Xenotransplantation: where do we stand in 2016?
DOI: https://doi.org/10.4414/smw.2017.14403
Gisella L.
Yung Pugaa, Robert
Riebenb, Leo
Bühlerc, Henk-Jan
Schuurmanc, Jörg D.
Seebacha
Xenotransplantation: where do we stand in 2016?
Summary
Worldwide, there is a constant rise in the number of patients with end-stage organ failure in critical need for transplants, but the number of organs/cells available from deceased or living human donors is limited. Xenotransplantation using pig organs/tissues represents a potential solution for this shortage; however, it has been hampered by a number of mainly immunological hurdles. Remarkable progress was presented at the latest biennial (13th) international congress of the International Xenotransplantation Association, November 2015 in Melbourne, Australia, and the American Transplant Congress, May 2016 in Boston, USA. Most importantly, the survival records of pig organ xenografts in nonhuman primate models have strikingly improved with the use of multitransgenic pigs. Moreover, no safety issues were encountered in clinical trials with porcine islets, and the removal of porcine endogenous retroviruses from the genome of a pig cell line by the CRISPR/Cas9 technology offers the perspective to overcome the perceived potential risk of xenozoonosis in the near future. For all these reasons, interest in xenotransplantation has been boosted. This review summarises the current status of xenotransplantation research, including Swiss contributions as well as regulatory and safety aspects in the light of upcoming clinical trials.
Abbreviations
- αGal
-
Galα1,3Galβ-R
- AVR
-
acute vascular rejection
- CRISPR/Cas9
-
clustered regularly interspaced short palindromic repeats and the associated protein 9
- CRP
-
complement regulatory proteins
- DXR
-
delayed xenograft rejection
- ECPR
-
endothelial protein C receptor
- GalT-KO
-
alpha1,3-galactosyltransferase knock-out
- HAR
-
hyperacute rejection
- IBMIR
-
instant blood-mediated inflammatory reaction
- IL
-
interleukin
- IXA
-
International Xenotransplantation Association
- KO
-
knock-out
- NHP
-
nonhuman primate
- NK
-
natural killer
- pEC
-
porcine endothelial cell
- PERV
-
porcine endogenous retroviruses
- TNF
-
tumour necrosis factor
- WHO
-
World Health Organization
Introduction
Worldwide, there is a constant rise in the number of patients with end-stage organ failure in critical need for transplants; however, the number of organs/cells available from deceased or living donors is limited [1]. In Switzerland alone, the number of patients on the waiting list has increased between 2011 and 2015 from 1 074 to 1 384 (29%), of whom 552 received an organ in 2015; approximately 60 patients die each year while waiting for an organ [2]. To mitigate this problem alternative approaches are needed and currently are in development, including stem cell technologies, tissue engineering, blastocyst complementation, medical devices and xenotransplantation [1, 3]. The World Health Organization (WHO) defines clinical xenotransplantation as "any procedure that involves the transplantation, implantation or infusion into a human recipient of either: (i) live cells, tissues, or organs from a nonhuman animal source; or (ii) human body fluids, cells, tissues or organs that have had ex vivo contact with live nonhuman animal cells, tissues or organs" [4]. This review focuses on the evolution and the current status of preclinical and clinical xenotransplantation research in nonhuman primates (NHP) and in patients receiving grafts from pigs. In particular, it presents the growing list of currently available genetically modified pigs with different targets to improve the immunological and physiological compatibility between pigs and humans, and the latest advances in preclinical pig-to-NHP models and ex vivo perfusion models. Regulatory and safety aspects of xenotransplantation are discussed in the light of upcoming clinical trials and, lastly, Swiss contributions to the field are briefly summarised.
The boost of xenotransplantation
Animal cells and organs used in documented attempted clinical transplantation to humans include: rabbit kidneys and livers in 1905; NHP testes in the 1920s and 1930s by Voronoff [5]; and several trials from the 1960s to the 1990s with kidneys, heart and livers stemming from NHP. None of these trials resulted in prolonged xenograft function in a human recipient. The focus of xenotransplantation thereafter shifted to the pig as potential donor [6–8], and several different immunological hurdles have been identified during subsequent decades of pig-to-human xenotransplantation research. Both humoral and cellular human immune responses against endothelial cells of vascularized pig xenografts trigger xenorejection, generally classified as: hyperacute rejection (HAR); acute vascular rejection (AVR) and delayed xenograft rejection (DXR). These mechanisms and the molecular incompatibilities leading to xenograft rejection are illustrated in figure 1A
. HAR occurring within minutes to hours was the first hurdle to overcome. The identification of the terminal carbohydrate residue Galα1,3Galβ-R (αGal), expressed by pigs but not by humans and NHPs, as the main target for HAR was a major breakthrough [9, 10]. In fact, both humans and the NHPs used in preclinical trials possess naturally formed anti-αGal antibodies that bind to the pig xenograft endothelium and induce complement-mediated damage. In the early 1990s, enthusiasm was sparked by the generation of the first transgenic pigs genetically engineered to express human complement-regulatory proteins (CRP) including membrane cofactor protein (CD46) [11], decay-accelerating factor (CD55) [12] and protectin (CD59) [13, 14]. Indeed, overexpression of CRP on the surface of vascular cells protects against natural antibody and complement-mediated injury, and prolongs the survival of transgenic pig heart and kidney grafts in NHP models to days or weeks. Large pharmaceutical companies, Sandoz/Novartis and Baxter, entered the field with considerable financial resources. Reflecting the growing interest and associated need to exchange results and to promote xenotransplantation, a first xenotransplantation congress was held in 1991 (Minneapolis, MN, USA), the journal Xenotransplantation was established in 1994, and the International Xenotransplantation Association (IXA) was established in 1998 as a section of the Transplantation Society [15]. At this time Sir Roy Calne stated that "clinical xenotransplantation is just around the corner, but it may be a very long corner", and indeed he proved to be right.
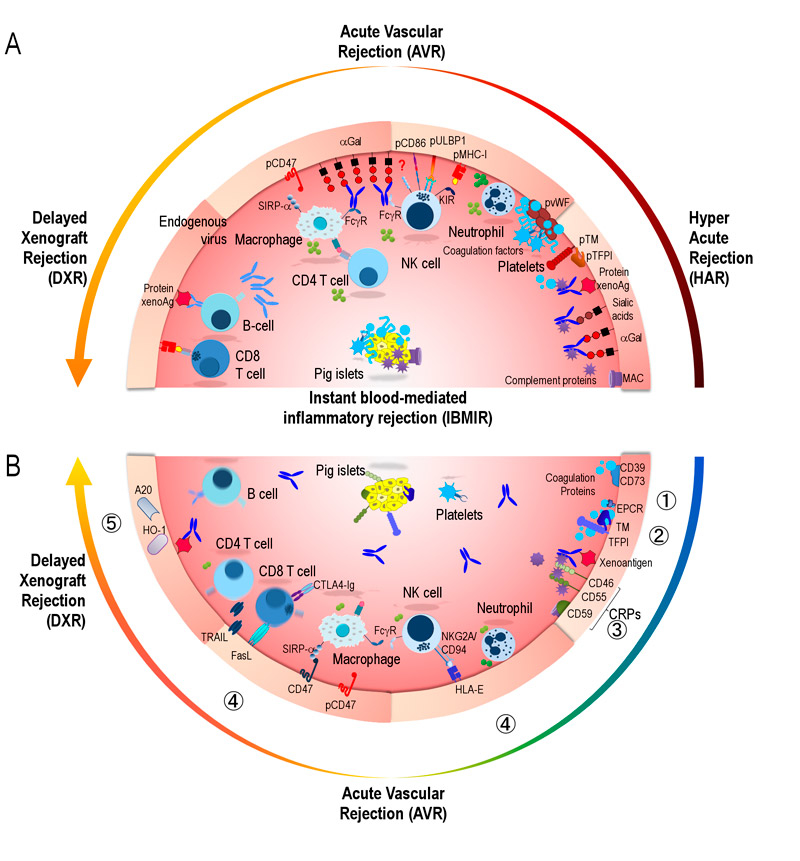
Figure 1 Immunological and coagulation hurdles in pig-to-human xenograft rejection and genetic modifications to overcome them.
(A) Hyperacute rejection, within minutes to hours, triggered by the binding of preformed natural antibodies (dark blue) to pig endothelial antigens, mainly αGal but also to sialic acids (Neu5Gc) and pig proteins. Antibody deposition leads to endothelial activation; activation of complement proteins and formation of the membrane attack complex (MAC; both in shown in purple). Activation of the coagulation cascade (in indigo blue) due to species incompatibilities with pig membrane-bound coagulation-regulatory proteins thrombomodulin (pTM) or tissue factor pathway inhibitor (pTFPI), leads to thrombosis and endothelial damage. Human platelets aggregate following interaction with pig von Willebrand factor (pvWF).
Acute vascular rejection, within hours to days, mediated by innate immune cells recruited by activated endothelia and proinflammatory signals. Neutrophils release oxygen-reactive species (dark green circles) and proinflammatory cytokines (light green circles); simultaneously, xenoantibodies bound to endothelia trigger antibody-dependent cell-mediated cytotoxicity by natural killer (NK) cells and macrophages. NK cells are activated by: (i) NKG2D responding to the pig activating ligand UL16 binding protein 1 (pULBP-1); (ii) the natural cytotoxicity triggering receptor 2 (NKp44) by an unknown pig ligand; (iii) a variant of CD28 to porcine CD86; (iv) lack of the inhibitory signals to killer-cell immunoglobulin-like receptors (KIR) by pig MHC class I due to species incompatibility. Macrophages secrete proinflammatory cytokines (light green circles), phagocytosis is triggered by species incompatibility of human signal regulatory protein alpha (SIRPα) and its inhibitory porcine ligand (pCD47).
Delayed xenograft rejection, within weeks to months, activation of the acquired immune response leading to induced anti-pig antibodies (light blue) to annexin A2, CD9, CD46, CD59, MHC, etc. by memory B cells and plasma cells (not shown); cytotoxic CD8 T cells react to pig MHCs and other proteins; and CD4 T cells provide help and secrete cytokines (light green circles).
Instant blood mediated inflammatory reaction occurs in response to cellular xenografts (islets), induces activation of complement, and the extrinsic pathway of the coagulation system, platelet aggregation. Leucocyte recruitment leads to thrombus formation and islet lysis.
(B) Strategies to overcome xenograft rejection: ① Modification of xenoantigens by either masking or deletion, most importantly αGal in GalT-KO pigs. ② Introduction of human coagulation regulatory proteins, CD39 and CD73, to avoid platelet aggregation; thrombomodulin (TM, CD141); and endothelial protein C receptor (EPCR, CD201) to promote the activation of human protein C which degrades clotting factors; tissue factor pathway inhibitor (TFPI). Deletion of pig von Willebrand factor inducing to human platelet aggregation. ③ Expression of human complement regulatory proteins (CRP), CD46 (MCP) and CD59 to inactivate complement factors, CD55 (DAF) to accelerate complement decay. ④ Control of cellular responses by induction of apoptosis using FasL (CD178) or TRAIL (CD253) expression; blocking of T cell co-stimulation with CTLA-4-Ig or LEA29Y; expression of HLA-E to inhibit NK cells via the inhibitory NKG2A/CD94 receptor; and human CD47 to regulate macrophages via SIRPα. ⑤ Expression of antiapoptotic A20 to protect porcine endothelial cells from TNF-mediated apoptosis or anti-inflammatory HO-1 to degrade free haem.
To illustrate indirectly the course of xenotransplantation research in relation to the general field of transplantation during the past twenty years, we have analysed the number of publications found in PubMed. There is a clear, steady and slow overall increase in the number of peer-reviewed "transplantation" publications per year (fig. 2). On the contrary, the numbers of articles in the field "xenotransplantation" follow a rather bumpy road, presumably reflecting achievements, newly identified obstacles, financing issues, and hopes and fears in the field, with a certain delay. Around the year 2000 an evident drop in publication numbers might have been related to the potential risk of pig-to-human transmission of porcine endogenous retrovirus (PERV), first published in 1996 [16–18]. This risk, together with the lack of progress in pig-to-NHP transplantation models, probably prompted Sandoz/Novartis to leave the field at that time. In the following years, the generation of knock-out (KO) pigs lacking the enzyme synthesising the αGal xenoantigen by several independent groups [19–21] allowed a substantial expansion of knowledge in xenotransplantation and stirred new hopes and publication numbers. Nevertheless, additional hurdles that limit the survival of organs from alpha1,3-galactosyltransferase KO (GalT-KO) animals in pig-to-NHP models became apparent, such as thrombotic microangiopathy, a consequence of species incompatibilities within the complement and coagulation systems [22]. The scientific community became aware that several additional molecular targets had to be modified to simultaneously address different pig-to-NHP/human incompatibilities. To improve the engineering efficiency of transgenic pigs and to obtain multiple genetic combinations more rapidly, novel technologies, such as the zinc finger and transcription activator-like effector nucleases, were applied. These approaches dramatically reduced the time from the identification of a molecular target to the generation of transgenic animals. Multitransgenic pigs became available with the introduction of multicistronic vector technologies by somatic-cell nuclear transfer, thus avoiding time-consuming breeding procedures [23, 24]. Notably, CRISPR/Cas9 technology has the potential to improve animal engineering in xenotransplantation as it speeds up the whole process of genetic engineering, and allows multiple genetic modifications with previously unknown precision and with high efficiency [25, 26] (CRISPR/Cas9: clustered regularly interspaced short palindromic repeats and the associated protein 9). Around the year 2010, reports of genetically modified pigs peaked, which was clearly paralleled by an increase in the number of overall publications in the field. However, since 2012 the numbers have dropped again and a plausible explanation might be that public funding assigned to research in Europe and the USA [27], as well as investments by the private sector, was dramatically cut down. Presumably, these budget issues affected high-risk projects such as xenotransplantation more than well-established topics such as general transplantation.
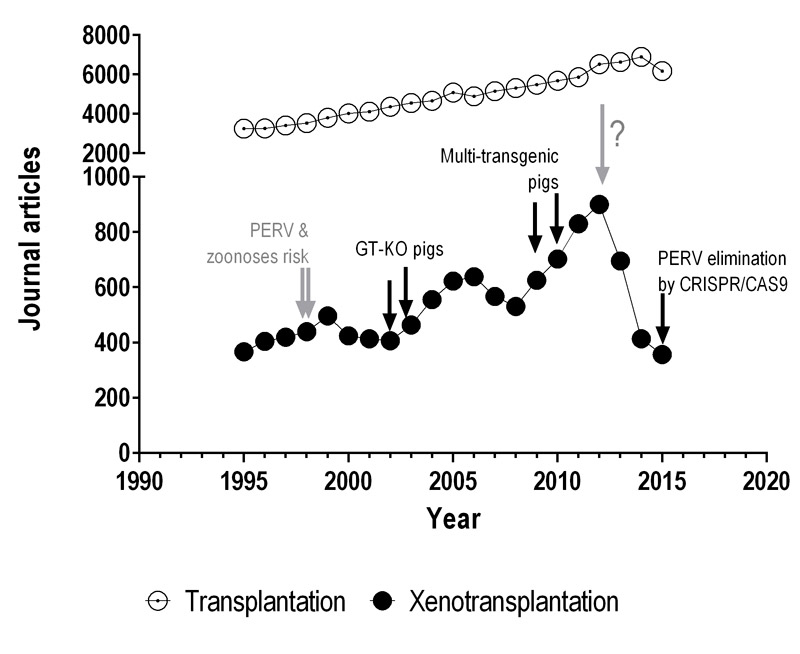
Figure 2 Chronology of the numbers of peer-reviewed publications in the field of xenotransplantation.
A PubMed search of original articles for the term "xenotransplantation" excluding (AND NOT) "tumor" was done for the years between 1995 and 2015 (●). The exclusion criterion was aimed to remove studies in which human tumour cells were injected into other animals, a model commonly used in cancer research. As control, "transplantation" AND NOT "tumor" search was also performed (○). Grey arrows indicate probable events that negatively impacted the field of xenotransplantation, whereas black arrows represent potentially positive impacts.
CRISPR/Cas9 = clustered regularly interspaced short palindromic repeats and the associated protein 9; GT-KO = α1,3-galactosyltransferase knock-out; PERV = porcine endogenous retroviruses
In recent years, xenotransplantation research has continued to show remarkable progress, as documented by presentations at the latest biennial (13th) IXA meeting in Melbourne, Australia, November 2015, and the American Transplant Congress in Boston, USA, May 2016. Most importantly, the survival of preclinical pig heart and kidney xenografts in NHP models have strikingly improved with use of multitransgenic pigs, proper costimulation-blocking agents, and anti-inflammatory biologicals such as tocilizumab (anti-interleukin 6 receptor, IL-6R) and etanacept (tumour necrosis factor [TNF] inhibitor) [28]. Moreover, the first clinical trials with encapsulated porcine islets [29–31] confirmed preclinical studies showing that the risk of pig-to-human transmission is lower than originally perceived, and also confirmed earlier reports that PERV did not pose a risk of xenozoonosis [32–35]. Finally, a recent paper on the permanent removal of multiple PERV copies from the genome of a pig cell line by the CRISPR/Cas9 technology indicated the possibility to delete PERV entirely from animals by genetic engineering [36]. Although this permanent removal is more complex and has not yet resulted in PERV-free animals, it might be preferred to the transgenic small interfering RNA approach to prevent PERV activation. The latter was reported by two groups about 10 years ago and yielded a significant reduction in PERV expression [37], or no clear effect because expression was already undetectable in targeted pigs [38]. For all these reasons, interest in xenotransplantation has been boosted, including the recommendation to prepare for clinical trials testing porcine heart and kidney grafts [39].
To illustrate the ongoing research efforts in the field we analysed the affiliation and geographical location of the speakers and poster presenters at the above-mentioned IXA meeting in Melbourne. A map showing the countries and numbers of groups active in xenotransplantation research demonstrates that Asia is the leading continent, followed by Europe and North America; South Korea alone seems to have a similar number of research groups as the US (fig. 3A). Within Europe, Germany is in the lead, (fig. 3B), but Switzerland stands out when data are adjusted for the number of inhabitants. However, the value of these data is limited, because participation of researchers at the Melbourne meeting depended on several factors, including country of origin and costs of registration and travel. Furthermore, venture capitalists and shareholders have renewed their interest in xenotransplantation, which is reflected by new companies investing in the field. Remarkably, the big pharmaceutical companies are still watching without participating, and industry is mainly represented by small- and medium-sized biotechnology companies.
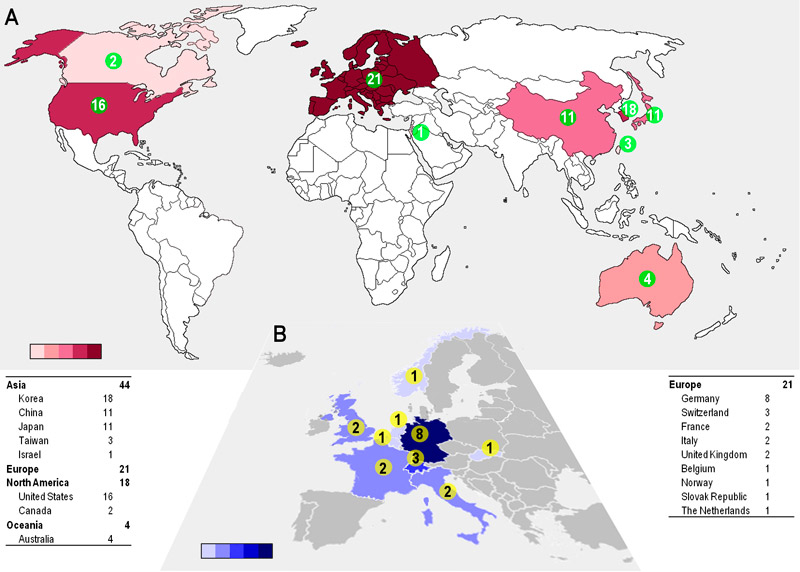
Figure 3 Where is xenotransplantation research taking place?
Number of research groups per country working in xenotransplantation. Data were extracted from the abstracts presented at the International Pancreas and Islet Transplantation Association-IXA 2015 joint conference, as according to criteria: (i) any type of research involving a xenotransplantation system; (ii) the number of different research groups per country. (A) Global view considering the European continent instead of individual countries. (B) Close up of Europe indicating the contributions by country. Colour gradients either in red or blue represent the increasing number of groups from light to dark colour.
Where do we stand with regards to clinical experience and the application of porcine xenotransplant products in 2016? A Swedish landmark pilot trial conducted in 1994 by Groth et al. showed survival of fetal porcine islets in 10 immunosuppressed kidney allograft recipients [40]. A clinical trial in China using nonencapsulated neonatal wild-type pig islets involving 22 patients highlighted the importance of the immunosuppressive protocol [31], and a highly disputed trial in Mexico involving 23 type-1 diabetic patients and using cotransplantation of porcine islets and Sertoli cells into preimplanted subcutaneous devices reported improvement of metabolic control and survival for more than 4 years [41]. Notably, none of these trials, which were all conducted without proper regulatory oversight, reported sustained insulin independence, and none reported transmission of PERV after long-term follow up [30, 35]. The New Zealand-based company Living Cell Technologies (LCT) was the first to obtain approval by the competent regulatory authority. There are five trials registered at ClinicalTrials.gov sponsored by LCT, which recently entered a joint venture with the Japanese company Otsuka to create Diatranz Otsuka Ltd (DOL); subsequently the activities of the DIABECELL porcine neonatal islet product moved to the US. Three trials using alginate-encapsulated wild-type neonatal pig islets in type-1 diabetes have already been completed, and first data on efficacy were presented in 2014 [42]. Two other clinical trials testing alginate-encapsulated wild-type porcine choroid plexus cells for the treatment of Parkinson's disease are ongoing at LCT [43]. To date, the authors are not aware of any registered clinical trial using living organs or free cells from wild-type or genetically-modified animals.
Genetically modified pigs available for xenotransplantation
Based on introductory studies, performed either in vitro or in vivo and identifying the molecular targets, genetically-modified pigs were generated to control cross-species incompatibilities and xenograft rejection. The introduction of new technologies has considerably sped-up the generation of multiple KO and transgenic pigs with multiple genetic modifications, i.e. KOs and/or transgenes, as well as the reduction of animal breeding times, to produce founders with more than one modification [24, 44, 45]. Several important immunological obstacles had to be addressed by either deletion of pig genes or introduction of human genes. These are: (i) HAR driven by naturally occurring xenoreactive antibodies and complement activation; (ii) AVR mediated by activated endothelium, innate immune cells, antibody binding, complement and coagulation factors; and (iii) DXR mainly mediated by T- and B-cell immune responses, macrophages and natural killer (NK) cells [44, 46]. In the particular case of nonencapsulated islet transplantation performed by injection into the portal vein, instant blood-mediated inflammatory reaction (IBMIR) has also to be considered, because this innate rejection results in a substantial loss of islets [47]. Moreover, several interspecies molecular incompatibilities in control of the coagulation system, leading to thrombotic microangiopathy in solid organ xenografts, have been identified [22]. The impressive achievement of the generation of an estimated 40 or more different genetic modifications of pigs in order to improve the immunological compatibility with humans, to control coagulation dysregulation and reduce inflammatory responses, has been summarised earlier [48]. The areas of intervention are presented in table 1 and figure 1B and include: ① modification of xenoantigens in pig organs by either knock-out of pig genes encoding enzymes involved in the synthesis of pig-specific carbohydrate residues, or introduction of human enzymes that add carbohydrate residues in order to mask the xenoantigens; ② addition of proteins regulating human coagulation to avoid thrombotic microangiopathy and intravascular coagulation; ③ introduction of human CRPs that inhibit complement activation or accelerate the degradation of activated complement factors; ④ factors alteration of cellular immune responses by the introduction of human molecules that control cellular xenograft rejection; and finally ⑤ introduction of genes encoding anti-apoptotic and/or anti-inflammatory [24, 49].
Table 1 Areas of intervention to avoid or control xenorejection.
|
Genetic modification
|
Mechanism of action
|
Rejection type
|
Xenoantigen
modification |
• α2FucT |
• Masking of xenoantigens by adding H blood-group antigen |
HAR |
| • β4GalNT2-KO |
• Deletion of xenoantigen (not yet characterised) |
| • CMAH-KO |
• Deletion of xenoantigen Neu5Gc |
| • cEndoGalC |
• Digestion of αGal xenoantigen by a Clostridium enzyme |
| • GLA |
• Partial degradation of αGal xenoantigen |
| • GnT-III |
• Masking of xenoantigens αGal and NeuGc |
| • GalT-KO and iGb3S-KO
|
• Deletion of xenoantigen αGal xenoantigen
|
Coagulation
regulation |
• CD39 |
• Avoiding platelet aggregation in activated/damaged EC |
HAR, AVR, IBMIR |
| • CD73 |
• Avoiding platelet aggregation in activated/damaged EC |
| • CD141 (TM) |
• Activation of human protein C leading to degradation of clotting factors Va and VIIIa |
| • CD201 (EPCR) |
• Enhancing the rate of human protein C activation |
| • TFPI |
• Inhibition the activated factor Xa and VIIa-TF proteases |
| • pvWF-deficient |
• Reduction of platelet aggregation triggered by human GPIb and pig vWF interactions |
Complement
regulation
proteins (CRP) |
• CD46 (MCP)
|
• Inactivation complement factors C3b and C4b
|
HAR, AVR, IBMIR |
| • CD55 (DAF) |
• Acceleration of complement decay |
| • CD59 |
• Inhibition of the complement membrane attack complex C5b-9 |
Cellular
immune
responses |
• ASGR1-KO |
• Decreases human platelet phagocytosis by pig sinusoidal endothelial cells |
AVR, DXR |
| • CIITA-DN |
• Transcription factor essential for SLA-II expression |
| • CD47 |
• Regulation of macrophage activation and phagocytosis |
| • CD178 (FasL) |
• Induction of apoptosis on activated T cells, NK cells, monocytes and neutrophils |
| • CD253 (TRAIL) |
• Induction of apoptosis of activated T cells |
| • CTLA4-Ig and pCTLA4-Ig |
• Inhibition of T cell co-stimulation via CD86/CD80 |
| • LEA29Y |
• Variant of CTLA4-Ig with higher affinity to CD86/CD80 |
| • HLA-E/β2-microglobulin |
• Inhibition of NK cells cytotoxicity |
Antiapoptotic
and anti-
inflammatory |
• A20 (TNFAIP3) |
• Inhibition of NF-κB activation and TNF-mediated apoptosis |
DXR, IBMIR |
| • HO-1 |
• Degradation of free haem |
| • sTNFRI-Fc |
• Inhibition of the binding of TNF to its receptors |
It is more than 20 years since "Astrid", the first transgenic pig expressing CD55, was born on Christmas Eve in 1992 in Cambridge, UK. Nowadays, GalT-KO pigs are generally used as a platform for other genetic modifications, and pigs expressing up to six [50–52] and even seven [25] modifications are available. However, the sole introduction of a human gene does not guarantee the survival of the genetically modified xenografts following transplantation. Depending on the promoter, some genes are not sufficiently expressed in the organs to be transplanted [50] or cause problems for the pig’s health [53]. Several companies like Revivicor (nowadays a division of the biotech company United Therapeutics) or public institutions in several countries have heavily invested in specialised facilities for the generation of genetically modified pigs. These include South Korea (Animal Science and Resources Research Center for Transgenic Cloned Pigs), China (Beijing Genomics Institute, originally a nonprofit organisation; State Key Laboratories of Agrobiotechnology, Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences), the USA (The National Swine Resource and Research Center (NSRRC) at the University of Missouri), Japan (Center for Advanced Biomedical science and Swine Research, Kagoshima University), and Germany (Transregio Collaborative Research Centre 127).
It is a well-established consensus in the scientific community that preclinical data stemming from large animal models, with pig-to-NHP models being most broadly used, are required before xenotransplantation can be applied in humans. However, it can be questioned to what degree these models are reliable indicators for the outcome in humans. There are several caveats, including the potential for insufficient function or lack of function of human gene products expressed by the transgenic pig organs or cells when transplanted into NHPs, although hard evidence supporting this point has not been published to our knowledge. Similarly, immunosuppressive and anti-inflammatory drugs used in these models, as well as pharmaceutical interventions to control coagulation dysregulation, have all been developed for humans and experience with their action and correct management in NHPs is limited. This was shown in a model of diabetes [54] and a life-supporting model of cardiac xenotransplantation [55]. The limitations of the model, such as complications in the long-term management of animals with experimentally-induced disease, become even more visible with increasing survival and function of xenografts in NHPs. In conclusion, testing the currently available genetically modified pigs in preclinical models of organ xenotransplantation using NHPs is extremely complex (besides being quite expensive), and requires specialised facilities, skilled surgeons, veterinary staff and sufficient financial resources. Also, it is essentially impossible to test separately all genetic modifications or individual genes in multitransgenic animals, as is done for medicines in drug combinations. Whereas in Europe the general belief is that NHP models are not absolutely necessary before moving to human trials, the opinion in the USA is that substantial results should be obtained with NHPs. Thus, it is hard to draw a conclusion in this topic before the outcome of the first clinical trials.
Survival of genetically-modified pig grafts in nonhuman primates
Remarkable progress has been made since the 1990s regarding survival of pig xenografts in NHPs. Table 2 summarises the most striking results and the longest survival times in cellular, tissue and solid organ pig-to-NHP models using genetically modified pigs. Evidently, we are aware of the selective nature of this list for space reasons, providing only a limited number of outstanding results and omitting many other important achievements, which were comprehensively summarised in 2013 by Cooper et al. [80].
Table 2 Survival of xenografts from transgenic pigs into nonhuman primates.
|
Type of graft
|
Xenograft*
|
Donor genetic background
[source]†
|
Recipient (n)‡
|
Immunosuppressive therapy§
|
Survival (days)¶
|
Year [Ref]
|
|
Maximum
|
Median (range)
|
| Cellular |
Islets |
CD46 |
M. fascicularis (5) |
ATG + CD154 + MMF |
396 |
ns |
2009 [56] |
CD46•TFPI•CTLA4-Ig
•GalT-KO [R] |
M. fascicularis (2) |
ATG + CD154 + MMF |
365 |
183
(0–365) |
2014 [57] |
| GalT-KO neonatal |
M. mulatta (4/5) |
CD154 + LFA-1 + CTLA4-Ig + MMF |
249 |
95
(50–249) |
2011 [58] |
| Neurones |
CTLA4-Ig |
Rhesus macaque (18) |
CsA + MMF + Cs |
521 |
ns |
2013 [59] |
| Organ |
hHeart |
CD46•TM•GalT-KO [R] update [60] |
P. anubis (5) |
ATG + CD20 + CD40 (50) + CVF + MMF + Cs |
945 |
298
(159–945) |
2016 [61] |
| CD46•TM•GalT-KO [R] |
P. anubis (5) |
ATG + CD20 + CD40 (50) + CVF + MMF + Cs |
550 |
230
(146–550) |
2014 [60] |
| CD46•TM•GalT-KO [R] |
Baboon (5) |
ATG + CD20 + CD40 + MMF + Cs |
380 |
91
(77–380) |
2014 [62] |
| CD46•GalT-KO |
Baboon (9) |
ATG + CD20 + CD154 + CVF + MMF + Cs |
236 |
71
(36–236) |
2012 [63] |
| CD46•GalT-KO [R] |
Baboon (8) |
ATG + CD20 + CD154 + CVF + MMF + Cs |
236 |
67
(ns–236) |
2014 [60] |
CD39•CD46•CD47•CD55•
EPCR•GalT-KO [R] |
Baboon (2) |
ATG + CD20 + CD40 + CVF + MMF + Cs |
143 |
94
(46–143) |
2016 [52] |
|
oHeart |
CD46 |
Baboon, ns (6) |
ATG + CD20 + TAC + Rapa + GAS914 or TCP |
57 |
18 (0–57) |
2011 [64] |
| CD55 |
P. anubis (1) |
CyP + CsA + MMF + Cs |
39 |
na |
2000 [65] |
| Kidney |
CD55•GalT-KO [NSRRC]•ATC update [66] |
Rhesus macaque (2) |
CD4 + CD8 + CD154 + MMF + Cs |
310 |
235
(160–310) |
2016 [67] |
| CD55 [I] (thymo-kidney) |
P. anubis (3) |
ATG or CD3 + TI + (CD2) + CyP + CVF + MMF
+ Cs, AS914 (1 animal) |
229 |
32
(32–229) |
2003 [68] |
| CD39•CD46•CD55•TM•EPCR•GalT-KO [R]**
|
Baboon (1) |
ATG + CD20 + CD40 + IL6R + aTNF + CVF + Rapa + LMWH + Cs |
136 |
na |
2015 [50] |
| CD55•GalT-KO [NSRRC] |
Rhesus macaque (2,1) |
CD4 + CD8 + CD154 + MMF + Cs |
133 |
130
(126–133),
6 (high titres) |
2015 [66] |
| CD46•TM•GalT-KO [R] /pTM promoter |
P. anubis, (2) |
ATG + CD40 +CTLA4-Ig + Rapa/TAC + LMWH + Cs |
130 |
115
(99–130) |
2015 [69] |
|
oLiver |
GalT-KO |
P. anubis (1) |
ATG + CVF + CTLA4-Ig + TAC + Cs |
25 |
na |
2016 [70] |
| GalT-KO |
P. hamadryas (3) |
ATG+ CD2 + CD154 + CVF + AZA + TAC + Cs |
9 |
7.7
(6–9) |
2012 [71] |
| CD55 |
P. anubis (2) |
CyP + CsA + Cs |
8 |
6 (4–8) |
2000 [72] |
| CD46•GalT-KO |
P. anubis (8) |
ATG + TAC + MMF + Cs or CyP + TAC + MMF+ Cs |
7 |
5
(1–7) |
2010 [73] |
| CD46•CD59•α2FucT |
P. anubis (5) |
CyP + CD20 + CD25 + CsA + MMF + Cs |
1 |
0.8
(0.5–1.0) |
2005 [74] |
|
aLiver |
GalT-KO |
P. hamadryas (3) |
ATG + CVF + TAC + Cs |
15 |
9 (6–15) |
2014 [75] |
|
oLung |
vWF-KO |
P. anubis (3) |
AIA + CsA + INN + AZA + Cs, Mφ |
109 h |
76
(19–109) h |
2007 [76] |
| CD46•GalT-KO |
P. anubis (2) |
Cs + CsA + AZA, Mφ |
48 h |
26
(3.5–48) h |
2011 [77] |
| CD46 [N] |
P. anubis (5) |
AIA + CsA + INN + AZA + Cs |
24 h |
23
(20–24) h |
2007 [76] |
| CD55•CD59 [N] |
Baboons (4) |
Splen.+ AIA + CsA + CyP + Cs |
24 h |
ns |
2000 [78] |
| Tissue |
Cornea |
CTLA4-Ig |
M. fascicularis (4) |
Local Cs |
120 |
70
(21–120) |
2014 [51] |
| CD39•CD55•CD59•α2FucT•GalT-KO |
M. fascicularis (2) |
Local Cs |
34 |
22
(21–34) |
2014 [51] |
Cellular xenotransplantation
Cellular xenotransplantation has been mainly performed with pig islets, but hepatocytes and neuronal cells have also been tested. Xenotransplantation of hepatocytes was recently reviewed [81], which showed that there are only a few pig-to-NHP studies, mostly using non-life-supporting systems; for example, Nagata et al. achieved survival of up to 253 days for wild-type porcine hepatocytes in cynomolgus monkeys [82]. There are no published transplantation data yet on hepatocytes from transgenic pigs. On the other hand, neuroblasts transferred from CTLA4-Ig transgenic pigs in a NHP model of Parkinson’s disease induced locomotion recovery that lasted for at least 6 months [59]. Concerning nonencapsulated adult pig islet transplantation into NHPs, promising results have been reported when islets from CD46 or CD46•TFPI•CTLA4-Ig•GalT-KO animals were used as source; these transplants lasted up to 396 and 365 days, respectively, [56, 57], whereas neonatal GalT-KO islets survived up to 249 days [58]. These data extend the 6 months survival of wild-type adult pig islets in NHPs with use of an immunosuppressive regimen reported by Park et al. [83]. Different strategies and biomaterials for encapsulation of pig islets are under investigation, with one highlight being 6-month survival of pig islets after alginate macroencapsulation in diabetic monkeys [84]. Also, different sites of implantation, mainly the peritoneal cavity and omentum, but also the bone marrow, are being investigated with respect to the loss of function and limited survival due to pericapsular fibrosis. The progress in the field of encapsulated porcine islets has been reviewed elsewhere in detail [79].
Solid organ xenotransplantation
In pig-to-NHP solid organ xenotransplantation, heterotopic and orthotopic transplant models have been established that are either life-supporting or not.
Heart
First attempts to overcome HAR in heterotopic pig-to-NHP heart transplantation were performed by the groups of Cooper, Platt and White [85–87], who showed the efficacy of transgenic expression of CRP [85, 86] or of blocking anti-Gal antibodies with soluble carbohydrates [87]. Since then HAR was definitively overcome, as evidenced by the 2.5-year maximum survival in baboons recently obtained by Mohiuddin et al. with heterotopic heart xenografts from CD46•TM•GalT-KO pigs and an impressive combination of immunosuppressive drugs consisting of antithymocyte globulin, anti-CD20 and anti-CD40 monoclonal antibodies, cobra venom factor, mycophenolate mofetil and ciclosporin [61]. More modest survival was initially reported in orthotopic and intrathoracic life-supporting heterotopic heart xenografts [85, 88, 89]. Nowadays, 2 months of survival have been achieved [65, 90], but these models are controversial owing to their complexity and technical failure rate [91].
Kidneys
Transplantation of life-supporting kidneys from genetically modified pigs to NHPs initially showed survival up to several weeks [92–95]. Now, maximum survival of 310 days has been achieved by using CD55•GalT-KO pigs as donors and immunosuppression with anti-CD4, anti-CD8 and anti-CD154 monoclonal antibodies in addition to mycophenolate mofetil and ciclosporin [67]. Another protocol using combined thymo-kidney grafts from human CD55 transgenic pigs achieved a survival of 229 days [68]. More recently, the use of kidneys from multitransgenic CD39•CD46•CD55•TM•EPCR•GalT-KO pigs showed a survival of 136 days, but the kidneys were actually only CD46•CD55•EPCR•GalT-KO because human CD39 and thrombomodulin (TM) were not expressed [50].
Liver
Survivals of pig liver xenografts range from a few hours or 1 week to a maximum of 25 days with use of livers from GalT-KO pigs [70]. Additional expression of human CD46 [73] gave an even better outcome, whereas the addition of extra genes in CD46•CD59•α2FucT pig livers did not [74]. A major problem in liver xenotransplantation is the development of severe post-transplant thrombocytopenia due to phagocytosis of platelets by porcine sinusoidal endothelial cells, Kupffer cells and macrophages. The causes of this phenomenon are protein and sugar incompatibilities between pigs and NHPs, including asialoglycoprotein receptor 1, von Willebrand factor / GPIb, CD47/SIRP-α and/or CD18, and tissue factor [96].
Lung
Lung xenotransplantation faces similar and even more severe complications; here success is measured in hours rather than days or months. The most promising results were obtained using von Willebrand factor-deficient pig donors in combination with macrophage depletion, which reached a maximum survival of 109 hours [76], whereas lung xenografts from CD46•GalT-KO pigs survived only 48 hours [77], and those containing only CD46 or CD55•CD59 were rejected after 1 day [76, 78].
Tissue and red blood cell xenotransplantation
Some studies using genetically-modified animals as donors for cell and tissue xenotransplantation have been reported. For pig corneas transplanted in NHPs, the sole introduction of CTLA4-Ig together with local corticosteroids allowed a maximum survival of 120 days [51]. A xenogeneic blood transfusion can be also considered to be a transplant. Indeed, erythrocytes obtained from blood type O pigs could be of great interest in transfusion medicine. Recently, the group of Tector et al. demonstrated the elimination of xenoantigenicity of swine erythrocytes via gene disruption of three enzymes implicated in the synthesis of carbohydrate xenoantigens (GalT, CMAH and β4GalNT2) [97]. In the light of the shortage of human blood donations, this finding opens an avenue for the potential application of porcine erythrocyte xenotransfusions.
Finally, it has been emphasised by the Pittsburgh group that, in addition to genetic modifications, anti-inflammatory biologicals such as tocilizumab (anti-IL-6R) and etanercept (TNF inhibitor) given in the early post-transplant period together with immunosuppressive drugs improve the transplant outcomes [98, 99]. This approach will probably soon become a standard in many pig-to-NHP models of xenotransplantation.
Ex vivo xenoperfusion systems to evaluate transgenic pig organs
Because every solid organ exhibits different features in terms of cellular anatomy, and blood or lymph circulation patterns, it is reasonable to expect organ-specific interactions upon circulation with human blood. Moreover, as mentioned above, pig-to-NHP models do not adequately replicate all the factors encountered in pig-to-human xenotransplantation, and human gene products expressed by the transplanted transgenic pig organs might function insufficiently or not at all in NHP. Therefore, ex-vivo perfusion models have been used in xenotransplantation for many years as a preclinical model to test early xenograft events and, more recently, to evaluate the potential of genetic modifications in pigs, in the USA, Canada, Norway, Japan, Korea and Australia, among others. Ex-vivo xenoperfusion models have a longstanding tradition in Germany, where experimental transplantation laboratories in Munich (heart, liver, kidney) [100–102] and Hannover (kidney, lung) [103–105] contributed substantially to the discovery of the mechanisms of xenograft rejection and to testing of genetically modified pigs. Perfusion of pig organs with whole human blood has the advantage of allowing direct study of human anti-pig responses, including soluble factors and cells, and assessment of the efficacy of pharmaceutical interventions [106]. A striking example of subtle differences between human anti-pig and NHP anti-pig responses resulting in substantially different outcomes has recently been reported by the group of Rees [107]. During extracorporeal pig liver perfusions, human blood, but not blood from NHPs (chimpanzee), resulted in phagocytosis of human erythrocytes by porcine Kupffer cells, causing the haematocrit to fall to 2.5% of the original value. This was due to the expression of a particular sugar residue (N-acetylneuraminic acid; Neu5Ac) on human erythrocytes, which is not present on the erythrocytes of NHPs. The Neu5Ac sugar residue is responsible for the observed clearance by porcine Kupffer cells involving sialoadhesin (siglec-1 or CD169). The presence of Neu5Ac should not be confused with the absence of N-glycolylneuraminic acid (Neu5Gc) in humans, which is related to the absence of the enzyme involved in the conversion of Neu5Ac in Neu5Gc [108]; the differential expression of Neu5Ac and Neu5Gc between humans and old-world NHPs (baboons, macaques) is nowadays a subject for discussions on the validity of NHP models in xenotransplantation [109]. Although very early reports by Bouhours et al. [110, 111] had revealed their importance in the generation of xenoantibodies, it took some time to become accepted knowledge.
Organs from genetically modified pigs have thus far not been used extensively in organ perfusion experiments. Regarding lung perfusion, the group of Pierson in Baltimore is clearly the leader in the field, with a broad experience and having fine-tuned the model to enable therapeutic interventions [112, 113]. A meta-analysis of their work including 157 independent lung perfusions of organs from various genetically modified pigs showed essentially that pig lungs perfused with human blood functioned longer when organs with a higher number of genetic modifications were used, and that the GalT-KO background combined with the expression of human CD46, CD55, endothelial protein C receptor (EPCR) and HO-1 was associated with better survival [114]. This meta-analysis also showed that, even in a lung perfusion model that is cheaper and less complicated than a whole-organ transplantation model, it is not possible to assess the value of each individual component of the multitransgenic donor animals.
Ex-vivo perfusion of transgenic pig hearts has also been performed by the group of Reichart in Munich [101, 115] and White in Cambridge, UK, and limited experience is available for other organs, including kidney [103, 116] and liver [117–119]. In Switzerland, the groups of Rieben in Bern and Seebach in Geneva have established pig forelimb xenoperfusions with human blood using HLA-E/CD46 double transgenic pigs, studying the effects on complement and coagulation activation as well as NK cell responses [120–123]. The interest of Seebach’s group in the role of NK cells in xenotransplantation is based on initial work of Inverardi [124], who showed preferential recruitment of human NK cells to rat hearts perfused with human peripheral blood lymphocytes, a finding later confirmed in pig kidney xenoperfusions with human peripheral blood lymphocytes [125, 126].
Swiss contribution to xenotransplantation
Switzerland has several links to the field of xenotransplantation. Historically, it is of note that Mary Shelley wrote her novel “Frankenstein or the modern Prometheus” almost 200 years ago in Geneva during a rainy summer. Although it is not clear what kind of material was actually used to create the body of the Creature, a concept combining (xeno)transplantation and tissue engineering must have been envisaged by the author [127]. In the following, we will not go into the doubtful activities of some private Swiss clinics offering “rejuvenating” cell therapies by injecting live animal cells, an option that was even embraced by the Pope in the 1950s and escaped the regulatory authority for a long time [128]. Clinical and academic researchers on one hand, and the Swiss pharmaceutical industry on the other, have been very active in the field of transplantation for many years, with many remarkable breakthroughs. For example, Novartis (formerly Sandoz before the merger with Ciba-Geigy in 1995) spearheaded the field with the development of ciclosporin in the 1970s [129], and then became very interested in xenotransplantation in the 1990s with a commercial interest to expand their franchise. Starting in 1992, Sandoz made huge investments in three groups led by: (i) David White and John Wallwork, who established Imutran as a spin-off of Cambridge University, UK, and worked on CRP-transgenic pigs; (ii) Fritz Bach at Deaconess Hospital, Boston, USA, working on endothelial cell activation and associated coagulation processes; and (iii) Biotransplant Inc., a spin-off of Harvard University and Massachusetts General Hospital, working in the field of tolerance. Imutran made major progress, with the birth of the first CD55-transgenic pig in 1992, followed by documentation of the absence of HAR in heterotopic heart transplants in NHPs. The company became part of Novartis in 1995; however, Novartis left the field by the end of 2000 for two reasons, namely the perceived xenozoonosis risk associated with the potential of PERV transmission [16], and lack of progress in the pig-to-NHP transplant models. To facilitate this exit, Novartis and Biotransplant formed the joint venture Immerge BioTherapeutics [130], which was in existence until 2004, and was quite successful in generating GalT-KO miniature swine and in research on PERV. Since then the Swiss biomedical industry has not been active in xenotransplantation, to our knowledge.
In academia, there are currently four research groups in Switzerland that have consistently contributed to the field. Each group focuses on different aspects of xenotransplantation, and shares common interests with an active collaboration between them.
The research unit of Leo Bühler is focusing on xenogeneic cell therapy, in particular porcine islet cells for the treatment of type 1 diabetes and porcine hepatocytes for the treatment of liver failure. From its early days, the unit collaborated in joint projects with the Ecole Polytechnique Fédérale de Lausanne, at first with the group of Christine Wandrey and more recently with the group of Sandrine Gerber-Lemaire [131–134]. The research at the Ecole Polytechnique centres around biomaterials for cell encapsulation, and novel products in encapsulation were then successfully tested in functional in-vitro tests, as well as in diabetes reversal and graft survival in rodent models. In a series of studies, porcine and human hepatocytes were investigated for their in-vitro potential to secrete albumin and metabolise drugs, and the in-vivo treatment of fulminant liver failure in rodents and in baboons. The progress in the field of xenogeneic cell therapy is such that the perspective of clinical operations has become visible. Since this requires important financial support that is not provided by research grants, several academic institutions have at this stage started spin-off companies. The Geneva group has, therefore, established such a company.
Robert Rieben’s group in Bern has been working on the role of antibodies and complement, as well as on regulators of the complement and coagulation cascades, in endothelial cell activation related to xenotransplantation. After initial studies on the use of synthetic carbohydrate antigens to block anti-αGal antibody binding to porcine endothelial cells [135], the group has pioneered the concept of endothelial cell protection using glycosaminoglycan analogues. They used low molecular weight dextran sulphate to prevent human serum cytotoxicity to porcine endothelial cells (pECs) and showed that this glycosaminoglycan analogue also delays HAR in ex vivo pig lung perfusion with human blood [136]. Hamster-to-rat cardiac xenotransplantation experiments, a model for delayed xenograft rejection, then showed that short-term use of dextran sulphate was able to induce accommodation of the hamster heart, which means that no vascular rejection occurred despite antibody and complement deposition on the graft endothelium [137]. More recently, the Bern team has analysed cells and tissues from pigs transgenic for human complement regulatory proteins as well as thrombomodulin. This was done in vitro, in a whole blood model with endothelial-coated microbeads in which the activation of the coagulation system can also be assessed, as well as ex vivo in a perfusion model of porcine limbs with human blood [120–123, 138]. In addition to the strong link with Jörg Seebach’s group, the Bern team participated in two European Union projects on xenotransplantation and is currently affiliated as an external partner with the German Transregio SFB 127 on xenotransplantation.
Jörg Seebach’s group, first in Zurich and now in Geneva, initially focused on in vitro interactions of human leucocyte subsets, in particular NK cells, and porcine endothelial cells (pEC) in order to characterise and overcome cell-mediated rejection mechanisms in pig-to-human xenotransplantation [139–141]. The group showed that transgenic expression of several human major histocompatibility class I molecules including HLA-A2, HLA-B27, HLA-Cw3, HLA-Cw4, HLA-E and HLA-G on pECs provided partial protection from xenogeneic NK cytotoxicity [142–147]. Thereafter, HLA-E transgenic pigs were generated in collaboration with the group of Eckhard Wolf in Munich and are now being tested in ex vivo perfusion models in collaboration with Robert Rieben’s team. These experiments demonstrated reduced human NK cell recruitment and tissue infiltration in HLA-E / human CD46 double-transgenic pig limbs perfused with human blood (manuscript submitted for publication). Moreover, it was shown that the lack of expression of αGal on pEC does not impair xenogeneic NK cytotoxicity, but reduces antibody-dependent cell cytotoxicity [148, 149]. Another line of research explored the adhesive interactions of pECs and leucocytes, showing that leucocyte rolling was dependent on CD49d/CD106 interactions [150], but not on αGal, whereas transmigration required human CD18 and CD99 [151]. More recently, regulatory dendritic and T cell-based strategies to overcome cell-mediated xenorejection were tested. It was shown in vitro that human regulatory T cells were recruited to pECs involving the chemokine receptors CXCR3, CCR4 and integrins CD18 and CD49d, whereas human CCL17 immobilised on pECs acts as a chemoattractant for human regulatory T cells [152, 153]. Finally, human and mouse dendritic cells differentiated in the presence of IL-10 (IL10-DC) showed modulatory properties for human-antipig xenoresponses mediated by both NK and CD8 T cells [154], and facilitated prolonged graft survival in a rat-to-mouse islet transplantation model, respectively (manuscript in revision).
Finally, Nicolas Mueller in Zurich brings broad expertise on the role of pig cytomegalovirus in the setting of xenotransplantation, which he acquired working with Jay Fishman's group in Boston, focusing on the activation of cytomegalovirus in solid organ xenotransplantation in NHP models [155–160]; and later it was shown together with Anne-Laure Millard that human cytomegalovirus can infect porcine xenografts [161–164].
Safety of xenotransplantation
As with any new medicinal product or procedure, “safety first” is pivotal and safety/tolerability is a crucial topic in research and development. Safety covers not only the xenogeneic cell, tissue or solid organ, but also the immunosuppression given to prevent rejection.
Specific to xenotransplantation is the safety of the porcine product. One major point is the possibility that, together with the xenograft, infectious pathogens are transmitted that can infect the recipient and cause disease. This is directly related to the characteristic of a xenograft, namely the presence of living cells: the material cannot be disinfected like acellular material (e.g., porcine heart valves or corneas). This has prompted regulatory agencies to issue guidelines describing the breeding and maintenance of donor animals in dedicated facilities with a so-called designated pathogen-free status [165, 166]. Apart from infectious pathogens that can affect the health status of the herd, animals should not contain microorganisms that have the potential of cross-species transmission, also called xenozoonosis [167, 168] such as herpes viruses (cytomegalovirus, gamma-lymphotropic herpes virus and hepatitis E virus). These pathogens are exogenous, and hence can be removed from the donor herd by specific breeding technologies.
This situation is different for endogenous viruses. The discovery that PERV can be transmitted from porcine to human cells in vitro [16–18] initiated intense research efforts [34]. Based on many literature reports, it now appears that the risk of pig-to-human cross-species transmission is quite low and manageable. For instance, in long-term studies on large cohorts of humans who were directly exposed to living porcine tissue, viral transmission from pig to humans has never been observed [30, 31, 35]. Also, pig-to-human transmission and replication during cell co-culture occurred notably in the human embryonic kidney 293 cell line, which lacks components of the intracellular machinery that protects against retroviruses, and not in primary cells; hence, this observation might reflect a laboratory artefact [34]. This aside, novel approaches in genetic engineering allow for the knock-out of multiple retroviral gene segments by the CRISPR/Cas9 technology [36], opening the possibility that PERV can be entirely removed from animals by genetic engineering.
Regulatory aspects of xenotransplantation
In 2004, the WHO urged member states to perform xenotransplantation in the clinic only under proper regulatory oversight [169]; this was followed by the first WHO global consultation on regulatory requirements for xenotransplantation clinical trials, held in Changsha in 2008, and several other consultations [170, 171]. From the scientific community, IXA has issued a “Consensus Statement on Conditions for Undertaking Clinical Trials of porcine islet products in type 1 diabetes”: the statement from 2009 has recently been updated [172]. IXA has also published a similar consensus statement regarding xenocorneal transplantation products [173]. These IXA documents are essentially not regulatory documents; their purpose is to propagate the opinion of the scientific community, particularly on efficacy requirements and the underlying status of scientific progress, and also the status of (microbial) safety in the field. They replace, to some extent, the discussions in governmental advisory committees, where mainly representatives from the scientific community debated progress in the field towards clinical application.
Regulatory agencies became interested in xenotransplantation and expressed concerns about xenozoonosis in the late 1990s. In October 1997, the US Food and Drug Administration put ongoing trials using a porcine product on hold until methods for the detection of PERV were developed and implemented in the monitoring of patients [174]. Shortly thereafter, regulations or guidelines for the clinical use of xenotransplantation products were established in a number of countries: in the USA the Public Health Service issued guidelines on infectious disease issues in 2001 [165], and the Food and Drug Administration issued in 2003 a Guidance for Industry covering the source of the animal, the product, and preclinical and clinical issues [166]. The European Medicines Agency issued a guideline on "Xenogeneic cell-based medicinal products" in 2009 [175]. These regulations and guidelines address both the porcine donor and the human recipient. Regarding the donor pig, the US guidelines describe accommodation in a "bio-secure" barrier facility maintaining a so-called designated pathogen-free status of a closed herd, for at least two generations. Regarding the human recipient, regulatory documents address both the individual patient and the public health aspect [176], with some major additions to what is presently used in clinical transplantation. Also recipients should be monitored for specific aspects of the porcine product, such as the transmission of infectious agents. This includes the assessment of PERV transmission [33, 177], and also that of “unknown” pathogens. To enable a retrospective analysis, tissue and cells from the donor pig and the human recipient need to be regularly sampled and archived of, in the USA for 50 years [166], and in the EU for 30 years [165, 175].
In Switzerland, the Transplantation Act was established in October 2004, defining a transplant product as "products manufactured from human or animal organs, tissue or cells that can be standardised or whose manufacturing process can be standardised" [178]. As an illustration, porcine pancreatic islets are a transplant product according to this definition, in contrast to islets from deceased human donors. The Transplant Act was followed in 2007 by a specific directive for transplantation of animal organs, tissues and cells [179]. This directive states very clearly that the clinical use of a xenotransplantation product is only allowed if there is no other therapeutic method with a similar benefit available. The directive includes specific instructions for clinical trials, (genetically modified) source animals, information to patients (including compulsory consent for life-long monitoring and autopsy), as well as archiving of samples and access to such archives.
Conclusions and perspectives
After a period of pioneering explorations, for instance the baboon liver transplants by Tom Starzl and the porcine islet transplants by Carl Groth (and a number of others), research efforts during the past 20 years have yielded tremendous progress and accumulated a huge amount of knowledge, both regarding biomedical knowledge in many scientific disciplines (including immunology, organ physiology and virology) and regarding technical aspects in many areas of support (pig genetic engineering and husbandry, assay development and product development from a commercial viewpoint). At IXA 2015 and American Transplant Congress 2016, several major advances in the field were highlighted, notably in heart, kidney and islet pig-to-NHP xenotransplantation. The rapid development of techniques for controlled and precise genetic engineering continues apace, with the promise of further prolongation of xenograft survival and further minimisation of infectious risk. In other words, it is time to start preparing for exploratory clinical studies in which the possible benefit for patients outweighs the risk of novel immunosuppressive strategies. The risk for patients related to the potential transmission of porcine pathogens to humans is not zero, but is no longer considered to be a reason to prohibit clinical trials. This phase transition in research and development has already been passed for islet transplantation (see the clinical trials at LCT and others mentioned above), whereas for other cells, tissues and organs there is still a great deal of work to be done before exploratory clinical studies can be initiated. For solid organs in particular, more basic research on efficacy and safety is needed in order to develop plans for first-in-human studies. As an alternative to immunosuppression, many efforts have been made to induce immune tolerance towards xenografts, or to encapsulate xenogeneic cells to protect them from rejection. The tolerance approach has not yet delivered robust protocols that can be applied clinically, whereas cell encapsulation has for some products already reached the stage of clinical development with minor success. A main difference between the present situation and the pioneering enterprises in the past is that clinical explorations are nowadays under oversight by competent regulatory authorities – which is absolutely in the interest of scientists, patients and society. Clinical trials with xenogeneic cells and organs will be possible, in Switzerland also, once the scientific basis for such studies is established.
The imminent generation of genetically modified pigs raised in germ-free facilities, in addition to the promising preclinical results using these pigs, heralds the next, promising phase of xenotransplantation, based on the huge investments made in the past. Noteworthy is the direction that Asian countries like South Korea, China and Japan are taking – strongly pursuing xenotransplantation, not only at the level of research but also by supporting biotechnology companies and clinical trials. Progress is speedy, with the outlook that in the future Europe and North America will buy xenotransplantation products from Asian companies. Europe and North America, still having a strong basis of experience and expertise in the field, can only continue to contribute to xenotransplantation research and development in the event of proper financial support by funding agencies and investment capital managers.
Prof. Dr. med. Jörg Seebach, Hopitaux Universitaires de Geneve, Rue Micheli-du-Crest 24, CH-1211 Geneva 14, joerg.seebach[at]hcuge.ch
References
1
Ekser
B
,
Cooper
DK
,
Tector
AJ
. The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int J Surg. 2015;23(Pt B):199–204. doi:.https://doi.org/10.1016/j.ijsu.2015.06.066
2Swisstransplant. Jahresbericht 2015 [2015 annual report]. Swisstransplant, the Swiss National Foundation for organdonation and transplantation. Bern, Switzerland. 2015. Available from: https://www.swisstransplant.org/fileadmin/user_upload/Swisstransplant/Jahresbericht/Neu_Jahresbericht2015_DE.pdf [accessed 2016 June 29].
3
Mou
L
,
Chen
F
,
Dai
Y
,
Cai
Z
,
Cooper
DK
. Potential alternative approaches to xenotransplantation. Int J Surg. 2015;23(Pt B):322–6. doi:.https://doi.org/10.1016/j.ijsu.2015.06.085
4World Health Organization (WHO). Xenotransplantation. WHO. Geneva, Switzerland. 2016. Available from: URL: http://www.who.int/transplantation/xeno/en/ [accessed 2016 June 29].
5
Bajic
P
,
Selman
SH
,
Rees
MA
. Voronoff to virion: 1920s testis transplantation and AIDS. Xenotransplantation. 2012;19(6):337–41. doi:.https://doi.org/10.1111/xen.12004
6
Deschamps
JY
,
Roux
FA
,
Saï
P
,
Gouin
E
. History of xenotransplantation. Xenotransplantation. 2005;12(2):91–109. doi:.https://doi.org/10.1111/j.1399-3089.2004.00199.x
7
Griesemer
A
,
Yamada
K
,
Sykes
M
. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev. 2014;258(1):241–58. doi:.https://doi.org/10.1111/imr.12152
8
Cooper
DK
,
Ekser
B
,
Tector
AJ
. A brief history of clinical xenotransplantation. Int J Surg. 2015;23(Pt B):205–10. doi:.https://doi.org/10.1016/j.ijsu.2015.06.060
9
Galili
U
,
Clark
MR
,
Shohet
SB
,
Buehler
J
,
Macher
BA
. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1----3Gal epitope in primates. Proc Natl Acad Sci USA. 1987;84(5):1369–73. doi:.https://doi.org/10.1073/pnas.84.5.1369
10
Cooper
DK
,
Good
AH
,
Koren
E
,
Oriol
R
,
Malcolm
AJ
,
Ippolito
RM
, et al.
Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1(3):198–205. doi:.https://doi.org/10.1016/0966-3274(93)90047-C
11
Diamond
LE
,
Quinn
CM
,
Martin
MJ
,
Lawson
J
,
Platt
JL
,
Logan
JS
. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001;71(1):132–42. doi:.https://doi.org/10.1097/00007890-200101150-00021
12
Schmoeckel
M
,
Bhatti
FN
,
Zaidi
A
,
Cozzi
E
,
Pino-Chavez
G
,
Dunning
JJ
, et al.
Xenotransplantation of pig organs transgenic for human DAF: an update. Transplant Proc. 1997;29(7):3157–8. doi:.https://doi.org/10.1016/S0041-1345(97)00823-3
13
Fodor
WL
,
Williams
BL
,
Matis
LA
,
Madri
JA
,
Rollins
SA
,
Knight
JW
, et al.
Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc Natl Acad Sci USA. 1994;91(23):11153–7. doi:.https://doi.org/10.1073/pnas.91.23.11153
14
Diamond
LE
,
McCurry
KR
,
Martin
MJ
,
McClellan
SB
,
Oldham
ER
,
Platt
JL
, et al.
Characterization of transgenic pigs expressing functionally active human CD59 on cardiac endothelium. Transplantation. 1996;61(8):1241–9. doi:.https://doi.org/10.1097/00007890-199604270-00021
15
Cooper
DK
,
Groth
CG
. A record of international meetings on xenotransplantation 1988-2010. Xenotransplantation. 2011;18(4):229–31. doi:.https://doi.org/10.1111/j.1399-3089.2011.00647.x
16
Patience
C
,
Takeuchi
Y
,
Weiss
RA
. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3(3):282–6. doi:.https://doi.org/10.1038/nm0397-282
17
Akiyoshi
DE
,
Denaro
M
,
Zhu
H
,
Greenstein
JL
,
Banerjee
P
,
Fishman
JA
. Identification of a full-length cDNA for an endogenous retrovirus of miniature swine. J Virol. 1998;72(5):4503–7.
18
Martin
U
,
Kiessig
V
,
Blusch
JH
,
Haverich
A
,
von der Helm
K
,
Herden
T
, et al.
Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet. 1998;352(9129):692–4. doi:.https://doi.org/10.1016/S0140-6736(98)07144-X
19
Phelps
CJ
,
Koike
C
,
Vaught
TD
,
Boone
J
,
Wells
KD
,
Chen
SH
, et al.
Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–4. doi:.https://doi.org/10.1126/science.1078942
20
Sharma
A
,
Naziruddin
B
,
Cui
C
,
Martin
MJ
,
Xu
H
,
Wan
H
, et al.
Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75(4):430–6. doi:.https://doi.org/10.1097/01.TP.0000053615.98201.77
21
Kolber-Simonds
D
,
Lai
L
,
Watt
SR
,
Denaro
M
,
Arn
S
,
Augenstein
ML
, et al.
Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101(19):7335–40. doi:.https://doi.org/10.1073/pnas.0307819101
22
Cowan
PJ
,
Robson
SC
. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg. 2015;23(Pt B):296–300. doi:.https://doi.org/10.1016/j.ijsu.2015.07.682
23
Butler
JR
,
Ladowski
JM
,
Martens
GR
,
Tector
M
,
Tector
AJ
. Recent advances in genome editing and creation of genetically modified pigs. Int J Surg. 2015;23(Pt B):217–22. doi:.https://doi.org/10.1016/j.ijsu.2015.07.684
24
Cooper
DK
,
Ekser
B
,
Ramsoondar
J
,
Phelps
C
,
Ayares
D
. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016;238(2):288–99. doi:.https://doi.org/10.1002/path.4635
25
Fischer
K
,
Kraner-Scheiber
S
,
Petersen
B
,
Rieblinger
B
,
Buermann
A
,
Flisikowska
T
, et al.
Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci Rep. 2016;6:29081. doi:.https://doi.org/10.1038/srep29081
26
Park
KE
,
Park
CH
,
Powell
A
,
Martin
J
,
Donovan
DM
,
Telugu
BP
. Targeted Gene Knockin in Porcine Somatic Cells Using CRISPR/Cas Ribonucleoproteins. Int J Mol Sci. 2016;17(6):810. doi:.https://doi.org/10.3390/ijms17060810
27
Wolinsky
H
. The crash reaches the universities. The global financial crisis threatens private and public university funding in the USA and Europe. EMBO Rep. 2009;10(3):209–11. doi:.https://doi.org/10.1038/embor.2009.17
28
Schuurman
HJ
. Pig-to-nonhuman primate solid organ xenografting: recent achievements on the road to first-in-man explorations. Xenotransplantation. 2016;23(3):175–8. doi:.https://doi.org/10.1111/xen.12244
29
Elliott
RB
,
Escobar
L
,
Tan
PL
,
Muzina
M
,
Zwain
S
,
Buchanan
C
. Live encapsulated porcine islets from a type 1 diabetic patient 9.5 yr after xenotransplantation. Xenotransplantation. 2007;14(2):157–61. doi:.https://doi.org/10.1111/j.1399-3089.2007.00384.x
30
Valdes-Gonzalez
R
,
Dorantes
LM
,
Bracho-Blanchet
E
,
Rodríguez-Ventura
A
,
White
DJ
. No evidence of porcine endogenous retrovirus in patients with type 1 diabetes after long-term porcine islet xenotransplantation. J Med Virol. 2010;82(2):331–4. doi:.https://doi.org/10.1002/jmv.21655
31
Wang
W
,
Mo
Z
,
Ye
B
,
Hu
P
,
Liu
S
,
Yi
S
. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36(12):1134–40.
32
Paradis
K
,
Langford
G
,
Long
Z
,
Heneine
W
,
Sandstrom
P
,
Switzer
WM
, et al.
Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. The XEN 111 Study Group. Science. 1999;285(5431):1236–41. doi:.https://doi.org/10.1126/science.285.5431.1236
33
Fishman
JA
,
Patience
C
. Xenotransplantation: infectious risk revisited. Am J Transplant. 2004;4(9):1383–90. doi:.https://doi.org/10.1111/j.1600-6143.2004.00542.x
34
Denner
J
,
Tönjes
RR
. Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev. 2012;25(2):318–43. doi:.https://doi.org/10.1128/CMR.05011-11
35
Wynyard
S
,
Nathu
D
,
Garkavenko
O
,
Denner
J
,
Elliott
R
. Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation. 2014;21(4):309–23. doi:.https://doi.org/10.1111/xen.12102
36
Yang
L
,
Güell
M
,
Niu
D
,
George
H
,
Lesha
E
,
Grishin
D
, et al.
Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science. 2015;350(6264):1101–4. doi:.https://doi.org/10.1126/science.aad1191
37
Dieckhoff
B
,
Petersen
B
,
Kues
WA
,
Kurth
R
,
Niemann
H
,
Denner
J
. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation. 2008;15(1):36–45. doi:.https://doi.org/10.1111/j.1399-3089.2008.00442.x
38
Ramsoondar
J
,
Vaught
T
,
Ball
S
,
Mendicino
M
,
Monahan
J
,
Jobst
P
, et al.
Production of transgenic pigs that express porcine endogenous retrovirus small interfering RNAs. Xenotransplantation. 2009;16(3):164–80. doi:.https://doi.org/10.1111/j.1399-3089.2009.00525.x
39
Reardon
S
. New life for pig-to-human transplants. Nature. 2015;527(7577):152–4. doi:.https://doi.org/10.1038/527152a
40
Groth
CG
,
Korsgren
O
,
Tibell
A
,
Tollemar
J
,
Möller
E
,
Bolinder
J
, et al.
Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994;344(8934):1402–4. doi:.https://doi.org/10.1016/S0140-6736(94)90570-3
41
Valdes-Gonzalez
R
,
Rodriguez-Ventura
AL
,
White
DJ
,
Bracho-Blanchet
E
,
Castillo
A
,
Ramírez-González
B
, et al.
Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin Exp Immunol. 2010;162(3):537–42. doi:.https://doi.org/10.1111/j.1365-2249.2010.04273.x
42
Matsumoto
S
,
Tan
P
,
Baker
J
,
Durbin
K
,
Tomiya
M
,
Azuma
K
, et al.
Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc. 2014;46(6):1992–5. doi:.https://doi.org/10.1016/j.transproceed.2014.06.008
43The U.S.National Institutes of Health DoHaHS. Open-label Investigation of the Safety and Clinical Effects of NTCELL in Patients With Parkinson's Disease. ClinicalTrials gov.; 2016. Available from: URL: https://clinicaltrials.gov/ct2/show/NCT01734733?term=xenotransplantation+AND+porcine+NOT+cancer&rank=6#wrapper [accessed 2016 September 6].
44
Niemann
H
,
Petersen
B
. The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 2016;25(3):361–74. doi:.https://doi.org/10.1007/s11248-016-9934-8
45
Yum
SY
,
Yoon
KY
,
Lee
CI
,
Lee
BC
,
Jang
G
. Transgenesis for pig models. J Vet Sci. 2016;17(3):261–8. doi:.https://doi.org/10.4142/jvs.2016.17.3.261
46
Cooper
DK
,
Ekser
B
,
Tector
AJ
. Immunobiological barriers to xenotransplantation. Int J Surg. 2015;23(Pt B):211–6. doi:.https://doi.org/10.1016/j.ijsu.2015.06.068
47
Goto
M
,
Tjernberg
J
,
Dufrane
D
,
Elgue
G
,
Brandhorst
D
,
Ekdahl
KN
, et al.
Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15(4):225–34. doi:.https://doi.org/10.1111/j.1399-3089.2008.00482.x
48
Ekser
B
,
Ezzelarab
M
,
Hara
H
,
van der Windt
DJ
,
Wijkstrom
M
,
Bottino
R
, et al.
Clinical xenotransplantation: the next medical revolution?
Lancet. 2012;379(9816):672–83. doi:.https://doi.org/10.1016/S0140-6736(11)61091-X
49
Klymiuk
N
,
Ludwig
B
,
Seissler
J
,
Reichart
B
,
Wolf
E
. Current concepts of using pigs as source for beta-cell replacement therapy of type 1 diabetes. Curr Mol Bio Rep. 2016.
50
Iwase
H
,
Liu
H
,
Wijkstrom
M
,
Zhou
H
,
Singh
J
,
Hara
H
, et al.
Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22(4):302–9. doi:.https://doi.org/10.1111/xen.12174
51
Vabres
B
,
Le Bas-Bernardet
S
,
Riochet
D
,
Chérel
Y
,
Minault
D
,
Hervouet
J
, et al.
hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation. 2014;21(5):431–43. doi:.https://doi.org/10.1111/xen.12107
52
Mohiuddin
M
,
Singh
A
,
Chan
J
,
Corcoran
P
,
Thomas Iii
M
,
Lewis
B
, et al.
Long Term heterotopic Cardiac Xenograft Survival from Donor Pigs with Six Gene Modifications
[abstract 1353].
Am J Transplant. 2016;16 (suppl 3).
53
Aigner
B
,
Klymiuk
N
,
Wolf
E
. Transgenic pigs for xenotransplantation: selection of promoter sequences for reliable transgene expression. Curr Opin Organ Transplant. 2010;15(2):201–6. doi:.https://doi.org/10.1097/MOT.0b013e328336ba4a
54
Graham
ML
,
Schuurman
HJ
. The usefulness and limitations of the diabetic macaque model in evaluating long-term porcine islet xenograft survival. Xenotransplantation. 2013;20(1):5–17. doi:.https://doi.org/10.1111/xen.12012
55
Abicht
JM
,
Mayr
T
,
Reichart
B
,
Buchholz
S
,
Werner
F
,
Lutzmann
I
, et al.
Pre-clinical heterotopic intrathoracic heart xenotransplantation: a possibly useful clinical technique. Xenotransplantation. 2015;22(6):427–42. doi:.https://doi.org/10.1111/xen.12213
56
van der Windt
DJ
,
Bottino
R
,
Casu
A
,
Campanile
N
,
Smetanka
C
,
He
J
, et al.
Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant. 2009;9(12):2716–26. doi:.https://doi.org/10.1111/j.1600-6143.2009.02850.x
57
Bottino
R
,
Wijkstrom
M
,
van der Windt
DJ
,
Hara
H
,
Ezzelarab
M
,
Murase
N
, et al.
Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am J Transplant. 2014;14(10):2275–87. doi:.https://doi.org/10.1111/ajt.12868
58
Thompson
P
,
Badell
IR
,
Lowe
M
,
Cano
J
,
Song
M
,
Leopardi
F
, et al.
Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11(12):2593–602. doi:.https://doi.org/10.1111/j.1600-6143.2011.03720.x
59
Aron Abdin
R
,
Vanhove
B
,
Vadori
M
,
Fante
F
,
Boldrin
M
,
De Benedictis
G
, et al.
Systemic immunosuppression plus local production of CTLA 4-Ig to control rejection of transgenic pig neuroblasts in non-human primate
[abstract]. Xenotransplantation. 2013;20:367–8.
60
Mohiuddin
MM
,
Singh
AK
,
Corcoran
PC
,
Hoyt
RF
,
Thomas
ML, 3rd
,
Ayares
D
, et al.
Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148(3):1106–13, discussion 1113–4. doi:.https://doi.org/10.1016/j.jtcvs.2014.06.002
61
Mohiuddin
MM
,
Singh
AK
,
Corcoran
PC
,
Thomas
ML, 3rd
,
Clark
T
,
Lewis
BG
, et al.
Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat Commun. 2016;7:11138. doi:.https://doi.org/10.1038/ncomms11138
62
Mohiuddin
MM
,
Singh
AK
,
Corcoran
PC
,
Hoyt
RF
,
Thomas
ML, 3rd
,
Lewis
BG
, et al.
One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14(2):488–9. doi:.https://doi.org/10.1111/ajt.12562
63
Mohiuddin
MM
,
Corcoran
PC
,
Singh
AK
,
Azimzadeh
A
,
Hoyt
RF, Jr
,
Thomas
ML
, et al.
B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12(3):763–71. doi:.https://doi.org/10.1111/j.1600-6143.2011.03846.x
64
Byrne
GW
,
Du
Z
,
Sun
Z
,
Asmann
YW
,
McGregor
CG
. Changes in cardiac gene expression after pig-to-primate orthotopic xenotransplantation. Xenotransplantation. 2011;18(1):14–27. doi:.https://doi.org/10.1111/j.1399-3089.2010.00620.x
65
Vial
CM
,
Ostlie
DJ
,
Bhatti
FN
,
Cozzi
E
,
Goddard
M
,
Chavez
GP
, et al.
Life supporting function for over one month of a transgenic porcine heart in a baboon. J Heart Lung Transplant. 2000;19(2):224–9. doi:.https://doi.org/10.1016/S1053-2498(99)00099-6
66
Higginbotham
L
,
Mathews
D
,
Breeden
CA
,
Song
M
,
Farris
AB, 3rd
,
Larsen
CP
, et al.
Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–30. doi:.https://doi.org/10.1111/xen.12166
67
Higginbotham
L
,
Kim
S
,
Mathews
D
,
Stephenson
A
,
Breeden
C
,
Larsen
C
, et al.
Late Renal Xenograft Failure Is Antibody-Mediated: Description of the Longest-Reported Survival in Pig-to-Primate Renal Xenotransplantation
[abstract 1515].
Am J Transplant. 2016;16(suppl 3).
68
Barth
RN
,
Yamamoto
S
,
LaMattina
JC
,
Kumagai
N
,
Kitamura
H
,
Vagefi
PA
, et al.
Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75(10):1615–24. doi:.https://doi.org/10.1097/01.TP.0000064335.50622.20
69
Iwase
H
,
Ekser
B
,
Satyananda
V
,
Bhama
J
,
Hara
H
,
Ezzelarab
M
, et al.
Pig-to-baboon heterotopic heart transplantation--exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22(3):211–20. doi:.https://doi.org/10.1111/xen.12167
70
Shah
JA
,
Navarro-Alvarez
N
,
DeFazio
M
,
Rosales
IA
,
Elias
N
,
Yeh
H
, et al.
A Bridge to Somewhere: 25-day Survival After Pig-to-Baboon Liver Xenotransplantation. Ann Surg. 2016;263(6):1069–71. doi:.https://doi.org/10.1097/SLA.0000000000001659
71
Kim
K
,
Schuetz
C
,
Elias
N
,
Veillette
GR
,
Wamala
I
,
Varma
M
, et al.
Up to 9-day survival and control of thrombocytopenia following alpha1,3-galactosyl transferase knockout swine liver xenotransplantation in baboons. Xenotransplantation. 2012;19(4):256–64. doi:.https://doi.org/10.1111/j.1399-3089.2012.00717.x
72
Ramirez
P
,
Chavez
R
,
Majado
M
,
Munitiz
V
,
Muñoz
A
,
Hernandez
Q
, et al.
Life-supporting human complement regulator decay accelerating factor transgenic pig liver xenograft maintains the metabolic function and coagulation in the nonhuman primate for up to 8 days. Transplantation. 2000;70(7):989–98. doi:.https://doi.org/10.1097/00007890-200010150-00001
73
Ekser
B
,
Long
C
,
Echeverri
GJ
,
Hara
H
,
Ezzelarab
M
,
Lin
CC
, et al.
Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. Am J Transplant. 2010;10(2):273–85. doi:.https://doi.org/10.1111/j.1600-6143.2009.02945.x
74
Ramírez
P
,
Montoya
MJ
,
Ríos
A
,
García Palenciano
C
,
Majado
M
,
Chávez
R
, et al.
Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant Proc. 2005;37(9):4103–6. doi:.https://doi.org/10.1016/j.transproceed.2005.09.186
75
Yeh
H
,
Machaidze
Z
,
Wamala
I
,
Fraser
JW
,
Navarro-Alvarez
N
,
Kim
K
, et al.
Increased transfusion-free survival following auxiliary pig liver xenotransplantation. Xenotransplantation. 2014;21(5):454–64. doi:.https://doi.org/10.1111/xen.12111
76
Cantu
E
,
Balsara
KR
,
Li
B
,
Lau
C
,
Gibson
S
,
Wyse
A
, et al.
Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7(1):66–75. doi:.https://doi.org/10.1111/j.1600-6143.2006.01603.x
77
Bush
EL
,
Barbas
AS
,
Holzknecht
ZE
,
Byrne
GW
,
McGregor
CG
,
Parker
W
, et al.
Coagulopathy in α-galactosyl transferase knockout pulmonary xenotransplants. Xenotransplantation. 2011;18(1):6–13. doi:.https://doi.org/10.1111/j.1399-3089.2011.00621.x
78
Lau
CL
,
Daggett
WC
,
Yeatman
MF
,
Chai
P
,
Lin
SS
,
Lodge
AJ
, et al.
The role of antibodies in dysfunction of pig-to-baboon pulmonary transplants. J Thorac Cardiovasc Surg. 2000;120(1):29–38. doi:.https://doi.org/10.1067/mtc.2000.106841
79
Cooper
DK
,
Matsumoto
S
,
Abalovich
A
,
Itoh
T
,
Mourad
NI
,
Gianello
PR
, et al.
Progress in clinical encapsulated islet xenotransplantation. Transplantation. 2016;100(11):2301–8. doi:.https://doi.org/10.1097/TP.0000000000001371
80
Cooper
DK
,
Satyananda
V
,
Ekser
B
,
van der Windt
DJ
,
Hara
H
,
Ezzelarab
MB
, et al.
Progress in pig-to-non-human primate transplantation models (1998-2013): a comprehensive review of the literature. Xenotransplantation. 2014;21(5):397–419. doi:.https://doi.org/10.1111/xen.12127
81
Zhou
H
,
Liu
H
,
Ezzelarab
M
,
Schmelzer
E
,
Wang
Y
,
Gerlach
J
, et al.
Experimental hepatocyte xenotransplantation--a comprehensive review of the literature. Xenotransplantation. 2015;22(4):239–48. doi:.https://doi.org/10.1111/xen.12170
82
Nagata
H
,
Nishitai
R
,
Shirota
C
,
Zhang
JL
,
Koch
CA
,
Cai
J
, et al.
Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology. 2007;132(1):321–9. doi:.https://doi.org/10.1053/j.gastro.2006.10.013
83
Shin
JS
,
Kim
JM
,
Kim
JS
,
Min
BH
,
Kim
YH
,
Kim
HJ
, et al.
Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am J Transplant. 2015;15(11):2837–50. doi:.https://doi.org/10.1111/ajt.13345
84
Dufrane
D
,
Goebbels
RM
,
Gianello
P
. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90(10):1054–62. doi:.https://doi.org/10.1097/TP.0b013e3181f6e267
85
Waterworth
PD
,
Dunning
J
,
Tolan
M
,
Cozzi
E
,
Langford
G
,
Chavez
G
, et al.
Life-supporting pig-to-baboon heart xenotransplantation. J Heart Lung Transplant. 1998;17(12):1201–7.
86
Lin
SS
,
Weidner
BC
,
Byrne
GW
,
Diamond
LE
,
Lawson
JH
,
Hoopes
CW
, et al.
The role of antibodies in acute vascular rejection of pig-to-baboon cardiac transplants. J Clin Invest. 1998;101(8):1745–56. doi:.https://doi.org/10.1172/JCI2134
87
Simon
PM
,
Neethling
FA
,
Taniguchi
S
,
Goode
PL
,
Zopf
D
,
Hancock
WW
, et al.
Intravenous infusion of Galalpha1-3Gal oligosaccharides in baboons delays hyperacute rejection of porcine heart xenografts. Transplantation. 1998;65(3):346–53. doi:.https://doi.org/10.1097/00007890-199802150-00009
88
Schmoeckel
M
,
Bhatti
FN
,
Zaidi
A
,
Cozzi
E
,
Waterworth
PD
,
Tolan
MJ
, et al.
Orthotopic heart transplantation in a transgenic pig-to-primate model. Transplantation. 1998;65(12):1570–7. doi:.https://doi.org/10.1097/00007890-199806270-00006
89
Xu
H
,
Gundry
SR
,
Hancock
WW
,
Matsumiya
G
,
Zuppan
CW
,
Morimoto
T
, et al.
Prolonged discordant xenograft survival and delayed xenograft rejection in a pig-to-baboon orthotopic cardiac xenograft model. J Thorac Cardiovasc Surg. 1998;115(6):1342–9. doi:.https://doi.org/10.1016/S0022-5223(98)70218-1
90
Byrne
GW
,
McGregor
CG
. Cardiac xenotransplantation: progress and challenges. Curr Opin Organ Transplant. 2012;17(2):148–54. doi:.https://doi.org/10.1097/MOT.0b013e3283509120
91
Cooper
DK
. Is successful orthotopic heart transplantation in the pig-to-non-human primate model required before proceeding to a clinical trial?
Xenotransplantation. 2016;23(4):328–9. doi:.https://doi.org/10.1111/xen.12251
92
Zaidi
A
,
Schmoeckel
M
,
Bhatti
F
,
Waterworth
P
,
Tolan
M
,
Cozzi
E
, et al.
Life-supporting pig-to-primate renal xenotransplantation using genetically modified donors. Transplantation. 1998;65(12):1584–90. doi:.https://doi.org/10.1097/00007890-199806270-00008
93
Meyer
C
,
Wolf
P
,
Romain
N
,
Ravanat
C
,
Roussi
J
,
Beller
JP
, et al.
Use of von Willebrand diseased kidney as donor in a pig-to-primate model of xenotransplantation. Transplantation. 1999;67(1):38–45. doi:.https://doi.org/10.1097/00007890-199901150-00006
94
Cowan
PJ
,
Aminian
A
,
Barlow
H
,
Brown
AA
,
Chen
CG
,
Fisicaro
N
, et al.
Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in non-immunosuppressed baboons. Transplantation. 2000;69(12):2504–15. doi:.https://doi.org/10.1097/00007890-200006270-00008
95
Pierson
RN, 3rd
,
Dorling
A
,
Ayares
D
,
Rees
MA
,
Seebach
JD
,
Fishman
JA
, et al.
Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16(5):263–80. doi:.https://doi.org/10.1111/j.1399-3089.2009.00534.x
96
Ekser
B
,
Markmann
JF
,
Tector
AJ
. Current status of pig liver xenotransplantation. Int J Surg. 2015;23(Pt B):240–6. doi:.https://doi.org/10.1016/j.ijsu.2015.06.083
97
Wang
ZY
,
Martens
GR
,
Blankenship
RL
,
Sidner
RA
,
Li
P
,
Estrada
JL
, et al.
Eliminating xenoantigen expression on swine RBC. Transplantation. 2016;1. doi:.https://doi.org/10.1097/TP.0000000000001302
98
Iwase
H
,
Ekser
B
,
Zhou
H
,
Liu
H
,
Satyananda
V
,
Humar
R
, et al.
Further evidence for sustained systemic inflammation in xenograft recipients (SIXR). Xenotransplantation. 2015;22(5):399–405. doi:.https://doi.org/10.1111/xen.12182
99
Ezzelarab
MB
,
Cooper
DK
. Systemic inflammation in xenograft recipients (SIXR): A new paradigm in pig-to-primate xenotransplantation?
Int J Surg. 2015;23(Pt B):301–5. doi:.https://doi.org/10.1016/j.ijsu.2015.07.643
100
Kornberg
A
,
Dietz
O
,
Mau
H
,
Pascher
A
,
Stangl
M
,
Scheele
J
, et al.
Impact of immunoadsorption on xenogeneic extracorporeal pig liver perfusion: assessment of organ function during autologous reperfusion. Xenotransplantation. 1999;6(3):187–93. doi:.https://doi.org/10.1034/j.1399-3089.1999.00027.x
101
Schmoeckel
M
,
Nollert
G
,
Shahmohammadi
M
,
Young
VK
,
Chavez
G
,
Kasper-König
W
, et al.
Prevention of hyperacute rejection by human decay accelerating factor in xenogeneic perfused working hearts. Transplantation. 1996;62(6):729–34. doi:.https://doi.org/10.1097/00007890-199609270-00005
102
Storck
M
,
Abendroth
D
,
Prestel
R
,
Pino-Chavez
G
,
Müller-Höker
J
,
White
DJ
, et al.
Morphology of hDAF (CD55) transgenic pig kidneys following ex-vivo hemoperfusion with human blood. Transplantation. 1997;63(2):304–10. doi:.https://doi.org/10.1097/00007890-199701270-00022
103
Ahrens
HE
,
Petersen
B
,
Ramackers
W
,
Petkov
S
,
Herrmann
D
,
Hauschild-Quintern
J
, et al.
Kidneys from α1,3-Galactosyltransferase knockout/human heme oxygenase-1/human A20 transgenic pigs are protected from rejection during ex vivo perfusion with human blood. Transplant Direct. 2015;1(6):e23.
104
Loss
M
,
Schmidtko
J
,
Przemeck
M
,
Kunz
R
,
Arends
H
,
Jalali
A
, et al.; M. Loss, J. Schmidtko, M. Przemeck. A primate model for discordant pig to primate kidney xenotransplantation without hyperacute graft rejection. J Invest Surg. 2001;14(1):21–9. doi:.https://doi.org/10.1080/089419301750072185
105
Pöling
J
,
Oezkur
M
,
Kogge
K
,
Mengel
M
,
Niemann
H
,
Winkler
M
, et al.
Hyperacute rejection in ex vivo-perfused porcine lungs transgenic for human complement regulatory proteins. Transpl Int. 2006;19(3):225–32. doi:.https://doi.org/10.1111/j.1432-2277.2006.00267.x
106
Grinyo
JM
,
Cruzado
JM
,
Torras
J
. Hyperacute rejection models: ex vivo xenoperfusion systems. Transplant Proc. 1999;31(1-2):970–1. doi:.https://doi.org/10.1016/S0041-1345(98)01862-4
107
Waldman
JP
,
Brock
LG
,
Rees
MA
. A human-specific mutation limits nonhuman primate efficacy in preclinical xenotransplantation studies. Transplantation. 2014;97(4):385–90. doi:.https://doi.org/10.1097/01.TP.0000441321.87915.82
108
Springer
SA
,
Diaz
SL
,
Gagneux
P
. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 2014;66(11):671–4. doi:.https://doi.org/10.1007/s00251-014-0795-0
109
Salama
A
,
Evanno
G
,
Harb
J
,
Soulillou
JP
. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22(2):85–94. doi:.https://doi.org/10.1111/xen.12142
110
Bouhours
D
,
Pourcel
C
,
Bouhours
JE
. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Gal alpha 1-3Gal), blood group H determinant and N-glycolylneuraminic acid. Glycoconj J. 1996;13(6):947–53. doi:.https://doi.org/10.1007/BF01053190
111
Bouhours
D
,
Liaigre
J
,
Lemoine
J
,
Mayer-Posner
F
,
Bouhours
JF
. Two novel isoneolacto-undecaglycosylceramides carrying Galalpha1-->3Lewis(x) on the 6-linked antenna and N-acetylneuraminic acidalpha2-->3 or Galactose alpha1-->3 on the 3-linked antenna, expressed in porcine kidney. Glycoconj J. 1998;15(10):1001–16. doi:.https://doi.org/10.1023/A:1006994126958
112
Nguyen
BN
,
Azimzadeh
AM
,
Schroeder
C
,
Buddensick
T
,
Zhang
T
,
Laaris
A
, et al.
Absence of Gal epitope prolongs survival of swine lungs in an ex vivo model of hyperacute rejection. Xenotransplantation. 2011;18(2):94–107. doi:.https://doi.org/10.1111/j.1399-3089.2011.00633.x
113
Burdorf
L
,
Stoddard
T
,
Zhang
T
,
Rybak
E
,
Riner
A
,
Avon
C
, et al.
Expression of human CD46 modulates inflammation associated with GalTKO lung xenograft injury. Am J Transplant. 2014;14(5):1084–95. doi:.https://doi.org/10.1111/ajt.12673
114
Harris
DG
,
Quinn
KJ
,
French
BM
,
Schwartz
E
,
Kang
E
,
Dahi
S
, et al.
Meta-analysis of the independent and cumulative effects of multiple genetic modifications on pig lung xenograft performance during ex vivo perfusion with human blood. Xenotransplantation. 2015;22(2):102–11. doi:.https://doi.org/10.1111/xen.12149
115
Brandl
U
,
Jöckle
H
,
Erhardt
M
,
Michel
S
,
Burdorf
L
,
Brenner
P
, et al.
Reduced fibrin deposition and intravascular thrombosis in hDAF transgenic pig hearts perfused with tirofiban. Transplantation. 2007;84(12):1667–76. doi:.https://doi.org/10.1097/01.tp.0000295742.45413.dc
116
Niemann
H
,
Verhoeyen
E
,
Wonigeit
K
,
Lorenz
R
,
Hecker
J
,
Schwinzer
R
, et al.
Cytomegalovirus early promoter induced expression of hCD59 in porcine organs provides protection against hyperacute rejection. Transplantation. 2001;72(12):1898–906. doi:.https://doi.org/10.1097/00007890-200112270-00006
117
Rees
MA
,
Butler
AJ
,
Chavez-Cartaya
G
,
Wight
DG
,
Casey
ND
,
Alexander
G
, et al.
Prolonged function of extracorporeal hDAF transgenic pig livers perfused with human blood. Transplantation. 2002;73(8):1194–202. doi:.https://doi.org/10.1097/00007890-200204270-00003
118
Matsushita
T
,
Ikai
I
,
Nishitai
R
,
Katsura
N
,
Yamanokuchi
S
,
Matsuo
K
, et al.
Suppressed complement activation in human decay accelerating factor transgenic porcine liver cross-circulated with nonhuman primates. Transplantation. 2003;75(11):1807–12. doi:.https://doi.org/10.1097/01.TP.0000063221.65123.49
119
LaMattina
JC
,
Burdorf
L
,
Zhang
T
,
Rybak
E
,
Cheng
X
,
Munivenkatappa
R
, et al.
Pig-to-baboon liver xenoperfusion utilizing GalTKO.hCD46 pigs and glycoprotein Ib blockade. Xenotransplantation. 2014;21(3):274–86. doi:.https://doi.org/10.1111/xen.12093
120
Bongoni
AK
,
Kiermeir
D
,
Jenni
H
,
Wünsch
A
,
Bähr
A
,
Ayares
D
, et al.
Activation of the lectin pathway of complement in pig-to-human xenotransplantation models. Transplantation. 2013;96(9):791–9. doi:.https://doi.org/10.1097/TP.0b013e3182a3a52b
121
Bongoni
AK
,
Kiermeir
D
,
Jenni
H
,
Bähr
A
,
Ayares
D
,
Klymiuk
N
, et al.
Complement dependent early immunological responses during ex vivo xenoperfusion of hCD46/HLA-E double transgenic pig forelimbs with human blood. Xenotransplantation. 2014;21(3):230–43. doi:.https://doi.org/10.1111/xen.12090
122
Bongoni
AK
,
Kiermeir
D
,
Schnider
J
,
Jenni
H
,
Garimella
P
,
Bähr
A
, et al.
Transgenic Expression of Human CD46 on Porcine Endothelium: Effect on Coagulation and Fibrinolytic Cascades During Ex Vivo Human-to-Pig Limb Xenoperfusions. Transplantation. 2015;99(10):2061–9. doi:.https://doi.org/10.1097/TP.0000000000000746
123
Bongoni
AK
,
Kiermeir
D
,
Denoyelle
J
,
Jenni
H
,
Burlak
C
,
Seebach
JD
, et al.
Porcine extrahepatic vascular endothelial asialoglycoprotein receptor 1 mediates xenogeneic platelet phagocytosis in vitro and in human-to-pig ex vivo xenoperfusion. Transplantation. 2015;99(4):693–701. doi:.https://doi.org/10.1097/TP.0000000000000553
124
Inverardi
L
,
Samaja
M
,
Motterlini
R
,
Mangili
F
,
Bender
JR
,
Pardi
R
. Early recognition of a discordant xenogeneic organ by human circulating lymphocytes. J Immunol. 1992;149(4):1416–23.
125
Khalfoun
B
,
Barrat
D
,
Watier
H
,
Machet
MC
,
Arbeille-Brassart
B
,
Riess
JG
, et al.
Development of an ex vivo model of pig kidney perfused with human lymphocytes. Analysis of xenogeneic cellular reactions. Surgery. 2000;128(3):447–57. doi:.https://doi.org/10.1067/msy.2000.107063
126
Ramos
A
,
Vega
A
,
Val
F
,
Chavez
G
,
López-Hoyos
M
,
Ruiz
JC
, et al.
Immunohistochemical study of a new experimental model of acute cellular xenograft rejection. Transplant Proc. 2000;32(5):960. doi:.https://doi.org/10.1016/S0041-1345(00)01060-5
127Shelley M. Introduction. In: Joseph MK, editor. Frankenstein or the modern Prometheus. 16th ed. Oxford: Oxford University Press; 1992.
128Chaisinthop N. What is Fresh Cell Therapy? Postdoctoral Researcher for the ESRC Bionetworking in Asia Project. WordPress / Academica WordPress Theme by WPZOOM.; 2013. 1-2. Available from: URL: http://www.centreforbionetworking.org/wp-content/uploads/2013/12/What-is-Fresh-Cell-Therapy.pdf [accessed 2016 July 15].
129
Borel
JF
. Jean-Francoişe Borel and the Origin of the World’s First Billion Dollar Molecule. Transplantation. 2015;99(10):2012–4. doi:.https://doi.org/10.1097/TP.0000000000000946
130Frontline. Organ farm. The business of xenotransplantation: Past and present. WGBH educational foundation.; 2001. Available from: URL: http://www.pbs.org/wgbh/pages/frontline/shows/organfarm/business/business.html [accessed 2016 June 29].
131
Mei
J
,
Sgroi
A
,
Mai
G
,
Baertschiger
R
,
Gonelle-Gispert
C
,
Serre-Beinier
V
, et al.
Improved survival of fulminant liver failure by transplantation of microencapsulated cryopreserved porcine hepatocytes in mice. Cell Transplant. 2009;18(1):101–10. doi:.https://doi.org/10.3727/096368909788237168
132
Muller
YD
,
Mai
G
,
Morel
P
,
Serre-Beinier
V
,
Gonelle-Gispert
C
,
Yung
GP
, et al.
Anti-CD154 mAb and rapamycin induce T regulatory cell mediated tolerance in rat-to-mouse islet transplantation. PLoS One. 2010;5(4):e10352. doi:.https://doi.org/10.1371/journal.pone.0010352
133
Meier
RP
,
Seebach
JD
,
Morel
P
,
Mahou
R
,
Borot
S
,
Giovannoni
L
, et al.
Survival of free and encapsulated human and rat islet xenografts transplanted into the mouse bone marrow. PLoS One. 2014;9(3):e91268. doi:.https://doi.org/10.1371/journal.pone.0091268
134
Meier
RP
,
Mahou
R
,
Morel
P
,
Meyer
J
,
Montanari
E
,
Muller
YD
, et al.
Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J Hepatol. 2015;62(3):634–41. doi:.https://doi.org/10.1016/j.jhep.2014.10.030
135
Rieben
R
,
Bovin
NV
,
Korchagina
EY
,
Oriol
R
,
Nifant’ev
NE
,
Tsvetkov
DE
, et al.
Xenotransplantation: in vitro analysis of synthetic alpha-galactosyl inhibitors of human anti-Galalpha1-->3Gal IgM and IgG antibodies. Glycobiology. 2000;10(2):141–8. doi:.https://doi.org/10.1093/glycob/10.2.141
136
Laumonier
T
,
Walpen
AJ
,
Maurus
CF
,
Mohacsi
PJ
,
Matozan
KM
,
Korchagina
EY
, et al.
Dextran sulfate acts as an endothelial cell protectant and inhibits human complement and natural killer cell-mediated cytotoxicity against porcine cells. Transplantation. 2003;76(5):838–43. doi:.https://doi.org/10.1097/01.TP.0000078898.28399.0A
137
Laumonier
T
,
Mohacsi
PJ
,
Matozan
KM
,
Banz
Y
,
Haeberli
A
,
Korchagina
EY
, et al.
Endothelial cell protection by dextran sulfate: a novel strategy to prevent acute vascular rejection in xenotransplantation. Am J Transplant. 2004;4(2):181–7. doi:.https://doi.org/10.1046/j.1600-6143.2003.00306.x
138
Bongoni
AK
,
Klymiuk
N
,
Wolf
E
,
Ayares
D
,
Rieben
R
,
Cowan
PJ
. Transgenic Expression of Human Thrombomodulin Inhibits HMGB1-Induced Porcine Aortic Endothelial Cell Activation. Transplantation. 2016;100(9):1871–9. doi:.https://doi.org/10.1097/TP.0000000000001188
139
Seebach
JD
,
Waneck
GL
. Natural killer cells in xenotransplantation. Xenotransplantation. 1997;4(4):201–11. doi:.https://doi.org/10.1111/j.1399-3089.1997.tb00184.x
140
Rieben
R
,
Seebach
JD
. Xenograft rejection: IgG1, complement and NK cells team up to activate and destroy the endothelium. Trends Immunol. 2005;26(1):2–5. doi:.https://doi.org/10.1016/j.it.2004.11.011
141
Schneider
MK
,
Seebach
JD
. Current cellular innate immune hurdles in pig-to-primate xenotransplantation. Curr Opin Organ Transplant. 2008;13(2):171–7. doi:.https://doi.org/10.1097/MOT.0b013e3282f88a30
142
Seebach
JD
,
Comrack
C
,
Germana
S
,
LeGuern
C
,
Sachs
DH
,
DerSimonian
H
. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J Immunol. 1997;159(7):3655–61.
143
Seebach
JD
,
Pazmany
L
,
Waneck
GL
,
Minja
F
,
Germana
S
,
LeGuern
C
, et al.
HLA-G expression on porcine endothelial cells protects partially against direct human NK cytotoxicity but not against ADCC. Transplant Proc. 1999;31(4):1864–5. doi:.https://doi.org/10.1016/S0041-1345(99)00190-6
144
Forte
P
,
Matter-Reissmann
UB
,
Strasser
M
,
Schneider
MK
,
Seebach
JD
. Porcine aortic endothelial cells transfected with HLA-G are partially protected from xenogeneic human NK cytotoxicity. Hum Immunol. 2000;61(11):1066–73. doi:.https://doi.org/10.1016/S0198-8859(00)00202-0
145
Forte
P
,
Pazmany
L
,
Matter-Reissmann
UB
,
Stussi
G
,
Schneider
MK
,
Seebach
JD
. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J Immunol. 2001;167(10):6002–8. doi:.https://doi.org/10.4049/jimmunol.167.10.6002
146
Forte
P
,
Baumann
BC
,
Weiss
EH
,
Seebach
JD
. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant. 2005;5(9):2085–93. doi:.https://doi.org/10.1111/j.1600-6143.2005.00987.x
147
Forte
P
,
Baumann
BC
,
Schneider
MK
,
Seebach
JD
. HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation. 2009;16(1):19–26. doi:.https://doi.org/10.1111/j.1399-3089.2009.00510.x
148
Baumann
BC
,
Forte
P
,
Hawley
RJ
,
Rieben
R
,
Schneider
MK
,
Seebach
JD
. Lack of galactose-alpha-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol. 2004;172(10):6460–7. doi:.https://doi.org/10.4049/jimmunol.172.10.6460
149
Baumann
BC
,
Schneider
MK
,
Lilienfeld
BG
,
Antsiferova
MA
,
Rhyner
DM
,
Hawley
RJ
, et al.
Endothelial cells derived from pigs lacking Gal alpha(1,3)Gal: no reduction of human leukocyte adhesion and natural killer cell cytotoxicity. Transplantation. 2005;79(9):1067–72. doi:.https://doi.org/10.1097/01.TP.0000157231.11083.7C
150
Schneider
MK
,
Strasser
M
,
Gilli
UO
,
Kocher
M
,
Moser
R
,
Seebach
JD
. Rolling adhesion of human NK cells to porcine endothelial cells mainly relies on CD49d-CD106 interactions. Transplantation. 2002;73(5):789–96. doi:.https://doi.org/10.1097/00007890-200203150-00023
151
Schneider
MK
,
Ghielmetti
M
,
Rhyner
DM
,
Antsiferova
MA
,
Seebach
JD
. Human leukocyte transmigration across Galalpha(1,3)Gal-negative porcine endothelium is regulated by human CD18 and CD99. Transplantation. 2009;87(4):491–9. doi:.https://doi.org/10.1097/TP.0b013e318195fb8d
152
Muller
YD
,
Ehirchiou
D
,
Golshayan
D
,
Buhler
LH
,
Seebach
JD
. Potential of T-regulatory cells to protect xenografts. Curr Opin Organ Transplant. 2012;17(2):155–61. doi:.https://doi.org/10.1097/MOT.0b013e3283508e17
153
Ehirchiou
D
,
Muller
YD
,
Chicheportiche
R
,
Heyrani Nobari
R
,
Madelon
N
,
Schneider
MK
, et al.
Chemoattractant Signals and Adhesion Molecules Promoting Human Regulatory T Cell Recruitment to Porcine Endothelium. Transplantation. 2016;100(4):753–62. doi:.https://doi.org/10.1097/TP.0000000000001034
154
Madelon
N
,
Puga Yung
GL
,
Seebach
JD
. Human anti-pig NK cell and CD8(+) T-cell responses in the presence of regulatory dendritic cells. Xenotransplantation. 2016 Nov 13. [Epub ahead of print] doi:.https://doi.org/10.1111/xen.12279
155
Gollackner
B
,
Mueller
NJ
,
Houser
S
,
Qawi
I
,
Soizic
D
,
Knosalla
C
, et al.
Porcine cytomegalovirus and coagulopathy in pig-to-primate xenotransplantation. Transplantation. 2003;75(11):1841–7. doi:.https://doi.org/10.1097/01.TP.0000065806.90840.C1
156
Mueller
NJ
,
Barth
RN
,
Yamamoto
S
,
Kitamura
H
,
Patience
C
,
Yamada
K
, et al.
Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002;76(10):4734–40. doi:.https://doi.org/10.1128/JVI.76.10.4734-4740.2002
157
Mueller
NJ
,
Livingston
C
,
Knosalla
C
,
Barth
RN
,
Yamamoto
S
,
Gollackner
B
, et al.
Activation of porcine cytomegalovirus, but not porcine lymphotropic herpesvirus, in pig-to-baboon xenotransplantation. J Infect Dis. 2004;189(9):1628–33. doi:.https://doi.org/10.1086/383351
158
Mueller
NJ
,
Kuwaki
K
,
Dor
FJ
,
Knosalla
C
,
Gollackner
B
,
Wilkinson
RA
, et al.
Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation. 2004;78(10):1449–53. doi:.https://doi.org/10.1097/01.TP.0000141361.68446.1F
159
Mueller
NJ
,
Kuwaki
K
,
Knosalla
C
,
Dor
FJ
,
Gollackner
B
,
Wilkinson
RA
, et al.
Early weaning of piglets fails to exclude porcine lymphotropic herpesvirus. Xenotransplantation. 2005;12(1):59–62. doi:.https://doi.org/10.1111/j.1399-3089.2004.00196.x
160
Mueller
NJ
,
Ezzelarab
M
,
Buhler
L
,
Haeberli
L
,
Ayares
D
,
Cooper
DK
. Monitoring of porcine and baboon cytomegalovirus infection in xenotransplantation. Xenotransplantation. 2009;16(6):535–6. doi:.https://doi.org/10.1111/j.1399-3089.2009.00536.x
161
Ghielmetti
M
,
Millard
AL
,
Haeberli
L
,
Bossart
W
,
Seebach
JD
,
Schneider
MK
, et al.
Human CMV infection of porcine endothelial cells increases adhesion receptor expression and human leukocyte recruitment. Transplantation. 2009;87(12):1792–800. doi:.https://doi.org/10.1097/TP.0b013e3181a75a41
162
Millard
AL
,
Häberli
L
,
Sinzger
C
,
Ghielmetti
M
,
Schneider
MK
,
Bossart
W
, et al.
Efficiency of porcine endothelial cell infection with human cytomegalovirus depends on both virus tropism and endothelial cell vascular origin. Xenotransplantation. 2010;17(4):274–87. doi:.https://doi.org/10.1111/j.1399-3089.2010.00594.x
163
Taveira
A
,
Ponroy
N
,
Mueller
NJ
,
Millard
AL
. Entry of human cytomegalovirus into porcine endothelial cells depends on both the cellular vascular origin and the viral strain. Xenotransplantation. 2014;21(4):324–40. doi:.https://doi.org/10.1111/xen.12097
164
Denner
J
,
Mueller
NJ
. Preventing transfer of infectious agents. Int J Surg. 2015;23(Pt B):306–11. doi:.https://doi.org/10.1016/j.ijsu.2015.08.032
165Centers for Disease Control and Prevention. U.S. Public Health Service Guideline on Infectious Disease Issues in Xenotransplantation. MMWR.50 (RR-15); 2001. 1-46. Available from: URL: http://www.cdc.gov/mmwr/PDF/rr/rr5015.pdf [accessed 2016 August 31].
166U.S.Department of Health and Human ServicesFood and Drug Administration. Guidance for industry: source animal, product, preclinical, and clinical issues concerning the use of xenotransplantation products in humans. Center for Biologics Evaluation and Research (CBER).; 2003. 1-60. Available from: URL: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm092707.pdf [accessed 2003 April 1].
167
Onions
D
,
Cooper
DK
,
Alexander
TJ
,
Brown
C
,
Claassen
E
,
Foweraker
JE
, et al.
An approach to the control of disease transmission in pig-to-human xenotransplantation. Xenotransplantation. 2000;7(2):143–55. doi:.https://doi.org/10.1034/j.1399-3089.2000.00047.x
168
Fishman
JA
. Infection in xenotransplantation. J Card Surg. 2001;16(5):363–73. doi:.https://doi.org/10.1111/j.1540-8191.2001.tb00536.x
169World Health Organization (WHO). Fifty-seventh World Health Assembly. Human organ and tissue transplantation. WHO.Agenda item 12.14. Resolution WHA57.18.; 2004. 1-3. Available from: URL: http://www.who.int/ethics/en/A57_R18-en.pdf [accessed 2004 May 22].
170WHO, International Xenotransplantation Association and The Transplantation Society. Second WHO global consultation on regulatory requirements for xenotransplantation clinical trials. 2011 Oct 17; http://www.who.int/transplantation/xeno/report2nd_global_consultation_xtx.pdf?ua=1.: WHO; 2011 p. 2-37.
171
Fishman
JA
,
Scobie
L
,
Takeuchi
Y
. Xenotransplantation-associated infectious risk: a WHO consultation. Xenotransplantation. 2012;19(2):72–81. doi:.https://doi.org/10.1111/j.1399-3089.2012.00693.x
172
Hering
BJ
,
Cozzi
E
,
Spizzo
T
,
Cowan
PJ
,
Rayat
GR
,
Cooper
DK
, et al.
First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Executive summary. Xenotransplantation. 2016;23(1):3–13. doi:.https://doi.org/10.1111/xen.12231
173
Kim
MK
,
Choi
HJ
,
Kwon
I
,
Pierson
RN, 3rd
,
Cooper
DK
,
Soulillou
JP
, et al.; International Xenotransplantation Association. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of xenocorneal transplantation. Xenotransplantation. 2014;21(5):420–30. doi:.https://doi.org/10.1111/xen.12129
174
Bloom
ET
. Xenotransplantation--federal regulatory considerations. Curr Top Microbiol Immunol. 2003;278:239–51. doi:.https://doi.org/10.1007/978-3-642-55541-1_9
175European Medicine Agency CfMPfHUC. Guideline on Xenogeneic Cell-Based Medicinal Products. EMEA.:1-14. Available from: URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003832.pdf [accessed 2009 February 27].
176
Chapman
LE
. Xenotransplantation, xenogeneic infections, biotechnology, and public health. Mt Sinai J Med. 2009;76(5):435–41. doi:.https://doi.org/10.1002/msj.20131
177
Denner
J
,
Tönjes
RR
,
Takeuchi
Y
,
Fishman
J
,
Scobie
L
. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Chapter 5: recipient monitoring and response plan for preventing disease transmission. Xenotransplantation. 2016;23(1):53–9. doi:.https://doi.org/10.1111/xen.12227
178The Federal Assembly of the Swiss Confederation. Federal Act on the Transplantation of Organs, Tissues and Cells (Transplantation Act). 810.21. 8-10-2004:1-26.
179The Federal Assembly of the Swiss Confederation. Verordnung über die Transplantation von tierischen Organen, Geweben und Zellen (Medizin und Menschenwürde). 810.213. 16-3-2007:1-17.


