The neuronal correlates of mirror illusion in children with spastic hemiparesis: a study with functional magnetic resonance imaging
DOI: https://doi.org/10.4414/smw.2017.14415
Christian
Weisstannera, Stefanie
Saxerbc, Roland
Wiesta, Alain
Kaelin-Langd, Christopher J
Newmane, Maja
Steinlinb, Sebastian
Gruntb
aSupport Centre for Advanced Neuroimaging (SCAN), University Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University of Berne,
bDepartment of Neuropaediatrics, Development and Rehabilitation, University Children’s Hospital, Inselspital, University of Bern,
cInstitute of Human Movement Sciences and Sport, ETH Zürich,
dNeurocentre of Southern Switzerland, Lugano,
ePaediatric Neurology and Neurorehabilitation Unit, Lausanne University Hospital,
The neuronal correlates of mirror illusion in children with spastic hemiparesis: a study with functional magnetic resonance imaging
Summary
AIM
To investigate the neuronal activation pattern underlying the effects of mirror illusion in children/adolescents with normal motor development and in children/adolescents with hemiparesis and preserved contralateral corticospinal organisation.
METHOD
The type of cortical reorganisation was classified according to results of transcranial magnetic stimulation. Only subjects with congenital lesions and physiological contralateral cortical reorganisation were included. Functional magnetic resonance imaging was performed to investigate neuronal activation patterns with and without a mirror box. Each test consisted of a unimanual and a bimanual motor task.
RESULTS
Seven children/adolescents with congenital hemiparesis (10–20 years old, three boys and four girls) and seven healthy subjects (8–17 years old, four boys and three girls) participated in this study. In the bimanual experiment, children with hemiparesis showed a significant effect of the mirror illusion (p<0.001 at voxel level, family-wise error corrected at cluster level) in the dorsolateral prefrontal cortex and anterior cingulate cortex of the affected and unaffected hemispheres, respectively. No significant effects of the mirror illusion were observed in unimanual experiments and in healthy participants.
INTERPRETATION
Mirror illusion in children/adolescents with hemiparesis leads to activation of brain areas involved in visual conflict detection and cognitive control to resolve this conflict. This effect is observed only in bimanual training. We consider that for mirror therapy in children and adolescents with hemiparesis a bimanual approach is more suitable than a unimanual approach.
Introduction
Ischaemic or haemorrhagic stroke, brain tumours, traumatic brain injury and congenital brain lesions/malformations are the most common causes of spastic hemiparesis in children. Children diagnosed with hemiparesis suffer from abnormalities of posture, tone and gait, and impaired hand function. Motor cortical representation of the affected hand can be observed in the contralateral hemisphere (contralateral organisation), the ipsilateral hemisphere (ipsilateral organisation) or in both hemispheres (mixed organisation) [1–3]. The type of cortical organisation depends on the timing, extent and location of brain lesions [3]. The impaired upper limb function results in difficulties with reaching, grasping, releasing and manipulating objects. It can impair self-care and restrict participation in school, leisure activities and professional education. Rehabilitation treatments aim to improve upper limb function and enhance participation. Different treatment strategies have been introduced to improve the effective use of the arm in children diagnosed with hemiparesis.
Mirror therapy is a rehabilitation strategy that provides a visual illusion of a functional paretic limb by using the mirror reflection of the nonparetic limb. During mirror therapy, a mirror is placed in the patient’s mid-sagittal plane between both hands so that the mirror reflection of the unaffected limb is superimposed on the affected limb. This provides the visual illusion of a normally moving affected limb. Mirror therapy was first described in 1995 for treatment of phantom limb pain after arm amputation [4]. Other studies investigated the effect of mirror therapy in adults with hemiparesis after stroke [5–10]. A systematic review concluded that mirror therapy is effective for improving motor function and possibly beneficial for daily activities and pain in adult stroke patients [11].
The effect of the mirror illusion on the motor system in upper limb rehabilitation has repeatedly been studied in healthy adults and in adults diagnosed with stroke. Functional magnetic resonance imaging (MRI) studies revealed increased activation in higher order visual regions, parts of the motor system, and areas associated with the awareness of self and spatial motion [12–16]. In adult stroke patients, training with mirror therapy of the upper limbs led to a shift in activation balance between the two M1 areas towards the affected cerebral hemisphere [8] and activation changes in supplementary motor areas [10].
Despite good clinical evidence for mirror therapy in upper limb rehabilitation of adults, there is only limited evidence regarding mirror therapy in children with hemiparesis. Since children have age-dependent mechanisms of neuronal plasticity, adult studies do not allow definite conclusions on the possible effects of the mirror illusion in children. It has been shown that self-rehabilitation with mirror therapy in children with hemiparesis is feasible and well accepted [17–19]. It has also been demonstrated that the mirror illusion in children with hemiparesis leads to a change in muscle activation patterns [20, 21]. A nonblinded pilot study showed that mirror therapy in children might improve strength, dynamic function and matching accuracy of the paretic arm [17]. In contrast, in a larger randomised controlled trial including 90 children with hemiparesis, the use of the mirror illusion during therapy had no significant effect on treatment outcomes [22]. However, this trial did not analyse the neuronal activation pattern during treatment and it remained unclear whether differences in reorganisation pattern and neuroplastic changes might influence the effectiveness of mirror therapy. Therefore, we aimed to investigate in more detail the neuronal mechanisms of the mirror illusion in children and adolescents. In a study using transcranial magnetic stimulation, we have previously shown that, depending on cortical reorganisation after early brain injury, the mirror illusion leads to an increase in cortical excitability [23]. In the present study we aimed to illustrate the neuronal mechanisms of the mirror illusion using functional MRI (fMRI).
Material and methods
This study was approved by the ethics committee of the canton Berne (KEK Nr. 029/12). All the participants and their parents gave their written informed consent.
Subjects
Children and adolescents with hemiparesis, between 8 and 20 years of age, and a convenience sample of typically developing peers were recruited at the Department of Neuropaediatrics, Development and Rehabilitation, University Children’s Hospital, Inselspital, University of Bern, Switzerland. Inclusion criteria for participants with hemiparesis were: hemiparesis due to a congenital nonprogressive brain lesion affecting the upper limb; no spasticity treatment (such as botulinum toxin injections) during the 6 months preceding inclusion; Manual Ability Classification System (MACS) level 1–3 [24]. The inclusion criterion for healthy participants was absence of neurological comorbidity. Exclusion criteria for all participants were: upper limb surgery during the 6 months preceding inclusion; comorbidities in upper limbs that inhibit active opening of the hand; moderate to severe visual disorders, such as hemianopsia or hemineglect; mental age <8 years and/or behavioural comorbidities that do not allow a safe and complete collaboration during the experiments; active epilepsy; pacemaker (for deep brain stimulation or heart disease); history of brain operation/injuries (possible foreign bodies); eye operation or injury with possible metal part in the eye; claustrophobia; metal implants or dental brace.
Transcranial magnetic stimulation
To assess the type of cortical organization transcranial magnetic stimulation (TMS) was performed in all subjects according to a previously described protocol [3]. Participants were seated with their hands in the neutral position with hands and forearms on a table surface. Silver-silver chloride surface electrodes (ALPINE, bioMed) were attached over the abductor pollicis brevis (APB) in a tendon-belly arrangement [25]. A Neurodata Amplifier System connected to an IPS230 Isolated Power System (Grass-Telefactor, Braintree, MA, USA) was used to preamplify electromyogram (EMG) signals. Data were postprocessed by a data acquisition system built with the Labview graphical programming language (sampling rate 5 kHz) [26]. We delivered monophasic TMS pulses at a frequency of 0.2 Hz on the M1 through a custom figure-of-8-shaped coil (diameter 5 cm) connected to a Magstim 200 (Magstim Co. Ltd., Whitland, Wales, UK). After identification of the hot spot [27], resting motor threshold (rMT) [28] of the unaffected APB was assessed. For measuring contralateral motor response, 20 stimulations at intensity of 120% rMT were applied. If stimulation of the hot spot also elicited an ipsilateral motor response, rMT of the ipsilateral APB was measured separately, 20 stimulations were applied and ipsilateral motor evoked potentials recorded at 120% rMT. The hemisphere was then switched and the same procedure repeated. Absence of ipsilateral/contralateral responses in the APB was determined by stimulation intensity up to 100% stimulator output or as high as the subject did not find too uncomfortable. For the present study, we only included patients with preserved contralateral organisation.
Functional magnetic resonance imaging
Imaging was performed on a 3-T MR system (Siemens Magnetom Trio, Erlangen, Germany). For functional imaging, a blood oxygenation level-dependent sequence (BOLD) was used with following parameters: TR 6000 ms; TE 50 ms; matrix size 64; time of acquisition 6:56. For the anatomical reference, a T1 magnetisation-prepared rapid gradient-echo imaging (MP RAGE) was acquired with a TR of 1950 ms, TE 2.2 ms, TI 900 ms, matrix size 256 and time of acquisition 4:33. For analysis, we performed slice time correction.
The acquired imaging data were analysed with SPM8 (statistical parametric mapping, Wellcome Departement of Cognitive Neurology, University of College London, UK), implemented in Matlab R2012a. First, on single-subject level, all functional images were slice time corrected, realigned to the first volume of the functional imaging series, co-registered to the subjects’ anatomical images. Secondly the functional images were normalised into standard space defined by the Montreal Neurological Institute template (MNI template) and smoothed with a Gaussian kernel (full-width at half maximum of 8 mm). For calculation of statistical parametric maps, we used the general linear model; the block design was convoluted with haemodynamic response function. Realignment parameters were modelled into the design matrix as regressors and a high-pass filter at 128 s for model estimation was used to remove low-frequency artefacts. Contrast maps were calculated for each experiment (movement with mirror, movement without mirror, bimanual and unimanual). To prevent image distortion, cost function masking (exclusion of lesioned voxels from the spatial normalisation algorithm) was performed: lesions were manually traced in native space onto the T1 images using MRIcron (http://www.cabiatl.com/mricro/mricron/index.html), which yielded binary lesion maps. Lesion maps and T1 images were then simultaneously spatially normalised to MNI stereotaxic space using the unified segmentation algorithm in SPM8. Global mean and motion outliers of fMRI data were detected with Artifact Detection Toolbox (http://www.nitrc.org/projects/artifact_detect/). A movement threshold of 2 mm was applied, and outliers removed from further analysis.
Experimental paradigm
Participants lay on their back in the scanner with their arms comfortably resting on the scanner table alongside their torso and with their elbows slightly flexed. A mirror was attached to the head coil above the head allowing the participants to view their hands. A customized mirror box was used to induce the mirror illusion (fig. 1). An experimental paradigm based on a previous study [14] was performed. Two separate tests (once with mirror and once without mirror), each consisting of a unimanual and a bimanual task (fig. 2), were completed. Participants either moved the unaffected hand (dominant hand in healthy subjects; unimanual task) or both hands simultaneously (bimanual task). In the mirror test, the mirror reflection of the unaffected (dominant) hand was observed. In the non-mirror test, the unaffected hand (dominant; unimanual task) or the affected hand (nondominant; bimanual task) was observed. For each of the four tasks a separate fMRI sequence was acquired. The four tasks were performed in random order. The onset and instructions for the subsequent part were communicated verbally through headphones. All four parts had a block design (alternating 30 s periods of active and inactive blocks). During active blocks participants opened and closed their hands, whereas during inactive blocks (rest) participants held their hands still. An auditory metronome (1 Hz cadence, 500 ms duration; 880 Hz / Audacity 2.0.0, Linux) was used to pace movements. Subjects were instructed to pace one complete open-close cycle of the hand to each beat of the metronome. Hand movements were observed and counted in the scanner by an examiner to ensure an approximately identical number in each different part. During inactive blocks a different tone was provided by the auditory metronome (440 Hz). Before the actual experiment within the scanner, all participants practiced the different tasks outside the scanner with the same mirror box to ensure that they fully understood the instructions.
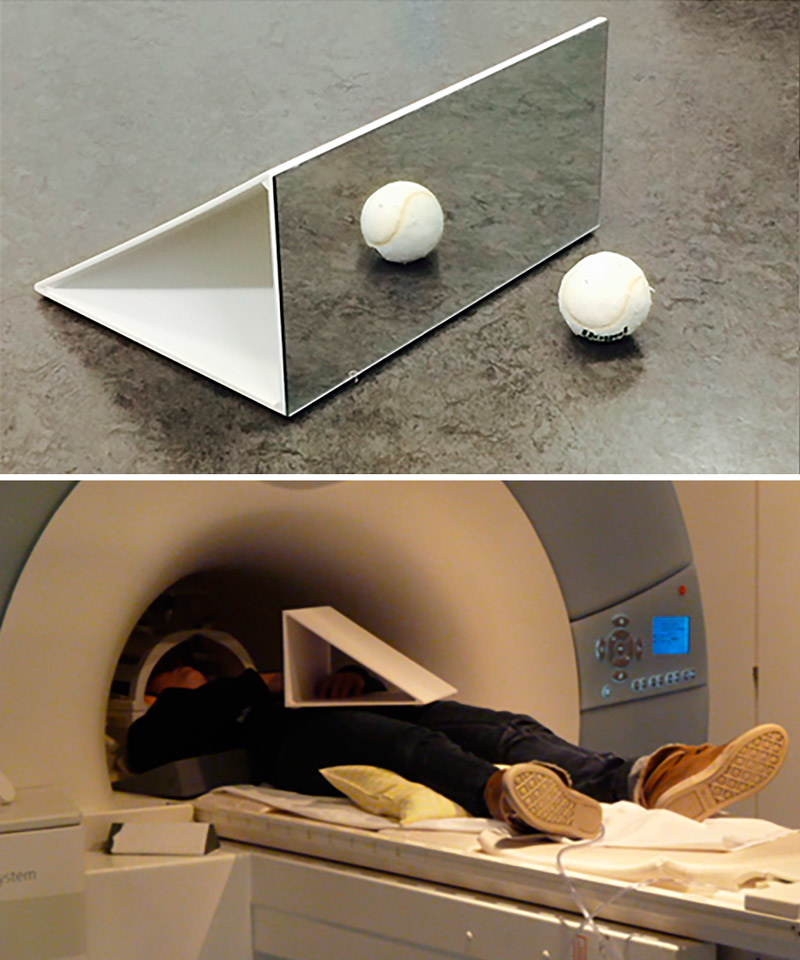
Figure 1 The experimental setting. A customised mirror box made out of plexiglas and wood was used for the experiment (left panel). Patients lay on their back in the scanner with their arms comfortably resting on the scanner table alongside their torso and with their elbows slightly flexed. A mirror was attached to the head coil above the head allowing the participants to view their hands (right panel).

Figure 2 The mirror paradigm used in the study. The paretic/nondominant is illustrated in blue. The arrow illustrates the gaze direction. The dotted line represents the mirror. The movement task consisted in a paced hand opening and closing. Participants either moved the unaffected/dominant hand (unimanual task, figs 2c and 2a) or both hands simultaneously (bimanual task, figs 2b and 2d). With the mirror, the mirror reflection of the unaffected/dominant hand was observed (figs 2a and 2b). Without the mirror, the unaffected/dominant hand (unimanual task, fig. 2a) or the affected/nondominant hand (bimanual task, fig. 2b) were observed.
Statistical analysis
EMG signals were stored for offline-analysis. A “playback” Labview program [26] was used to review waveforms of all measurements. Study participants were classified into contralateral and ipsilateral/mixed cortical organisation. Participants with ipsilateral/mixed cortical organisation (n = 3) were excluded from further data analysis.
Before second-level group analysis, contrast maps of patients with right-sided lesions and of right-handed controls were flipped about the midsagittal plane so that the lesioned side would be the same for all patients and handedness for controls. The individual contrast maps obtained from single-subject analysis were used for a t-Test. A one-sample t-Test was used to assess for group effects of movement, for this both unimanual and both bimanual conditions were grouped together. Differences between the mirror and the no-mirror experiments, presumably induced by the mirror reflection of the moving hand, were assessed using a two-sample t-test for bimanual and unimanual experiments separately. For all t-tests, a primary threshold of p<0.001 at voxel level and a cluster-extent threshold at family-wise error rate (FWER) for multiple comparison correction was used. Age and MACS were used as covariates for patients, age for controls.
Results
Participants
Twelve children and adolescents with hemiparesis and eight healthy participants were recruited for this study. Three participants with hemiparesis were excluded from data analysis because they showed ipsilateral or mixed reorganisation on TMS, constituting a group too small for meaningful analyses. One participant with hemiparesis and one healthy participant were not able to follow appropriately the instructions of the experimental paradigm in the scanner owing to attentional problems and were therefore excluded. One child with arterial ischaemic stroke within the vascular territory of the medial cerebral artery at the age of 5 years was also excluded in order to have a group with only congenital lesions and reduce bias. Thus, for the final data analysis we included seven participants with hemiparesis (three boys and four girls, 10–20 years old, median age 13.5 years, standard deviation [SD] 3.4 years) and seven healthy participants (four boys and three girls, 8–17 years, median age 11 years, SD 3.4 years). For detailed information on all participants, see table 1.
Table 1 Detailed information on all participants.
|
Subject
|
Age
|
Gender
|
Hemi-
paresis
|
Handedness
|
MACS
|
Origin
|
MRI
|
|
Group: healthy control
|
| 1 |
8 |
Male |
NA |
Right |
NA |
NA |
Normal |
| 6 |
15 |
Male |
NA |
Left |
NA |
NA |
Normal |
| 15 |
11 |
Male |
NA |
Right |
NA |
NA |
Normal |
| 16 |
11 |
Male |
NA |
Left |
NA |
NA |
Normal |
| 17 |
16 |
Female |
NA |
Right |
NA |
NA |
Normal |
| 21 |
10 |
Female |
NA |
Left |
NA |
NA |
Normal |
| 22 |
17 |
Female |
NA |
Right |
NA |
NA |
Normal |
|
Group: hemiparesis
|
| 5 |
17 |
Female |
Left |
Right |
1 |
Congenital |
Minor right periventricular defect |
| 7 |
13 |
Male |
Right |
Left |
1 |
Congenital |
Minor left periventricular defect |
| 13 |
17 |
Female |
Right |
Left |
1 |
Congenital |
Minor left periventricular defect |
| 14 |
20 |
Male |
Left |
Right |
1 |
Congenital |
Right periventricular defect |
| 18 |
11 |
Female |
Right |
Left |
2 |
Congenital |
Left opercular, insular defect |
| 19 |
14 |
Male |
Right |
Left |
1 |
Congenital |
Left periventricular defect |
| 20 |
11 |
Female |
Left |
Right |
1 |
Congenital |
Right periventricular defect |
| MACS= manual ability classification system; MRI = magnetic resonance imaging; NA = not applicable |
Functional magnetic resonance imaging
Observed effects for movement for both bimanual and unimanual motor tasks and effect of mirror are listed separately for healthy participants and patients with contralateral organisation in table 2. Regions were determined with wfu pickatlas v 3.0 (http://www.ansir.wfubmc.edu).
Table 2 Detailed information on all affected regions.
|
Anatomical location
|
Hemisphere
|
Cluster size
|
MNI
|
|
Z
|
x
|
y
|
z
|
|
Areas of activation, healthy participants
|
Areas of activation, unimanual experiment, effect of movement
p<0.001, k at FWER |
| Precentral gyrus (BA4) |
Nondominant |
2039 |
6.28 |
36 |
−27 |
57 |
| Cerebellum, dentate |
Dominant |
1074 |
5.53 |
−18 |
−54 |
−27 |
| Inferior occipital gyrus (BA 19) |
Nondominant |
179 |
4.49 |
48 |
−78 |
-9 |
| Supramarginal gyrus |
Dominant |
205 |
4.25 |
−48 |
−33 |
24 |
| Cerebellum, declive |
Nondominant |
83 |
3.97 |
30 |
−72 |
−27 |
Areas of activation, bimanual experiment, effect of movement
p<0.001, k at FWER |
| Precentral gyrus |
Nondominant |
1134 |
7.37 |
33 |
−24 |
60 |
| Postcentral gyrus (BA3) |
Dominant |
643 |
6.67 |
−39 |
−24 |
57 |
| Middle temporal gyrus (BA18) |
Nondominant |
1711 |
5.46 |
36 |
−84 |
−15 |
| Supramarginal gyrus |
Dominant |
151 |
5.31 |
−54 |
−24 |
18 |
| Postcentral gyrus (BA40) |
Nondominant |
75 |
5.04 |
51 |
−27 |
18 |
| Thalamus (VPLN) |
Dominant |
87 |
4.42 |
−15 |
−18 |
6 |
| Precentral gyrus |
Nondominant |
75 |
4.09 |
54 |
3 |
9 |
|
Areas of activation, patients, contralateral organised
|
Areas of activation, unimanual experiment, effect of movement
p<0.001, k at FWER |
| Precentral gyrus (BA4) |
Unaffected |
626 |
6.07 |
39 |
−21 |
63 |
| Cerebellum, culmen |
Affected |
328 |
5.86 |
−12 |
−54 |
−21 |
Areas of activation, bimanual experiment, effect of movement
p<0.001, k at FWER |
| Postcentral gyrus (BA3) |
Affected |
396 |
5.75 |
−39 |
−30 |
63 |
| Precentral gyrus (BA4) |
Unaffected |
333 |
5.10 |
33 |
−24 |
54 |
| Cerebellum, culmen |
Unaffected |
206 |
4.75 |
15 |
−54 |
−21 |
Areas of activation, bimanual experiment, effect of mirror
p<0.001, k at FWER |
| Dorsolateral prefrontal cortex (BA9) |
Affected |
279 |
4.33 |
−36 |
33 |
33 |
| Anterior cingulate gyrus |
Unaffected |
192 |
4.25 |
6 |
30 |
30 |
In healthy participants (unimanual experiment), significant effects of movement (contrast between movement and rest) were observed in the precentral gyrus (nondominant side), inferior occipital gyrus (nondominant side), supramarginal gyrus (dominant gyrus) and cerebellum (both sides). In bimanual experiments, significant effects of movement (contrast between movement and rest) were observed in the precentral gyrus (nondominant side), postcentral gyrus (both sides), middle temporal gyrus (nondominant side) and thalamus (dominant side). Analysis of the effect of mirror (mirror vs no mirror) showed no significant clusters for the unimanual or bimanual experiments.
In patients with contralateral organisation, activation due to movement (contrast between movement and rest) in the unimanual experiment was found in the precentral gyrus (unaffected hemisphere) and cerebellum (affected hemisphere). In the bimanual experiment, activation due to movement (contrast between movement and rest) was found in the postcentral gyrus (affected hemisphere), precentral gyrus (unaffected hemisphere) and cerebellum (unaffected hemisphere). Effects of the mirror were observed in the dorsolateral prefrontal cortex, BA 9 (affected hemisphere) and anterior cingulate cortex (unaffected hemisphere) in the bimanual experiment (fig. 3). No effect of the mirror was found in the unimanual experiment.
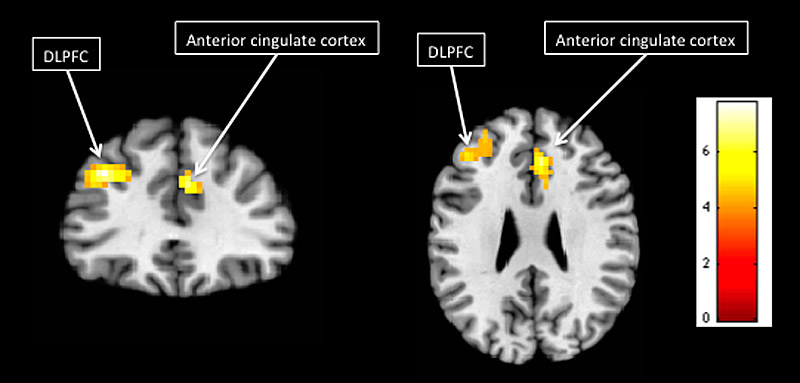
Figure 3 Statistical parametric maps of effect of mirror in children with hemiparesis, bimanual experiment observed in the dorsolateral prefrontal cortex (DLPFC; affected hemisphere) and anterior cingulate cortex (unaffected hemisphere).
In the bimanual experiment with mirror, patients showed more activation in the left striatum in comparison with healthy participants. In contrast, healthy participants showed more activation in right inferior parietal lobule, right and left occipital lobe than did the patients. In the bimanual task without mirror, healthy controls showed more activation in the left striatum. In the unimanual task with mirror, patients activated more in the left striatum, right inferior precentral gyrus (BA 4) and left inferior frontal gyrus when compared with healthy controls.
Discussion
The aim of the study was to identify the specific neuronal activation patterns underlying the potential effects of mirror therapy in children. In experiments with the mirror during bimanual movement in children with hemiparesis, we found increased activation in the dorsolateral prefrontal cortex (DLPFC) on the affected side and the right anterior cingulate cortex (ACC) on the unaffected side. No significant effect as a consequence of the mirror illusion was observed during the unimanual motor task. In healthy controls, the mirror illusion did not increase brain activation, during neither the uni- nor the bimanual motor task.
Literature regarding the effect of mirror illusion feedback and mirror therapy in children and adolescents with hemiplegia is scarce [17, 18]. One previous study examined brain activation in patients with unilateral cerebral palsy during movement observation of the paretic and nonparetic hand using fMRI. In this study, participants observed a video displaying an opening-closing hand movement from a third-person perspective. It was found that observing hand movements is associated with relevant activations in temporo-parieto-fronto-occipital networks [29]. This study, however, did not use a mirror box to examine the effect of mirror visual feedback. The present study is, to our knowledge, the first fMRI study including children and adolescents to investigate the neuronal correlates of mirror illusion using a mirror box. A direct comparison with previous research in the paediatric population is therefore not possible. Various studies have investigated the effect of mirror visual feedback on brain activity in healthy adults [7, 8, 12, 13, 30–40]. Cortical areas mainly belonging to attentional networks have been reported to be activated; these areas comprise dorsolateral prefrontal cortex [36], postcentral sulcus, posterior wall (S1/S2) [33], lateral sulcus, upper wall (S2) [33, 34] superior posterior parietal cortex [36], precuneus (V6) [12, 13, 37, 41], cuneus/lingual gyrus (V1/V2) [13, 41], superior/middle occipital gyrus (V2, 3, 5) [13, 16, 41], fusiform gyrus (V4) [13, 41] and the insular cortex, posterior region [35]. Also, in adult patients diagnosed with stroke, brain areas in networks belonging to the attentional system (precuneus [V6] [12, 14], posterior cingulate cortex [14]) showed increased activation due to mirror visual feedback [30].
It was hypothesised that one of the mechanisms of mirror visual feedback on primary and secondary somatosensory areas, in healthy subjects as well as in patients with hemiparesis due to stroke, is the result of increased involvement of attentional resources to resolve perceptional incongruence [30]. In the present study we found a different pattern, with increased activation of the ACC only in children with hemiparesis. The ACC plays a role in motor control, cognition and the arousal/drive state of the organism [42]. As an example in cognition control, the ACC is thought to play an important role in detecting conflict between competing representations [42]. In our case, the conflict lies within the mirror illusion of the healthy hand representing the paretic hand. The ACC is densely connected with the DLPFC. The prefrontal cortex in general deals with nonroutine operations [43], with the DLPFC modulating lower level systems [44]. In this situation, its main task is to resolve the conflict detected by the ACC. Increased activation of the same cortical area due to mirror visual feedback has also been reported in a positron emission tomography study including healthy adult participants [36]. Fink et al. described that the mismatch condition created by the mirror illusion led to an increased activation in the DLPFC. It was considered that enhanced monitoring of movement due to mirror visual feedback was associated with the involvement of the DLPFC [30, 36]. These authors concluded that increased neural activity in the DLPFC was due to increased attentional demand for the integration of vision and proprioception and increased hand-eye coordination [36].
Cortical areas belonging to networks responsible for attentional aspects of the mirror neurone system (superior temporal gyrus, premotor cortex) have been reported to be activated by mirror visual feedback [16, 30]. The mirror neurone system (MNS) has raised great interest over the past two decades and has been studied widely in humans with stroke. The core structures of the MNS are the ventral premotor cortex, posterior inferior frontal gyrus and rostral inferior parietal lobule [45]. Several studies have addressed the question of the development of the MNS and if it is present at birth. It is not known whether a functioning MNS exists in newborns, but imitation behaviours exist from birth [46, 47]. The superior temporal gyrus is involved in the visual identification of biological motion. Together with the premotor cortex it forms a network that enables the imitation of movement and motor learning. In the present study, we did not find increased activation in these parts of the MNS. The potential effects of mirror visual feedback in children and adolescents do not seem to rely on the MNS.
Interestingly, we found increased activation in the ACC and the DLPFC only during the bimanual motor task, and no increased activation could be found during the unimanual motor task. Previous literature has reported an effect of mirror visual feedback on cortical activity during both bimanual and unimanual motor tasks [30]. Although the majority of the studies examining the effect of mirror visual feedback on cortical activity featured an experimental setup that included bimanual motor tasks, it is difficult to draw a conclusion on the comparative therapeutic effect of mirror therapy using a bimanual versus a unimanual approach. However, as to date no therapeutic study exists in children diagnosed with hemiparesis, we consider that a bimanual approach should be preferred in children and adolescents.
In the present study we did not find increased cortical activation due to mirror visual feedback in healthy patients. This is in contrast to various studies that previously found increased activation in various brain areas in healthy adults. In a previous study using fMRI, it was shown that active movements are associated with significantly more brain activation in children with unilateral cerebral palsy than in children with normal motor development [48].This may explain why in the present study we were not able to detect similar effects of mirror visual feedback on cortical activation in healthy subjects compared with patients with hemiparesis. However, since we found a clear and specific activation pattern including the AAC in hemiparetic children, we think that the difference we found between children and adults reflected an underlying physiological difference between adults and children.
The main finding when the patients and healthy participant groups were compared was that patients showed, in both bi- and unimanual experiments with the mirror, more activation in the striatum, which is involved in motor and certain executive functions, and this on the left side, the side involved in movement of the paretic hand.
In summary, to date the majority of evidence regarding the effect of mirror visual feedback on brain activity stems from studies in healthy adult individuals and the few studies that have examined a patient population considered only adults after stroke. The finding that mirror therapy may have an impact on multiple functional networks might mean it can serve as a versatile tool to promote motor recovery. Large-scale clinical trials that include measurement of brain function and structure are required to examine the efficacy and the underlying mechanisms of mirror therapy in children. Further research is needed to fully understand and exploit the potential of mirror therapy in paediatric neurorehabilitation, since mirror visual feedback can exert a modulatory influence on the motor system. In children and adolescents with hemiparesis due to a congenital brain lesion and contralateral cortical reorganisation, mirror visual feedback seems to generate a specific activation pattern, in which the increased attentional demand for integration of vision and proprioception leads to an activation of the DLPC and the ACC.
The present study is subject to potential bias that limits the generalisability of its results. In general, detecting study-related effects in fMRI experiments in children with focal brain lesions is difficult, as the neuronal circuits are still developing and may be distorted and heterogeneous between subjects. This makes a comparison with adult studies difficult. We included a limited number of participants with large age range, and the number of patients with ipsilateral reorganisation was small. We therefore decided to not perform a separate analysis in patients with ipsilateral cortical reorganisation and excluded them to guarantee a homogenous population with similar corticospinal motor networks. The exclusion of patients with ipsilateral cortical reorganisation resulted in a smaller sample size and the small sample size represents a limitation of the study. We compared patients with hemiparesis with a group of typically developing peers; however, gender and handedness were not matched. Therefore the results of the present study should be interpreted with caution and future studies should include larger samples.
Conclusion
In this study of neuronal correlates of mirror visual feedback in children and adolescents with hemiparesis, in the bimanual experiment the mirror illusion increased activity in the anterior cingulate cortex and DLPFC. The activation in these structures is probably a result of conflict detection, i.e., mirror illusion, and increased cognitive control to resolve this conflict. We conclude that, as in adults, mirror illusion increases attention in order to resolve perceptional incongruence of hand motion. In summary, the results of the present study provide insights into the mechanisms underlying the mirror illusion in children and adolescents, and seem to show a specific pattern in the paediatric population. However, future studies need to clarify the therapeutic effect of mirror therapy and the possible neuroplastic changes underlying mirror therapy in the paediatric population.
Acknowledgements
We are grateful to all children and parents who participated in this study. We furthermore would like to thank all the foundations which supported this study financially (Inselspital-Stiftung, Batzebär, Vinetum and Johanna Durmüller-Bol). A special thank also goes to the staff of the Institute of Diagnostic and Interventional Neuroradiology and especially Martin Zbinden.
Christian Weisstanner, MD, Support Centre for Advanced Neuroimaging (SCAN), University Institute of Diagnostic and Interventional Neuroradiology, Inselspital, University of Bern, CH-3010 Bern, christian.weisstanner[at]insel.ch
References
1
Carr
LJ
,
Harrison
LM
,
Evans
AL
,
Stephens
JA
. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116(5):1223–47. Published online October 01, 1993. doi:.https://doi.org/10.1093/brain/116.5.1223
2
Guzzetta
A
,
Bonanni
P
,
Biagi
L
,
Tosetti
M
,
Montanaro
D
,
Guerrini
R
, et al.
Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol. 2007;118(5):1110–21. Published online March 27, 2007. doi:.https://doi.org/10.1016/j.clinph.2007.02.014
3
Staudt
M
,
Grodd
W
,
Gerloff
C
,
Erb
M
,
Stitz
J
,
Krägeloh-Mann
I
. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125(10):2222–37. doi:.https://doi.org/10.1093/brain/awf227
4
Ramachandran
VS
,
Rogers-Ramachandran
D
,
Cobb
S
. Touching the phantom limb. Nature. 1995;377(6549):489–90. Published online October 12, 1995. doi:.https://doi.org/10.1038/377489a0
5
Altschuler
EL
,
Wisdom
SB
,
Stone
L
,
Foster
C
,
Galasko
D
,
Llewellyn
DM
, et al.
Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353(9169):2035–6. Published online June 22, 1999. doi:.https://doi.org/10.1016/S0140-6736(99)00920-4
6
Cacchio
A
,
De Blasis
E
,
De Blasis
V
,
Santilli
V
,
Spacca
G
. Mirror therapy in complex regional pain syndrome type 1 of the upper limb in stroke patients. Neurorehabil Neural Repair. 2009;23(8):792–9. Published online May 26, 2009. doi:.https://doi.org/10.1177/1545968309335977
7
Dohle
C
,
Püllen
J
,
Nakaten
A
,
Küst
J
,
Rietz
C
,
Karbe
H
. Mirror therapy promotes recovery from severe hemiparesis: a randomized controlled trial. Neurorehabil Neural Repair. 2009;23(3):209–17. Published online December 17, 2008. doi:.https://doi.org/10.1177/1545968308324786
8
Michielsen
ME
,
Selles
RW
,
van der Geest
JN
,
Eckhardt
M
,
Yavuzer
G
,
Stam
HJ
, et al.
Motor recovery and cortical reorganization after mirror therapy in chronic stroke patients: a phase II randomized controlled trial. Neurorehabil Neural Repair. 2011;25(3):223–33. Published online November 06, 2010. doi:.https://doi.org/10.1177/1545968310385127
9
Yavuzer
G
,
Selles
R
,
Sezer
N
,
Sütbeyaz
S
,
Bussmann
JB
,
Köseoğlu
F
, et al.
Mirror therapy improves hand function in subacute stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2008;89(3):393–8. Published online February 26, 2008. doi:.https://doi.org/10.1016/j.apmr.2007.08.162
10
Hamzei
F
,
Läppchen
CH
,
Glauche
V
,
Mader
I
,
Rijntjes
M
,
Weiller
C
. Functional plasticity induced by mirror training: the mirror as the element connecting both hands to one hemisphere. Neurorehabil Neural Repair. 2012;26(5):484–96. Published online January 17, 2012. doi:.https://doi.org/10.1177/1545968311427917
11
Thieme
H
,
Mehrholz
J
,
Pohl
M
,
Behrens
J
,
Dohle
C
. Mirror therapy for improving motor function after stroke. Cochrane Database Syst Rev. 2012;3(3):CD008449. Published online March 16, 2012. doi:.https://doi.org/10.1002/14651858.CD008449.pub2
12
Wang
J
,
Fritzsch
C
,
Bernarding
J
,
Krause
T
,
Mauritz
KH
,
Brunetti
M
, et al.
Cerebral activation evoked by the mirror illusion of the hand in stroke patients compared to normal subjects. NeuroRehabilitation. 2013;33(4):593–603. Published online September 11, 2013. doi:.https://doi.org/10.3233/NRE-130999
13
Wang
J
,
Fritzsch
C
,
Bernarding
J
,
Holtze
S
,
Mauritz
KH
,
Brunetti
M
, et al.
A comparison of neural mechanisms in mirror therapy and movement observation therapy. J Rehabil Med. 2013;45(4):410–3. Published online March 12, 2013. doi:.https://doi.org/10.2340/16501977-1127
14
Michielsen
ME
,
Smits
M
,
Ribbers
GM
,
Stam
HJ
,
van der Geest
JN
,
Bussmann
JB
, et al.
The neuronal correlates of mirror therapy: an fMRI study on mirror induced visual illusions in patients with stroke. J Neurol Neurosurg Psychiatry. 2011;82(4):393–8. Published online September 24, 2010. doi:.https://doi.org/10.1136/jnnp.2009.194134
15
Fritzsch
C
,
Wang
J
,
Dos Santos
LF
,
Mauritz
KH
,
Brunetti
M
,
Dohle
C
. Different effects of the mirror illusion on motor and somatosensory processing. Restor Neurol Neurosci. 2014;32(2):269–80. Published online November 19, 2013. doi:.https://doi.org/10.3233/RNN-130343
16
Matthys
K
,
Smits
M
,
Van der Geest
JN
,
Van der Lugt
A
,
Seurinck
R
,
Stam
HJ
, et al.
Mirror-induced visual illusion of hand movements: a functional magnetic resonance imaging study. Arch Phys Med Rehabil. 2009;90(4):675–81. Published online April 07, 2009. doi:.https://doi.org/10.1016/j.apmr.2008.09.571
17
Gygax
MJ
,
Schneider
P
,
Newman
CJ
. Mirror therapy in children with hemiplegia: a pilot study. Dev Med Child Neurol. 2011;53(5):473–6. Published online March 18, 2011. doi:.https://doi.org/10.1111/j.1469-8749.2011.03924.x
18
Smorenburg
AR
,
Ledebt
A
,
Deconinck
FJ
,
Savelsbergh
GJ
. Practicing a matching movement with a mirror in individuals with spastic hemiplegia. Res Dev Disabil. 2013;34(9):2507–13. doi:.https://doi.org/10.1016/j.ridd.2013.05.001
19
Pasquet
T
,
Gaillard
F
,
Newman
CJ
,
Jequier Gygax
M
,
Le Cornec
C
,
Bonan
I
, et al.
Feasibility of a self-rehabilitation program by mirror therapy in children with hemiplegic cerebral palsy. Ann Phys Rehabil Med. 2016;59S:e9. doi:.https://doi.org/10.1016/j.rehab.2016.07.023
20
Smorenburg
AR
,
Ledebt
A
,
Feltham
MG
,
Deconinck
FJ
,
Savelsbergh
GJ
. The positive effect of mirror visual feedback on arm control in children with spastic hemiparetic cerebral palsy is dependent on which arm is viewed. Exp Brain Res. 2011;213(4):393–402. doi:.https://doi.org/10.1007/s00221-011-2789-6
21
Feltham
MG
,
Ledebt
A
,
Deconinck
FJ
,
Savelsbergh
GJ
. Mirror visual feedback induces lower neuromuscular activity in children with spastic hemiparetic cerebral palsy. Res Dev Disabil. 2010;31(6):1525–35. doi:.https://doi.org/10.1016/j.ridd.2010.06.004
22
Bruchez
R
,
Jequier Gygax
M
,
Roches
S
,
Fluss
J
,
Jacquier
D
,
Ballabeni
P
, et al.
Mirror therapy in children with hemiparesis: a randomized observer-blinded trial. Dev Med Child Neurol. 2016;58(9):970–8. doi:.https://doi.org/10.1111/dmcn.13117
23
Grunt
S
,
Newman
CJ
,
Saxer
S
,
Steinlin
M
,
Weisstanner
C
,
Kaelin-Lang
A
. The Mirror Illusion Increases Motor Cortex Excitability in Children With and Without Hemiparesis. Neurorehabil Neural Repair. 2016:1545968316680483. [e-pub ahead of print.] doi:.https://doi.org/10.1177/1545968316680483
24
Eliasson
AC
,
Krumlinde-Sundholm
L
,
Rösblad
B
,
Beckung
E
,
Arner
M
,
Ohrvall
AM
, et al.
The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006;48(7):549–54. Published online June 20, 2006. doi:.https://doi.org/10.1017/S0012162206001162
25
Z’Graggen
WJ
,
Conforto
AB
,
Wiest
R
,
Remonda
L
,
Hess
CW
,
Kaelin-Lang
A
. Mapping of direction and muscle representation in the human primary motor cortex controlling thumb movements. J Physiol. 2009;587(9):1977–87. Published online March 18, 2009. doi:.https://doi.org/10.1113/jphysiol.2009.171066
26
Kaelin-Lang
A
,
Cohen
LG
. Enhancing the quality of studies using transcranial magnetic and electrical stimulation with a new computer-controlled system. J Neurosci Methods. 2000;102(1):81–9. Published online September 23, 2000. doi:.https://doi.org/10.1016/S0165-0270(00)00284-3
27
Brasil-Neto
JP
,
Cohen
LG
,
Panizza
M
,
Nilsson
J
,
Roth
BJ
,
Hallett
M
. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol. 1992;9(1):132–6. Published online January 01, 1992. doi:.https://doi.org/10.1097/00004691-199201000-00014
28
Rossini
PM
,
Barker
AT
,
Berardelli
A
,
Caramia
MD
,
Caruso
G
,
Cracco
RQ
, et al.
Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92. Published online August 01, 1994. doi:.https://doi.org/10.1016/0013-4694(94)90029-9
29
Dinomais
M
,
Lignon
G
,
Chinier
E
,
Richard
I
,
Ter Minassian
A
,
Tich
SN
. Effect of observation of simple hand movement on brain activations in patients with unilateral cerebral palsy: an fMRI study. Res Dev Disabil. 2013;34(6):1928–37. doi:.https://doi.org/10.1016/j.ridd.2013.03.020
30
Deconinck
FJ
,
Smorenburg
AR
,
Benham
A
,
Ledebt
A
,
Feltham
MG
,
Savelsbergh
GJ
. Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil Neural Repair. 2015;29(4):349–61. doi:.https://doi.org/10.1177/1545968314546134
31
Shinoura
N
,
Suzuki
Y
,
Watanabe
Y
,
Yamada
R
,
Tabei
Y
,
Saito
K
, et al.
Mirror therapy activates outside of cerebellum and ipsilateral M1. NeuroRehabilitation. 2008;23(3):245–52. Published online June 19, 2008.
32
Merians
AS
,
Tunik
E
,
Fluet
GG
,
Qiu
Q
,
Adamovich
SV
. Innovative approaches to the rehabilitation of upper extremity hemiparesis using virtual environments. Eur J Phys Rehabil Med. 2009;45(1):123–33.
33
Wasaka
T
,
Kakigi
R
. Conflict caused by visual feedback modulates activation in somatosensory areas during movement execution. Neuroimage. 2012;59(2):1501–7. doi:.https://doi.org/10.1016/j.neuroimage.2011.08.024
34
Wasaka
T
,
Kakigi
R
. The effect of unpredicted visual feedback on activation in the secondary somatosensory cortex during movement execution. BMC Neurosci. 2012;13(1):138. doi:.https://doi.org/10.1186/1471-2202-13-138
35
Dohle
C
,
Stephan
KM
,
Valvoda
JT
,
Hosseiny
O
,
Tellmann
L
,
Kuhlen
T
, et al.
Representation of virtual arm movements in precuneus. Exp Brain Res. 2011;208(4):543–55. doi:.https://doi.org/10.1007/s00221-010-2503-0
36
Fink
GR
,
Marshall
JC
,
Halligan
PW
,
Frith
CD
,
Driver
J
,
Frackowiak
RS
, et al.
The neural consequences of conflict between intention and the senses. Brain. 1999;122(3):497–512. doi:.https://doi.org/10.1093/brain/122.3.497
37
Mehnert
J
,
Brunetti
M
,
Steinbrink
J
,
Niedeggen
M
,
Dohle
C
. Effect of a mirror-like illusion on activation in the precuneus assessed with functional near-infrared spectroscopy. J Biomed Opt. 2013;18(6):066001. doi:.https://doi.org/10.1117/1.JBO.18.6.066001
38
Carson
RG
,
Ruddy
KL
. Vision modulates corticospinal suppression in a functionally specific manner during movement of the opposite limb. J Neurosci. 2012;32(2):646–52. doi:.https://doi.org/10.1523/JNEUROSCI.4435-11.2012
39
Garry
MI
,
Loftus
A
,
Summers
JJ
. Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res. 2005;163(1):118–22. doi:.https://doi.org/10.1007/s00221-005-2226-9
40
Fukumura
K
,
Sugawara
K
,
Tanabe
S
,
Ushiba
J
,
Tomita
Y
. Influence of mirror therapy on human motor cortex. Int J Neurosci. 2007;117(7):1039–48. doi:.https://doi.org/10.1080/00207450600936841
41
Dohle
C
,
Kleiser
R
,
Seitz
RJ
,
Freund
HJ
. Body scheme gates visual processing. J Neurophysiol. 2004;91(5):2376–9. Published online December 19, 2003. doi:.https://doi.org/10.1152/jn.00929.2003
42
Paus
T
. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2(6):417–24. doi:.https://doi.org/10.1038/35077500
43
Miller
EK
,
Cohen
JD
. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24(1):167–202. Published online April 03, 2001. doi:.https://doi.org/10.1146/annurev.neuro.24.1.167
44
Heekeren
HR
,
Marrett
S
,
Ungerleider
LG
. The neural systems that mediate human perceptual decision making. Nat Rev Neurosci. 2008;9(6):467–79. doi:.https://doi.org/10.1038/nrn2374
45
Iacoboni
M
,
Dapretto
M
. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–51. Published online November 23, 2006. doi:.https://doi.org/10.1038/nrn2024
46
Bertenthal
BI
,
Longo
MR
. Is there evidence of a mirror system from birth?
Dev Sci. 2007;10(5):526–9. doi:.https://doi.org/10.1111/j.1467-7687.2007.00633.x
47
Simpson
EA
,
Murray
L
,
Paukner
A
,
Ferrari
PF
. The mirror neuron system as revealed through neonatal imitation: presence from birth, predictive power and evidence of plasticity. Philos Trans R Soc Lond B Biol Sci. 2014;369(1644):20130289. doi:.https://doi.org/10.1098/rstb.2013.0289
48
Van de Winckel
A
,
Klingels
K
,
Bruyninckx
F
,
Wenderoth
N
,
Peeters
R
,
Sunaert
S
, et al.
How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res Dev Disabil. 2013;34(1):183–97. doi:.https://doi.org/10.1016/j.ridd.2012.07.030


