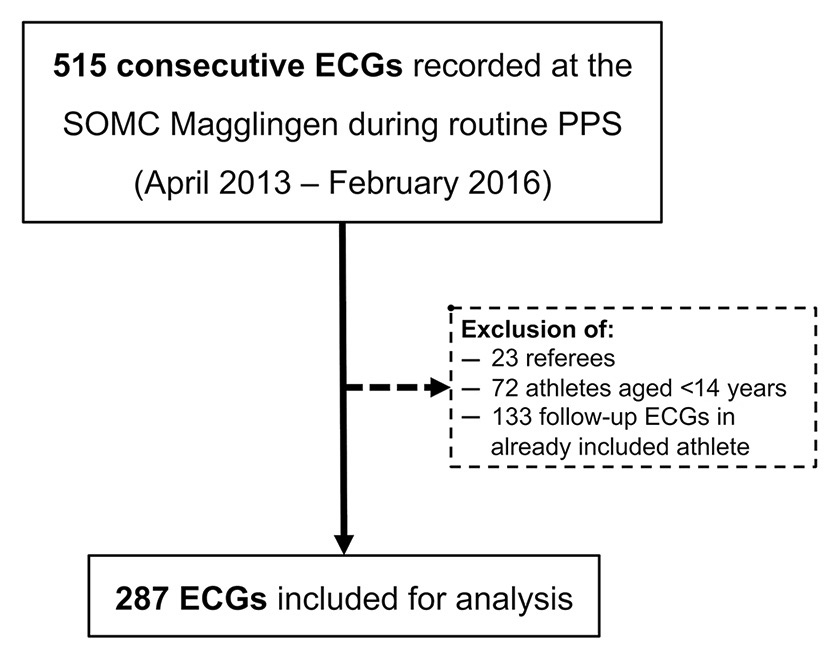
Figure 1
Flow-chart for ECG inclusion during the study period.
PPS = pre-participation screening; SOMC = Swiss Olympic Medical Centre.
DOI: https://doi.org/10.4414/smw.2016.14376
Although the cost-effectiveness of electrocardiogram (ECG) screening to prevent sudden cardiac arrest (SCA) in athletes is still intensely debated [1, 2], evidence supporting its accuracy for detecting athletes needing further cardiological work-up is growing. Performance of a resting ECG during cardiovascular preparticipation screening (PPS) has been shown to be 5 times more sensitive than personal history and even 10 times more sensitive than physical examination alone to detect cardiovascular disease in athletes, together with a higher positive likelihood ratio [3]. Although the International Olympic Committee (IOC) and the European Society of Cardiology (ESC) recommend routine ECG screening of all competitive athletes [4, 5], the American Heart Association and the American College of Cardiology do not [6]. For Switzerland, the European PPS strategy has been adapted by the Swiss Society of Sports Medicine (SSSM) to include a specified medical history, a cardiac examination and a 12-lead resting ECG, recommended to start from the age of 14 years and to be repeated every 1 to 2 years until the end of the sports career [7].
Besides financial concerns, controversy persists on the ability to distinguish the patterns associated with cardiomyopathies from those resulting from regular training on resting ECG, resulting in false positive abnormal ECG patterns requiring further cardiological work-up. Over recent years, a number of recommendations for ECG interpretation in athletes have been defined with the aim to improve specificity without losing sensitivity. Compared with the 2010 ESC criteria [8] and the original Seattle criteria established in 2013 [9], the refined Seattle criteria published in 2014 improved the positive predictive value of abnormal ECG findings from 3.0% and 9.3%, respectively, to 12.0%, while preserving a sensitivity of 100% for the detection of major cardiac abnormalities in white athletes [10]. The original Seattle criteria allocated the ECGs to three categories (normal, training-related, or abnormal), whereas the refined criteria allocate them to only two categories (normal or abnormal), with some of the originally abnormal patterns considered as normal if isolated (borderline criteria) [10]. Finally, the revised Seattle criteria were presented in a conference paper in 2015 with the aim to further improve the accuracy of screening. The most relevant change compared with the refined Seattle criteria is that complete right bundle-branch block with a QRS duration of 120–139 ms is also considered to be a borderline criterion. Additionally, in contrast to the refined Seattle criteria, T-wave inversion (TWI) up to lead V4 in black athletes and isolated QRS voltage criteria for right ventricular hypertrophy are considered to be normal findings in the revised Seattle criteria [11].
The aim of our study was to assess the prevalence of abnormal ECGs in the Swiss elite athlete population in accordance with the most recent ECG interpretation criteria, and to evaluate potential differences in the prevalence of ECG modifications according to sports discipline.
Swiss Olympic is the umbrella organisation of Olympic and non-Olympic sports federations in Switzerland. It not only promotes sports participation of the general population but also provides structures supporting elite athletes competing at national and international level. Among other activities, Swiss Olympic set up a network of nine Swiss Olympic Medical Centres (SOMC) in Switzerland with multidisciplinary expertise in sports medicine, available to the different sports federations for routine PPS and also for treatment of injuries. Each federation chooses one principal SOMC for their athletes. All SOMCs are under the responsibility of a physician certified in sports medicine (federal SSSM certificate), who performs the PPS. In 2013 the Swiss Olympic Medical Centre (SOMC) in Magglingen and the Interdisciplinary Centre for Sports Medicine at the Inselspital (University Hospital) Bern started a collaboration, and the athletes’ ECGs were evaluated by experienced sports cardiologists from the University Hospital.
For the purpose of this study we evaluated the ECGs from a cohort of consecutive male and female Swiss elite athletes, competing at national or international level, aged 14 years or older, who underwent a routine medical check-up at the SOMC Magglingen between 2013 and 2016. If an athlete had more than one ECG recorded during this period, only the first one was considered for analysis. The exclusion criteria were known cardiovascular disease, history of underperformance, abnormal tiredness, acute infectious disease or cardiac symptoms, and an abnormal physical examination.
The resting 12-lead ECGs were recorded according to the current recommendation with a Schiller AT- 10® automat (voltage 10 mm/mV, paper speed 25 mm/s) by the attending sport physician from the SOMC Magglingen [12]. The ECGs were then anonymised and scanned in high resolution. An advanced cardiology fellow certified in sports medicine (TP) and a senior cardiologist with several years of expertise in sports cardiology (AM) independently analysed the blinded ECGs. The RR interval, P-wave duration, PR interval, QRS interval, QT and QTc interval, QRS-axis and T-axis were measured with manual calipers if not obviously normal. The lead with the longest interval or complex duration was considered for analysis. In particular, the QT interval was measured with the tangent method in lead II or V5 [13], and the QTc interval calculated with Bazett’s formula [14]. To select athletes requiring further cardiological work-up, we assessed the ECGs according to the original Seattle criteria [9] from April 2013 to April 2014, according to the refined Seattle criteria [10] from May 2014 until August 2015, and finally according to the revised Seattle criteria [11] from September 2015 to February 2016. The ECGs were allocated to one of the following groups: (a) normal, (b) training-related (original criteria only), or (c) abnormal (changes suggestive of potential cardiac disease). Additionally, for the purpose of the analyses of this study, all ECGs were categorised according to both the original and revised Seattle criteria. TWI were further characterised as right precordial (leads V1–V3), lateral (leads V4–V6, I and aVL), inferior (leads II and aVF), or inferolateral (combination of inferior and lateral). In the event of disagreement between the two readers, allocation was determined by consensus, including two experienced sports cardiologists (LT and MW).
In the case of abnormal resting ECG findings, the attending sports physician at the SOMC Magglingen was informed about further recommended cardiological work-up.
During the routine PPS, a detailed history including information on age, gender, ethnicity, sport discipline and training habits was obtained and recorded prospectively in a database.
As proposed by Mitchell et al., the various sports disciplines were categorised according to their static (estimated percentage of maximal voluntary contraction: I. low <20%; II. moderate 20–50%, III. high >50%) and dynamic (estimated percentage of maximal oxygen uptake: A. low <40%; B. moderate 40–70%; C. high >70%) components [15].
The study protocol complied with the Declaration of Helsinki and was approved by the local Ethics Committee of the Canton Berne. All enrolled athletes signed an informed consent form allowing the scientific use of their ECGs and clinical data.
The results are presented as means ± standard deviation or counts (percentages). Fisher’s exact test was used to compare frequencies of ECG abnormalities between the sports classes by Mitchell [15]. A p-value <0.05 was considered statistically significant. All data were analysed using the SPSS (Version 21) software (SPSS Inc, Chicago, IL, USA).
During the inclusion period (April 2013 to February 2016), 515 ECGs were recorded during routine annual PPS at the SOMC Magglingen. As shown in figure 1, a total of 287 ECGs were finally considered for analysis. No athlete had to be excluded because of known cardiovascular disease, or abnormal history or physical examination. Details of athletes are reported in table 1. Briefly, 64.1% of the athletes were male and all but one (99.7%) were Caucasian. Median age was 20.4 ± 4.9 years. Median weekly training volume was 17.7 ± 7.1 h and 237 (82.6%) athletes competed internationally. Twenty-two different sports disciplines were represented in our cohort. Although most of our athletes competed in high dynamic and/or high static disciplines, all sport classes in the classification by Mitchell et al. were represented [15].

Figure 1
Flow-chart for ECG inclusion during the study period.
PPS = pre-participation screening; SOMC = Swiss Olympic Medical Centre.
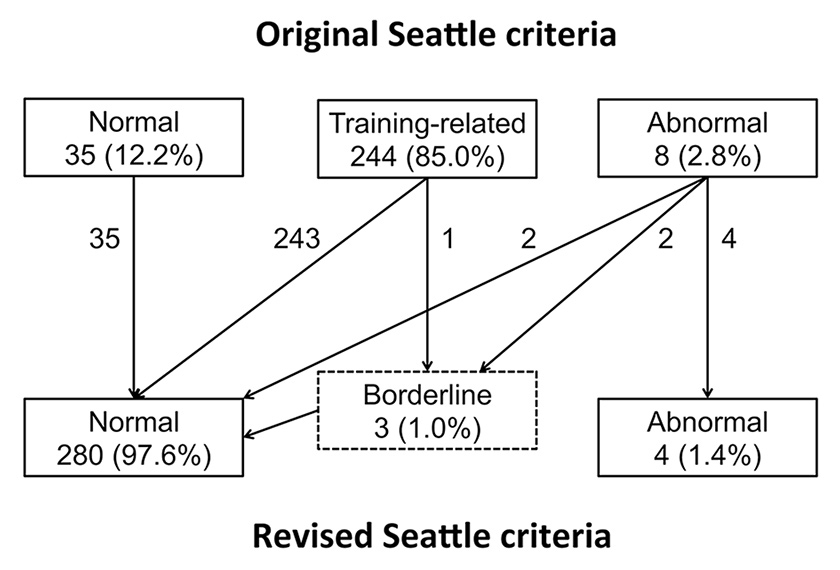
Figure 2
Allocation of ECG changes according to the original and revised Seattle criteria.
The different types of ECG modifications are described in table 2. Based on the original Seattle criteria, 35 (12.2%) ECGs were classified as “normal”, 244 (85.0%) as “training-related” and 8 (2.8%) as “abnormal”. One athlete showed right precordial TWI, two athletes inferolateral TWI, one athlete a Q-wave duration of 46 ms in lateral leads, two athletes a Q-wave amplitude of more than 3 mm and two athletes a QRS left axis deviation.
The categorisation according to the revised Seattle criteria reduced the prevalence of abnormal ECGs to 4 (1.4%), including all cases of TWI and the athlete with a prolonged Q-wave duration. The ECGs of the two athletes with QRS left axis deviation (borderline criterion) were finally reclassified as normal, as the modification was isolated. The Q-wave amplitudes in the two remaining athletes were far less than 25% of the height of the ensuing R-wave, with direct reclassification of their ECGs to the normal group. Finally, one ECG showed an isolated complete right bundle-branch block with a QRS duration of 130 ms (borderline criterion) and was also reclassified in the normal group. Allocations made with both categorisations are summarised in figure 2. All of the abnormal ECGs according to the revised Seattle criteria showed sinus bradycardia or sinus arrhythmia, and two of the three ECG with TWI presented significant lateral early repolarisation. No other electrocardiographic abnormality was detected.
Further cardiological work-up revealed underlying structural heart disease in only one athlete (<1% of our cohort) (table 3). Because a localised reduction in longitudinal strain was detected on transthoracic echocardiography, cardiac magnetic resonance imaging was performed, which showed a small inferoapical mid-wall scar, suggestive of former myocarditis. No restriction on participation in competition was proposed by the treating sports cardiologist.
All abnormal ECGs occurred in Caucasian males (p = 0.03 for gender difference with regard to the original Seattle criteria, p = 0.13 for revised Seattle criteria).
| Table 1:Athletes characteristics. | |
| Characteristics | n = 287 |
| Age (years) | 20.4 ± 4.9 |
| Male gender | 184 (64.1) |
| Caucasian ethnicity | 286 (99.7) |
| Body mass index (kg/m2) | 22.4 ± 4.9 |
| Training duration (years) | 8.7 ± 4.9 |
| Average weekly training time (hours) | 17.7 ± 7.1 |
| Level of competition | |
| International | 237 (82.6) |
| National | 50 (17.4) |
| Sport disciplines (sports category*) | |
| Gymnastics (IIIA) | 51 (17.8) |
| Alpine skiing (IIIB) | 40 (13.9) |
| Cross country (IIC) | 35 (12.2) |
| Ice hockey (IIC) | 33 (11.5) |
| Triathlon (IIIC) | 29 (10.2) |
| 99 (34.5%) athletes performed the following other sports: golf (IA), volleyball (IB), badminton/soccer (IC), equestrian (IIA), field event(jumping)/figure skating/ motorcycling/running(sprint) (IIB), handball/swimming (IIC), bobsledding/martial arts (IIIA), snowboarding (IIIB), cycling/decathlon/kayaking (IIIC). Values are reported as number of athletes (percent), mean ± standard deviation. * Based on Mitchell classification [15] | |
When the nine different sports classes according to Mitchell were compared, there was a significant difference in occurrence of abnormal ECGs (p = 0.018). As shown in table 4, we found three abnormal ECGs in highly dynamic and highly static sports (class IIIC), one in highly dynamic and medium static (class IIC), and zero abnormal ECG in all other classes.
| Table 2:Electrocardiographic characteristics. | |||
| ECG Modification | Original SC | Revised SC | N (%) |
| Sinus bradycardia | T | N | 112 (39) |
| Sinus arrhythmia | T | N | 189 (65.9) |
| Ectopic atrial or junctional rhythm | T | N | 13 (4.5%) |
| First degree AV block | T | N | 3 (1.0) |
| Mobitz Type I second degree AV block | T | N | 0 (0) |
| Voltage criteria for LVH | T | N | 24 (8.4) |
| Incomplete RBBB | T | N | 46 (16) |
| Early repolarisation | T | N | 59 (20.6) |
| TWI V1–V3 ≤ age 16 years | NA | N | 0 (0) |
| ST elevation followed by TWI V1–V4 in black athletes | T | N | 0 (0) |
| Left axis deviation | A | B | 2 (0.7) |
| Left atrial enlargement | A | B | 0 (0) |
| Right ventricular hypertrophy pattern | A | NA | 0 (0) |
| Right axis deviation | NA | B | 1 (0.3) |
| Right atrial enlargement | NA | B | 0 (0) |
| Complete RBBB with QRS duration 120-139 ms | NA | B | 1 (0.3) |
| TWI right precordial infero-lateral | A A A | A A A | 4 (1.4) 1 (0.3) 2 (0.7) |
| Pathological Q-Waves >3 mm ≥40 ms or ≥25% of the height of the ensuing R-wave | A A | NA A | 2 (0.7) 1 (0.3) |
| A = abnormal ECG; AV = atrioventricular; B = borderline ECG; LBBB = left bundle-branch block; N = normal or training-related ECG; NA = not available; PVC = premature ventricular complex; RBBB = right bundle-branch block; SC = Seattle criteria; T = training-related; TWI = T-wave inversion. ST segment depression, complete LBBB, QRS ≥140 ms duration, ventricular pre-excitation, prolonged QT interval, Brugada Type 1 pattern, profound sinus bradycardia <30/min, PR interval ≥400 ms, Mobitz Type II 2 AV block, 3 AV block, ≥2 PVCs, atrial tachyarrhythmias, and ventricular tachyarrhythmias were all considered as abnormal findings in both the original and the revised Seattle criteria (not present in our population). | |||
| Table 3:Results of the cardiological workup of the athletes with an abnormal ECG. | ||||||
| Gender | Age | Sport | Abnormal ECG | Modification | Cardiological workup | |
| (years) | Original SC | Revised SC | ||||
| Male | 16.2 | Swimming | Yes | Yes | TWI V1-V4 | TTE normal |
| Male | 35.9 | Triathlon | Yes | Yes | TWI V5-V6 and II-III-aVF | TTE normal |
| Male | 16.7 | Triathlon | Yes | Yes | TWI V2-V5 and II-III-aVF | TTE with localised reduced longitudinal strain Cardiac MRI: small inferoapical mid-wall scar |
| Male | 23.7 | Cycling | Yes | Yes | Q-wave >40 ms | Declined further examinations. Normal Q-wave duration on follow-up ECG 4 months later |
| Male | 17.9 | Triathlon | Yes | No | Q-wave >3 mm | TTE normal |
| Male | 15.0 | Soccer | Yes | No | Q-wave >3 mm | No workup done (according to the refined Seattle criteria [10]) |
| Male | 19.0 | Gymnastics | Yes | No | Left axis deviation | No workup done (according to the refined Seattle criteria [10]) |
| Male | 14.4 | Equestrian | Yes | No | Left axis deviation | TTE normal |
| DD = differential diagnosis; SC = Seattle criteria; TTE = transthoracic echocardiography; TWI = T-wave inversion | ||||||
| Table 4:Distribution of ECG modifications, as defined in the revised Seattle criteria, according to sports classes by Mitchell [15]. | ||
| High static, low dynamic (IIIA) Total: 61 Normal ECG: 61 (100.0) Abnormal ECG: 0 (0.0) | High static, medium dynamic (IIIB) Total: 41 Normal ECG: 41 (100.0) Abnormal ECG: 0 (0.0) | High static, high dynamic (IIIC) Total: 48 Normal ECG: 45 (93.8) Abnormal ECG: 3 (6.3) |
| Medium static, low dynamic (IIA) Total: 3 Normal ECG: 3 (100.0) Abnormal ECG: 0 (0.0) | Medium static, medium dynamic (IIB) Total: 10 Normal ECG: 10 (100.0) Abnormal ECG: 0 (0.0) | Medium static, high dynamic (IIC) Total: 97 Normal ECG: 96 (99.0) Abnormal ECG: 1 (1.0) |
| Low static, low dynamic (IA) Total: 2 Normal ECG: 2 (100.0) Abnormal ECG: 0 (0.0) | Low static, medium dynamic (IB) Total: 5 Normal ECG: 5 (100.0) Abnormal ECG: 0 (0.0) | Low static, high dynamic (IC) Total: 20 Normal ECG: 20 (100.0) Abnormal ECG: 0 (0.0) |
| ECG = electrocardiogram. Values are reported as number (percent). There was a significant difference in the occurrence of abnormal ECGs according to the nine sport classes using Fisher’s exact test (p = 0.018), with athletes competing in high static high dynamic sports more often presenting abnormal ECGs. The static component of sport is evaluated according to estimated percentage of maximal voluntary contraction (<20%, 20–50%, >50%) and the dynamic component according to the estimated percentage of maximal oxygen uptake (<40%, 40–70%, >70%) [15]. | ||
Our main result shows that the prevalence of “abnormal” ECGs according to modern screening criteria is very low in consecutive elite Caucasian athletes, and further reduced by the use of the most recent revised Seattle criteria (1.4%) rather than the original Seattle criteria (2.8%). The low prevalence of abnormal ECGs in our cohort contrasts with those reported in the large-scale international registers from Sheikh et al. [10] and Riding and colleagues (7.1% and even 8.5% in white athletes, based on original Seattle criteria) [16]. A selection bias might account for this difference, as their athletes were not declared consecutive and could have been referred to these expert centres for evaluation of a previously detected ECG abnormality. Our study supports the conclusion that further cardiological work-up is rarely required in elite athletes. Consequently, the cost of a screening programme including an ECG in elite athletes may even be lower than the 147 Swiss francs (151 US dollars) per athlete estimated by Menafoglio et al. [17]
Three of the four abnormal ECGs in our population were due to isolated TWI. The prevalence of TWI in our cohort (1.0%) was comparable to previous large-scale international [18–20] or Swiss [17] reports. Of these three athletes, only one (33.3%) presented a structural myocardial pathology in the cardiological work-up, consisting in a small inferoapical mid-wall scar, which was finally interpreted as resulting from former myocarditis. This is in agreement with the study by Pelliccia et al., who described structural abnormalities in 31.7% of athletes with repolarisation abnormalities [19]. However, as in the reports of Jacob et al. and Menafoglio et al. [17, 18], none of our athletes with isolated TWI presented echocardiographic findings associated with a clear underlying cardiomyopathy. The significance of TWI in athletes remains unclear, as it could be the manifestation of underlying subclinical cardiac pathology, such as hypertrophic cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy, but might also be the consequence of electrical remodelling due to regular high-volume training [18, 21]. For example, Pelliccia et al. reported a rate of development of overt cardiomyopathies during a mean follow-up of 9 years in 6.0% of athletes presenting with TWI without initial structural abnormalities [19]. Schnell et al. stressed the need for annual ECG follow-up, as 5.8% of their athletes with initial TWI had already developed a clear cardiac pathology after a mean follow-up of 12 months [20]. More specifically, the localisation of TWI on ECG may be relevant for the electrocardiographic differentiation between the athlete’s heart and an underlying cardiomyopathy. In support of this, Calo et al. reported that in a cohort of young soccer players the majority of inferolateral TWIs were associated with underlying structural abnormality, as opposed to TWIs confined to the anterior or inferior leads [22]. Also, all athletes in the study by Pelliccia et al. who developed a cardiomyopathy during follow-up presented TWI in inferior and lateral leads [19]. Therefore, it has been proposed that TWI affecting the lateral leads should always be considered as potentially pathological in athletes, and requiring further work-up [23, 24]. Consistent with this, the only athlete of our population with a structural abnormality presented with inferolateral TWI.
To the best of our knowledge, no previous study using modern screening criteria and including consecutive athletes has evaluated the potential difference in prevalence of ECG modifications according to sports classes. Interestingly, our pilot analysis shows a difference in the occurrence of abnormal ECGs according to the sports classes as defined by Mitchell et al. [15] Moreover, all TWI in our cohort occurred in athletes performing highly dynamic sports (IIC and IIIC), known for the most important myocardial remodelling [25]. This trend was also present in the study by Jacob et al., with 12/14 isolated TWIs occurring with highly dynamic sports in white athletes [18], and in the multi-ethnic study by Schnell et al., where all TWIs occurred in athletes performing highly dynamic sports [20]. More specifically, Brosnan et al. showed that right precordial TWIs were three times more common in endurance athletes than in nonendurance athletes [26]. In contrast, Calo et al. reported no association between exercise-induced cardiac remodelling and TWI in any regional distribution in young soccer players [22, 27]. Larger studies correlating the structural changes of the heart chambers with electrical remodelling according to the performed sport disciplines are therefore needed, ideally with periodic recording of ECGs, both in- and off-season. Supporting the necessity of follow-up ECGs in those with abnormal ECG findings, our only athlete with a pathological Q-wave (duration >40 ms) initially declined further work-up and was found to have a normalised Q-wave in a follow-up ECG 4 months later.
The principal strength of our study was the inclusion of consecutive high-level elite athletes of both genders performing diverse sports disciplines. More than 80% of our cohort competed at international level. We acknowledge some limitations. The relatively small size of our cohort was insufficient to enable us to detect all kind of ECG abnormalities indexed by the Seattle criteria. Also, we did not perform a complete cardiological work-up in all athletes independently of the ECG findings. However, as the sensitivity of the Seattle criteria has already been demonstrated in large multi-ethnic cohorts, it is unlikely that we missed any relevant cardiac pathology. We acknowledge that our cohort included only one athlete of African or Afro-Caribbean ethnicity, who are known to present ECG alterations more often. Moreover, another explanation for the low prevalence of ECG abnormalities in our population may be the low number of screened cyclists and rowers, who are reported to exhibit the most important cardiac adaptations among athletes [25]. Finally, as in most other studies dealing with ECG screening, the prevalence of ECG abnormalities may be underestimated because athletes with abnormal ECGs may have been already detected during previous screening and excluded from competitive sport.
In our cohort of high-level elite athletes, the prevalence of abnormal ECGs was very low according to the most recent ECG interpretation criteria. The most frequent relevant ECG modification was isolated T-wave inversion. Only one athlete was diagnosed with a new significant cardiac disease (0.3%). All our athletes with abnormal ECGs performed high dynamic sports (classes IIC and IIIC). Further large-scale studies addressing the correlation between structural modifications in the hearts of athletes in different sport classes and ECG modifications are needed.
Acknowledgements:We thank the medical team from the Swiss Olympic Medical Centre in Magglingen for the recording of the ECG and the excellent collaboration.
1 Halkin A, Steinvil A, Rosso R, Adler A, Rozovski U, Viskin S. Preventing sudden death of athletes with electrocardiographic screening: what is the absolute benefit and how much will it cost? J Am Coll Cardiol. 2012;60(22):2271–6. doi:http://dx.doi.org/10.1016/j.jacc.2012.09.003.
2 Harmon KG, Drezner JA, O’Connor FG, Asplund C, Finnoff JT. Should Electrocardiograms Be Part of the Preparticipation Physical Examination? PM R. 2016;8(3, Suppl):S24–35. doi:http://dx.doi.org/10.1016/j.pmrj.2016.01.001.
3 Harmon KG, Zigman M, Drezner JA. The effectiveness of screening history, physical exam, and ECG to detect potentially lethal cardiac disorders in athletes: a systematic review/meta-analysis. J Electrocardiol. 2015;48(3):329–38. doi:http://dx.doi.org/10.1016/j.jelectrocard.2015.02.001.
4 Bille K, Figueiras D, Schamasch P, Kappenberger L, Brenner JI, Meijboom FJ, et al. Sudden cardiac death in athletes: the Lausanne Recommendations. Eur J Cardiovasc Prev Rehabil. 2006;13(6):859–75. doi:http://dx.doi.org/10.1097/01.hjr.0000238397.50341.4a.
5 Corrado D, Pelliccia A, Bjørnstad HH, Vanhees L, Biffi A, Borjesson M, et al.; Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology; Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Eur Heart J. 2005;26(5):516–24. doi:http://dx.doi.org/10.1093/eurheartj/ehi108.
6 Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 2: Preparticipation Screening for Cardiovascular Disease in Competitive Athletes: A Scientific Statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356–61. doi:http://dx.doi.org/10.1016/j.jacc.2015.09.034.
7 Villiger B, Hintermann M, Goerre S, Schmied C. Task Force “Prevention Sudden Death in Elite Sport” SGSM/SSMS 2010: The sudden cardiac death of a young athlete: Recommendations for a sensible and effective preventive exam. SGSM/SSMS. 2010:59-60.
8 Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, et al.; Section of Sports Cardiology, European Association of Cardiovascular Prevention and Rehabilitation. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31(2):243–59. doi:http://dx.doi.org/10.1093/eurheartj/ehp473.
9 Drezner JA, Ackerman MJ, Anderson J, Ashley E, Asplund CA, Baggish AL, et al. Electrocardiographic interpretation in athletes: the ‘Seattle criteria’. Br J Sports Med. 2013;47(3):122–4. doi:http://dx.doi.org/10.1136/bjsports-2012-092067.
10 Sheikh N, Papadakis M, Ghani S, Zaidi A, Gati S, Adami PE, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129(16):1637–49. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.113.006179.
11 Corrado D. Reading an athlete’s ECG: from ESC to Seattle and beyond. 1st International course in Sports Cardiology, St George’s University of London, UK- August 28, 2015;Assessed the 10th of May 2016 at: http://www.c-r-y.org.uk/wp-content/uploads/2015/10/Reading-the-athletes-ECG_Corrado-D.pdf.
12 Kligfield P, Gettes LS, Bailey JJ, Childers R, Deal BJ, Hancock EW, et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. Recommendations for the standardization and interpretation of the electrocardiogram: part I: the electrocardiogram and its technology a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2007;49(10):1109–27. doi:http://dx.doi.org/10.1016/j.jacc.2007.01.024.
13 Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5(7):1015–8. doi:http://dx.doi.org/10.1016/j.hrthm.2008.03.037.
14 Napolitano C, Bloise R, Priori SG. Long QT syndrome and short QT syndrome: how to make correct diagnosis and what about eligibility for sports activity. J Cardiovasc Med (Hagerstown). 2006;7(4):250–6. doi:http://dx.doi.org/10.2459/01.JCM.0000219317.12504.5f.
15 Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: classification of sports. J Am Coll Cardiol. 2005;45(8):1364–7. doi:http://dx.doi.org/10.1016/j.jacc.2005.02.015.
16 Riding NR, Sheikh N, Adamuz C, Watt V, Farooq A, Whyte GP, et al. Comparison of three current sets of electrocardiographic interpretation criteria for use in screening athletes. Heart. 2015;101(5):384–90. doi:http://dx.doi.org/10.1136/heartjnl-2014-306437.
17 Menafoglio A, Di Valentino M, Segatto JM, Siragusa P, Pezzoli R, Maggi M, et al. Costs and yield of a 15-month preparticipation cardiovascular examination with ECG in 1070 young athletes in Switzerland: implications for routine ECG screening. Br J Sports Med. 2014;48(15):1157–61. doi:http://dx.doi.org/10.1136/bjsports-2013-092929.
18 Jacob D, Main ML, Gupta S, Gosch K, McCoy M, Magalski A. Prevalence and significance of isolated T wave inversion in 1755 consecutive American collegiate athletes. J Electrocardiol. 2015;48(3):407–14. doi:http://dx.doi.org/10.1016/j.jelectrocard.2015.03.005.
19 Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli G, et al. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358(2):152–61. doi:http://dx.doi.org/10.1056/NEJMoa060781.
20 Schnell F, Riding N, O’Hanlon R, Axel Lentz P, Donal E, Kervio G, et al. Recognition and significance of pathological T-wave inversions in athletes. Circulation. 2015;131(2):165–73. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.114.011038.
21 Wilson MG, Sharma S, Carré F, Charron P, Richard P, O’Hanlon R, et al. Significance of deep T-wave inversions in asymptomatic athletes with normal cardiovascular examinations: practical solutions for managing the diagnostic conundrum. Br J Sports Med. 2012;46(Suppl 1):i51–8. doi:http://dx.doi.org/10.1136/bjsports-2011-090838.
22 Calò L, Sperandii F, Martino A, Guerra E, Cavarretta E, Quaranta F, et al. Echocardiographic findings in 2261 peri-pubertal athletes with or without inverted T waves at electrocardiogram. Heart. 2015;101(3):193–200. doi:http://dx.doi.org/10.1136/heartjnl-2014-306110.
23 Papadakis M, Carre F, Kervio G, Rawlins J, Panoulas VF, Chandra N, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur Heart J. 2011;32(18):2304–13. doi:http://dx.doi.org/10.1093/eurheartj/ehr140.
24 Sharma S, Papadakis M. Interpreting the Athlete’s EKG: are all repolarization anomalies created equal? Circulation. 2015;131(2):128–30. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.114.013739.
25 Maron BJ, Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114(15):1633–44. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.106.613562.
26 Brosnan M, La Gerche A, Kalman J, Lo W, Fallon K, MacIsaac A, et al. Comparison of frequency of significant electrocardiographic abnormalities in endurance versus nonendurance athletes. Am J Cardiol. 2014;113(9):1567–73. doi:http://dx.doi.org/10.1016/j.amjcard.2014.01.438.
27 Wasfy MM, Baggish AL. T-wave inversions in athletes: a sheep in wolf’s clothing? Heart. 2015;101(3):167–8. doi:http://dx.doi.org/10.1136/heartjnl-2014-306988.
Author contributions: T. Perrin and L. Trachsel contributed equally to the manuscript
Disclosure statement:The present study did not benefit from any external funding. All authors have reported that they have no conflict of interest relevant to the contents of this paper to disclose.