Biofilm formation on ureteral stents – incidence, clinical impact and prevention
DOI: https://doi.org/10.4414/smw.2017.14408
Valentin
Zumsteina, Patrick
Betscharta, Werner C.
Albrichb, Matthias T.
Buhmannc, Qun
Renc, Hans-Peter
Schmida, Dominik
Abta
aDepartment of Urology, Cantonal Hospital St Gallen,
bDivision of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen,
cLaboratory for Biointerfaces, Empa, Swiss Federal Laboratories for Materials Science and Technology, St Gallen,
Biofilm formation on ureteral stents – incidence, clinical impact and prevention
Summary
Ureteral stents are a simple, minimally invasive method of maintaining ureteral drainage to assure renal function, treat pain caused by ureteral obstruction and avoid external or visible devices. Ureteral stenting is, however, associated with a clear side-effect profile, including irritation on voiding, pain and haematuria. Complications such as stent dysfunction and clinically significant urinary tract infections are also regularly observed. Although this has not yet thoroughly researched, it appears that biofilm formation on ureteral stents plays a key role in the associated morbidity. In this review, we summarise the current evidence and identify areas that should be further studied to reduce the morbidity associated with ureteral stenting.
Introduction
Internal drainage of the upper urinary tract by ureteral stents is used for different purposes in urology [1]. They are a simple and effective method of maintaining ureteral drainage to assure renal function, treat pain caused by ureteral obstruction, and avoid external or visible devices.
However, ureteral stenting has a well-defined side effect profile. Most patients suffer pain, as well as irritation on voiding, and haematuria often while the stent is in situ [2, 3]. Complications such as stent dysfunction and clinically significant urinary tract infections (UTIs) are regularly observed. The procedure therefore also constitutes a relevant economic burden [4]. In view of the prevalence of ureteral stenting and the associated symptoms, Joshi et al. made an important step forward by developing and validating a specially designed questionnaire, the Ureteral Stent Symptoms Questionnaire (USSQ), which analyses the various domains of health affected by stents [5].
Despite this, the possibilities of preventing and treating stent-associated morbidity are still limited. Alpha-blockers [6], antimuscarinics [7] and good patient education [8] can reduce symptoms caused by ureteral stents, whereas the influence of the intravesical stent position is still controversial [9, 10]. So far, none of the materials or designs tested has reduced symptoms significantly [11]. Further studies in this field and novel concepts to reduce stent-associated morbidity are urgently required.
The role of biofilm formation on ureteral stents and its prevention have been discussed as a possible approach. Biofilms are defined as an accumulation of microorganisms and extracellular biopolymers that form a structured community on a surface [12]. Ureteral stents offer an ideal surface substrate for such microbial colonisation and biofilm formation [13, 14]. Biofilms have been suspected to be the main reason for stent obstruction, stent dysfunction and clinically relevant infections leading to premature or emergency stent changes, antibiotic treatment and hospitalisation. Moreover, it has been proposed that biofilms lead to irritation and inflammation of the urothelium, which may aggravate the symptoms described.
In this review, we give an overview of the topic and describe the current evidence. We provide information on the incidence and development of biofilms, including the process and timeframes of biofilm formation, and the distribution and composition of biofilms. We also review methods for biofilm assessment, such as bacterial involvement, mineralogical composition and quantification of biofilms. We discuss the clinical impact of biofilms on stent-associated symptoms, infections, encrustation and obstruction. Moreover, we report on the most recent concepts for preventing and treating biofilms, including antimicrobials and different materials and coatings.
Methods
We performed this systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement [15]. The protocol for the review is available on PROSPERO, the international prospective register of systematic reviews (CRD42016037872, http://www.crd.york.ac.uk/PROSPERO). MEDLINE and SCOPUS were independently searched by two authors (VZ and PB), screening for eligibility was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions, followed by crosschecking and clarification of any differences by a third author (DA). The following search terms including the relevant MeSH terms were used: ((ureteral catheter OR ureteral stent OR DJ stent OR double J stenting) AND ((lower urinary tract symptoms OR LUTS) OR morbidity OR incidence OR (prophylaxis or prevention) OR treatment OR (dysuria OR symptoms OR pain) OR (complications OR problems) OR biofilm OR infection OR (stent material OR stent design) OR questionnaire OR encrustation)) AND (etiology OR pathogenesis OR causality OR causes). Results were limited to English language, abstract and full-text availability. In addition, references of relevant articles were screened for additional important papers.
Results
We identified 17 503 records through database searching, with 160 articles finally included in this systematic review. The PRISMA flow diagram is shown in figure 1.
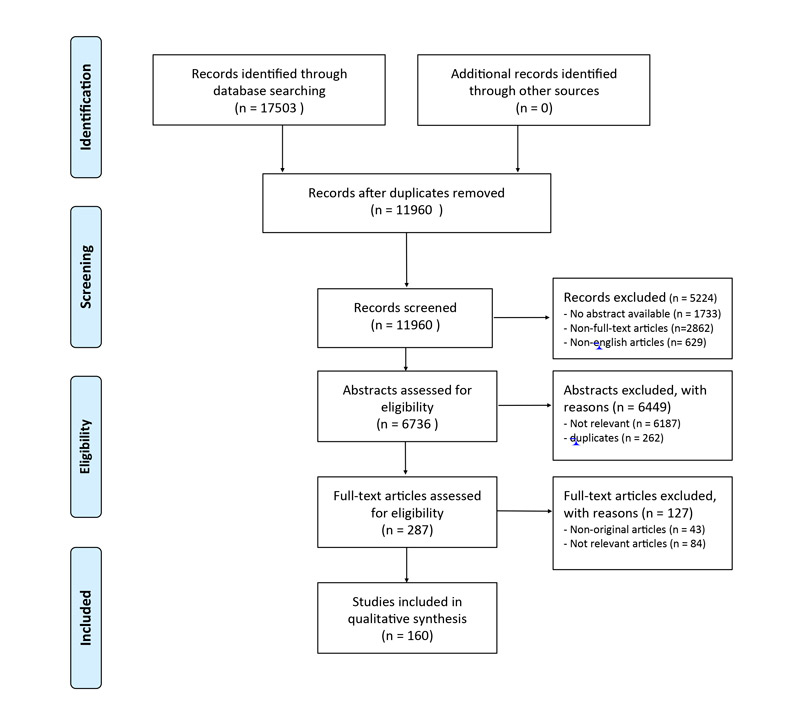
Figure 1 PRISMA flow diagram. Modified from: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. For more information, visit www.prisma-statement.org.
Development and incidence of biofilms on ureteral stents
Process of biofilm formation
In general, the development of a biofilm is regarded as a multistep process, where the first step involves the formation of a conditioning film made up of extracellular molecules [16, 17]. After insertion, the stent material comes into contact with body fluids such as urine and blood, and with uroepithelial tissue. As a result of the complex and variable composition of human urine, information on the composition of the conditioning film on stents is still limited. Elwood and co-workers examined the in vitro formation of conditioning films on stents after incubation in urine and found adsorbed cytokeratins in particular. These are glycosylated cell-surface proteins abundantly present on the surface of uroepithelial cells. Additionally, blood proteins, such as haemoglobin and fibrinogen, and inflammatory proteins appear to be involved in conditioning film formation, possibly owing to injuries and inflammation often associated with the surgical insertion of ureteral stents [18].
Even though uromodulin (Tamm-Horsfall protein), one of the most abundant urinary antimicrobial proteins, has been found in stent biofilms in most patients, it appeared not to be among the relevant proteins for conditioning film formation in the first 72 hours after insertion and seems to play only a marginal role in further biofilm development and encrustation [19]. In contrast, it has previously been shown to be a key factor in the development of conditioning films [16, 20].
It is assumed that, in a second step, these conditioning film proteins facilitate the adsorption of various molecules from the surrounding fluids and tissues, such as collagen, fibrinogen and albumin [17], which then alter the surface of the ureteral stent and may allow attachment of microorganisms [21]. However, a recent study indicates that the presence of a conditioning film might not increase bacterial adhesion and colonisation of stents by uropathogens [18]. The mechanisms of attachment of microorganisms to ureteral stent surfaces therefore still remain unclear. It has been shown that urinary pH, ionic strength, and electrostatic and hydrophobic interactions play an important role [12, 22–24]. The investigation of proteins present in encrustations and biofilms showed that five different proteins are present in high numbers: alpha-1 antitrypsin, immunoglobulin kappa (Ig kappa), immunoglobulin heavy chain G1 (IgH G1), and histones H2b, and H3a.
Bacteria attach to foreign body surfaces via various species-specific strategies that define the biofilm-building potential of an organism. For example, bacterial cell-surface appendages, such as type 3 fimbriae, are regarded as important virulence factors as they play a large role in surface attachment and biofilm formation by Klebsiella pneumoniae [25, 26] and Escherichia coli [27]. Other adhesion strategies include adhesion to secreted bacterial extracellular polymeric substances that may also contribute to conditioning-film formation [28]. Regarding the formation of mineralised biofilms on ureteral stents, urease-secreting strains, such as Pseudomonas aeruginosa and Proteus mirabilis in particular, have been extensively investigated. They secrete urease, which increases the urine pH resulting in the precipitation of struvite and hydroxyapatite crystals, adhesion factors, transporters, transcription factors, enzymes and two component systems. Of note, co-infection with two bacteria leads to synergistic induction of urease activity [29–33]. Molecular research and mutagenesis analysis are ongoing and promise a better understanding of the mechanisms of biofilm formation by bacteria [25, 32, 34–40] and fungi [41–45].
Many authors have emphasised the importance of flow dynamics in the stented ureter and the implications of vesicoureteral reflux, and several in vitro models have been developed to further investigate the process of biofilm formation [17, 23, 46–52]. However, many of the in vitro bacterial adhesion assays have been derived mostly from classic microbiological approaches, and often do not reflect important in vivo factors such as stasis vs flow, rich medium vs physiological or pathological urine environment, or the involvement of multiple species which may be synergistic or inhibitory.
In the last stage of stent biofilm development, complex biofilm structures are formed, where groups of bacteria are divided by spaces filled with surrounding fluid, and open water channels allow the transport of oxygen and nutrients to assure further cellular growth. Depending on the microorganisms involved, ureteral stent biofilms are composed 10 to 25% of cells and 75 to 90% of exopolysaccharide matrix [16], mostly with a rough, often mineralised, surface (figures 2, 3
).
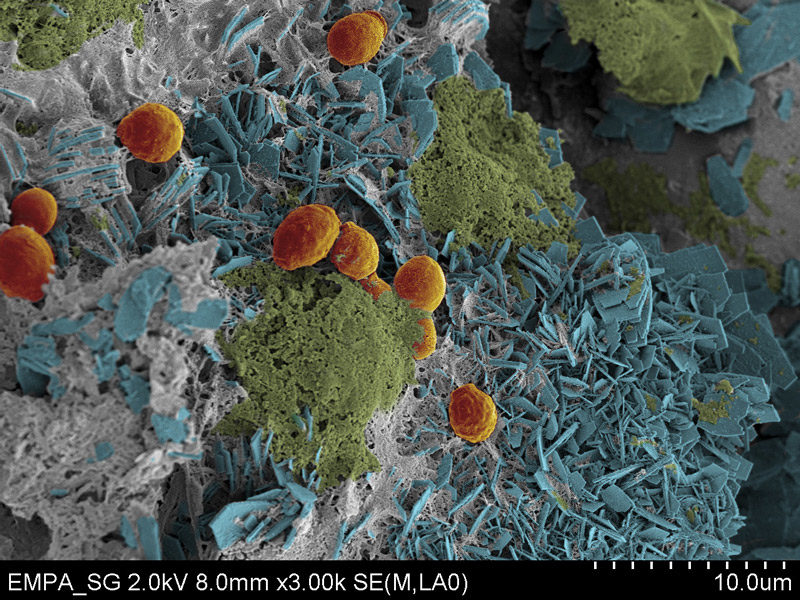
Figure 2 Colourised scanning electron micrograph of a stent biofilm showing microbes (highlighted in orange), hexagonal carbonate hydroxyapatite crystals (blue) and amorphous crystal-like structures (green). For imaging, stent sections were fixed with glutaraldehyde and formaldehyde in phosphate-buffered saline, followed by Au/Pd sputtering after chemical dehydration.

Figure 3 Example of a cross-section of a ureteral stent (left image) and a scanning electron micrograph of the stent biofilm (right image).
Timeframes of biofilm development and encrustation
Discussions about the optimum indwelling time for ureteral stents are still ongoing. One reason for this might be that only sparse data on the temporal development of stent biofilms are available. The initial steps of biofilm formation – conditioning film formation – occur immediately after stent insertion, whereas clinically significant encrustation seems to require longer indwelling times. It has been shown that after short-term antibiotic prophylaxis of 2 to 3 days, bacterial colonisation of the stent is detectable 2 weeks after implantation, and that stent colonisation precedes urine colonisation, with detection of planktonic bacteria [53, 54]. Kawahara et al. [55] described an encrustation rate of 27% at less than 6 weeks, 57% at 6 to 12 weeks, and 76% at more than 12 weeks. They did not, however, quantify the biofilm mass in detail. Rahman et al. [56] reported on colonisation rates of 24% before 4 weeks, 33% after 4 to 6 weeks, and 71% after 6 weeks. Riedl et al. [14] reported 100% ureteral stent colonisation in permanently stented patients (mean stent indwelling time 39.5 days) and 69% in the temporarily stented (mean 11 days). In a retrospective study of severely impacted ureteral stents requiring advanced removal procedures, 43% of the stents had become encrusted within 4 months and 76% within 6 months [57]. In patients with risk factors, such as diabetes mellitus, chronic renal failure and diabetic nephropathy, shorter stent indwelling times have thus been recommended because of a significantly higher risk of colonisation and bacteriuria [58].
Distribution and composition of biofilms
In spatial distribution, the biofilm mass seems to decrease towards the distal tip of the stent [59], and inner deposits seem to be very rare, even in “obstructed” stents [60]. Calcium oxalate appears to be the predominant type of encrustation, followed by struvite, in the mineralised biofilms [61], and it has been shown that the mineral composition on ureteral stents significantly correlates with stone analysis in patients with urinary stones [59, 62].
Enterococcus faecalis and E. coli seem to be the most commonly involved microbial colonizers on ureteral stents [63]. Bacteria expressing urease, such as Proteus spp., Providencia or Pseudomonas, are also involved and can induce rapid growth of biofilms. Urease activity causes alkalisation of the urine, leading to undersaturation of magnesium and calcium with resulting precipitation on the stent [61]. Other bacteria that have been associated with stent biofilm formation are Staphylococcus and Edwardsiella spp. [64–66]. However, the type of microbes identified on ureteral stents strongly depends on the method of detection, and information enabling comparisons is lacking at present. Aydin et al. [66] recently suggested that pathogens identified in urine cultures are the same as those colonising the stent. In contrast, other authors have reported that urine culture has a low sensitivity (40%) for stent colonisation [67]. In the study by Riedl et al. [14], pathogens colonising the stent were correctly identified by means of urine cultures in only 21% of patients.
Assessment of biofilms
As previously described, the stent biofilm composition and the process of biofilm development are complex, and include organic and inorganic components. Different approaches are therefore used to assess biofilms on ureteral stents.
Assessment of bacteria involved
Although several methods have been described, the most appropriate examination procedure still needs to be defined. The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines 2015 propose assessment by use of microscopy, and with culture or culture-independent techniques, preferably after sonication to investigate microbial diversity [68]. However, these recommendations do not guarantee complete release of biofilm from stents. Bonkat et al. [69] showed advantages over sonication with a roll-plate technique in the diagnosis of microbial ureteral stent colonisation. They also reported that urine culture is less sensitive than both sonication and the roll-plate technique.
Recently, Choe et al. [64] showed that different techniques must be applied simultaneously to increase the detection of bacterial species in a urinary catheter biofilm. They compared four different 16S ribosomal RNA analysis techniques: capillary electrophoresis, terminal restriction fragment length polymorphism, denaturing gradient gel electrophoresis and pyrosequencing. All showed different bacteria. Wilks et al. [70] described the combination of an advanced light microscopy technique, episcopic differential interference contrast microscopy with epifluorescence, as a real-time imaging method to track all stages of biofilm development.
Mineral composition
Both X-ray diffraction [59, 61] and optical coherence tomography [71] are feasible and effective methods [62] to determine the specific mineralogy of encrustations. Furthermore, Bithelis et al. [61] reported that Fourier-transform infrared spectroscopy was superior to classical scanning electron microscopy.
Quantification of biofilms
Little has been published on quantitative biofilm analysis of ureteral stents. As mentioned above, the overall spatial distribution of the stent biofilms is somewhat inhomogeneous. Bithelis et al. [61] analysed the mean mass of encrustation per stent, finding 71 mg on average in stone-forming patients compared with 1 mg in patients with no history of stone formation. Sighinolfi et al. [59] assessed the weight of encrustations separately at different positions along the ureteral stent. The median weight of encrustation was 6 mg/cm at the proximal end and 3 mg/cm at the distal end of the stent. However, no well-defined methods to assess total biofilm mass on ureteral stents or to reliably quantify bacterial load have been published.
Clinical impact of biofilms
Impact on stent-associated symptoms
Ureteral stents have a well-defined side effect profile. Amongst other complaints, irritation on voiding and stent-related pain affecting daily activities have been reported in 78 and 80% of patients [2], and 42% of patients suffer from haematuria [3]. Only two studies have assessed the influence of biofilms on stent-associated symptoms. Bonkat et al. showed a significant association between biofilms on ureteral stents and the incidence of lower urinary tract symptoms [72]. Moreover, a longer stent indwelling time and positive urine cultures have been reported to be significantly associated with patient discomfort [73]. Nevertheless, a negative impact of biofilms on stent-associated symptoms seems to be plausible, even though the correlation of biofilms and stent comfort has not yet been investigated sufficiently. In particular, studies using the USSQ [5], a validated questionnaire assessing the whole spectrum of stent-associated morbidity, are not available to date.
Impact on urinary tract infections and bacteriuria
Stent colonisation has been reported to precede urine colonisation [54]. However, stent colonisation does not always entail symptomatic UTI, particularly given that the bacteria may live the biofilm lifestyle instead of detaching and living as planktonic swarmers [66, 74]. As urine cultures have a low sensitivity (40%) for stent colonisation, a negative culture does not rule out a colonised stent [67]. On the day of stent removal, only 13% of patients with colonised stents showed bacteria (>105 colony-forming units/ml) in culture of urine obtained prior to stent removal (mean indwelling time of stent 33.9 ± 22.4 days) [66]. However, these are controversial findings, as other authors found sensitivities of 21 to 93% [75, 76]. Moreover, bacteria from stented patients, even when cultured from urine, are more resistant to antibiotics than those cultured from urine before stent insertion. This is probably associated with the expression of biofilm-specific genes [67]. Thus, even in the case of sterile urine cultures, secondary endoscopic procedures after stent removal are likely to put the patients at an increased risk of infectious complications.
UTIs associated with indwelling ureteral stents are most frequently caused by E. coli, Enterococcus spp., Staphylococcus spp., Pseudomonas spp. and Candida spp. [67, 74]. Comorbidities such as chronic renal failure, diabetes and pregnancy have been shown to increase the risk of lower UTIs associated with ureteral stents [77]. A study assessing complications after renal transplantation concluded that early removal of the stent 2 weeks after renal transplantation decreases morbidity and UTI rates [78].
Impact on stent encrustation and obstruction
Markedly encrusted ureteral stents can pose a serious challenge to the urologist when the stent has to be removed or changed. In serious cases, a multimodal approach using extracorporeal shock wave lithotripsy, ureteroscopy or even more invasive techniques may be necessary [57]. As the grade of encrustation is related to the indwelling time [55], “forgotten stents” in particular often give rise to problems [79].
Obstruction of the stent can lead to upper urinary tract retention resulting in flank pain, deterioration of renal function, obstructive pyelonephritis and even sepsis, and therefore often requires surgical intervention. However, even completely obstructed stents often pass unnoticed as the urine is still often able to pass along the dysfunctional stent [79, 80].
Prevention and treatment
Antimicrobials
Several studies have emphasised the efficacy of fluoroquinolones in preventing biofilm formation. However, the use of antibiotics at the time of stent insertion can only postpone but not prevent biofilm formation [14], and eradication of already preformed biofilms cannot be achieved by antibiotics [81–83]. To reinforce the antibacterial activity of fluoroquinolones, various combinations have shown promising effects. Pentacyclic triterpenes [84], N-acetylcysteine [85] or rifampin [81] may enhance the antibiotic activity of fluoroquinolones, even against preformed mature biofilms [85]. However, none of these combinations have found their way into clinical practice, and bacterial diversity and antibiotic resistance present an increasing problem in clinical practice.
Besides antibiotics, cis-2-decenoic acid, an unsaturated fatty acid, showed promising results in the prevention of biofilm formation on catheter biofilms [86]. Along with the ability to inhibit biofilm formation by P. aeruginosa, cis-2-decenoic acid is capable of inducing the dispersion of established biofilms formed by multiple types of microorganisms.
Although some of the experimental approaches described above showed promising results against specific microbes, clinical implementation is difficult due to the variety of bacteria involved in the process of biofilm formation.
Stent materials
Important properties of an ideal stent are easy insertion, resistance to compression and migration, biodurability and biocompatibility [87]. Although numerous types of stent have attempted to meet these requirements, the ideal stent has yet to be created.
While soft materials – synthetic polymeric compounds such as silicone or polyurethane – might be associated with a lower incidence of irritation [87, 88], metal stents seem to perform better in patients with ureteral compression due to extrinsic malignant obstruction. However, the problem of encrustation and bacterial adhesion remains the same [89].
New promising designs, such as novel, gel-based ureteral stents [90] or the use of an electrical microcurrent to prevent biofilm formation [91, 92] are still at the developmental stage, and clinical data have not yet been published. At the moment, none of the materials on the market can prevent or reduce biofilm formation on ureteral stents to a clinically relevant extent. An overview of different stent materials and their performance is given in table 1.
Table 1 Ureteral stent materials and their performance in preventing biofilm formation.
|
Stent material
|
Specification
|
Performance
|
Clinical stage /
availability
|
| Polyurethane stents |
– “Soft” ureteral double-pigtail catheter (Sof-Flex®)
– “Firm” ureteral double-pigtail catheter (classic polyurethane stent) |
– No differences in encrustation and bacterial adhesion of the stents in vivo [88]
– Less stent related dysuria and pain in patients with “soft” stent type [88] |
Both stent types are commercially available |
| Metal stents |
– Self-expandable ureteral stent (Wallstent®, cobalt-based alloy)
– Thermo-expandable ureteral stent (Memokath®, nickel-titanium alloy)
– “Coil” design ureteral double-pigtail catheter (Resonance®, nickel-cobalt-chromium-molybdenum alloy) |
– No advantages regarding encrustation in vivo
– Fewer urinary tract infections reported in vivo
– Good performance in malignant ureteric obstruction (especially Memokath®)
– Poor performance in benign ureteral obstruction [89] |
All three stents are commercially available |
| Gel-based stents |
– Gel-based stent (pAguaMedicina®, highly hydrated, partially hydrolysed polyacrylonitrile polymer) |
– Significant reduction (43–71%) of bacterial adhesion and biofilm formation in vitro, not yet tested in vivo [90] |
Commercially available for paediatric use. |
Coatings
A further approach to preventing biofilm formation is the principle of coating ureteral stents. Although heparin-coated stents significantly reduced ureteral stent encrustation, no positive effect against bacterial adhesion was seen [89, 93, 94]. In the past, hydrogel-based coatings raised expectations that they would effectively inhibit hydroxyapatite encrustation and bacterial biofilm colonisation, and reduce general stent-related morbidity [95, 96]. However, in 2007, John et al. dampened these expectations when they showed that bacterial adhesions were similar using stents with and without hydrogel-based coatings [97]. Laube et al. examined the effect of diamond-like carbon coatings in vivo and reported a decrease in encrustation and biofilm formation [98]. In principle, stents can also be coated with various active compounds such as antimicrobials or enzymes. After initial encouraging results, a recently published study showed that triclosan-eluting stents had no significant impact on biofilm formation, encrustation or infection development in short-term stented patients [99]. Some combinations of antimicrobials effectively inhibit biofilm-forming properties of UTI-specific bacteria [100–102]. However, the (co-)induction of antibiotic resistance and the wide range of biofilm-forming bacteria limited the effect of such coatings and their implementation in clinical practice. Nevertheless, initial results indicate that an enzyme-based approach might be an alternative to conventional antibiotic coatings. Watterson et al. [103] found less encrustation on oxalate degrading enzyme-coated silicone, and the most recent findings showed that the use of nanoscale bodies might be promising. Francesko et al. [104] described nanoscale structures in bacteria-responsive surface coatings on medical indwelling devices acting via antibacterial and self-defensive properties. An overview of coating techniques and their influence on biofilm formation is shown in table 2.
Table 2 Coating techniques and their influence on biofilm formation.
|
Coating technique
|
Performance
|
Clinical stage /
availability
|
| Heparin coating |
– Significant reduction of encrustation in vivo
– No effect on bacterial adhesion in vivo [93–95] |
Commercially available product (Radiance®) |
| Hydrogel-based coatings |
– Significant reduction of encrustation in vitro
– No effect on bacterial adhesion in vitro [97] |
Commercially available product (HydroPlus® coating) |
| Diamond-like carbon coatings |
– Decrease of encrustation and biofilm formation in vitro and in vivo [98] |
Commercially available product (VisioSafe DIAMOND®) |
| Triclosan-eluting stents |
– No significant reduction of encrustation or biofilm formation in vitro and in vivo
– Significant reduction of stent-related flank pain, abdominal pain and urethral pain [99] |
Formerly commercially available (Triumph®); Withdrawn from market because of concerns about the development of bacterial resistance |
| Oxalate degrading enzyme coatings |
– Significant reduction of encrustation, no results on bacterial adhesion in vitro and in vivo (animal study) [103] |
Not yet commercially available |
| Nanoscale-body coating |
– Antibacterial effects resulting in effective prevention of Pseudomonas aeruginosa biofilm formation in vitro [104] |
Not yet commercially available |
As with stent materials, one of the problems in the development of more effective stent coatings seems to be the absence of standardised testing with well-elaborated in vitro models. High manufacturing costs are another limitation for the implementation of promising materials and coatings in clinical practice.
Other methods of biofilm prevention
Correction of metabolic alterations in urine such as hyperoxaluria, hypocitruria or reduced volume can have a positive effect on encrustation and biofilm formation. Bithelis et al. [61] showed that these factors, which are often responsible for stone formation, might also enhance the risk of biofilm forming on ureteral stents.
Discussion
Ureteral stents are a simple, minimally invasive method to ensure urinary transport through the upper urinary tract. They also offer an ideal surface for biofilm formation.
The process of biofilm formation on ureteral stents is complex and includes the early formation of a conditioning film and subsequent accumulation of organic and inorganic molecules, as well as adhesion and colonisation by a variety of uropathogens. Finally, an extracellular polymeric matrix can become the main component of the biofilm, and a structure is formed that ensures nutrition and protection of the microorganisms involved, which show reduced growth rate together with increased resistance to antibiotic therapy.
Great efforts have been made to understand the processes and timeframes of biofilm formation and its composition and distribution. Further research is, however, imperative, as a better understanding of biofilm formation would provide novel approaches for its prevention and treatment.
A lack of well-defined and validated examination methods, especially for the identification and quantification of the bacteria involved, is an important limitation at present.
Existing evidence clearly suggests that stents more resistant to biofilm formation might significantly reduce the incidence of stent encrustation, obstruction and dysfunction, as well as associated UTI. This would probably improve quality of life and also reduce the economic burden of ureteral stenting. It is surprising that the influence of biofilms and encrustation on patient symptoms has hardly been investigated. This should be subject of future clinical trials, where the use of the validated Ureteral Stent Symptom Questionnaire (USSQ) is strongly recommended to assess the whole spectrum of stent-associated morbidity and facilitate better comparisons of findings.
So far, the possibilities for preventing and treating biofilms on ureteral stents are limited. The main reasons for the absence of stents more resistant to biofilm formation are the complexity of biofilm formation with several different bacterial species involved, as well as the formation of host urinary conditioning films that additionally compromise efforts to develop effective coatings and surfaces. To overcome this, more elaborated in vitro biofilm models simulating in vivo conditions as closely as possible are required so that promising novel stent materials and coatings can be more effectively tested.
Conclusion
Even though many aspects are still unclear, biofilms on ureteral stents appear to be a key factor in the associated morbidity. Thus, further research in this field seems to be worthwhile to reduce the morbidity associated with ureteral stenting.
Authors’ contribution
VZ and PB contributed equally to this work.
Valentin Zumstein, MD, Department of Urology, Rorschacherstrasse 95, Kantonsspital St Gallen, CH-9007 St Gallen, valentin.zumstein[at]kssg.ch
References
1
Liatsikos
E
,
Kallidonis
P
,
Stolzenburg
JU
,
Karnabatidis
D
. Ureteral stents: past, present and future. Expert Rev Med Devices. 2009;6(3):313–24. doi:.https://doi.org/10.1586/erd.09.5
2
Joshi
HB
,
Stainthorpe
A
,
Keeley
FX, Jr
,
MacDonagh
R
,
Timoney
AG
. Indwelling ureteral stents: evaluation of quality of life to aid outcome analysis. J Endourol. 2001;15(2):151–4. doi:.https://doi.org/10.1089/089277901750134421
3
Leibovici
D
,
Cooper
A
,
Lindner
A
,
Ostrowsky
R
,
Kleinmann
J
,
Velikanov
S
, et al.
Ureteral stents: morbidity and impact on quality of life. Isr Med Assoc J. 2005;7(8):491–4.
4
Staubli
SE
,
Mordasini
L
,
Engeler
DS
,
Sauter
R
,
Schmid
HP
,
Abt
D
. Economic Aspects of Morbidity Caused by Ureteral Stents. Urol Int. 2016;97(1):91–7. doi:.https://doi.org/10.1159/000443379
5
Joshi
HB
,
Newns
N
,
Stainthorpe
A
,
MacDonagh
RP
,
Keeley
FX, Jr
,
Timoney
AG
. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169(3):1060–4. doi:.https://doi.org/10.1097/01.ju.0000049198.53424.1d
6
Lamb
AD
,
Vowler
SL
,
Johnston
R
,
Dunn
N
,
Wiseman
OJ
. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int. 2011;108(11):1894–902. doi:.https://doi.org/10.1111/j.1464-410X.2011.10170.x
7
Park
SC
,
Jung
SW
,
Lee
JW
,
Rim
JS
. The effects of tolterodine extended release and alfuzosin for the treatment of double-j stent-related symptoms. J Endourol. 2009;23(11):1913–7. doi:.https://doi.org/10.1089/end.2009.0173
8
Abt
D
,
Warzinek
E
,
Schmid
HP
,
Haile
SR
,
Engeler
DS
. Influence of patient education on morbidity caused by ureteral stents. Int J Urol. 2015;22(7):679–83. doi:.https://doi.org/10.1111/iju.12782
9
Abt
D
,
Mordasini
L
,
Warzinek
E
,
Schmid
HP
,
Haile
SR
,
Engeler
DS
, et al.
Is intravesical stent position a predictor of associated morbidity?
Korean J Urol. 2015;56(5):370–8. doi:.https://doi.org/10.4111/kju.2015.56.5.370
10
Giannarini
G
,
Keeley
FX, Jr
,
Valent
F
,
Manassero
F
,
Mogorovich
A
,
Autorino
R
, et al.
Predictors of morbidity in patients with indwelling ureteric stents: results of a prospective study using the validated Ureteric Stent Symptoms Questionnaire. BJU Int. 2011;107(4):648–54. doi:.https://doi.org/10.1111/j.1464-410X.2010.09482.x
11
Walker
NA
,
Bultitude
MF
,
Brislane
K
,
Thomas
K
,
Glass
JM
. Management of stent symptoms: what a pain!
BJU Int. 2014;114(6):797–8. doi:.https://doi.org/10.1111/bju.12534
12
Tenke
P
,
Köves
B
,
Nagy
K
,
Hultgren
SJ
,
Mendling
W
,
Wullt
B
, et al.
Update on biofilm infections in the urinary tract. World J Urol. 2012;30(1):51–7. doi:.https://doi.org/10.1007/s00345-011-0689-9
13
Farsi
HMA
,
Mosli
HA
,
Al-Zemaity
MF
,
Bahnassy
AA
,
Alvarez
M
. Bacteriuria and colonization of double-pigtail ureteral stents: long-term experience with 237 patients. J Endourol. 1995;9(6):469–72. doi:.https://doi.org/10.1089/end.1995.9.469
14
Riedl
CR
,
Plas
E
,
Hübner
WA
,
Zimmerl
H
,
Ulrich
W
,
Pflüger
H
. Bacterial colonization of ureteral stents. Eur Urol. 1999;36(1):53–9. doi:.https://doi.org/10.1159/000019927
15
Moher
D
,
Liberati
A
,
Tetzlaff
J
,
Altman
DG
,
Group
P
. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. doi:.https://doi.org/10.1016/j.jclinepi.2009.06.005
16
Tenke
P
,
Kovacs
B
,
Jäckel
M
,
Nagy
E
. The role of biofilm infection in urology. World J Urol. 2006;24(1):13–20. doi:.https://doi.org/10.1007/s00345-005-0050-2
17
Venkatesan
N
,
Shroff
S
,
Jeyachandran
K
,
Doble
M
. Effect of uropathogens on in vitro encrustation of polyurethane double J ureteral stents. Urol Res. 2011;39(1):29–37. doi:.https://doi.org/10.1007/s00240-010-0280-7
18
Elwood
CN
,
Lo
J
,
Chou
E
,
Crowe
A
,
Arsovska
O
,
Adomat
H
, et al.
Understanding urinary conditioning film components on ureteral stents: profiling protein components and evaluating their role in bacterial colonization. Biofouling. 2013;29(9):1115–22. doi:.https://doi.org/10.1080/08927014.2013.829049
19
Canales
BK
,
Higgins
L
,
Markowski
T
,
Anderson
L
,
Li
QA
,
Monga
M
. Presence of five conditioning film proteins are highly associated with early stent encrustation. J Endourol. 2009;23(9):1437–42. doi:.https://doi.org/10.1089/end.2009.0389
20
Santin
M
,
Motta
A
,
Denyer
SP
,
Cannas
M
. Effect of the urine conditioning film on ureteral stent encrustation and characterization of its protein composition. Biomaterials. 1999;20(13):1245–51. doi:.https://doi.org/10.1016/S0142-9612(99)00026-5
21
Busscher
HJ
,
van der Mei
HC
,
Schakenraad
JM
. Analogies in the two-dimensional spatial arrangement of adsorbed proteins and adhering bacteria: bovine serum albumin and Streptococcus sanguis 12. J Biomater Sci Polym Ed. 1992;3(1):85–94. doi:.https://doi.org/10.1163/156856292X00097
22
Choong
S
,
Whitfield
H
. Biofilms and their role in infections in urology. BJU Int. 2000;86(8):935–41. doi:.https://doi.org/10.1046/j.1464-410x.2000.00949.x
23
Jones
DS
,
Djokic
J
,
Gorman
SP
. Characterization and optimization of experimental variables within a reproducible bladder encrustation model and in vitro evaluation of the efficacy of urease inhibitors for the prevention of medical device-related encrustation. J Biomed Mater Res B Appl Biomater. 2006;76B(1):1–7. doi:.https://doi.org/10.1002/jbm.b.30230
24
Hedelin
H
. Uropathogens and urinary tract concretion formation and catheter encrustations. Int J Antimicrob Agents. 2002;19(6):484–7. doi:.https://doi.org/10.1016/S0924-8579(02)00095-X
25
Boddicker
JD
,
Anderson
RA
,
Jagnow
J
,
Clegg
S
. Signature-tagged mutagenesis of Klebsiella pneumoniae to identify genes that influence biofilm formation on extracellular matrix material. Infect Immun. 2006;74(8):4590–7. doi:.https://doi.org/10.1128/IAI.00129-06
26
Schroll
C
,
Barken
KB
,
Krogfelt
KA
,
Struve
C
. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010;10(1):179. doi:.https://doi.org/10.1186/1471-2180-10-179
27
Ong
CL
,
Ulett
GC
,
Mabbett
AN
,
Beatson
SA
,
Webb
RI
,
Monaghan
W
, et al.
Identification of type 3 fimbriae in uropathogenic Escherichia coli reveals a role in biofilm formation. J Bacteriol. 2008;190(3):1054–63. doi:.https://doi.org/10.1128/JB.01523-07
28
Petrova
OE
,
Sauer
K
. Sticky situations: key components that control bacterial surface attachment. J Bacteriol. 2012;194(10):2413–25. doi:.https://doi.org/10.1128/JB.00003-12
29
Jacobsen
SM
,
Shirtliff
ME
. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence. 2011;2(5):460–5. doi:.https://doi.org/10.4161/viru.2.5.17783
30
Armbruster
CE
,
Smith
SN
,
Yep
A
,
Mobley
HL
. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. J Infect Dis. 2014;209(10):1524–32. doi:.https://doi.org/10.1093/infdis/jit663
31
Hola
V
,
Peroutkova
T
,
Ruzicka
F
. Virulence factors in Proteus bacteria from biofilm communities of catheter-associated urinary tract infections. FEMS Immunol Med Microbiol. 2012;65(2):343–9. doi:.https://doi.org/10.1111/j.1574-695X.2012.00976.x
32
Holling
N
,
Lednor
D
,
Tsang
S
,
Bissell
A
,
Campbell
L
,
Nzakizwanayo
J
, et al.
Elucidating the genetic basis of crystalline biofilm formation in Proteus mirabilis. Infect Immun. 2014;82(4):1616–26. doi:.https://doi.org/10.1128/IAI.01652-13
33
Jones
SM
,
Yerly
J
,
Hu
Y
,
Ceri
H
,
Martinuzzi
R
. Structure of Proteus mirabilis biofilms grown in artificial urine and standard laboratory media. FEMS Microbiol Lett. 2007;268(1):16–21. doi:.https://doi.org/10.1111/j.1574-6968.2006.00587.x
34
d’Enfert
C
,
Janbon
G
. Biofilm formation in Candida glabrata: What have we learnt from functional genomics approaches?
FEMS Yeast Res. 2016;16(1):fov111. doi:.https://doi.org/10.1093/femsyr/fov111
35
Fattahi
S
,
Kafil
HS
,
Nahai
MR
,
Asgharzadeh
M
,
Nori
R
,
Aghazadeh
M
. Relationship of biofilm formation and different virulence genes in uropathogenic Escherichia coli isolates from Northwest Iran. GMS Hyg Infect Control. 2015;10:Doc11.
36
Hadjifrangiskou
M
,
Gu
AP
,
Pinkner
JS
,
Kostakioti
M
,
Zhang
EW
,
Greene
SE
, et al.
Transposon mutagenesis identifies uropathogenic Escherichia coli biofilm factors. J Bacteriol. 2012;194(22):6195–205. doi:.https://doi.org/10.1128/JB.01012-12
37
Newman
JA
,
Rodrigues
C
,
Lewis
RJ
. Molecular basis of the activity of SinR protein, the master regulator of biofilm formation in Bacillus subtilis. J Biol Chem. 2013;288(15):10766–78. doi:.https://doi.org/10.1074/jbc.M113.455592
38
Orme
R
,
Douglas
CW
,
Rimmer
S
,
Webb
M
. Proteomic analysis of Escherichia coli biofilms reveals the overexpression of the outer membrane protein OmpA. Proteomics. 2006;6(15):4269–77. doi:.https://doi.org/10.1002/pmic.200600193
39
Orlando
F
,
Ghiselli
R
,
Cirioni
O
,
Minardi
D
,
Tomasinsig
L
,
Mocchegiani
F
, et al.
BMAP-28 improves the efficacy of vancomycin in rat models of gram-positive cocci ureteral stent infection. Peptides. 2008;29(7):1118–23. doi:.https://doi.org/10.1016/j.peptides.2008.03.005
40
Schembri
MA
,
Klemm
P
. Biofilm formation in a hydrodynamic environment by novel fimh variants and ramifications for virulence. Infect Immun. 2001;69(3):1322–8. doi:.https://doi.org/10.1128/IAI.69.3.1322-1328.2001
41
Girish Kumar
CP
,
Menon
T
. Biofilm production by clinical isolates of Candida species. Med Mycol. 2006;44(1):99–101. doi:.https://doi.org/10.1080/13693780500338084
42
Marcus
RJ
,
Post
JC
,
Stoodley
P
,
Hall-Stoodley
L
,
McGill
RL
,
Sureshkumar
KK
, et al.
Biofilms in nephrology. Expert Opin Biol Ther. 2008;8(8):1159–66. doi:.https://doi.org/10.1517/14712598.8.8.1159
43
Mathé
L
,
Van Dijck
P
. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet. 2013;59(4):251–64. doi:.https://doi.org/10.1007/s00294-013-0400-3
44
Negri
M
,
Silva
S
,
Breda
D
,
Henriques
M
,
Azeredo
J
,
Oliveira
R
. Candida tropicalis biofilms: effect on urinary epithelial cells. Microb Pathog. 2012;53(2):95–9. doi:.https://doi.org/10.1016/j.micpath.2012.05.006
45Nett JE, Andes DR. Fungal Biofilms: In Vivo Models for Discovery of Anti-Biofilm Drugs. Microbiol Spectr 3 (2015).
46
Carugo
D
,
Elmahdy
M
,
Zhao
X
,
Drake
MJ
,
Zhang
X
,
Clavica
F
. An artificial model for studying fluid dynamics in the obstructed and stented ureter. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5335–8.
47
Clavica
F
,
Zhao
X
,
ElMahdy
M
,
Drake
MJ
,
Zhang
X
,
Carugo
D
. Investigating the flow dynamics in the obstructed and stented ureter by means of a biomimetic artificial model. PLoS One. 2014;9(2):e87433. doi:.https://doi.org/10.1371/journal.pone.0087433
48
Gorman
SP
,
Garvin
CP
,
Quigley
F
,
Jones
DS
. Design and validation of a dynamic flow model simulating encrustation of biomaterials in the urinary tract. J Pharm Pharmacol. 2003;55(4):461–8. doi:.https://doi.org/10.1211/002235702856
49
Robert
M
,
Boularan
AM
,
El Sandid
M
,
Grasset
D
. Double-J ureteric stent encrustations: clinical study on crystal formation on polyurethane stents. Urol Int. 1997;58(2):100–4. doi:.https://doi.org/10.1159/000282959
50
Shaheen
T
,
Edirisinghe
T
,
Gabriel
M
,
Bourdoumis
A
,
Buchholz
N
,
Knight
M
. In vitro encrustation of a semi-permanent polymer-covered nitinol ureter stent: an artificial urine model. Urolithiasis. 2014;42(3):203–7. doi:.https://doi.org/10.1007/s00240-014-0652-5
51
Tunney
MM
,
Keane
PF
,
Jones
DS
,
Gorman
SP
. Comparative assessment of ureteral stent biomaterial encrustation. Biomaterials. 1996;17(15):1541–6. doi:.https://doi.org/10.1016/0142-9612(96)89780-8
52
Waters
SL
,
Heaton
K
,
Siggers
JH
,
Bayston
R
,
Bishop
M
,
Cummings
LJ
, et al.
Ureteric stents: investigating flow and encrustation. Proc Inst Mech Eng H. 2008;222(4):551–61. doi:.https://doi.org/10.1243/09544119JEIM317
53
Paick
SH
,
Park
HK
,
Oh
SJ
,
Kim
HH
. Characteristics of bacterial colonization and urinary tract infection after indwelling of double-J ureteral stent. Urology. 2003;62(2):214–7. doi:.https://doi.org/10.1016/S0090-4295(03)00325-X
54
Lojanapiwat
B
. Colonization of internal ureteral stent and bacteriuria. World J Urol. 2006;24(6):681–3. doi:.https://doi.org/10.1007/s00345-006-0135-6
55
Kawahara
T
,
Ito
H
,
Terao
H
,
Yoshida
M
,
Matsuzaki
J
. Ureteral stent encrustation, incrustation, and coloring: morbidity related to indwelling times. J Endourol. 2012;26(2):178–82. doi:.https://doi.org/10.1089/end.2011.0385
56
Rahman
MA
,
Alam
MM
,
Shamsuzzaman
SM
,
Haque
ME
. Evaluation of bacterial colonization and bacteriuria secondary to internal ureteral stent. Mymensingh Med J. 2010;19(3):366–71.
57
Bultitude
MF
,
Tiptaft
RC
,
Glass
JM
,
Dasgupta
P
. Management of encrusted ureteral stents impacted in upper tract. Urology. 2003;62(4):622–6. doi:.https://doi.org/10.1016/S0090-4295(03)00506-5
58
Kehinde
EO
,
Rotimi
VO
,
Al-Awadi
KA
,
Abdul-Halim
H
,
Boland
F
,
Al-Hunayan
A
, et al.
Factors predisposing to urinary tract infection after J ureteral stent insertion. J Urol. 2002;167(3):1334–7. doi:.https://doi.org/10.1016/S0022-5347(05)65294-9
59
Sighinolfi
MC
,
Sighinolfi
GP
,
Galli
E
,
Micali
S
,
Ferrari
N
,
Mofferdin
A
, et al.
Chemical and Mineralogical Analysis of Ureteral Stent Encrustation and Associated Risk Factors. Urology. 2015;86(4):703–6. doi:.https://doi.org/10.1016/j.urology.2015.05.015
60
Laube
N
,
Kleinen
L
,
Avrutin
V
,
Böde
U
,
Meissner
A
,
Fisang
C
. The distribution of crystalline material in obstructed stents--in need for intra-luminal surface modification?
J Biomed Mater Res B Appl Biomater. 2008;87B(2):590–7. doi:.https://doi.org/10.1002/jbm.b.31132
61
Bithelis
G
,
Bouropoulos
N
,
Liatsikos
EN
,
Perimenis
P
,
Koutsoukos
PG
,
Barbalias
GA
. Assessment of encrustations on polyurethane ureteral stents. J Endourol. 2004;18(6):550–6. doi:.https://doi.org/10.1089/end.2004.18.550
62
Rouprêt
M
,
Daudon
M
,
Hupertan
V
,
Gattegno
B
,
Thibault
P
,
Traxer
O
. Can ureteral stent encrustation analysis predict urinary stone composition?
Urology. 2005;66(2):246–51. doi:.https://doi.org/10.1016/j.urology.2005.03.054
63
Brotherhood
H
,
Lange
D
,
Chew
BH
. Advances in ureteral stents. Transl Androl Urol. 2014;3(3):314–9.
64
Choe
HS
,
Son
SW
,
Choi
HA
,
Kim
HJ
,
Ahn
SG
,
Bang
JH
, et al.
Analysis of the distribution of bacteria within urinary catheter biofilms using four different molecular techniques. Am J Infect Control. 2012;40(9):e249–54. doi:.https://doi.org/10.1016/j.ajic.2012.05.010
65
Keane
PF
,
Bonner
MC
,
Johnston
SR
,
Zafar
A
,
Gorman
SP
. Characterization of biofilm and encrustation on ureteric stents in vivo. Br J Urol. 1994;73(6):687–91. doi:.https://doi.org/10.1111/j.1464-410X.1994.tb07557.x
66
Aydin
HR
,
Irkilata
L
,
Aydin
M
,
Gorgun
S
,
Demirel
HC
,
Adanur
S
, et al.
Incidence of bacterial colonisation after indwelling of double-J ureteral stent. Arch Ital Urol Androl. 2016;87(4):291–4. doi:.https://doi.org/10.4081/aiua.2015.4.291
67
Kehinde
EO
,
Rotimi
VO
,
Al-Hunayan
A
,
Abdul-Halim
H
,
Boland
F
,
Al-Awadi
KA
. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18(9):891–6. doi:.https://doi.org/10.1089/end.2004.18.891
68
Høiby
N
,
Bjarnsholt
T
,
Moser
C
,
Bassi
GL
,
Coenye
T
,
Donelli
G
, et al.; ESCMID Study Group for Biofilms and Consulting External Expert Werner Zimmerli. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21(Suppl 1):S1–25. doi:.https://doi.org/10.1016/j.cmi.2014.10.024
69
Bonkat
G
,
Braissant
O
,
Rieken
M
,
Müller
G
,
Frei
R
,
van der Merwe
A
, et al.
Comparison of the roll-plate and sonication techniques in the diagnosis of microbial ureteral stent colonisation: results of the first prospective randomised study. World J Urol. 2013;31(3):579–84. doi:.https://doi.org/10.1007/s00345-012-0963-5
70
Wilks
SA
,
Fader
MJ
,
Keevil
CW
. Novel Insights into the Proteus mirabilis Crystalline Biofilm Using Real-Time Imaging. PLoS One. 2015;10(10):e0141711. doi:.https://doi.org/10.1371/journal.pone.0141711
71
Bader
MJ
,
Zilinberg
K
,
Weidlich
P
,
Waidelich
R
,
Püls
M
,
Gratzke
C
, et al.
Encrustation of urologic double pigtail catheters-an ex vivo optical coherence tomography (OCT) study. Lasers Med Sci. 2013;28(3):919–24. doi:.https://doi.org/10.1007/s10103-012-1177-1
72
Bonkat
G
,
Rieken
M
,
Müller
G
,
Roosen
A
,
Siegel
FP
,
Frei
R
, et al.
Microbial colonization and ureteral stent-associated storage lower urinary tract symptoms: the forgotten piece of the puzzle?
World J Urol. 2013;31(3):541–6. doi:.https://doi.org/10.1007/s00345-012-0849-6
73
El-Nahas
AR
,
El-Assmy
AM
,
Shoma
AM
,
Eraky
I
,
El-Kenawy
MR
,
El-Kappany
HA
. Self-retaining ureteral stents: analysis of factors responsible for patients’ discomfort. J Endourol. 2006;20(1):33–7. doi:.https://doi.org/10.1089/end.2006.20.33
74
Ben-Meir
D
,
Golan
S
,
Ehrlich
Y
,
Livne
PM
. Characteristics and clinical significance of bacterial colonization of ureteral double-J stents in children. J Pediatr Urol. 2009;5(5):355–8. doi:.https://doi.org/10.1016/j.jpurol.2009.01.001
75
Al-Ghazo
MA
,
Ghalayini
IF
,
Matani
YS
,
El-Radaideh
KM
,
Haddad
HI
. The risk of bacteriuria and ureteric stent colonization in immune-compromised patients with double J stent insertion. Int Urol Nephrol. 2010;42(2):343–7. doi:.https://doi.org/10.1007/s11255-009-9607-0
76
Yeniyol
CO
,
Tuna
A
,
Yener
H
,
Zeyrek
N
,
Tilki
A
,
Coskuner
A
. Bacterial colonization of double J stents and bacteriuria frequency. Int Urol Nephrol. 2002;34(2):199–202. doi:.https://doi.org/10.1023/A:1023285422278
77
Akay
AF
,
Aflay
U
,
Gedik
A
,
Sa̧hin
H
,
Bircan
MK
. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39(1):95–8. doi:.https://doi.org/10.1007/s11255-006-9150-1
78
Coskun
AK
,
Harlak
A
,
Ozer
T
,
Eyitilen
T
,
Yigit
T
,
Demirbaş
S
, et al.
Is removal of the stent at the end of 2 weeks helpful to reduce infectious or urologic complications after renal transplantation?
Transplant Proc. 2011;43(3):813–5. doi:.https://doi.org/10.1016/j.transproceed.2010.11.016
79
Ahallal
Y
,
Khallouk
A
,
El Fassi
MJ
,
Farih
MH
. Risk factor analysis and management of ureteral double-j stent complications. Rev Urol. 2010;12(2-3):e147–51.
80
Singh
I
,
Gupta
NP
,
Hemal
AK
,
Aron
M
,
Seth
A
,
Dogra
PN
. Severely encrusted polyurethane ureteral stents: management and analysis of potential risk factors. Urology. 2001;58(4):526–31. doi:.https://doi.org/10.1016/S0090-4295(01)01317-6
81
Minardi
D
,
Montanari
MP
,
Tili
E
,
Cochetti
I
,
Mingoia
M
,
Varaldo
PE
, et al.
Effects of fluoroquinolones on bacterial adhesion and on preformed biofilm of strains isolated from urinary double J stents. J Chemother. 2008;20(2):195–201. doi:.https://doi.org/10.1179/joc.2008.20.2.195
82
Balagué
C
,
Fernández
L
,
Pérez
J
,
Grau
R
. Effect of ciprofloxacin on adhesive properties of non-P mannose-resistant uropathogenic Escherichia coli isolates. J Antimicrob Chemother. 2003;51(2):401–4. doi:.https://doi.org/10.1093/jac/dkg048
83
Reid
G
,
Habash
M
,
Vachon
D
,
Denstedt
J
,
Riddell
J
,
Beheshti
M
. Oral fluoroquinolone therapy results in drug adsorption on ureteral stents and prevention of biofilm formation. Int J Antimicrob Agents. 2001;17(4):317–9, discussion 319–20. doi:.https://doi.org/10.1016/S0924-8579(00)00353-8
84
Wojnicz
D
,
Tichaczek-Goska
D
,
Kicia
M
. Pentacyclic triterpenes combined with ciprofloxacin help to eradicate the biofilm formed in vitro by Escherichia coli. Indian J Med Res. 2015;141(3):343–53. doi:.https://doi.org/10.4103/0971-5916.156631
85
El-Feky
MA
,
El-Rehewy
MS
,
Hassan
MA
,
Abolella
HA
,
Abd El-Baky
RM
,
Gad
GF
. Effect of ciprofloxacin and N-acetylcysteine on bacterial adherence and biofilm formation on ureteral stent surfaces. Pol J Microbiol. 2009;58(3):261–7.
86
Davies
DG
,
Marques
CN
. A fatty acid messenger is responsible for inducing dispersion in microbial biofilms. J Bacteriol. 2009;191(5):1393–403. doi:.https://doi.org/10.1128/JB.01214-08
87Al-Bareeq R. Denstedt J.D. Urinary Tract Stone Disease. London: Springer; 2011. pp 543–551.
88
Lennon
GM
,
Thornhill
JA
,
Sweeney
PA
,
Grainger
R
,
McDermott
TE
,
Butler
MR
. ‘Firm’ versus ‘soft’ double pigtail ureteric stents: a randomised blind comparative trial. Eur Urol. 1995;28(1):1–5.
89
Lange
D
,
Chew
BH
. Update on ureteral stent technology. Ther Adv Urol. 2009;1(3):143–8. doi:.https://doi.org/10.1177/1756287209341306
90
Rosman
BM
,
Barbosa
JA
,
Passerotti
CP
,
Cendron
M
,
Nguyen
HT
. Evaluation of a novel gel-based ureteral stent with biofilm-resistant characteristics. Int Urol Nephrol. 2014;46(6):1053–8. doi:.https://doi.org/10.1007/s11255-013-0636-3
91
Gabi
M
,
Hefermehl
L
,
Lukic
D
,
Zahn
R
,
Vörös
J
,
Eberli
D
. Electrical microcurrent to prevent conditioning film and bacterial adhesion to urological stents. Urol Res. 2011;39(2):81–8. doi:.https://doi.org/10.1007/s00240-010-0284-3
92
Chakravarti
A
,
Gangodawila
S
,
Long
MJ
,
Morris
NS
,
Blacklock
AR
,
Stickler
DJ
. An electrified catheter to resist encrustation by Proteus mirabilis biofilm. J Urol. 2005;174(3):1129–32. doi:.https://doi.org/10.1097/01.ju.0000168618.79096.cb
93
Tenke
P
,
Riedl
CR
,
Jones
GL
,
Williams
GJ
,
Stickler
D
,
Nagy
E
. Bacterial biofilm formation on urologic devices and heparin coating as preventive strategy. Int J Antimicrob Agents. 2004;23(Suppl 1):S67–74. doi:.https://doi.org/10.1016/j.ijantimicag.2003.12.007
94
Cauda
F
,
Cauda
V
,
Fiori
C
,
Onida
B
,
Garrone
E
. Heparin coating on ureteral Double J stents prevents encrustations: an in vivo case study. J Endourol. 2008;22(3):465–72. doi:.https://doi.org/10.1089/end.2007.0218
95Lange D, Chew BH. in Biomaterials and Tissue Engineering in Urology 85-103 (Elsevier Ltd., 2009).
96
Stickler
DJ
,
Evans
A
,
Morris
N
,
Hughes
G
. Strategies for the control of catheter encrustation. Int J Antimicrob Agents. 2002;19(6):499–506. doi:.https://doi.org/10.1016/S0924-8579(02)00091-2
97
John
T
,
Rajpurkar
A
,
Smith
G
,
Fairfax
M
,
Triest
J
. Antibiotic pretreatment of hydrogel ureteral stent. J Endourol. 2007;21(10):1211–6. doi:.https://doi.org/10.1089/end.2007.9904
98
Laube
N
,
Kleinen
L
,
Bradenahl
J
,
Meissner
A
. Diamond-like carbon coatings on ureteral stents--a new strategy for decreasing the formation of crystalline bacterial biofilms?
J Urol. 2007;177(5):1923–7. doi:.https://doi.org/10.1016/j.juro.2007.01.016
99
Mendez-Probst
CE
,
Goneau
LW
,
MacDonald
KW
,
Nott
L
,
Seney
S
,
Elwood
CN
, et al.
The use of triclosan eluting stents effectively reduces ureteral stent symptoms: a prospective randomized trial. BJU Int. 2012;110(5):749–54. doi:.https://doi.org/10.1111/j.1464-410X.2011.10903.x
100
Cirioni
O
,
Ghiselli
R
,
Silvestri
C
,
Minardi
D
,
Gabrielli
E
,
Orlando
F
, et al.
Effect of the combination of clarithromycin and amikacin on Pseudomonas aeruginosa biofilm in an animal model of ureteral stent infection. J Antimicrob Chemother. 2011;66(6):1318–23. doi:.https://doi.org/10.1093/jac/dkr107
101
Cirioni
O
,
Silvestri
C
,
Ghiselli
R
,
Kamysz
W
,
Minardi
D
,
Castelli
P
, et al.
In vitro and in vivo effects of sub-MICs of pexiganan and imipenem on Pseudomonas aeruginosa adhesion and biofilm development. Infez Med. 2013;21(4):287–95.
102
Darouiche
RO
,
Mansouri
MD
,
Gawande
PV
,
Madhyastha
S
. Efficacy of combination of chlorhexidine and protamine sulphate against device-associated pathogens. J Antimicrob Chemother. 2008;61(3):651–7. doi:.https://doi.org/10.1093/jac/dkn006
103
Watterson
JD
,
Cadieux
PA
,
Beiko
DT
,
Cook
AJ
,
Burton
JP
,
Harbottle
RR
, et al.
Oxalate-degrading enzymes from Oxalobacter formigenes: a novel device coating to reduce urinary tract biomaterial-related encrustation. J Endourol. 2003;17(5):269–74. doi:.https://doi.org/10.1089/089277903322145431
104
Francesko
A
,
Fernandes
MM
,
Ivanova
K
,
Amorim
S
,
Reis
RL
,
Pashkuleva
I
, et al.
Bacteria-responsive multilayer coatings comprising polycationic nanospheres for bacteria biofilm prevention on urinary catheters. Acta Biomater. 2016;33:203–12. doi:.https://doi.org/10.1016/j.actbio.2016.01.020


