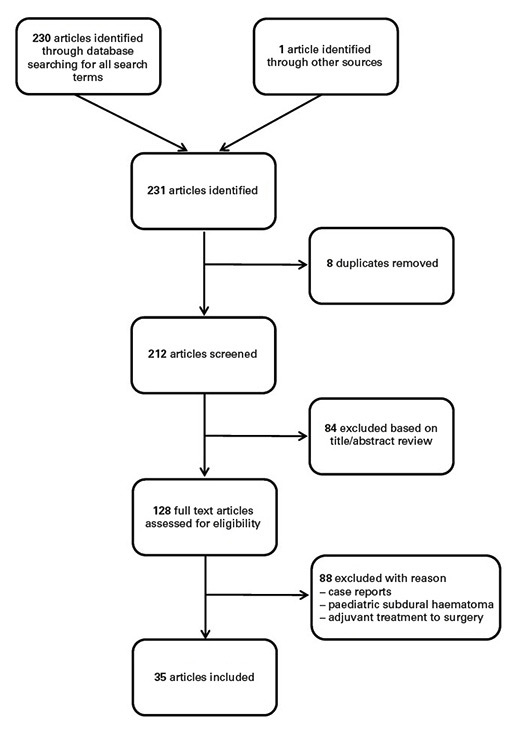
Figure 1 Selection of articles included in this review.
DOI: https://doi.org/10.4414/smw.2017.14398
Chronic subdural haematoma (cSDH) is a common neurosurgical entity, often caused by head trauma and occurring mostly in elderly patients. The incidence in people 70 years of age and older is estimated at 58/100 000 persons a year [1]. In 41% of the cases, patients are treated with oral anticoagulation or antiplatelet therapy at the time of diagnosis [2]. Since the incidence is expected to double by the year 2030, owing to the continuous aging of the population, there is a need for evidence-based management guidelines for both general and specialised healthcare practitioners [3]. Surgical treatment is considered the gold standard for symptomatic cSDH, but may become complex because most of the patients are advanced in age and/or are being treated with anticoagulants. Even though the outcome of surgical treatment is considered good, conservative treatment may be highly valuable in some situations.
An understanding of the pathophysiology is crucial for the development of medical agents in order to treat cSDHs conservatively. In healthy individuals, the subdural space is a virtual space [3]. A layer of dural border cells with enlarged extracellular space containing bridging veins is tethered between the dura mater and the arachnoid mater [4]. In individuals with brain atrophy (e.g., elderly, alcoholics), the bridging veins are stretched since the arachnoid is separated from the dura mater. These stretched veins can be easily torn, typically as a result of minor trauma, leading to acute bleeding into the virtual subdural space. Thereafter, fibrin deposition and fibrinolytic organisation occurs, and the blood in the subdural space triggers inflammation [5–8]. After weeks, neomembrane with fragile neocapillaries is formed, typically leading to further microbleeding and the maintenance or enlargement of the SDH. Other factors influencing the enlargement of the SDH are: fragility of the neocapillaries [4], acceleration of fibrinolysis [9], high concentration of fibrin degradation products [10, 11] and high concentrations of vascular endothelial growth factor (VEGF) [12–14] in the subdural space.
Chronic SDH is also described as a circumscribed chronic self-perpetuating inflammatory disorder. Stanisic et al. showed significantly higher levels of proinflammatory mediators (interleukin-2 receptor, and interleukins 5, 6 and 7) and anti-inflammatory mediators in the cSDH fluid compared with systemic levels, as well as a higher ratio of pro- to anti-inflammatory mediators [15]. The contact between cSDH fluid and the dural border cells seems to evoke a local aseptic inflammatory and inflammation-induced angiogenic reaction [16]. This angiogenic reaction leads to the formation of neomembranes that cause repeated microbleeds into the haematoma cavity. Consequently, cSDH may be considered as an angiogenic disease, where inflammatory phenomena play a major role [5].
A clinical classification, namely the Markwalder grading score (MGS) [17], might help to decide which therapy modality might be more appropriate. However, no consensus exists about the best treatment for each grade and the treatment modality that should be chosen individually for each patient. Symptomatic patients with a confirmed radiological appearance of a haematoma are usually treated surgically, whereas patients with asymptomatic haematomas and small non-space-occupying haematomas can be managed conservatively with a medical agent or through careful observation [18].
We provide a systematic review summarising the conservative treatment modalities of cSDH and their indications. The emphasis was on studies investigating stand-alone pharmacological treatment of cSDH; if for a specific agent none was available, adjunct treatment to surgery is described.
References for this review were identified by searching PubMed from 1960 up to and including 2016. Search terms were: “cSDH conservative treatment”, “cSDH nonsurgical or nonoperative treatment”, “cSDH steroids”, “cSDH corticosteroids”, “cSDH dexamethasone”, “cSDH ACE-inhibitors”, “cSDH tranexamic acid”, “cSDH mannitol”, “cSDH platelet activating factor receptor antagonist” and “cSDH statins OR atorvastatin” with no restrictions to a particular language, or to human studies, case reports, clinical trials, controlled clinical trials, meta-analyses, randomised controlled trials, reviews or systematic reviews. Abstracts were independently reviewed by two of the authors (JS and FN), duplicates were removed and the final list of references was generated (fig. 1). The review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [19]. The classification of evidence was conducted in accordance with the US Preventive Services Task Forces, the National Health Service Centre Reviews and Dissemination (UK) and the Cochrane Collaboration (table 1) [20, 21]. Recommendations type A, B or C were formulated according to the levels of evidence I, II, or III, type A reflecting a high degree of clinical certainty, type B moderate certainty, and type C recommendations meaning either a lack of certainty or even harmfulness.

Figure 1 Selection of articles included in this review.
Table 1 Classification of evidence (adapted from Soleman et al. [4] and Lawrence at al [20, 21].).
| Class I | Evidence provided by one or more well-designed RCTs |
| Class II | Evidence provided by moderate quality RCTs, good quality cohort or case studies. |
| Class III | Evidence obtained expert’s opinion based on clinical experiences |
RCT = randomised controlled trial
After searching for all terms, 230 records were identified by the database and 1 additional record was identified through other sources. After removal of 18 duplicates, 212 records were screened. Based on title or abstract review, 84 records were excluded. Out of the remaining 128 records, 93 were excluded with reason, resulting in 35 articles (fig. 1).
The surgical drainage of a symptomatic cSDH is still considered the preferred treatment. In general, three primary surgical techniques are used: (1) craniotomy with an opening of >30 mm, (2) burr hole craniostomy with opening of 10–30 mm, and (3) twist drill craniostomy with small openings of <10 mm of the skull. Twist drill and burr hole craniostomy are suggested for primary treatment with or without symptoms; twist drill craniostomy is used especially in patients with multiple comorbidities [3, 4]. Craniotomy permits a good exposure to the brain and is the most effective technique to manage complicated cases [3]. Class I evidence studies are practically nonexistent and there are still many debates about the surgical treatment of cSDH [22].
From the literature, it is apparent that conservative therapy for cSDH could be used in some cases. Conservative management is reserved for asymptomatic small-sized haematomas, patients refusing operation, or those with high operative risk [3, 23]. Various medical therapy options such as corticosteroids, angiotensin converting-enzyme (ACE) inhibitors, mannitol 20%, tranexamic acid, platelet activating factor receptor antagonists and statins are discussed in the literature, but only in small observational studies. In addition some authors described spontaneous resolution while conducting a “wait and watch” or “wait and scan” regimen [24, 25]. Two surveys among neurosurgeons showed that conservative management is rarely used and therefore more evidence-based prospective studies are needed to evaluate its value and efficacy in patients with cSDH [26, 27].
The natural history of cSDH remains unclear. A few small case series and several case reports describe spontaneous resolution of traumatic and nontraumatic cSDHs, even in patients under antiplatelet therapy [24, 28–31]. Clear indications for a wait and watch or wait and scan regimen do not exist. According to Lee et al., sufficient potential subdural space is the premorbid condition leading to progression of cSDHs. Any force to shrink the brain can be the precipitating factor for the progression of cSDH; the opposite, namely an expansion, leads to spontaneous resolution of the haematoma [32]. In addition, the interaction between the premorbid status, the maturation of neomembranes, and the dynamics of absorption and expansion influence the progression or regression of SDHs [32]. Unfortunately, there are no clear clinical or radiological signs that indicate whether the cSDH will resolve spontaneously or not. Some authors advocate that patients who have minimal neurological deficits and lesions of small size with low or isodensity and ventricular dilatation on computed tomography (CT) have a greater chance for spontaneous resolution of their haematoma [33]. Others postulate that for patients with mild symptoms and frontal haematomas [34], or for patients older than 70 years with worsening mental function and brain atrophy, a wait and scan regimen should be followed [29]. However these conclusions are based on case reports or very small case series (type C recommendation). To conclude, wait and watch or wait and scan management is indicated in patients with no or minor symptoms (Markwald score 0–1), or in patients with a premorbid condition not allowing surgical evacuation of cSDH (type C recommendation).
Corticosteroids inhibit the synthesis of various proinflammatory mediators, immune system cells, proinflammatory enzymes, and the synthesis of nitric oxid and cyclooxygenase. Vascular endothelial growth factor (VEGF), a potent inducer of vascular permeability, was shown to be suppressed by corticosteroids [8]. In addition, corticosteroids stimulate inflammatory inhibitors like lipocortin, which blocks phospholipase A2 and ACE [35]. VEGF and ACE, as well as other inflammatory and angiogenic mediators, were shown to play a crucial role in the pathogenesis and maintenance of cSDH [6]. Therefore, it is postulated that corticosteroids reduce or even disrupt the inflammation-induced angiogenic reaction in cSDH through the inhibition of these inflammatory and angiogenetic factors [3]. This theory is supported by a study in which cSDH was induced in rats and thereafter treated with corticosteroids. The group of rats treated with intramuscular injection of dexamethasone showed histologically no formation of neomembranes, in contrast to the untreated group where neomembranes were present [7].
The first study evaluating the nonsurgical treatment of cSDH with corticosteroids was performed in 1974 by Bender et al. [36]. This study, from the era before CT (diagnosis was based on angiography), compared medical treatment (n = 75; prednisone 60 mg orally for a few days, thereafter reduction of dose by 10 mg every 3 days) to medical and surgical treatment (n = 22) and surgical treatment only (n = 88). Of the 75 patients treated medically, 22 were considered as medical failure and therefore underwent operation. The medically treated group showed no mortality and poor outcome in 2 cases, whereas in the surgical group 12 patients died and 7 had poor outcome. Based on these results the authors recommend surgery as the first choice of treatment in comatose or progressively worsening patients, whereas treatment with corticosteroids should be started in all other patients and when the response is good, should remain the only treatment (type C recommendation). A retrospective study by Pichert et al. in 56 patients treated medically with corticosteroids (regimen unknown) showed a favourable outcome in 83%, whereas 8 patients eventually required surgical drainage [37]. They concluded that conservative treatment with corticosteroids can be recommended in patients without distinct focal symptoms and nonsoporose or noncomatose patients (type C recommendation). In 2005, a prospective cohort study of 112 patients with cSDH was conducted, in which 26 patients were treated with corticosteroids (dexamethasone 4 mg four times a day for 21 days), 69 patients were treated with burr hole drainage and postoperative corticosteroids, 13 patients had with surgical drainage alone, and 4 patients received neither surgery nor corticosteroids [38]. In the corticosteroid group, one patient (4%) required burr hole drainage after 1 month and in surgical group recurrence was seen in three patients (4%), with no significant difference between the groups. In the group without any treatment, two out of four patients ultimately required surgical drainage. Hospitalisation time, outcome and mortality were comparable in the steroid group, the steroid plus surgical drainage group, and the surgical drainage only group. Significant steroid-related complications were not reported, and two diabetic patients treated with steroids required additional insulin therapy for optimal diabetic control only during the treatment period. The authors conclude that corticosteroids might be a valid alternative for patients with cSDH not suitable for surgical drainage. However, large randomised studies are needed to confirm these suggestions (type C recommendation). In 2009, Delgado-López et al. compared, in a retrospective manner, a conservative regimen of corticosteroids (patients with MGS 0–2, dexamethasone 4 mg every 8 hours for 48–72 hours, if patient showed improvement discharge and dose reduction by 1 mg/day every 3 days) with a surgical regimen with twist drill craniostomy (patients with MGS 3–4) [5]. Patients treated with steroids showed a good outcome in 96% of the cases, whereas 93% of patients treated operatively showed favourable outcome. The median hospital stay was shorter in the dexamethasone group (6 vs 8 days). Medical complications occurred in 27% of the patients treated with corticosteroids, most commonly mild hyperglycaemia and nosocomial infections. In two thirds of the patients treated conservatively surgery was avoided. The authors concluded that corticosteroids were effective in 67% of the cases and are therefore a safe and feasible conservative treatment for patients presenting with cSDH and a MGS of 0–2 (type C recommendation) [5]. A meta-analysis by Almenaver et al. showed no significant difference in recurrence rate, morbidity and mortality for patients treated with corticosteroids when compared with surgical treatment (type B recommendation) [39]. The authors commented, however, that these findings should be interpreted cautiously since the number of studies included was small and further studies are warranted. Lastly, a recent prospective study evaluated the role of corticosteroids (dexamethasone 4 mg every 8 hours for 3 days, if successful dose tapering for 4 weeks) as a medical treatment for cSDH in 26 patients [40]. In 15 cases (10 patients after 3 days, 5 patients at 3–6 weeks) burr-hole drainage was eventually needed, whereas the remaining 11 patients were completely relieved of symptoms and showed near total or total resolution of the cSDH on CT after 6–8 weeks. Two patients developed steroid-related complications. Gender, midline shift, thickness of haematoma and Hounsfield units of the haematoma on CT showed statistically significant differences when the failure group was compared with the success group. The authors then proposed a radiological grading scale (table 2) that can help predict the chance of successful treatment with corticosteroids. They conclude that in patients with low grades (0–2) corticosteroid treatment might be more successful than in high grades (4 and 5) [40]. A pilot randomised controlled trial recently initiated in Canada enrolled 10 patients treated with steroids (dexamethasone 4 mg three times a day for 3 weeks with tapering for a week) and 10 patients who received placebo. The study was terminated after 20 patients had been recruited because 80% of the patients receiving steroids suffered a serious adverse event as opposed to 10% of the patients in the placebo group [41]. Table 3 summarises the studies evaluating corticosteroids as a medical treatment modality for cSDH. In addition, multiple case reports have been published suggesting corticosteroids for the treatment of cSDH [42].
Table 2 Proposed radiological grading system of cSDH for evaluating the success of corticosteroid treatment (adapted from Thotakura et al. [40]).
| Size based on midline shift* |
| Small (no midline shift) | 0 |
|---|---|
| Medium (<5 mm) | 1 |
| Large (5–10 mm) | 2 |
| Massive (>10mm) | 3 |
| Density based on HU in CT scan | |
| <30 | 0 |
| 31–40 | 1 |
| >40 | 2 |
cSDH = chronic subdural haematoma; CT = computed tomography; HU = Hounsfield units * Bilateral cSDH – one extra point to be added
Table 3 Studies evaluating the treatment of cSDH with corticosteroids: results and recommendations.
| Study | Dosage |
Treatment
groups |
Eventually
surgical (n (%) |
Recommendations | Type |
|---|---|---|---|---|---|
| Bender et al. 1974 [36] |
Prednisone 60 mg orally for a few days, thereafter reduction of dose by 10mg every 3 days | C (n = 75) CS (n = 22) S (n = 88) |
22 (29.3%) | Surgery first choice of treatment in comatose or progressively worsening patients, for the rest treatment with corticosteroids | C |
| Pichert et al. 1987 [37] |
Unknown | C (n = 56) | 8 (14.3%) | Recommended in patients without distinct focal symptoms and nonsoporose or noncomatose patients | C |
| Sun et al. 2005 [38] |
Dexamethasone 4 mg four times a day for 21 days | C (n = 26) CS (n = 96) S (n = 13) N (n = 4) |
1 (4%) | Corticosteroids might be a valid alternative for patients with cSDH not suitable for surgical drainage | C |
| Delgado-López et al. 2009 [5] |
Dexamethasone 4 mg every 8 hours for 48–72 hours; if patient showed improvement, discharge and dose reduction by 1 mg/day every 3 days | C (n = 101) S (n = 19) N (n = 2) |
22 (21.8%) | Safe and feasible conservative treatment for patients presenting with cSDH and a MGS of 0–2 | C |
| Almenawer et al. 2013 [39] |
n.a. (meta-analysis) | C,S (total n = 315) |
RR for surgery 1.07 (n.s.) |
No significant difference in recurrence rate, morbidity, and mortality for corticosteroids when compared with surgical treatment | B |
| Thotakura et al. 2015 [40] |
Dexamethasone 4 mg every 8 hours for 3 days; if patient showed improvement, discharge and dose reduction for 4 weeks | C (n = 26) | 15 (57.7%) | Recommended in patients with radiological grading of 0–2 (sometimes 3) | C |
cSDH = chronic subdural haematoma; C = corticosteroids only; CS = corticosteroids plus surgery; S = surgery only; N = no treatment; MGS = Markwalder grading score; n.a. = not applicable; n.s. = non-significant; RR = relative risk;
Corticosteroids as an adjuvant treatment to surgical drainage of cSDH have been explored in one meta-anlysis [39] and a few observational studies [27, 43–45].
In summary, although it appears that corticosteroids might play a role in the conservative treatment of cSDH, well designed studies supporting their use are lacking. Moreover, the ideal dosage and duration of treatment is still unclear, and the ideal patient group for this particular treatment is still to be determined in large prospective studies [3, 5, 40]. It seems that in patients with lower MGS (e.g., 0–2), patients with a low radiological grading (0–2), patients refusing operation, or inoperable patients, medical treatment with corticosteroids (dexamethasone 4 mg every 8 hours) might be a valid alternative to surgery (type C recommendation). The role and efficacy of corticosteroids as a medical treatment still need to be ascertained through large prospective randomised studies [46].
The effect of ACE inhibitors on the course of cSDH remains unclear. ACE inhibitors are used for the treatment of arterial hypertension, but also for pathologies with increased angiogenic activity such as Kaposi’s syndrome and diabetic retinopathy [47]. On the basis of the angiogenic hypothesis of cSDH, it is assumed that ACE inhibitors might lower the risk for developing a cSDH and for its recurrence [47, 48]. However, contrary data have been presented, since ACE inhibitors are known to increase the levels of bradykinin, the end-product of the kallikrein-kinin system. Bradykinin, a vasoactive peptide, induces both permeability and vasodilatation, and leads to blood extravasation from the neomembranes in cSDH, leading to an enlargement of the haematoma [49]. Studies evaluating ACE inhibitors as a conservative medical treatment for cSDH do not exist. However, three studies evaluated the effect of ACE inhibitors during the postsurgical course of cSDH. Weigel et al. showed a lower recurrence rate of cSDH after surgical drainage in patients treated with ACE inhibitors for hypertension. They concluded that ACE inhibitors lower recurrence rate and might even lower the risk of developing a cSDH [47] (type C recommendation). In a randomised controlled study in which an ACE inhibitor (perindopril) was given for 3 months postoperatively and compared with placebo, no significant difference in recurrence rates was seen [48]. The authors concluded that ACE inhibitors do not lower recurrence rates after surgical drainage of cSDH (type B recommendation). Unfortunately, other studies evaluating the effect of ACE inhibitors as adjuvant treatment with the surgical evacuation of cSDH and as stand-alone conservative treatment do not exist. Based on the available data, the benefit of ACE inhibitors as an adjuvant treatment to the surgical evacuation of cSDH is ambiguous. ACE inhibitors do not seem to reduce the recurrence rate of cSDH and cannot be recommended at this point (type B recommendation). Studies examining the efficacy of ACE inhibitors as a stand-alone conservative treatment do not exist and therefore no recommendation can be made (table 4). Further studies evaluating the benefit of ACE inhibitors as a conservative treatment option for cSDH are warranted [4].
Table 4 Studies evaluating the treatment of cSDH with various conservative treatment modalities: results and recommendations.
| Treatment | Recommendation | Type |
Reference
(number of patients included) |
|---|---|---|---|
| ACE inhibitors | - ACE inhibitors only evaluated as adjuvant treatment to surgery - Effect on recurrence remain debatable - Role as sole conservative treatment unknown |
B/C | Weigel et al. [47] (310) Poulsen et al. [48] (47) Neidert et al. [49] (203) |
| Tranexamic acid | - Good efficacy - Unknown risk for thromboembolic events |
C | Kageyama et al. [50] (21) |
| Mannitol | - Seems an effective conservative treatment modality - Long treatment duration |
C | Suzuki et al. [23] (20) Kinjo et al. [51] (23) |
| Platelet activating factor receptor inhibitor | - Seems to promote resolution of cSDH - In patients with hygromas and no paresis |
C | Hirashima et al. [52, 53] (53, 39) |
| Statins | - Atorvastatin seems to be a safe and cost-effective alternative so surgery - In asymptomatic or mild symptomatic patients |
C | Li et al. [54] (n.a.) Wang et al. [55] (23) Min et al. [56] (7) |
cSDH= chronic subdural haematoma; n.a.: not applicable
Fibrinolytic and coagulative hyperactivity seem to play a role in the liquefaction and progression of cSDH [50]. In addition, increased permeability of the capillaries in the outer haematoma membrane lead to microbleeds that influence haematoma growth [4]. The kallikrein system, which is activated by plasmin, induces inflammation and therefore increases vascular permeability and leucocyte migration, and was found to be present in the outer membrane of cSDH [50]. Tranexamic acid has an antifibrinolytic effect via inhibition of plasminogen activator and plasmin. Therefore, it is hypothesised that tranexamic acid might inhibit the hyperfibrinolytic activity and the increased vascular permeability in cSDH, leading to a gradual absorption of the haematoma [50]. Based on these assumptions, a retrospective study analysing the influence of tranexamic acid on cSDH managed conservatively was conducted by Kageyama et al [50]. They showed, in 18 patients treated with 750 mg of tranexamic acid once a day, a complete resorption of all haematomas and therefore concluded that tranexamic acid might be a valid conservative treatment for cSDH (type C recommendation). Adverse events associated with tranexamic acid were not seen in their cohort. However, patients with anticoagulation were excluded from the study, and therefore the effect of tranexamic acid on anticoagulated patients remains unknown; in these patients tranexamic acid should be applied with caution. To date the role of tranexamic acid in the treatment of cSDH is still uncertain; in particular, its effect on the resorption of cSDH and the adverse event rate, especially in patients at risk for thromboembolic events, is still to be evaluated in larger studies and therefore recommendations cannot be made (table 4). A placebo-controlled phase IIB randomised controlled (TRACS) aiming to determine whether tranexamic acid can increase the rate of resolution and lower the need for surgical evacuation of cSDH when treated conservatively is currently ongoing [57].
Mannitol is an osmotic diuretic used for the treatment of increased intracranial pressure [23]. In cSDH it was hypothesised that the haematoma increases as a result of an osmotic gradient within the haematoma capsule. Consequently, increase of haematoma produces strain to the capsule, leading to microbleeds and further enlargement of the haematoma. Reduction of haematoma pressure using mannitol might prevent this chain of events, stopping the continuous rebleeding inside the haematoma cavity and ultimately allowing spontaneous resorption of the haematoma [23]. Based on these assumptions, Suzuki et al. administered a 500–1000 ml mannitol drip (20%) daily as a conservative treatment modality for cSDH [23]. Of 23 consecutive patients, 22 showed decreasing haematoma volume and complete neurological recovery at follow up, whereas in one case burr hole drainage was needed after 12 days of mannitol treatment. The duration of mannitol treatment ranged from 12 to 106 days, which is definitely a drawback of this treatment. The authors concluded that mannitol can be used for the conservative treatment of cSDH. They recommend using a dose of 1000 ml, since it showed better results than a dose of 500 ml, and if the treatment does reduce symptoms after 3 to 4 days, surgical evacuation should be undertaken immediately (type C recommendation). In another study from Japan, 20 patients treated with mannitol (20%) 1000 ml daily intravenously for 2 weeks were screened. During the first and second week of treatment, four patients showed aggravated symptoms. However, at the end of follow-up all, cases were asymptomatic and after 5 months there was complete resolution of all haematomas on CT [51]. These results suggest that mannitol may have a role in the conservative treatment of cSDH, but on the basis of available data recommendations cannot be made (table 4).
Inflammation seems to play a major role in the pathogenesis of cSDH [52, 53]. Platelet activating factor (PAF), a highly potent lipid mediator of inflammation, has been shown to be involved in the formation and growth of cSDH [53]. Etizolam, a PAF receptor antagonist, has been shown to attenuate the recurrence rate after burr hole drainage of cSDH [52]. A study including 62 patients diagnosed with cSDH and showing only mild symptomatic (no paresis, no herniation), were alternately assigned to a treatment group (etizolam 3 mg per day for 14 days) and to a control group in a prospective manner. Because of drop-outs, 53 patients were included in the final analysis, 24 in the treatment group and 29 in the control group. Surgery was needed in 54% and 93% of the treatment and control groups, respectively, a significant difference (p = 0.001). Multivariate logistic regression analysis showed significant negative correlation between etizolam treatment and low density of the haematoma on CT and significant positive correlation between preoperative paresis and the requirement for surgery. The authors concluded that etizolam can promote the resolution of cSDH, especially in patients with hygromas or low-density haematomas on CT and without paresis at presentation (type C recommendation) (table 4) [52]. To date, there are no further studies investigating the efficacy of a PFA receptor antagonist as conservative treatment for cSDH.
Previous reports indicate that statins promote angiogenesis and restrain inflammation [54, 55]. A study in rats showed that resolution of the SDH and neurological recovery of the rats treated with atorvastatin was faster and better than in rats treated with saline [54]. In addition, microscopic examination of the membranes showed a higher density of CD31+ neovasculature, lower levels of interleukin-6, tumour necrosis factor-α and VEGF, and lower number of neutrophil granulocytes in the atorvastatin group. The authors concluded that the inflammatory modulation induced by statins in rats eliminated the SDH and improved neurological recovery [54]. Based on similar assumptions, Wang et al. conducted a prosepective study including 23 patients who were treated conservatively for cSDH with atorvastatin (oral dose of 20 mg once a day for 1–6 months), [55]. Of the 23 patients, 1 underwent surgery because of neurological deterioration after 4 weeks of conservative treatment, and in the remaining 22 patients a significant reduction in haematoma volume was seen within the first month of treatment [55]. None of these 22 patients had a relapse during the 36 months of follow-up and no atorvastatin adverse effects were documented [55]. The results suggest that atorvastatin is a safe and cost-effective alternative to surgical treatment (type C recommendation) [55]. An additional study analysed the effect of atorvastatin as a conservative treatment (oral dose of 20 mg once a day for 1–6 months) and as an adjuvant treatment to surgery. In the seven patients treated conservatively a significant haematoma reduction was seen after 1 month; at 6 months all haematomas had disappeared. None of the patients required surgical evacuation [56]. In addition, when atorvastatin was used as adjuvant treatment in 39 of the 102 surgical patients, a significant difference in outcome in favour of the atorvastatin group was seen [56].
To conclude, it seems that atorvastatin is a valid and safe option for the conservative treatment of asymptomatic or mildly symptomatic cSDH patients (type C recommendation) [55, 56]. Further prospective studies with bigger cohorts are needed in order to determine the true value of statins in the conservative treatment of cSDH (table 4). A multicentre, randomised, placebo controlled, double-blind clinical trial evaluating the efficacy and safety of atorvastatin as a conservative treatment of cSDH is planned by Jiang et al [58].
The natural history of cSDH remains unknown. In addition, we cannot predict which patients may be valid candidates for conservative treatment. It seems that patients presenting with cSDH and a MGS of 0–2 might benefit from conservative treatment. In the case of large asymptomatic haematomas and small symptomatic haematomas, the preferred treatment remains surgery, even though the effect of conservative treatment in these cases is poorly analysed. Therefore, further studies elaborating on the natural history and pathophysiology of cSDH, exploring the factors effecting its growth and its clinical evolution, and evaluating conservative treatment modalities are essential.
In general, studies evaluating the natural history and conservative treatment of cSDH remain sparse and are predominantly of low evidence level. Steroids and tranexamic acid seem to be promising conservative modalities to treat cSDH. However, the efficacy of these medications is still based on a poor grade of evidence (mostly evidence grade III). Steroids have been shown to be a good alternative to surgery even in symptomatic patients who are not progressing or present in a comatose state (type C recommendation). Series evaluating tranexamic acid show a good efficacy, but these series are very small and it remains unknown whether tranexamic acid increases the risk of thromboembolic events in patients treated with antithrombotic or anticoagulant medication. ACE inhibitors were evaluated as only adjuvant therapy to surgery, and their effect on recurrence rate remains debatable. Even though ACE inhibitors lower VEGF levels in the haematoma cavity their efficacy in treating cSDH remains unclear. Mannitol seems an effective conservative treatment modality; however, these data too are based on small retrospective case series and the long treatment duration is a major drawback. A platelet activating factor receptor antagonist was shown, with a low evidence grade, to promote the resolution of cSDH, especially in patients with hygromas or low-density haematomas on CT and without paresis at presentation. Lastly, atorvastatin seems to be a valid and safe option for the conservative treatment of asymptomatic or mildly symptomatic cSDH patients.
The recurrence rate after surgical evacuation of cSDH still remains relatively high (7–20%) [22]. With the help of an adjuvant postoperative treatment these rates might be lowered, although the role of medications (e.g., steroids, mannitol, etc.) as adjuvant therapy to the surgical evacuation of cSDH still remains ambiguous. This aspect of conservative treatment is still to be explored.
It seems that, especially in patients with few symptoms or in polymorbid patients at a high operation risk, conservative treatment might be a good alternative to surgery. However, further evidence-based research in this field is needed before definitive recommendations can be made. Recently, a number of prospective studies, which will hopefully provide us with high level evidence for the various conservative treatment modalities of cSDH, have been initiated (table 5).
Table 5 Ongoing studies investigating various conservative treatment modalities for cSDH.
| Rationale | Study design | Status | Anticipated number of patients | Trial registration ID |
|---|---|---|---|---|
| Treatment of chronic subdural haematoma by corticosteroids | PR, DB, PC | NYR | 202 | NCT02650609 |
| Dexamethasone versus burr hole craniostomy for symptomatic chronic subdural haematoma | PR, SB | R | 300 | NCT02111785 |
| Effect and safety of atorvastatin to treat chronic subdural haematoma | PR, DB, PC | R | 200 | NCT02024373 |
| The clinical study of atorvastatin and dexamethasone on treatment for chronic subdural haematoma in the patients with coagulation disorders | PR, SB | NYR | 60 | NCT02192320 |
| Tranexamic acid in chronic subdural haematomas (TRACS) | PR, DB, PC | R | 130 | NCT02568124 |
| Interest of oral corticosteroids in the treatment of chronic subdural haematomas (hemacort) | PR, DB, PC | R | 340 | NCT01380028 |
| A study on the safety of tranexamic acid for the chronic subdural haematoma population | Open label | R | 50 | NCT02618382 |
| The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural haematoma – the DRESH study | PR, DB PC |
R | 820 | EudraCT 201100354442 |
| A randomised, double blind, placebo-controlled trial of a 2-week course of dexamethasone for adult patients with a symptomatic chronic subdural haematoma (Dex-CSDH trial) | PR, DB, PC | R | 750 | ISRCTN 80782810 |
cSDH = chronic subdural haematoma; DB = double blind; NYR = not yet recruiting; PC = placebo controlled; PR = prospective randomised; R = recruiting; SB = single blind
No financial support and no other potential conflict of interest relevant to this article was reported.
1 Kudo H , Kuwamura K , Izawa I , Sawa H , Tamaki N . Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992;32(4):207–9. doi:.https://doi.org/10.2176/nmc.32.207
2 Chen JC , Levy ML . Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11(3):399–406.
3 Santarius T , Kirkpatrick PJ , Kolias AG , Hutchinson PJ . Working toward rational and evidence-based treatment of chronic subdural hematoma. Clin Neurosurg. 2010;57:112–22.
4Soleman J, Taussky P, Fandino J, Muroi C. Evidence Based Treatment of Chronic Subdural Hematoma. In: Sadaka DF, editor. Traumatic Brain Injury: In Tech; 2014.
5 Delgado-López PD , Martín-Velasco V , Castilla-Díez JM , Rodríguez-Salazar A , Galacho-Harriero AM , Fernández-Arconada O . Dexamethasone treatment in chronic subdural haematoma. Neurocirugia (Astur). 2009;20(4):346–59. doi:.https://doi.org/10.1016/S1130-1473(09)70154-X
6 Frati A , Salvati M , Mainiero F , Ippoliti F , Rocchi G , Raco A , et al. Inflammation markers and risk factors for recurrence in 35 patients with a posttraumatic chronic subdural hematoma: a prospective study. J Neurosurg. 2004;100(1):24–32. doi:.https://doi.org/10.3171/jns.2004.100.1.0024
7 Glover D , Labadie EL . Physiopathogenesis of subdural hematomas. Part 2: Inhibition of growth of experimental hematomas with dexamethasone. J Neurosurg. 1976;45(4):393–7. doi:.https://doi.org/10.3171/jns.1976.45.4.0393
8 Nagatani K , Wada K , Takeuchi S , Nawashiro H . Corticosteroid suppression of vascular endothelial growth factor and recurrence of chronic subdural hematoma. Neurosurgery. 2012;70(5):E1334 . [Author reply E-6.].
9 Labadie EL , Glover D . Local alterations of hemostatic-fibrinolytic mechanisms in reforming subdural hematomas. Neurology. 1975;25(7):669–75. doi:.https://doi.org/10.1212/WNL.25.7.669
10 Katano H , Kamiya K , Mase M , Tanikawa M , Yamada K . Tissue plasminogen activator in chronic subdural hematomas as a predictor of recurrence. J Neurosurg. 2006;104(1):79–84. doi:.https://doi.org/10.3171/jns.2006.104.1.79
11 Fujisawa H , Ito H , Saito K , Ikeda K , Nitta H , Yamashita J . Immunohistochemical localization of tissue-type plasminogen activator in the lining wall of chronic subdural hematoma. Surg Neurol. 1991;35(6):441–5. doi:.https://doi.org/10.1016/0090-3019(91)90177-B
12 Hohenstein A , Erber R , Schilling L , Weigel R . Increased mRNA expression of VEGF within the hematoma and imbalance of angiopoietin-1 and -2 mRNA within the neomembranes of chronic subdural hematoma. J Neurotrauma. 2005;22(5):518–28. doi:.https://doi.org/10.1089/neu.2005.22.518
13 Suzuki K , Takano S , Nose T , Doi M , Ohashi N . Increased concentration of vascular endothelial growth factor (VEGF) in chronic subdural hematoma [letter]. J Trauma. 1999;46(3):532–3. doi:.https://doi.org/10.1097/00005373-199903000-00040
14 Weigel R , Schilling L , Schmiedek P . Specific pattern of growth factor distribution in chronic subdural hematoma (CSH): evidence for an angiogenic disease. Acta Neurochir (Wien). 2001;143(8):811–8, discussion 819. doi:.https://doi.org/10.1007/s007010170035
15 Stanisic M , Aasen AO , Pripp AH , Lindegaard KF , Ramm-Pettersen J , Lyngstadaas SP , et al. Local and systemic pro-inflammatory and anti-inflammatory cytokine patterns in patients with chronic subdural hematoma: a prospective study. Inflamm Res. 2012;61(8):845–52. doi:.https://doi.org/10.1007/s00011-012-0476-0
16 Xu XP , Liu C , Liu J , Pang YG , O XD , Fu J , et al. Local application of corticosteroids combined with surgery for the treatment of chronic subdural hematoma. Turk Neurosurg. 2015;25(2):252–5. doi:. https://doi.org/http://dx.doi.org.10.5137/1019-5149.JTN.8989-13.3
17 Markwalder TM , Steinsiepe KF , Rohner M , Reichenbach W , Markwalder H . The course of chronic subdural hematomas after burr-hole craniostomy and closed-system drainage. J Neurosurg. 1981;55(3):390–6. doi:.https://doi.org/10.3171/jns.1981.55.3.0390
18 Kolias AG , Chari A , Santarius T , Hutchinson PJ . Chronic subdural haematoma: modern management and emerging therapies. Nat Rev Neurol. 2014;10(10):570–8. doi:.https://doi.org/10.1038/nrneurol.2014.163
19 Moher D , Liberati A , Tetzlaff J , Altman DG ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. doi:.https://doi.org/10.1016/j.ijsu.2010.02.007
20Guide to clinical preventive services. report of the U.S. Preventive Services Task Force. In: Force USPST, editor.: DIANE Publishing; 1989. p. 24, Appendix A.
21Lawrence R. Guide to Clinical Preventive Services. In: Edition USPSTF, editor.: DIANE Publishing; 1989.
22 Soleman J , Lutz K , Schaedelin S , Mariani L , Fandino J . Use of Subperiosteal Drain Versus Subdural Drain in Chronic Subdural Hematomas Treated With Burr-Hole Trepanation: Study Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2016;5(2):e38. doi:.https://doi.org/10.2196/resprot.5339
23 Suzuki J , Takaku A . Nonsurgical treatment of chronic subdural hematoma. J Neurosurg. 1970;33(5):548–53. doi:.https://doi.org/10.3171/jns.1970.33.5.0548
24 Göksu E , Akyüz M , Uçar T , Kazan S . Spontaneous resolution of a large chronic subdural hematoma: a case report and review of the literature. Ulus Travma Acil Cerrahi Derg. 2009;15(1):95–8.
25 Voelker JL . Nonoperative treatment of chronic subdural hematoma. Neurosurg Clin N Am. 2000;11(3):507–13.
26 Cenic A , Bhandari M , Reddy K . Management of chronic subdural hematoma: a national survey and literature review. Can J Neurol Sci. 2005;32(4):501–6. doi:.https://doi.org/10.1017/S0317167100004510
27 Santarius T , Lawton R , Kirkpatrick PJ , Hutchinson PJ . The management of primary chronic subdural haematoma: a questionnaire survey of practice in the United Kingdom and the Republic of Ireland. Br J Neurosurg. 2008;22(4):529–34. doi:.https://doi.org/10.1080/02688690802195381
28 Juković M , Kojadinović Z , Popovska B , Till V . Complete spontaneous resolution of compressive chronic subdural hematoma in a patient with liver failure. Med Glas (Zenica). 2012;9(2):417–20.
29 Parlato C , Guarracino A , Moraci A . Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 2000;53(4):312–5, discussion 315–7. doi:.https://doi.org/10.1016/S0090-3019(00)00200-7
30 Tiwari AR , Maheshwari S , Balasubramaniam S , Devendra T , Savant H . Spontaneous Resolution of Non Traumatic Chronic Subdural Haematoma Despite Continued Antiplatelet Therapy: A Case Report. J Clin Diagn Res. 2015;9(6):PD01–02.
31 Yang S , Zhang X , Jin Y . Spontaneous resolution of nontraumatic chronic subdural hematoma associated with anti-aggregation therapy. J Craniofac Surg. 2014;25(4):e363–5. doi:.https://doi.org/10.1097/SCS.0000000000000814
32 Lee KS . Natural history of chronic subdural haematoma. Brain Inj. 2004;18(4):351–8. doi:.https://doi.org/10.1080/02699050310001645801
33 Naganuma H , Fukamachi A , Kawakami M , Misumi S , Nakajima H , Wakao T . Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 1986;19(5):794–8. doi:.https://doi.org/10.1227/00006123-198611000-00013
34 Horikoshi T , Naganuma H , Fukasawa I , Uchida M , Nukui H . Computed tomography characteristics suggestive of spontaneous resolution of chronic subdural hematoma. Neurol Med Chir (Tokyo). 1998;38(9):527–32, discussion 532–3. doi:.https://doi.org/10.2176/nmc.38.527
35 Emich S , Richling B , McCoy MR , Al-Schameri RA , Ling F , Sun L , et al. The efficacy of dexamethasone on reduction in the reoperation rate of chronic subdural hematoma--the DRESH study: straightforward study protocol for a randomized controlled trial. Trials. 2014;15(1):6. doi:.https://doi.org/10.1186/1745-6215-15-6
36 Bender MB , Christoff N . Nonsurgical treatment of subdural hematomas. Arch Neurol. 1974;31(2):73–9. doi:.https://doi.org/10.1001/archneur.1974.00490380021001
37 Pichert G , Henn V . [Conservative therapy of chronic subdural hematomas]. Schweiz Med Wochenschr. 1987;117(47):1856–62.
38 Sun TF , Boet R , Poon WS . Non-surgical primary treatment of chronic subdural haematoma: Preliminary results of using dexamethasone. Br J Neurosurg. 2005;19(4):327–33. doi:.https://doi.org/10.1080/02688690500305332
39 Almenawer SA , Farrokhyar F , Hong C , Alhazzani W , Manoranjan B , Yarascavitch B , et al. Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg. 2014;259(3):449–57. doi:.https://doi.org/10.1097/SLA.0000000000000255
40 Thotakura AK , Marabathina NR . Nonsurgical Treatment of Chronic Subdural Hematoma with Steroids. World Neurosurg. 2015;84(6):1968–72. doi:.https://doi.org/10.1016/j.wneu.2015.08.044
41Role of Dexamethasone in the Conservative Treatment of Chronic Subdural Hematoma. 2015 01.02.2016 [cited 26 Aug 2016]; Available from: https://clinicaltrials.gov/ct2/show/study/NCT02362321?term=chronic+subdural+hematoma&rank=15§=X370156
42 Rudiger A , Ronsdorf A , Merlo A , Zimmerli W . Dexamethasone treatment of a patient with large bilateral chronic subdural haematomata. Swiss Med Wkly. 2001;131(25-26):387.
43 Dran G , Berthier F , Fontaine D , Rasenrarijao D , Paquis P . Efficacité de la corticothérapie dans le traitement adjuvant des hématomes sous-duraux chroniques. Étude rétrospective sur 198 cas. [Effectiveness of adjuvant corticosteroid therapy for chronic subdural hematoma: a retrospective study of 198 cases]. Neurochirurgie. 2007;53(6):477–82. doi:.https://doi.org/10.1016/j.neuchi.2007.09.146
44 Berghauser Pont LM , Dammers R , Schouten JW , Lingsma HF , Dirven CM . Clinical factors associated with outcome in chronic subdural hematoma: a retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery. 2012;70(4):873–80, discussion 880. doi:.https://doi.org/10.1227/NEU.0b013e31823672ad
45 Berghauser Pont LM , Dirven CM , Dippel DW , Verweij BH , Dammers R . The role of corticosteroids in the management of chronic subdural hematoma: a systematic review. Eur J Neurol. 2012;19(11):1397–403. doi:.https://doi.org/10.1111/j.1468-1331.2012.03768.x
46 Zarkou S , Aguilar MI , Patel NP , Wellik KE , Wingerchuk DM , Demaerschalk BM . The role of corticosteroids in the management of chronic subdural hematomas: a critically appraised topic. Neurologist. 2009;15(5):299–302. doi:.https://doi.org/10.1097/NRL.0b013e3181b65558
47 Weigel R , Hohenstein A , Schlickum L , Weiss C , Schilling L . Angiotensin converting enzyme inhibition for arterial hypertension reduces the risk of recurrence in patients with chronic subdural hematoma possibly by an antiangiogenic mechanism. Neurosurgery. 2007;61(4):788–92, discussion 792–3. doi:.https://doi.org/10.1227/01.NEU.0000298907.56012.E8
48 Poulsen FR , Munthe S , Søe M , Halle B . Perindopril and residual chronic subdural hematoma volumes six weeks after burr hole surgery: a randomized trial. Clin Neurol Neurosurg. 2014;123:4–8. doi:.https://doi.org/10.1016/j.clineuro.2014.05.003
49 Neidert MC , Schmidt T , Mitova T , Fierstra J , Bellut D , Regli L , et al. Preoperative angiotensin converting enzyme inhibitor usage in patients with chronic subdural hematoma: Associations with initial presentation and clinical outcome. J Clin Neurosci. 2016;28:82–6. doi:.https://doi.org/10.1016/j.jocn.2015.09.022
50 Kageyama H , Toyooka T , Tsuzuki N , Oka K . Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg. 2013;119(2):332–7. doi:.https://doi.org/10.3171/2013.3.JNS122162
51 Kinjo T , Sakurai Y , Ogawa A , Komatsu S , Suzuki J . [Nonsurgical treatment of chronic subdural hematoma. Sequential changes of computed tomography findings]. Neurol Med Chir (Tokyo). 1985;25(8):645–53. doi:.https://doi.org/10.2176/nmc.25.645
52 Hirashima Y , Kurimoto M , Nagai S , Hori E , Origasa H , Endo S . Effect of platelet-activating factor receptor antagonist, etizolam, on resolution of chronic subdural hematoma--a prospective study to investigate use as conservative therapy. Neurol Med Chir (Tokyo). 2005;45(12):621–6, discussion 626. doi:.https://doi.org/10.2176/nmc.45.621
53 Hirashima Y , Kuwayama N , Hamada H , Hayashi N , Endo S . Etizolam, an anti-anxiety agent, attenuates recurrence of chronic subdural hematoma--evaluation by computed tomography. Neurol Med Chir (Tokyo). 2002;42(2):53–5, discussion 56. doi:.https://doi.org/10.2176/nmc.42.53
54 Li T , Wang D , Tian Y , Yu H , Wang Y , Quan W , et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J Neurol Sci. 2014;341(1-2):88–96. doi:.https://doi.org/10.1016/j.jns.2014.04.009
55 Wang D , Li T , Tian Y , Wang S , Jin C , Wei H , et al. Effects of atorvastatin on chronic subdural hematoma: a preliminary report from three medical centers. J Neurol Sci. 2014;336(1-2):237–42. doi:.https://doi.org/10.1016/j.jns.2013.11.005
56 Min X , Pin C , Xun Z , Cun-Zu W , Xue-Qiang S , Bo Y . Effects of atorvastatin on conservative and surgical treatments of chronic subdural hematoma in patients. World Neurosurg. 2016;91:23–8. doi:http://dx.doi.org/10.1016/j.wneu.2016.03.067.
57 Iorio-Morin C , Blanchard J , Richer M , Mathieu D . Tranexamic Acid in Chronic Subdural Hematomas (TRACS): study protocol for a randomized controlled trial. Trials. 2016;17(1):235. doi:.https://doi.org/10.1186/s13063-016-1358-5
58 Jiang R , Wang D , Poon WS , Lu YC , Li XG , Zhao SG , et al. Effect of ATorvastatin On Chronic subdural Hematoma (ATOCH): a study protocol for a randomized controlled trial. Trials. 2015;16(1):528. doi:.https://doi.org/10.1186/s13063-015-1045-y