic reviews, examining the association between physical activity and the risk of cognitive impairment and dementia, yielded promising results, with moderate to strong effects of physical activity on brain health. These results indicate that individuals with a physically active lifestyle exhibit less cognitive decline [33–36], reduced brain atrophy [37] and increased hippocampal volume [38] compared with people with a sedentary lifestyle. A third important protective factor for the maintenance of brain health is cognitive activity. This aspect will be reviewed in more detail below and a new module within the Swiss Health Coaching programme [39], the “BrainCoach”, which focuses on cognitive activity as a preventive measure of cognitive decline, is introduced.
Cognitive activity as a modifiable protective factor for cognitive decline
As shown in figure 1, low educational attainment [11] or cognitive inactivity [10] constitutes the largest single risk factor for Alzheimer’s disease. Certainly, high educational attainment will not prevent pathological brain changes; however, a number of studies indicate that higher educational attainment is related with higher levels of cognitive performance and seems to buffer negative effects and mitigate clinical symptoms in everyday life [2, 40–44]. The rationale behind this observation is the hypothesis that higher educational attainment results in an increased cognitive reserve [43, 45]. This so-called cognitive reserve hypothesis implies that individuals with higher levels of brain activity (e.g., through individual or synergistic contributions of high educational/occupational attainment and maintained cognitive activity up until old age) may better cope with brain pathology and are able to compensate for brain damage much longer because of increased synaptic densities and a more complex and efficient structure of neural networks [23, 43]. Thus, people with the same extent of pathological brain changes may exhibit different clinical manifestations of the disease depending on their level of cognitive reserve [40] (fig. 3, [45]). Importantly, a person with a high cognitive reserve will have a steeper decline once symptoms are manifest, as a result of higher pathological accumulations in the brain, until cognitive dysfunctions are clinically perceivable [45].
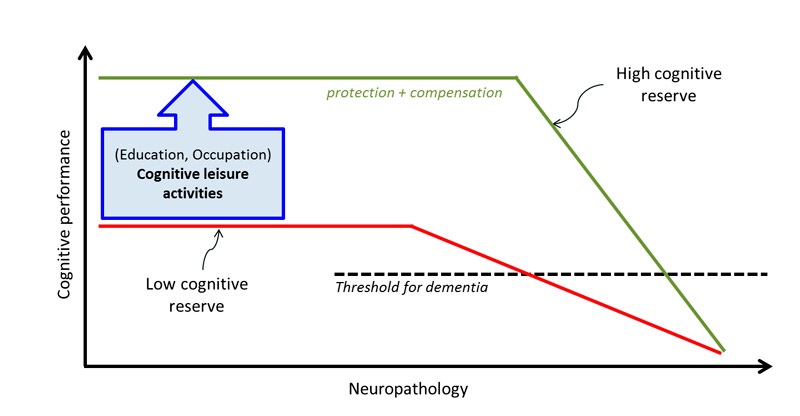
Figure 3 Illustration of the association between the emergence of dementia-associated neuropathology, its clinical expression and cognitive reserve. Figure adapted from [45]; reproduced with permission. Educational attainment and occupational challenges can usually no longer be changed in individuals aged 50 years and older. However, cognitive leisure activities may increase their cognitive reserve and thus lead to a delay in the emergence of cognitive decline.
These findings are supported by several studies reporting reduced incidence rates of cognitive impairment and dementia in older adults with a high educational attainment [2, 40–44]. Additionally, an analysis from Barnes and Yaffe [10] indicates that the relative risk of developing dementia (the ratio of the probability of developing dementia in an at-risk group compared with a no-risk group) in people with a low education (primary education only) is 59% higher than in people with a higher education.
However, older adults experiencing a cognitive decline have obviously already completed their formal and occupational education. The cognitive reserve, however, is not influenced by education alone. Retrospective studies indicate that significant associations exist between cognitively activating leisure activities, engagement in social activities and the level of cognitive performance, and the risk of dementia [46–48]. Moreover, studies show that performing cognitively stimulating leisure activities later in life may somewhat compensate for a low educational attainment [49, 50]. Thus, cognitive reserve is not a static condition, but can be influenced and enhanced at any point in someone’s lifetime
The great diversity of cognitive tasks and the variability in the durations of exposure to cognitive tasks in different studies investigating the association between cognitive activity and risk of dementia meant that some studies also had inconclusive findings (see for example [51]). Nevertheless, there is substantial evidence that participating in cognitive activities conveys beneficial effects for the maintenance of brain health and may delay cognitive decline [47, 48]. Various experimental studies on rodents [51, 52] and imaging studies on humans [53–55] suggest that a mentally stimulating environment promotes neurotrophic changes in the hippocampal formation, neurogenesis and synaptic density. For example, a study by Lazarov et al. [56] with transgenic mice corroborated the idea that a cognitively stimulating environment (housing in a cave with running wheels, colourful tunnels and assorted toys) results in an increased clearance of amyloid-β deposits compared with mice held in standard housing. Additionally, an imaging study by Valenzuela et al. [53] with healthy older human adults showed that participants with high levels of mental activity (in the domains of education, occupation, creative arts, reading, writing, socialising and day-to-day habits) across their lifespan (young adulthood, middle age and late life) exhibited a reduced rate of hippocampal atrophy compared with those with low levels.
A Cochrane review [57] examining the effect of cognitive training (the structured practice of tasks targeting specific domains of cognitive functioning) with 36 included studies involving healthy older participants or people with mild cognitive impairment provided evidence for an improvement in immediate and delayed verbal recall compared with participants without training. However, this positive effect did not exceed the improvements in active control groups receiving “only” nonspecific cognitive stimulation, such as reading, playing board games or dancing, which may significantly reduce the risk for cognitive decline or dementia (see also for example [48, 49, 58]). Moreover, various studies revealed that musical activities (playing an instrument or singing) enhances performance in different cognitive domains (attention, executive functions) by promoting neural plasticity and increasing grey matter volume in frontal, motor, parietal and temporal (e.g., hippocampal) areas [54, 55, 58–60].
In summary, there is hopeful evidence that nonpharmacological interventions in stages with no or very little cognitive impairment may be effective in delaying (further) cognitive decline. Optimally, such a programme should address all possible preventive aspects: dietary habits, physical activity, intake of toxic substances (smoking behaviour and alcohol consumption) and cognitive activity.
Prevention studies with a multidimensional approach
Although observational studies confirm the association between the modifiable risk factors mentioned and Alzheimer’s disease, results from intervention studies investigating the effect of these factors in delaying the onset of cognitive decline or Alzheimer’s disease are mixed. These inconsistent findings may have resulted from various methodological problems including small samples, short intervention periods with short follow-up, inappropriate timing (too late to obtain a significant intervention effect [24]) or a mono-interventional approach investigating only one risk factor [19]. However, as the underlying pathology of Alzheimer’s disease is multifactorial, measures addressing multiple target areas aiming to modify vascular and lifestyle factors simultaneously seem more appropriate. To date, there are three large ongoing European intervention studies targeting simultaneously multiple risk factors: the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER [61, 62], the Prevention of Dementia by Intensive Vascular Care (PreDIVA [63, 64], study and the study Multidomain Alzheimer Preventive Trial (MAPT [65–67]). Until now, only preliminary results from these studies have been published. In 2011, the European Dementia Prevention Initiative (EDPI; http://www.edpi.org/) was launched to combine the valuable information collected in the three ongoing European trials mentioned above. The aim of this initiative is to improve and promote the collaboration between researchers involved in the field of dementia prevention to combine experience and datasets, and better to define target populations, intervention strategies and methodological challenges in large dementia prevention trials [24]. Based on the studies mentioned above, an innovative and interactive Internet intervention platform for the treatment of cardiovascular disease in older people, called Healthy Ageing Through Internet Counselling in the Elderly (HATICE), was initiated as an additional project by the members of the EDPI (http://www.hatice.eu/). An additional initiative from Switzerland named “EviPrev” is based on current scientific data and focuses on evidence-based prevention and early detection of potentially chronic diseases (short interventions on physical activity, nutrition, smoking, alcohol consumption and screening for high blood pressure, dyslipidaemia, diabetes, breast cancer, colon cancer, and others) in the primary healthcare setting. Although this project is not considered to be a prevention study with a multidimensional approach, it has an important impact on cognitive health by systematically recording cardiovascular risk factors. A systematic assessment of these risk factors, which significantly affect cognitive health, is crucial for effective treatments and successful lifestyle interventions [68, 69].
Health Coaching: a multidimensional counselling programme to promote health behaviour in the primary care setting
Based on the findings summarised so far, an additional multidimensional and structured health programme of counselling in primary care practice called “Health Coaching” was developed by the Swiss College of Primary Care Medicine (http://www.gesundheitscoaching-khm.ch/ [39]). Designed for general practitioners (GPs), the primary aim of this programme is to promote health behaviour in the Swiss population and prevent chronic diseases (e.g., stroke, coronary heart disease, cardiovascular disease) by targeting the most important contributors to disease burden including smoking, alcohol consumption, body weight, dietary habits, level of physical activity, and coping strategies with stress [39, 70]. The aim is to give GPs the tools to motivate their patients and support them during the implementation of a healthier lifestyle. Patients are involved in the decision process and share the responsibility for their health with the practitioner. Thus, an important aspect of Health Coaching is that patient and health professional meet on equal terms and jointly plan a step-by-step health programme based on the patient’s individual preferences and abilities [39]. The programme also emphasises the high importance of the GPs’ communication skills and offers specific training programmes in “motivational interviewing” (see below) within this programme [71]. In a nonrandomised study, the programme showed high acceptability, feasibility and improvements in health-related behaviour [39]. Although Health Coaching as a nonpharmacological programme is quite comprehensive and probably sufficient for individuals who are still in the workforce, it could benefit from an additional module focusing on cognitive activity for patients who are beyond retirement or who exhibit subtle cognitive problems.
BrainCoach: a programme to promote cognitive activity
Cognitive activity
Based on scientific data reviewed above we have developed a cognitive activity module to complement the Health Coaching programme. This programme, named “BrainCoach”, was specifically created for older adults at risk for cognitive impairment who might be, for example, in the so-called “silent phase” of Alzheimer’s disease [22]. It is meant to be implemented in the primary care setting and to be conducted by the GPs and other healthcare professionals (e.g., psychologists). The primary purpose of this module is – in accordance with the theory of cognitive reserve described above and the rule of “Use it or lose it!” – to support older adults and promote their motivation to maintain and increase brain health by increasing their cognitive activity. The programme addresses especially older adults feeling cognitively “bored” in everyday life, individuals shortly before retirement or individuals with subtle cognitive alterations. The promotion of the activity will be achieved by use of a specific folder (A4-format) including information about cognitive activity and its effects on brain health, communication skills (motivational interviewing) and a structured questionnaire as a guideline (working sheet) to evaluating the patients’ current cognitive activity and increase their motivation to find and implement a cognitive activity in their daily life. The BrainCoach intentionally does not include specific cognitive exercises, but highlights the importance of eliciting the patients’ motivation to find a cognitive activity that they like to perform regularly (e.g., something they performed earlier in their life). In case the patients cannot think of any cognitive activity they would like to engage in, the “BrainCoach” module offers a range of different cognitive activities – a “cognitive buffet” (different activities depicted in colour photographs) – from which the patients may choose the ones they loved to perform. Possible activities include those that can be performed individually, such as doing artwork, solving crossword puzzles, singing, playing a musical instrument, or reading books/newspapers. Activities carried out in groups have an additional stimulating component, especially due to the need of social interactions. Examples of these kinds of activities include language courses, dancing classes, singing in a choir, reading circles, attending university for seniors, playing board games, or attending cultural events with friends (e.g., theatre, cinema, and concerts). This list included in the BrainCoach programme cannot be exhaustive, since a variety of additional activities may positively affect brain health. The patients are free to choose something from this list or to engage in another cognitively stimulating activity. The BrainCoach programme is cost-free; however, costs associated with the chosen activities have to be covered by the patients.
Motivational interviewing
Mathematically speaking, it would make the most sense to especially inspire patients with a low cognitive reserve to (newly) engage in cognitive activities, since these individuals have the largest room for improvement (see fig. 3). Thus, the technique used to motivate patients will be critical. The main technique used in the module BrainCoach, analogous to Health Coaching, is motivational interviewing [72], a well-known client-centred and collaborative counselling technique that has frequently and successfully been applied in general healthcare settings and health promotion [73]. Briefly, eliciting people’s intrinsic motivation appears to be a key factor to achieve long-lasting behavioural change [74–76]. For example, in BrainCoach a cognitive activity is chosen from the cognitive buffet because of the patient’s personal interest in the activity. Its motivation is more likely to be intrinsic, because the patient probably chose this activity for being enjoyable, pleasurable, or giving satisfaction. Importantly, because patient and health practitioner work together at ground level to identify a suitable cognitive activity, engagement in this activity is not extrinsically motivated: the patient does not engage in this activity to receive a reward, or because of feeling obliged. Importantly, motivational interviewing is based on the principles of a good and equal partnership/collaboration between the GP / healthcare professional and the client, the evocation of a client’s personal values and intrinsic motivation, and the client’s autonomy to decide what to do (or not). Motivational interviewing can be used in a brief intervention format, making it suitable for the primary care setting. As motivating and supporting patients to make health-related behavioural changes represent a challenge for GPs, profound communication skills are crucial for successful counselling. Within BrainCoach, a training programme for GPs in motivational interviewing will be developed.
The BrainCoach module, including the folder, the “cognitive buffet” and a structured questionnaire (worksheet) based on the communication skills included in motivational interviewing is currently being tested in a pilot study (feasibility, acceptance of the documents and the concept), which will be finalised and evaluated by March 2017. Its acceptance and feasibility by the patients and the impact on cognition and dementia incidence will be evaluated more extensively afterwards, in a comprehensive study with an adequate and robust study design.
Conclusions
Because of demographic change, dementia represents a major healthcare issue for our society. Although the underlying pathogenesis of Alzheimer’s disease is not fully understood, a number of observational studies provide strong evidence for an adverse effect of multiple cardiovascular and lifestyle risk factors [12]. In this regard, prevention strategies are needed to manage and lower the increase of dementia cases influenced by these risk factors. Delaying cognitive decline and dementia would have a huge impact on its incidence and prevalence. Specifically, a 10–20% reduction of the seven main risk factors for Alzheimer’s disease (diabetes, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, low educational attainment) would decrease Alzheimer’s disease prevalence by 8–15% by 2050 [11]. Additionally, estimates from a projection model imply that interventions with the potential to delay disease onset or progression by only 1 year would reduce the number of Alzheimer’s disease patients by about 11% (9 million cases [76]). Understanding the contribution and the impact of different lifestyle factors on disease development will have an important influence on future disease management and treatment since many of these risk factors are modifiable. Thus, prevention programmes and strategies targeting the modification of these factors seem a viable and reasonable approach to mitigate and delay cognitive decline and dementia [13]. Various factors including a healthy diet, smoking cessation, a physically active lifestyle and cognitive activity have been proposed to exert protective effects on both physical and mental health. The Health Coaching programme developed by the Swiss College of Primary Care Medicine represents a comprehensive concept simultaneously targeting multiple risk factors. However, scientific evidence supports the importance of cognitive activity to maintain or increase cognitive reserve. Therefore, the module BrainCoach may represent a promising tool. Motivational interviewing as an empathetic, nonconfrontational and collaborative communication method has been shown to be effective for long-term behavioural change in several areas. The Health Coaching programme together with the BrainCoach, using the motivation interviewing counselling technique, could be a valuable, effective and low-cost approach to maintain both, physical and mental health. These programmes have important and promising implications for preventive intervention against cognitive decline and dementia.
Andreas U. Monsch, Memory Clinic, University Centre for Medicine of Aging Basel, Felix Platter Hospital, Burgfelderstrasse 101, CH-4012 Basel, Andreas.Monsch[at]unibas.ch
References
1Alzheimer’s Disease International. World Alzheimer Report 2015. The Global Impact of Dementia. [Internet]. 2015. Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf
2
Satizabal
CL
Beiser
AS
Chouraki
V
Chêne
G
Dufouil
C
Seshadri
S
. Incidence of Dementia over Three Decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523–32. doi:.https://doi.org/10.1056/NEJMoa1504327
3
Matthews
FE
Stephan
BCM
Robinson
L
Jagger
C
Barnes
LE
Arthur
A
Cognitive Function and Ageing Studies (CFAS) Collaboration
. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun. 2016;7:11398. doi:.https://doi.org/10.1038/ncomms11398
4Alzheimervereinigung S. Zahlen und Fakten zur Demenz [Internet]. 2015. Available from: http://www.alz.ch/index.php/zahlen-zur-demenz.html
5
Kraft
E
Marti
M
Werner
S
Sommer
H
. Cost of dementia in Switzerland. Swiss Med Wkly. 2010;140:w13093.
6
McKhann
GM
Knopman
DS
Chertkow
H
Hyman
BT
Jack
CR
Jr
Kawas
CH
The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. doi:.https://doi.org/10.1016/j.jalz.2011.03.005
7
Salmon
DP
. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. Curr Top Behav Neurosci. 2012;10:187–212.
8
Braak
H
Braak
E
. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. doi:.https://doi.org/10.1007/BF00308809
9
Tiraboschi
P
Hansen
LA
Thal
LJ
Corey-Bloom
J
. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62(11):1984–9. doi:.https://doi.org/10.1212/01.WNL.0000129697.01779.0A
10
Barnes
DE
Yaffe
K
. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–28. doi:.https://doi.org/10.1016/S1474-4422(11)70072-2
11
Norton
S
Matthews
FE
Barnes
DE
Yaffe
K
Brayne
C
. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–94. doi:.https://doi.org/10.1016/S1474-4422(14)70136-X
12
Reitz
C
Brayne
C
Mayeux
R
. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–52. doi:.https://doi.org/10.1038/nrneurol.2011.2
13
Xu
W
Tan
L
Wang
H-F
Jiang
T
Tan
M-S
Tan
L
Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015;86(12):1299–306.
14
Braak
H
Braak
E
. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–7. doi:.https://doi.org/10.1016/S0197-4580(97)00056-0
15
Corder
EH
Saunders
AM
Strittmatter
WJ
Schmechel
DE
Gaskell
PC
Small
GW
Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–3. doi:.https://doi.org/10.1126/science.8346443
16
Farrer
LA
Cupples
LA
Haines
JL
Hyman
B
Kukull
WA
Mayeux
R
APOE and Alzheimer Disease Meta Analysis Consortium
. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. JAMA. 1997;278(16):1349–56. doi:.https://doi.org/10.1001/jama.1997.03550160069041
17
Fratiglioni
L
Paillard-Borg
S
Winblad
B
. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–53. doi:.https://doi.org/10.1016/S1474-4422(04)00767-7
18
Fratiglioni
L
Winblad
B
von Strauss
E
. Prevention of Alzheimer’s disease and dementia. Major findings from the Kungsholmen Project. Physiol Behav. 2007;92(1-2):98–104. doi:.https://doi.org/10.1016/j.physbeh.2007.05.059
19Mangialasche F, Xu W, Kivipelto M. Prevention of Alzheimer’s Disease: Intervention Studies. In: Zerr I, editor. Understanding Alzheimer’s Disease [Internet]. InTech; 2013 [cited 2015 Dec 21]. Available from: http://www.intechopen.com/books/understanding-alzheimer-s-disease/prevention-of-alzheimer-s-disease-intervention-studies
20
Andrieu
S
Coley
N
Lovestone
S
Aisen
PS
Vellas
B
. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14(9):926–44. doi:.https://doi.org/10.1016/S1474-4422(15)00153-2
21
Barnett
J
Bahar-Fuchs
A
Cherbuin
N
Herath
P
Anstey
K
. Interventions to prevent cognitive decline and dementia in adults without cognitive impairment: a systematic review. J Prev Alzheimers Dis. 2015;2:38–45. doi:.https://doi.org/10.14283/jpad.2015.36
22
Dubois
B
Hampel
H
Feldman
HH
Scheltens
P
Aisen
P
Andrieu
S
Proceedings of the Meeting of the International Working Group (IWG) and the American Alzheimer’s Association on “The Preclinical State of AD”; July 23, 2015; Washington DC, USA
. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12(3):292–323. doi:.https://doi.org/10.1016/j.jalz.2016.02.002
23Alzheimer’s Disease International. World Alzheimer Report 2014. Dementia and risk reduction - An analysis of protective and modifiable factors. London: Alzheimer’s Disease International; 2014.
24
Richard
E
Andrieu
S
Solomon
A
Mangialasche
F
Ahtiluoto
S
van Charante
EPM
Methodological challenges in designing dementia prevention trials - the European Dementia Prevention Initiative (EDPI). J Neurol Sci. 2012;322(1-2):64–70. doi:.https://doi.org/10.1016/j.jns.2012.06.012
25
Solomon
A
Mangialasche
F
Richard
E
Andrieu
S
Bennett
DA
Breteler
M
Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med. 2014;275(3):229–50. doi:.https://doi.org/10.1111/joim.12178
26American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association Press; 2013.
27
Biessels
GJ
. Capitalising on modifiable risk factors for Alzheimer’s disease. Lancet Neurol. 2014;13(8):752–3. doi:.https://doi.org/10.1016/S1474-4422(14)70154-1
28
Morris
MC
Tangney
CC
Wang
Y
Sacks
FM
Bennett
DA
Aggarwal
NT
. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–14. doi:.https://doi.org/10.1016/j.jalz.2014.11.009
29
Scarmeas
N
Luchsinger
JA
Schupf
N
Brickman
AM
Cosentino
S
Tang
MX
Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–37. doi:.https://doi.org/10.1001/jama.2009.1144
30
Scarmeas
N
Stern
Y
Tang
M-X
Mayeux
R
Luchsinger
JA
. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006;59(6):912–21. doi:.https://doi.org/10.1002/ana.20854
31
Gillette-Guyonnet
S
Secher
M
Vellas
B
. Nutrition and neurodegeneration: epidemiological evidence and challenges for future research. Br J Clin Pharmacol. 2013;75(3):738–55. doi:.https://doi.org/10.1111/bcp.12058
32
Canevelli
M
Lucchini
F
Quarata
F
Bruno
G
Cesari
M
. Nutrition and Dementia: Evidence for Preventive Approaches?
Nutrients. 2016;8(3):144. doi:.https://doi.org/10.3390/nu8030144
33
Hayes
SM
Alosco
ML
Hayes
JP
Cadden
M
Peterson
KM
Allsup
K
Physical Activity Is Positively Associated with Episodic Memory in Aging. J Int Neuropsychol Soc. 2015;21(10):780–90. doi:.https://doi.org/10.1017/S1355617715000910
34
Lautenschlager
NT
Cox
KL
Flicker
L
Foster
JK
van Bockxmeer
FM
Xiao
J
Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–37. doi:.https://doi.org/10.1001/jama.300.9.1027
35
Rockwood
K
Middleton
L
. Physical activity and the maintenance of cognitive function. Alzheimers Dement. 2007;3(2, Suppl):S38–44. doi:.https://doi.org/10.1016/j.jalz.2007.01.003
36
Rovio
S
Kåreholt
I
Helkala
E-L
Viitanen
M
Winblad
B
Tuomilehto
J
Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 2005;4(11):705–11. doi:.https://doi.org/10.1016/S1474-4422(05)70198-8
37
Best
JR
Chiu
BK
Liang Hsu
C
Nagamatsu
LS
Liu-Ambrose
T
. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J Int Neuropsychol Soc. 2015;21(10):745–56. doi:.https://doi.org/10.1017/S1355617715000673
38
Erickson
KI
Voss
MW
Prakash
RS
Basak
C
Szabo
A
Chaddock
L
Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–22. doi:.https://doi.org/10.1073/pnas.1015950108
39
Neuner-Jehle
S
Schmid
M
Grüninger
U
. The “Health Coaching” programme: a new patient-centred and visually supported approach for health behaviour change in primary care. BMC Fam Pract. 2013;14(1):100. doi:.https://doi.org/10.1186/1471-2296-14-100
40
Katzman
R
Terry
R
DeTeresa
R
Brown
T
Davies
P
Fuld
P
Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23(2):138–44. doi:.https://doi.org/10.1002/ana.410230206
41
Andel
R
Vigen
C
Mack
WJ
Clark
LJ
Gatz
M
. The effect of education and occupational complexity on rate of cognitive decline in Alzheimer’s patients. J Int Neuropsychol Soc. 2006;12(1):147–52. doi:.https://doi.org/10.1017/S1355617706060206
42
Scarmeas
N
Albert
SM
Manly
JJ
Stern
Y
. Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2006;77(3):308–16. doi:.https://doi.org/10.1136/jnnp.2005.072306
43
Stern
Y
. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–12. doi:.https://doi.org/10.1016/S1474-4422(12)70191-6
44
Langa
KM
Larson
EB
Crimmins
EM
Faul
JD
Levine
DA
Kabeto
MU
A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2016 Nov 21. [Epub ahead of print] doi:10.1001/jamainternmed.2016.6807.https://doi.org/http://dx.doi.org.
45
Stern
Y
. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–28. doi:.https://doi.org/10.1016/j.neuropsychologia.2009.03.004
46
Sattler
C
Toro
P
Schönknecht
P
Schröder
J
. Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2012;196(1):90–5. doi:.https://doi.org/10.1016/j.psychres.2011.11.012
47
Akbaraly
TN
Portet
F
Fustinoni
S
Dartigues
J-F
Artero
S
Rouaud
O
Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854–61. doi:.https://doi.org/10.1212/WNL.0b013e3181b7849b
48
Verghese
J
Lipton
RB
Katz
MJ
Hall
CB
Derby
CA
Kuslansky
G
Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–16. doi:.https://doi.org/10.1056/NEJMoa022252
49
Lachman
ME
Agrigoroaei
S
Murphy
C
Tun
PA
. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 2010;18(1):4–10. doi:.https://doi.org/10.1097/JGP.0b013e3181ab8b62
50
Reed
BR
Dowling
M
Tomaszewski Farias
S
Sonnen
J
Strauss
M
Schneider
JA
Cognitive activities during adulthood are more important than education in building reserve. J Int Neuropsychol Soc. 2011;17(4):615–24. doi:.https://doi.org/10.1017/S1355617711000014
51
Brown
J
Cooper-Kuhn
CM
Kempermann
G
Van Praag
H
Winkler
J
Gage
FH
Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–6. doi:.https://doi.org/10.1046/j.1460-9568.2003.02647.x
52
Kempermann
G
Kuhn
HG
Gage
FH
. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–5. doi:.https://doi.org/10.1038/386493a0
53
Valenzuela
MJ
Sachdev
P
Wen
W
Chen
X
Brodaty
H
. Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One. 2008;3(7):e2598. doi:.https://doi.org/10.1371/journal.pone.0002598
54
Belleville
S
Mellah
S
de Boysson
C
Demonet
JF
Bier
B
. The pattern and loci of training-induced brain changes in healthy older adults are predicted by the nature of the intervention. PLoS One. 2014;9(8):e102710. doi:.https://doi.org/10.1371/journal.pone.0102710
55
Barrett
KC
Ashley
R
Strait
DL
Kraus
N
. Art and science: how musical training shapes the brain. Front Psychol. 2013;4:713. doi:.https://doi.org/10.3389/fpsyg.2013.00713
56
Lazarov
O
Robinson
J
Tang
Y-P
Hairston
IS
Korade-Mirnics
Z
Lee
VM-Y
Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120(5):701–13. doi:.https://doi.org/10.1016/j.cell.2005.01.015
57
Martin
M
Clare
L
Altgassen
AM
Cameron
MH
Zehnder
F
. Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Syst Rev. 2011;(1):CD006220.
58
Bugos
JA
Perlstein
WM
McCrae
CS
Brophy
TS
Bedenbaugh
PH
. Individualized piano instruction enhances executive functioning and working memory in older adults. Aging Ment Health. 2007;11(4):464–71. doi:.https://doi.org/10.1080/13607860601086504
59
Gaser
C
Schlaug
G
. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23(27):9240–5.
60
Fotuhi
M
Do
D
Jack
C
. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8(4):189–202.
61
Kivipelto
M
Ngandu
T
Laatikainen
T
Winblad
B
Soininen
H
Tuomilehto
J
. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–41. doi:.https://doi.org/10.1016/S1474-4422(06)70537-3
62
Ngandu
T
Lehtisalo
J
Solomon
A
Levälahti
E
Ahtiluoto
S
Antikainen
R
A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. doi:.https://doi.org/10.1016/S0140-6736(15)60461-5
63
Ligthart
SA
van den Eerenbeemt
KD
Pols
J
van Bussel
EF
Richard
E
van Charante
EPM
. Perspectives of older people engaging in nurse-led cardiovascular prevention programmes: a qualitative study in primary care in the Netherlands. Br J Gen Pract. 2015;65(630):e41–8. doi:.https://doi.org/10.3399/bjgp15X683149
64
Richard
E
Van den Heuvel
E
van Charante
EPM
Achthoven
L
Vermeulen
M
Bindels
PJ
Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord. 2009;23(3):198–204. doi:.https://doi.org/10.1097/WAD.0b013e31819783a4
65
Gillette-Guyonnet
S
Andrieu
S
Dantoine
T
Dartigues
J-F
Touchon
J
Vellas
B
MAPT Study Group
. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer’s disease. Alzheimers Dement. 2009;5(2):114–21. doi:.https://doi.org/10.1016/j.jalz.2009.01.008
66
Lilamand
M
Cesari
M
del Campo
N
Cantet
C
Soto
M
Ousset
P-J
MAPT Study Group
. Brain Amyloid Deposition Is Associated With Lower Instrumental Activities of Daily Living Abilities in Older Adults. Results From the MAPT Study. J Gerontol A Biol Sci Med Sci. 2016;71(3):391–7. doi:.https://doi.org/10.1093/gerona/glv155
67
Vellas
B
Carrie
I
Gillette-Guyonnet
S
Touchon
J
Dantoine
T
Dartigues
JF
MAPT Study: a multidomain approach for preventing Alzheimer’s disease: design and baseline data. J Prev Alzheimers Dis. 2014;1(1):13–22.
68
Cornuz
J
Rodondi
N
Ospelt
R
Zoller
M
Durrer
D
Tschirky
E
EviPrev, ein Programm zu Prävention und Gesundheitsförderung in der Hausarztpraxis. Schweiz Ärztezeitung. 2010;91:60–3. Article in German.
69
Cornuz
J
Auer
R
Neuner-Jehle
S
Humair
J-P
Jacot-Sadowski
I
Cardinaux
R
Schweizer Empfehlungen für den Check-up in der Arztpraxis. Swiss Med Forum. 2015;15(43):974–80. Article in German.
70
Neuner-Jehle
S
Schmid
M
Grüninger
U
. Kurzberatung in der Arztpraxis zur Verbesserung des Gesundheitsverhaltens: Probleme und Lösungen. Praxis (Bern). 2014;103(5):271–7. Article in German. doi:.https://doi.org/10.1024/1661-8157/a001572
71
Neuner-Jehle
S
Grüninger
U
Schmid
M
. Efficacy of a communication skill training fostering health promotion in primary care: a mixed method analysis. J Community Med Health Educ. 2016;6(2):1000413.
72Rollnick S, Miller W, Butler C. Motivational Interviewing in Health Care. New York: The Guilford Press; 2008.
73
Söderlund
LL
Madson
MB
Rubak
S
Nilsen
P
. A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns. 2011;84(1):16–26. doi:.https://doi.org/10.1016/j.pec.2010.06.025
74
Ng
JYY
Ntoumanis
N
Thøgersen-Ntoumani
C
Deci
EL
Ryan
RM
Duda
JL
Self-Determination Theory Applied to Health Contexts: A Meta-Analysis. Perspect Psychol Sci. 2012;7(4):325–40. doi:.https://doi.org/10.1177/1745691612447309
75Patrick H, Resnicow K, Teixeira PJ, Williams GC. Communication skills to elicit physical activity behavior change: How to talk to the client. In Nigg CR (editor). ACSM’s Behavioral Aspects of Physical Activity and Exercise. Philadelphia: Lippincott Williams & Wilkins; 2013. pp 129–52).
76
Teixeira
PJ
Silva
MN
Mata
J
Palmeira
AL
Markland
D
. Motivation, self-determination, and long-term weight control. Int J Behav Nutr Phys Act. 2012;9(1):22. doi:.https://doi.org/10.1186/1479-5868-9-22


