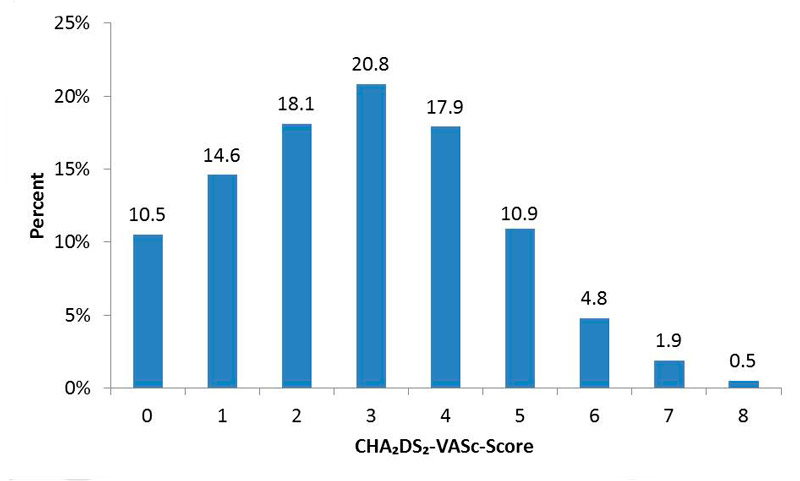
Figure 1 CHA2DS2-VaSc score distribution at baseline.
DOI: https://doi.org/10.4414/smw.2017.14410
Atrial fibrillation is the most common cardiac arrhythmia in the general population [1] and affected patients face an increased risk of stroke, heart failure, cognitive decline and death [2–5]. Thus, treatments reducing the risk of these complications will have a substantial public health impact.
One of the few therapies in this context is oral anticoagulation (OAC) for stroke prevention in atrial fibrillation patients with additional cardiovascular risk factors. Vitamin K antagonists (VKAs) reduce the risk of stroke by 64% compared with placebo [6], but are cumbersome to use and associated with an increased risk of major bleeding. Non-VKA oral anticoagulants (NOACs) are much easier to use, and at least as effective and at least as safe as VKA treatment [7–10]. Accordingly, these treatments have been approved for stroke prevention in many countries worldwide, and are the preferred treatment strategy in current European guidelines [11].
However, few data are available about the uptake of NOACs in clinical practice and whether there are patient characteristics associated with a preferential switch from VKA to NOAC treatment. One study, performed between 2010 and 2013, showed that NOACs accounted for up to 62% of new prescriptions for patients with recently diagnosed nonvalvular atrial fibrillation, and that female sex, lower income, higher haemorrhagic risk according to the HAS-BLED score and higher CHA2DS2-VASc score were associated with lower odds of receiving a NOAC [12]. However, this study had no prospective follow-up, considered only de novo anticoagulation and provided no data on switching from VKA to NOAC therapy.
Aspirin is less effective for stroke prevention in atrial fibrillation patients than standard OAC [6]. Studies comparing apixaban with low-dose aspirin in atrial fibrillation patients demonstrated clear superiority and similar safety in favour of apixaban treatment [13]. Accordingly, current European guidelines discourage the use of low-dose aspirin for stroke prevention in patients with atrial fibrillation, and NOACs may help to reduce the high prescription of aspirin in contemporary atrial fibrillation patients [11].
In this context, we assessed the uptake of NOACs in a large, prospective and unselected cohort of patients with atrial fibrillation, evaluated frequency of and predictors for a switch from VKA to NOAC therapy, and assessed whether the prevalence of aspirin monotherapy has changed over time.
The Basel Atrial Fibrillation Cohort Study (BEAT-AF) is a prospective observational, multicentre cohort study. Between 2010 and 2014, 1550 patients with atrial fibrillation documented on an electrocardiogram (ECG) or rhythm strip were enrolled across seven centres in Switzerland. Participating centres are the University Hospital Basel, Kantonsspital St. Gallen, Kantonsspital Luzern, Regional Hospital Lugano, Regional Hospital Bellinzona, University Hospital Lausanne (CHUV) and University Hospital Geneva (HUG). Patients were recruited from in- and outpatient clinics. We strongly encouraged all centres to comprehensively screen patients in in- and outpatient clinics and to include consecutive patients. The enrolment of patients with acute illnesses was postponed until their health status had stabilised. Exclusion criteria were the inability to sign informed consent and the presence of short, transient forms of atrial fibrillation. For the purpose of this study, we excluded five (0.3%) patients because of missing information on medical therapy at baseline, such that 1545 patients remained in the current analysis. The study protocol was approved by the local ethics committees, and informed written consent was obtained from each participant.
At baseline, patients in collaboration with trained study persons completed detailed questionnaires about personal, medical, nutritional and lifestyle factors, current atrial fibrillation symptoms, and comorbidities. Information on current medications was also recorded, including details about OAC and antiplatelet treatment. Body mass index (BMI) was calculated as weight in kg divided by height in metres squared. All patients underwent venous blood sampling at the local study centre. Glomerular filtration rate (GFR) was calculated with the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula.
All patients were followed up yearly with mailed questionnaires and phone interviews in order to collect similar information to that recorded at baseline. Medication intake was updated at all yearly follow-up visits. For the present analysis, data of all completed follow-up visits that were available at the time of data export in August 2015 were used. At that time, a total of 57 (3.7%) drop outs and 14 (0.9%) patients lost to follow-up were registered.
Stroke risk was categorised using the CHA2DS2-VASc score as recommended by current guidelines [11]. Specifically, one point was scored for a history of congestive heart failure, hypertension, diabetes, vascular disease, age between 65 and 74 years and female sex, and two points for a history of stroke or transient ischaemic attack or an age ≥75 years. For the purpose of this study, men with a CHA2DS2-VASc score of 1 or higher and women with a CHA2DS2-VASc score of 2 or higher were considered to have an indication for OAC [11].
Categorical variables are presented as numbers (percentages) and continuous variables are expressed as means ± standard deviation. Prevalence and type of antithrombotic treatment over time were plotted by calendar year in which the corresponding visit occurred. OAC according to CHA2DS2-VASc score and differences in anticoagulation over time were compared with standard chi-square tests. In order to evaluate independent predictors of a switch from VKA to NOAC treatment, we performed multivariable logistic regression analyses adjusting for a predefined set of covariates, including age, sex, GFR, BMI, atrial fibrillation type (paroxysmal vs nonparoxysmal), diabetes mellitus, history of heart failure and history coronary heart disease. All statistical analyses were performed using SAS 9.4 (SAS Corporation, Cary, North Carolina, USA).
Baseline characteristics are presented in table 1. Women represented 29.5% of the study population, mean age was 68 ± 11 years and BMI was 27 ± 4 kg/m2; 55.7% of patients had paroxysmal atrial fibrillation and 73% were on OAC. The mean heart rate was 71 ± 18 beats per minute and the mean systolic blood pressure 134 ± 20 mm Hg. Most of the patients did not have recent-onset atrial fibrillation. The mean period between first atrial fibrillation diagnosis and the baseline visit was 14 ± 9 months.
Table 1 Baseline characteristics.
| Female sex (n = 1545) | 456 (29.5%) |
| Age (years) (n = 1545) | 68.1 ± 11.7 |
| Body mass index (kg/m2) (n = 1539) | 27.1 ± 4.7 |
| Heart rate (beats/min) (n = 1538) | 71.0 ± 18.3 |
| Systolic blood pressure (mm Hg) (n = 1528) | 134.6 ± 19.7 |
| Diastolic blood pressure (mm Hg) (n = 1528) | 78.9 ± 13.0 |
| History of hypertension (n = 1545) | 1044 (67.6%) |
| History of heart failure (n = 1545) | 314 (20.3%) |
| History of coronary heart disease (n = 1545) | 325 (21.0%) |
| History of peripheral artery disease (n = 1545) | 95 (6.1%) |
| History of heart valve surgery (n = 1545) | 133 (8.6%) |
| History of stroke/TIA (n = 1545) | 194 (12.6%) |
| History of diabetes mellitus (n = 1545) | 212 (13.7%) |
| History of major bleeding (n = 1545) | 150 (9.7%) |
| Intracranial | 24 (16%) |
| Gastrointestinal | 41 (27.3%) |
| Current smoking (n = 1536) | 138 (8.9%) |
| Atrial fibrillation type (n = 1543) | |
| Paroxysmal | 860 (55.7%) |
| Persistent | 368 (23.8%) |
| Permanent | 315 (20.4%) |
| Oral anticoagulation (n = 1545) | 1129 (73.1%) |
| Vitamin K antagonists | 971 (86.0%) |
| Rivaroxaban | 139 (12.3%) |
| Dabigatran | 19 (1.7%) |
| Aspirin (n = 1538) | 357 (23.2%) |
| Other platelet inhibitors (n = 1545) | 40 (2.6%) |
TIA = transient ischemic attack Data are mean ± standard deviation or count (percentage)
Between 2010 and 2015, a total of 161, 449, 871, 1309, 1268 and 806 study visits with complete anticoagulation data available were performed in each calendar year, respectively. Table 2 shows the prevalence of the CHA2DS2-VASc risk factors at the baseline visit. The most common risk factors were hypertension (67.6%) and age (67.1% being ≥65 years).
Table 2 Prevalence of CHA2DS2-VASc risk factors at baseline.
| CHA2DS2-VASc risk factor | Number(%) |
|---|---|
| History of heart failure | 314 (20.3%) |
| History of hypertension | 1044 (67.6%) |
| Age | |
| 65–74 years | 550 (35.6%) |
| ≥75 years | 486 (31.5%) |
| Total >65 years | 1036 (67.1%) |
| Diabetes mellitus | 212 (13.7%) |
| Thromboembolic events: | |
| Stroke/TIA | 194 (12.6%) |
| Systemic embolisms | 68 (4.4%) |
| Vascular disease* | 370 (24.0%) |
| Female sex | 456 (29.5%) |
TIA = transient ischaemic attack * Vascular disease was defined as history of peripheral artery disease, myocardial infarction, aortic plaque, percutaneous coronary intervention or aortocoronary bypass surgery.
As presented in figure 1, 74.9% participants had a CHA2DS2-VASc score of at least 2. A total of 86.9% of the participants (85.1% men, 91% women) had an indication for OAC based on the criteria specified above.

Figure 1 CHA2DS2-VaSc score distribution at baseline.
The prevalence of OAC increased with increasing CHA2DS2-VASc score, as shown in figure 2. Whereas 28% of patients with a CHA2DS2-VASc score of 0 were anticoagulated, this number increased to 65.3% among those with a score of 1 or 2 and to 85.8% among those with a score >2 (p <0.0001).
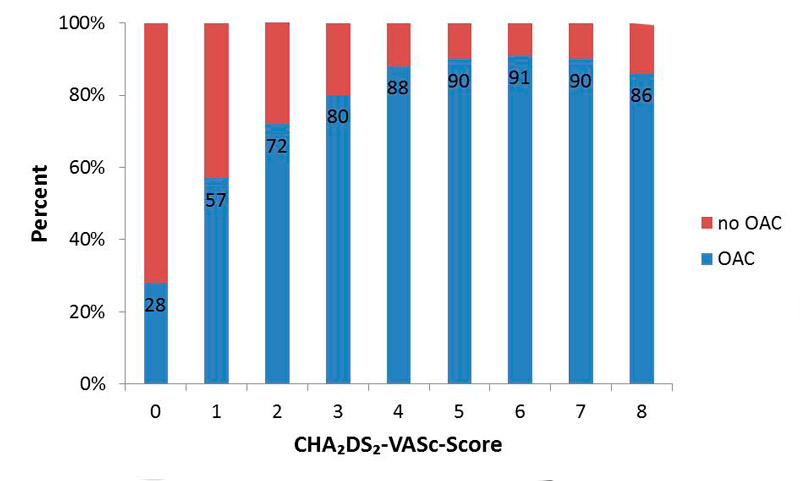
Figure 2 Oral anticoagulation (OAC) according to CHA2DS2-VASc score at baseline.
Figure 3 shows the prevalence of orally anticoagulated study participants between 2010 and 2015 among those with an indication for OAC. The prevalence of OAC was already high in 2010 and increased nonsignificantly afterwards (75%, 73%, 77%, 76%, 78% and 80%, p = 0.2).
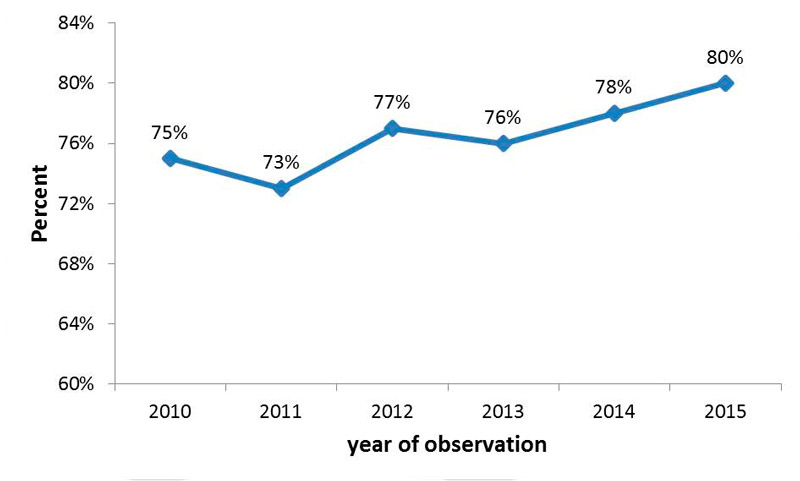
Figure 3 Prevalence of oral anticoagulated patients over time from 2010 to 2015; 203 patients with CHA2DS2-VaSc score of 0 (men) or 1 (women) were excluded from the analyses.
Regulatory approval in Switzerland for stroke prevention among atrial fibrillation patients was obtained in April 2012 for rivaroxaban, in May 2012 for dabigatran, in October 2013 for apixaban and in March 2015 for edoxaban. NOAC uptake, starting in 2011, is shown in figure 4. In 2010, all participants were anticoagulated with VKAs, and the percentage decreased to 70.2% in 2015 (p <0.0001). The most frequently used NOAC during all periods was rivaroxaban (83.2% of all NOACs in 2015). In 2015, the corresponding proportions were 14.4% for dabigatran and 2.4% for apixaban (fig. 4).
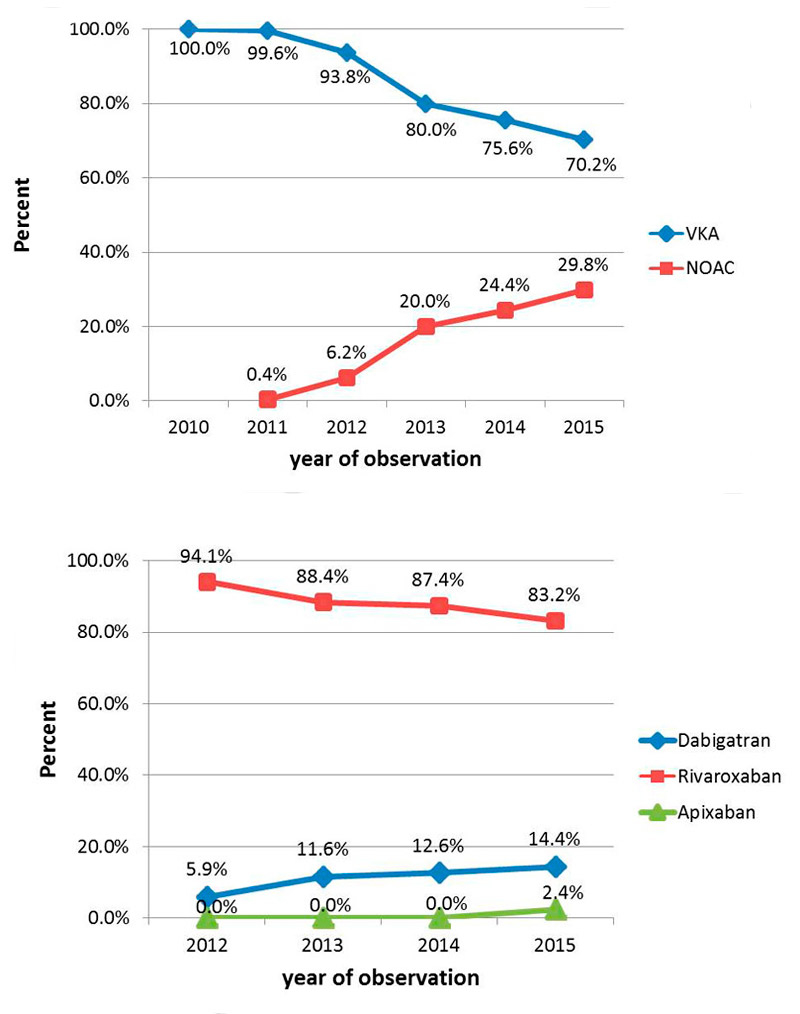
Figure 4 Uptake of non-vitamin K antagonist oral anticoagulants over time from 2010 to 2015; 203 patients with CHA2DS2-VaSc score of 0 (men) or 1 (women) were excluded from the analyses.
NOAC = non-vitamin K antagonist oral anticoagulant; VKA = vitamin K antagonist
The use of low-dose aspirin as a monotherapy significantly decreased over time, as demonstrated in figure 5. In 2010, 23% of the study participants had monotherapy with aspirin, and this proportion decreased to 10.5% in 2015 (p <0.0001).
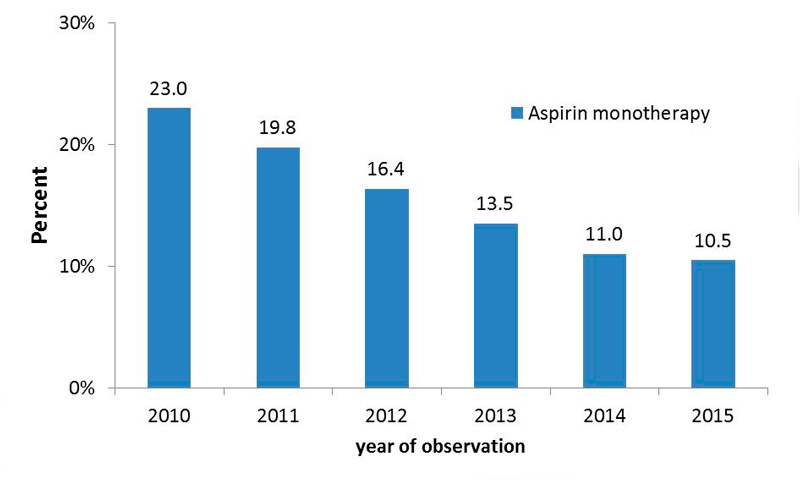
Figure 5 Use of aspirin monotherapy over time.
We excluded 83 patients on VKAs at baseline and 22 on NOACs at baseline from the switch analyses because of missing follow-up data. Most patients stayed on the same type of OAC during follow-up (76% of those on a VKA and 77% of those on an NOAC). Out of 888 patients who received VKA treatment at baseline, 86 (9.7%) were switched to an NOAC during follow-up, as shown in figure 6. Among the 136 patients taking an NOAC at the baseline visit, 4 (2.9%) were switched to VKA therapy.
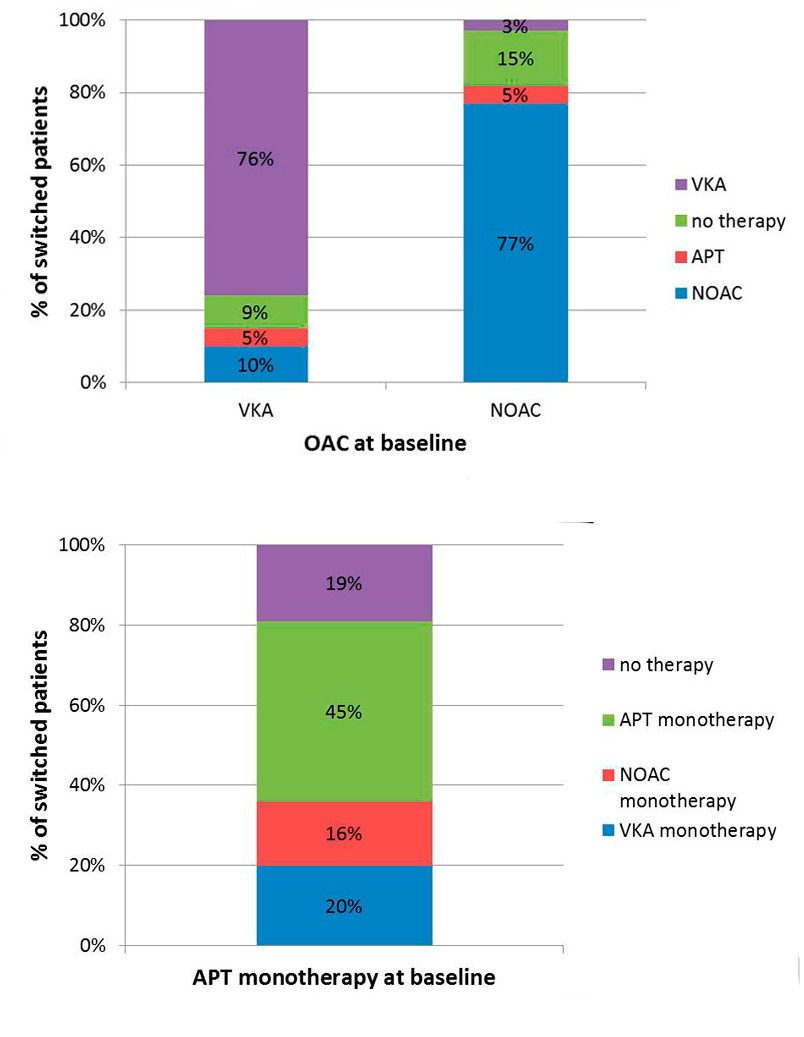
Figure 6 Switch of oral anticoagulation over time (percentage of switched patients over the follow-up period by type of baseline anticoagulation).
APT = antiplatelet therapy; NOAC = non-vitamin K antagonist oral anticoagulant; VKA = vitamin K antagonist
A logistic regression analysis was performed on the 888 patients with VKA treatment at baseline. We found that older patients (odds ratio [OR] per 1 year increase 0.97, 95% confidence interval [CI] 0.95–0.99; p = 0.0006) and patients with a history of heart failure (OR 0.5, 95% CI 0.3–0.96; p = 0.04) were significantly less likely to be switched to NOAC therapy, whereas patients with a higher BMI were more frequently switched from VKA to NOAC therapy (OR per 1 kg/m2 increase 1.05, 95% CI 1.0–1.1; p = 0.02). After adjustment for clinically relevant covariates, age (OR 0.98, 95% CI 0.95–0.99; p = 0.02 and BMI (OR 1.05, 95%CI 1.0–1.1; p = 0.05) remained independent predictors, as shown in table 3.
Table 3 Predictors for switch from vitamin K antagonists to non-vitamin K antagonist oral anticoagulants.
| Predictor (n = 86) | Univariate model | Multivariate model | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Female sex | 1.0 (0.6–1.6) | 1.0 | 1.1 (0.7–1.8) | 0.8 |
| GFR | 1.3 (0.9–1.9) | 0.2 | 1.1 (0.6–2.0) | 0.8 |
| BMI | 1.05 (1.0–1.1) | 0.02 | 1.05 (1.0–1.1) | 0.05 |
| Paroxysmal AF | 1.2 (0.8–1.9) | 0.4 | 1.2 (0.7–1.8) | 0.5 |
| History of diabetes | 1.2 (0.7–2.1) | 0.6 | 1.4 (0.8–2.6) | 0.3 |
| History of heart failure | 0.5 (0.3–0.96) | 0.04 | 0.6 (0.3–1.1) | 0.09 |
| Coronary heart disease | 0.6 (0.4–1.1) | 0.1 | 0.7 (0.4–1.3) | 0.2 |
| Age | 0.97 (0.95–0.99) | 0.0006 | 0.98 (0.95–0.99) | 0.02 |
AF = atrial fibrillation; BMI = body mass index; CI = confidence interval; GFR = glomerular filtration rate; OR = odds ratio p-values were based on logistic regression, models were adjusted for sex, GFR (over/below 60ml/min), BMI, AF type (paroxysmal/nonparoxysmal), diabetes mellitus, heart failure, coronary heart disease (myocardial infarction or percutaneous transluminal coronary angioplasty or aortocoronary bypass) and age
In this large prospective study of patients with documented atrial fibrillation, several important findings were observed. First, prevalence of OAC use was high at baseline and nonsignificantly increased during follow-up. Second, a steady increase of NOAC use was observed from the period prior to regulatory approval until 2015, when approximately 30% of anticoagulated patients received NOAC treatment. Third, whereas most patients stayed on their initial form of OAC, the use of low-dose aspirin monotherapy significantly decreased. Finally, no strong predictors for switching from VKA to NOAC therapy over time emerged from this study.
Prior studies suggested a low prevalence of OAC among atrial fibrillation patients with additional risk factors for stroke, even in highly developed countries [14, 15]. In contrast, our study documented a high use of OAC before and after the introduction of NOACs. There are inconsistent data on whether the introduction of NOACs has helped to reduce the number of atrial fibrillation patients who do not receive OAC. Two different studies regarding the specific adoption of dabigatran presented a rapid uptake without evidence for increased overall anticoagulation [16, 17], whereas a more recent study showed that the availability of NOACs has been associated with an increased use of OAC [18]. The higher rate of OAC in Switzerland compared with other healthcare settings could result from a better implementation, but we cannot completely exclude some selection bias of our cohort, as most of the patient recruitment took part in large hospital centres with potentially higher guideline adherence.
There has been a steady increase of NOAC use during the last 5 years. Our study has the advantage of directly assessing the transition period before and after regulatory approval of NOACs. Most published studies were conducted during the first years after approval of the new agents. In the transition period between 2011 and 2013, the OAC distribution pattern in our cohort is in line to other western European countries [19, 20]. Studies that focused on atrial fibrillation newly diagnosed after NOAC approval reported a higher proportion of NOAC use [12, 17, 21], which is in line with our study showing that patients on VKA treatment are relatively rarely switched to NOAC therapy.
We observed a steady decrease of low-dose aspirin monotherapy from 23.0% in 2010 to 10.5% in 2015. This trend is highly encouraging, given that low-dose aspirin is less effective in reducing stroke among atrial fibrillation patients and that the bleeding risk may be similar to that of NOAC therapy [6, 22]. In contrast, a recent cross-sectional registry from the United States showed that more than 25% of atrial fibrillation patients still received aspirin treatment without OAC [23]. The fact that current European, but not American, guidelines strongly discourage aspirin monotherapy may be one reason why its use may still be higher in the United States [11, 24]. Overall, the availability of NOAC therapy as a safe and easy-to-use alternative to VKA treatment may also help to reduce the prescription of aspirin in this context. Despite the continuous reduction, one out of ten patients still received aspirin monotherapy in 2015. Increased efforts are therefore needed to further improve adherence to guideline-directed management of patients with atrial fibrillation.
Patients on a VKA who could benefit from a switch to an NOAC are those with poor control of the prothrombin time international normalised ratio (INR) [25, 26] and those with a history of stroke or transient ischaemic attack [25]. Additional appropriate candidates are patients at higher risk for (intracranial) bleeding [25, 26]. Real world data show that switching from VKAs to NOACs is safe [27]. However, in contrast to the preferential recommendation to use NOACs in new-onset atrial fibrillation, current guidelines recommend a switch to NOACs only in patients with a poorly controlled INR, unable to attend monitoring visits, or those who had adverse side effects on VKA treatment [11, 28]. In our study, few patients were switched from VKA to NOAC therapy, and a higher BMI and younger age were significantly associated with a higher likelihood to be switched to NOACs. However, the switching group seems to be heterogeneous, as the significance of these predictors was relatively modest. More studies with larger sample sizes are needed to evaluate which patients should be switched from VKA to NOAC therapy.
Main strengths of this study are the large sample size of an unselected and well-characterised atrial fibrillation population. Furthermore the study period encompassed the time just before and after approval of NOAC agents. Some limitations need to be taken into account in the interpretation of our findings. As a result of the observational study design we are not able to prove any causality. The generalisability of our findings to other care settings is unknown, particularly to those where the prescription of NOACs is restricted. Furthermore, we cannot exclude some selection bias as most of our patients were recruited at large hospitals, and those patients who are exclusively treated in medical general practices might therefore be underrepresented. However, to minimise selection bias we strongly encouraged all centres to consecutively screen and enrol eligible patients. Finally, the group of patients who were switched from VKA to NOAC treatment was relatively small, and our analysis may have lacked power to identify more modest predictors.
In conclusion, the rate of OAC use in eligible patients in Switzerland is high and the proportion treated with NOACs is steadily increasing. One of the most important findings is that switching is infrequent, as the majority of patients remained on VKA treatment during prospective follow-up. Given the strong data for safety and efficacy of NOACs, future efforts may therefore be directed to switching from VKA to NOACs in appropriate patients. Importantly, the prescription of aspirin as a monotherapy was more than halved within recent years, suggesting important guideline concordant improvements in OAC use among patients with atrial fibrillation in Switzerland.
This study was supported by the Swiss National Science Foundation (PP00P3_159322), the Swiss Heart Foundation, the University of Basel, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dome, Bayer, Daiichi-Sankyo and Pfizer/Bristol-Myers Squibb.
DC has received research support from Bayer, Bristol-Myers-Squibb, Pfizer and Daiichi-Sankyo; he also received consultant and/or speaker fees from Bayer, Bristol-Myers-Squibb, Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. JS served in the advisory boards for Daiichi-Sankyo, Bayer & Boehringer-Ingelheim. DS received honoria from Daiichi-Sankyo and Pfizer. MK has served on the speakers’ bureau for Boston Scientific, St. Jude Medical and Biotronik. He has received lecture/consulting fees from Sorin, Boehringer Ingelheim, Bayer, Sanofi Aventis, Novartis and MSD and has received unrestricted grants from Sanofi Aventis, Bayer and Boehringer Ingelheim. He is a proctor for Medtronic (Cryoballoon).
1 Krijthe BP , Kunst A , Benjamin EJ , Lip GY , Franco OH , Hofman A , et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–51. doi:.https://doi.org/10.1093/eurheartj/eht280
2 Conen D , Chae CU , Glynn RJ , Tedrow UB , Everett BM , Buring JE , et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305(20):2080–7. doi:.https://doi.org/10.1001/jama.2011.659
3 Wang TJ , Larson MG , Levy D , Vasan RS , Leip EP , Wolf PA , et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–5. doi:.https://doi.org/10.1161/01.CIR.0000072767.89944.6E
4 Wolf PA , Abbott RD , Kannel WB . Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. doi:.https://doi.org/10.1161/01.STR.22.8.983
5 Kalantarian S , Stern TA , Mansour M , Ruskin JN . Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158(5 Pt 1):338–46. doi:.https://doi.org/10.7326/0003-4819-158-5-201303050-00007
6 Hart RG , Pearce LA , Aguilar MI . Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–67. doi:.https://doi.org/10.7326/0003-4819-146-12-200706190-00007
7 Connolly SJ , Ezekowitz MD , Yusuf S , Eikelboom J , Oldgren J , Parekh A , et al.; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi:.https://doi.org/10.1056/NEJMoa0905561
8 Granger CB , Alexander JH , McMurray JJ , Lopes RD , Hylek EM , Hanna M , et al.; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi:.https://doi.org/10.1056/NEJMoa1107039
9 Patel MR , Mahaffey KW , Garg J , Pan G , Singer DE , Hacke W , et al.; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi:.https://doi.org/10.1056/NEJMoa1009638
10 Giugliano RP , Ruff CT , Braunwald E , Murphy SA , Wiviott SD , Halperin JL , et al.; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. doi:.https://doi.org/10.1056/NEJMoa1310907
11 Camm AJ , Lip GY , De Caterina R , Savelieva I , Atar D , Hohnloser SH , et al.; ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation--developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–413. doi:.https://doi.org/10.1093/europace/eus305
12 Desai NR , Krumme AA , Schneeweiss S , Shrank WH , Brill G , Pezalla EJ , et al. Patterns of initiation of oral anticoagulants in patients with atrial fibrillation- quality and cost implications. Am J Med. 2014;127(11):1075–82.e1. doi:.https://doi.org/10.1016/j.amjmed.2014.05.013
13 Connolly SJ , Eikelboom J , Joyner C , Diener HC , Hart R , Golitsyn S , et al.; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–17. doi:.https://doi.org/10.1056/NEJMoa1007432
14 Alamneh EA , Chalmers L , Bereznicki LR . Suboptimal Use of Oral Anticoagulants in Atrial Fibrillation: Has the Introduction of Direct Oral Anticoagulants Improved Prescribing Practices? Am J Cardiovasc Drugs. 2016;16(3):183–200. doi:.https://doi.org/10.1007/s40256-016-0161-8
15 Berti D , Moors E , Moons P , Heidbuchel H . Prevalence and antithrombotic management of atrial fibrillation in hospitalised patients. Heart. 2015;101(11):884–93. doi:.https://doi.org/10.1136/heartjnl-2014-307059
16 Kirley K , Qato DM , Kornfield R , Stafford RS , Alexander GC . National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. 2012;5(5):615–21. doi:.https://doi.org/10.1161/CIRCOUTCOMES.112.967299
17 Steinberg BA , Holmes DN , Piccini JP , Ansell J , Chang P , Fonarow GC , et al.; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators and Patients. Early adoption of dabigatran and its dosing in US patients with atrial fibrillation: results from the outcomes registry for better informed treatment of atrial fibrillation. J Am Heart Assoc. 2013;2(6):e000535. doi:.https://doi.org/10.1161/JAHA.113.000535
18 Barnes GD , Lucas E , Alexander GC , Goldberger ZD . National Trends in Ambulatory Oral Anticoagulant Use. Am J Med. 2015;128(12):1300–5.e2. doi:.https://doi.org/10.1016/j.amjmed.2015.05.044
19 Kirchhof P , Ammentorp B , Darius H , De Caterina R , Le Heuzey JY , Schilling RJ , et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014;16(1):6–14. doi:.https://doi.org/10.1093/europace/eut263
20 Le Heuzey JY , Ammentorp B , Darius H , De Caterina R , Schilling RJ , Schmitt J , et al. Differences among western European countries in anticoagulation management of atrial fibrillation. Data from the PREFER IN AF registry. Thromb Haemost. 2014;111(5):833–41. doi:.https://doi.org/10.1160/TH13-12-1007
21 Huisman MV , Rothman KJ , Paquette M , Teutsch C , Diener HC , Dubner SJ , et al.; GLORIA-AF Investigators. Antithrombotic Treatment Patterns in Patients with Newly Diagnosed Nonvalvular Atrial Fibrillation: The GLORIA-AF Registry, Phase II. Am J Med. 2015;128(12):1306–13.e1. doi:.https://doi.org/10.1016/j.amjmed.2015.07.013
22 Flaker GC , Eikelboom JW , Shestakovska O , Connolly SJ , Kaatz S , Budaj A , et al. Bleeding during treatment with aspirin versus apixaban in patients with atrial fibrillation unsuitable for warfarin: the apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment (AVERROES) trial. Stroke. 2012;43(12):3291–7. doi:.https://doi.org/10.1161/STROKEAHA.112.664144
23 Hsu JC , Maddox TM , Kennedy KF , Katz DF , Marzec LN , Lubitz SA , et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: Insights from the ncdr pinnacle registry. JAMA Cardiol. 2016;1(1):55–62. doi:.https://doi.org/10.1001/jamacardio.2015.0374
24 January CT , Wann LS , Alpert JS , Calkins H , Cigarroa JE , Cleveland JC, Jr , et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. doi:.. Erratum in: J Am Coll Cardiol. 2014 Dec 2;64(21):2305–7. https://doi.org/10.1016/j.jacc.2014.03.022
25 Guimarães PO , Kaatz S , Lopes RD . Practical and clinical considerations in assessing patients with atrial fibrillation for switching to non-vitamin K antagonist oral anticoagulants in primary care. Int J Gen Med. 2015;8:283–91.
26 Verdecchia P , Angeli F , Aita A , Bartolini C , Reboldi G . Why switch from warfarin to NOACs? Intern Emerg Med. 2016;11(3):289–93. doi:.https://doi.org/10.1007/s11739-016-1411-0
27 Bouillon K , Bertrand M , Maura G , Blotière PO , Ricordeau P , Zureik M . Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2(4):e150–9. doi:.https://doi.org/10.1016/S2352-3026(15)00027-7
28 Lip GY . Recommendations for thromboprophylaxis in the 2012 focused update of the ESC guidelines on atrial fibrillation: a commentary. J Thromb Haemost. 2013;11(4):615–26. doi:.https://doi.org/10.1111/jth.12140
Steffen Blum and Matylda Zimny contributed equally to this manuscript and are shared first authors.
This study was supported by the Swiss National Science Foundation (PP00P3_159322), the Swiss Heart Foundation, the University of Basel, Boehringer Ingelheim, Sanofi-Aventis, Merck Sharp & Dome, Bayer, Daiichi-Sankyo and Pfizer/Bristol-Myers Squibb.
DC has received research support from Bayer, Bristol-Myers-Squibb, Pfizer and Daiichi-Sankyo; he also received consultant and/or speaker fees from Bayer, Bristol-Myers-Squibb, Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. JS served in the advisory boards for Daiichi-Sankyo, Bayer & Boehringer-Ingelheim. DS received honoria from Daiichi-Sankyo and Pfizer. MK has served on the speakers’ bureau for Boston Scientific, St. Jude Medical and Biotronik. He has received lecture/consulting fees from Sorin, Boehringer Ingelheim, Bayer, Sanofi Aventis, Novartis and MSD and has received unrestricted grants from Sanofi Aventis, Bayer and Boehringer Ingelheim. He is a proctor for Medtronic (Cryoballoon).