DOI: https://doi.org/10.4414/smw.2016.14393
Treatments targeting the human epidermal growth factor receptor 2 (HER2) have drastically changed the natural disease course of HER2-positive breast cancer, particularly in the presence of metastases [1–3]. HER2 gene amplification is found in about 15 to 20% of breast cancers and was associated with poor prognosis before the advent of HER2-targeted treatments [2, 4].
Trastuzumab, the first humanised monoclonal antibody against the extracellular domain of HER2 [5], inhibits the growth of breast tumour cells by receptor internalisation, inhibition of cell-cycle progression and recruitment of immune-effector cells [6]. Trastuzumab has been approved for the treatment of HER2-overexpressing metastatic breast cancer [6] since 1998 in the United States and since 2000 in Switzerland and the European Union. A Cochrane collaboration study in 2014 on “trastuzumab-containing regimens for metastatic breast cancer” analysed seven randomised trials (1497 patients) and showed longer overall survival and progression-free survival in HER2-positive women with metastatic breast cancer receiving a trastuzumab treatment regimen [7]. Overall survival has even been shown to be superior in oestrogen receptor (ER) negative disease [3]. Moreover, patients with ER-positive/HER2-positive breast cancer have shown the longest survival after diagnosis of distant metastases among all breast cancer subtypes [8].
At present, there is no evidence that treatment with trastuzumab can safely be stopped after a certain time [9]. The practical guideline of the American Society of Clinical Oncology in 2014 recommends HER2-targeted therapy until progression or significant toxicities [10]. The resultant high costs of the therapy and possible adverse effects on the cardiovascular system show the need for more data on this subject [11]. Some authors have reported stopping trastuzumab maintenance after several years of complete remission [12, 13]. Knowledge of prognostic factors could optimise treatment approaches and guide prognostication for this the population of long-term responders.
Some case reports have described complete remission of patients with HER2-positive metastatic breast cancer treated with trastuzumab in combination with chemotherapy [12]. The longest relapse-free time mentioned was 8.5 years [9]. All of these cases had continued anti-HER2 maintenance therapy. Many were associated with liver metastases [11, 14], whereas early central nervous system (CNS) metastases are related to an unfavourable prognosis [15]. It is also hypothesised that the impact of trastuzumab in patients with ER-negative disease is higher than in patients with ER mutation [16]. Cross-talk between activation of epidermal growth factor / HER2 and ER activation seems to play a role in resistance to hormonal therapy [14]. Combination therapy with anti-HER2 agents and endocrine treatment offers only limited clinical benefit [17]. However, only a few biomarkers or clinicopathological predictive factors for overall survival are known to date.
This study was approved by the Ethics Committees of Zurich and registered at the Swiss National Clinical Trials Portal (SNCTP000001431) and on clinical trials.gov (NCT02560311).
This was a retrospective single-centre cohort study. Included were all patients treated for HER2-positive metastatic breast cancer between January 2004 and August 2014 at the University Hospital of Zurich, Switzerland. Patients receiving prior trastuzumab in the neoadjuvant or adjuvant setting were not excluded.
All patients were identified using the clinical database. Clinical follow-up data were recorded up to October 2015. Each patient’s medical record was reviewed to create a database of demographic and clinical characteristics. Histological information about the primary breast cancer tissue was taken. HER2 positivity was determined as a grade 3+ in immunohistochemistry or by a positive result of fluorescence in situ hybridization (value ≥2.0). ER and progesterone receptor (PR) status were documented as described in the pathology report. Cardiac toxicity was defined as the occurrence of left ventricular dysfunction of grade 2 or higher (as defined by the National Cancer Institute [NCI] version 3.0), confirmed on echocardiogram.
The primary endpoint of the study was progression-free survival (PFS), defined as the time from diagnosis of metastatic breast cancer to the time of disease progression or death from any cause. Secondary endpoints were overall survival (OS) and response rate. Response rates were determined with fluordeoxyglucose-positron emission tomography, positron emission tomography / computed tomography, or computed tomography performed after the first-line chemotherapy. Criteria for progression or response were based on an interdisciplinary tumour board decision that considered imaging, tumour marker and clinical assessment.
For patients who had not reached the endpoint under consideration at the time of the analysis, time-points were censored at the time of last tumour assessment for PFS and at the time of last contact for the overall survival OS.
Descriptive statistics were used for patient characteristics, tumour characteristics and details of the received therapy. Overall survival and other time-to-event endpoints were evaluated using Kaplan-Meier curves. To evaluate factors predicting long-term outcomes, the log-rank (Mantel-Cox) test was used to calculate the hazard ratio (HR) and p-values.
A total of 81 patients with HER2-positive metastatic breast cancer were identified in the hospital’s clinical database for further analysis. Patients and tumour characteristics are shown in table 1. The median age at the diagnosis of metastatic disease was 52 years (range 32–85 years). The median follow-up duration was 2.9 years (range 0.03–11.46 years) from the time of metastatic breast cancer diagnosis to death, loss to follow up or cut-off point. At the time of the last follow-up, 36 deaths (44.4%) were confirmed.
Twenty-six patients (32.1%) had primary (de novo) metastatic disease. Of the remaining 55 patients with secondary (not de novo) metastatic breast cancer, 3 had only neoadjuvant, 7 neoadjuvant plus adjuvant and 45 only adjuvant therapy. Twenty-five patients had trastuzumab-based therapy, mostly in combination with an anthracycline and a taxane, 30 patients had chemotherapy or endocrine therapy without an anti-HER2 treatment owing to lack of approval of trastuzuamb for adjuvant use. The median time from initial breast cancer diagnosis to diagnosis of metastatic disease was 3.4 years (range 0.17–13.40 years).
The median number of initial sites of metastatic disease was two (range one to four). Bone (40 patients, 49.4%) was the most common site, followed by the liver (36 patients, 44.4%) and nodal metastases (29 patients, 35.8%). Only 6 patients (7.4%) had CNS involvement at first diagnosis of metastatic disease.
As first-line therapy, most patients were treated with trastuzumab (69 patients, 86.3%) as anti-HER2 treatment, in combination with chemotherapy. Five patients received lapatinib (6.3%) plus chemotherapy. The chemotherapy most commonly consisted of docetaxel (51.4%) or vinorelbine (33.8%). The other agents used were capecitabine (10.8%), anthracycline (2.7%) and gemcitabine (1.4%). The remaining seven patients received either trastuzumab as monotherapy (two patients, 2.4%), or trastuzumab in combination with endocrine therapy (five patients, 6.2%).
After disease progression most patients received trastuzumab in combination with chemotherapy: second-line in 37 patients (71.2%), third-line in 25 patients (71.4%). As second-line therapy Trastuzumab was combined with a newer anti-HER2 therapy for five patients as second-line therapy and for six patients as third-line therapy. The remaining patients received either lapatinib plus chemotherapy or chemotherapy only (table 1).
| Table 1: Patients’ characteristics (n = 81). | |
| Age at diagnosis of MBC (years), median (range) | 52 (32–85) |
| Menopausal status | |
| Premenopausal | 29 (35.8%) |
| Postmenopausal | 42 (51.9%) |
| Data missing | 10 (12.3%) |
| Disease status | |
| De novo (primary) | 26 (32.1%) |
| Not de novo (secondary) | 55 (67.9%) |
| Therapy | |
| Neoadjuvant only | 3 |
| Neoadjuvant + adjuvant | 7 |
| Adjuvant only | 45 |
| Tumour stage (n = 77) | |
| I | 9 (11.7%) |
| II | 13 (16.9%) |
| III | 19 (37.7%) |
| IV | 26 (33.8%) |
| Tumour grade (n = 64) | |
| G 2 | 16 (25%) |
| G 3 | 48 (75%) |
| ER/PR status | |
| ER+/PR+ | 32 (39.5%) |
| ER+/PR− | 20 (24.7%) |
| ER−/PR− | 29 (35.8%) |
| Prior adjuvant therapy (n = 55) | |
| Trastuzumab-based (n = 25) | |
| Anthracycline + taxane | 15 (25%) |
| Anthracycline | 2 (3.6%) |
| Other chemotherapy | 1 (1.8%) |
| Anti-HER2 alone | 6 (11.0%) |
| Anti-HER2 + endocrine | 1 (1.8%) |
| Without anti-HER2 treatment (n = 30) | |
| Anthracycline+ docetaxel | 7 (12.7%) |
| CMF1 | 6 (10.9%) |
| Anthracycline | 7 (12.7%) |
| Other chemotherapy | 3 (5.4%) |
| Endocrine alone | 7 (12.7%) |
| Time to first metastatic relapse (years, n = 52) | |
| Mean | 3.8 |
| Median(range) | 3.4 (0.17–13.40) |
| Number of site(s) of initial recurrence | |
| 1 | 42 (51.9%) |
| 2 | 25 (30.9%) |
| 3 | 12 (14.8%) |
| 4 | 2 (0.2%) |
| Site of first metastatic disease2 | |
| Liver | 36 (44.4%) |
| Lung | 20 (24.7%) |
| Bone | 40 (49.4%) |
| Brain | 6 (7.4%) |
| Lymph nodes | 29 (35.8%) |
| Skin | 1 (1.2%) |
| Medulla | 1 (1.2%) |
| Soft tissue | 3 (3.7%) |
| First-line treatment of metastatic disease | |
| Trastuzumab + chemotherapy3 | 69 (86.3%) |
| Trastuzumab + endocrine (no chemotherapy) | 5 (6.3%) |
| Trastuzumab only4 | 2 (2.5%) |
| Lapatinib + chemotherapy5 | 5 (6.3%) |
| First-line chemotherapy with anti-HER2 treatment (n = 74) | |
| Vinorelbine | 25 (33.8%) |
| Docetaxel | 38 (51.4%) |
| Anthracycline | 2 (2.7%) |
| Gemcitabine | 1 (1.4) |
| Capecitabine | 8 (10.8%) |
| Anti-HER2 treatment after progression | |
| Second-line treatment (n = 52) | |
| Trastuzumab + chemotherapy (incl. 5 + Pertuzumab, Trastuzumab-Emtansin) | 37 (71.2%) |
| Lapatinib + capecitabine | 8 (15.4%) |
| Trastuzumab-entamsin only | 1 (1.9%) |
| Chemotherapy only (1 doxorubicin, 2 capecitabine, 2 vinorelbine 1 cyclophosphamide) | 6 (11.5%) |
| Third-line treatment (n = 35) | |
| Trastuzumab + chemotherapy (incl. 6 + Pertuzumab, Trastuzumab-Emtansin) | 25 (71.4%) |
| Lapatinib + capecitabine | 3 (8.6%) |
| Trastuzumab-entamsin only | 3 (8.6%) |
| Chemo only (1 doxorubicin, 1 capecitabine, 1 gemcitabine, 1 docetaxel) | 4 (11.4%) |
| ER = oestrogen receptor; MBC = metastatic breast cancer; PR = progesterone receptor; 1 Cyclophosphymide, methotrexate, 5-fluorouracil; 2 Multiple sites can apply; 3 3 plus pertuzumab, 2 plus lapatinib; 4 1 plus lapatinib, 1 intrathecal application; 5 All with capecitabine | |
Only six (7.4%) patients were found to have brain metastases at first diagnosis of metastatic disease. However, 34 of 81 patients (42%) developed brain metastases at some point during follow-up. Almost all of these 34 patients (91.2%) received brain radiotherapy. The treatment of brain metastases and leptomeningeal spread is summarised in table 2. Median OS after diagnosis of brain metastases was 26 months (95% CI 19.9–32.0 months). The OS for patients with de novo brain metastases was 1.9 years (95% CI 1.7–2.2 years).
The objective response rate (ORR) was 60.5%, with 16 (19.8%) complete responses and 33 (40.7%) partial responses (table 3). Four patients had a stable disease and 28 had progressive disease on anti-HER2 treatment.
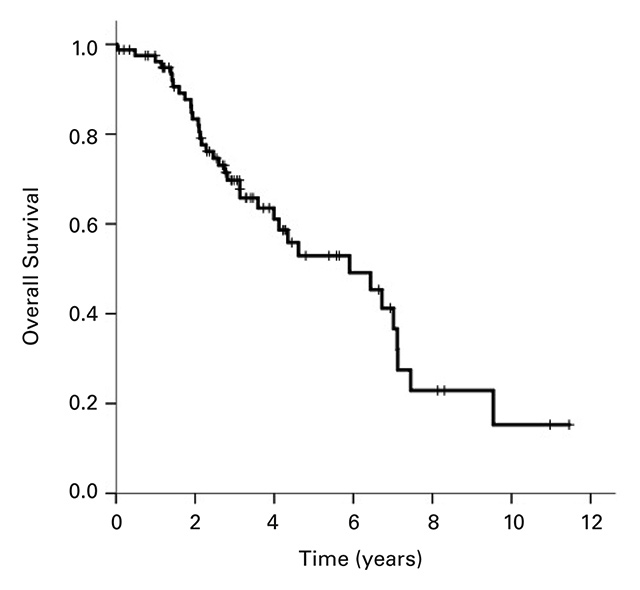
The median OS for all 81 patients was 5.90 years (95% CI 3.5–8.3; Fig. 1a). Median PFS) for the 70 patients available for analysis was 13.6 months (95% CI 9.0–18.3; Fig. 1b). Twenty patients (28.6%) remained in remission after 1 year, 11 (15.7%) after 2 years and 4 (5.7%) beyond 5 years. In patients with primary (de novo) metastases the PFS was 25 months (95% CI 16.8–33.2 months), whereas patients with secondary metastases had a PFS of 11.4 months (95% CI 7.8–15.0 months; Fig. 1c). The difference between these two patient groups was significant with a p-value of 0.006. There was no significant difference between the PFS of patients with positive hormone receptor status (17.1 months, 95% CI 12.0–22.4) compared with hormone receptor-negative patients (11.0 months, 95% CI 8.4–9.4; p = 0.77).

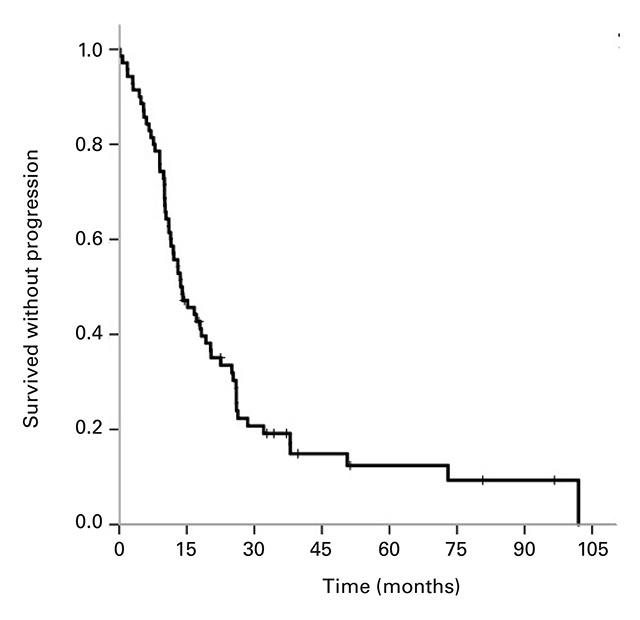
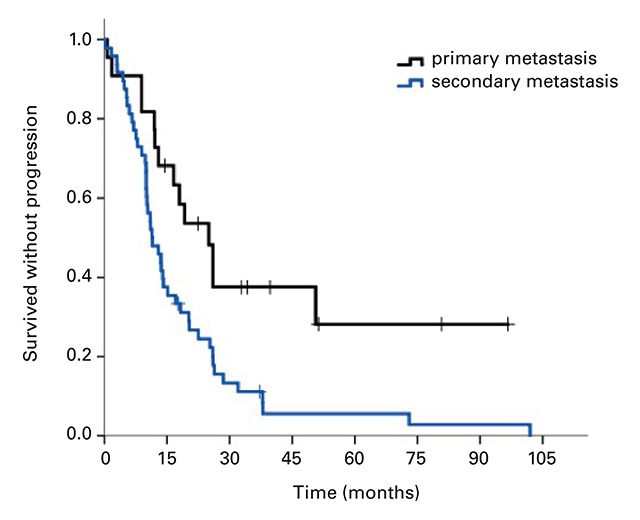
Figure 1
Kaplan-Meier curves for (a) overall survival for all patients, (b) progression-free survival (n = 70), and (c) progression-free survival of patients with primary metastatic disease vs patients with secondary metastatic disease, p = 0.006.
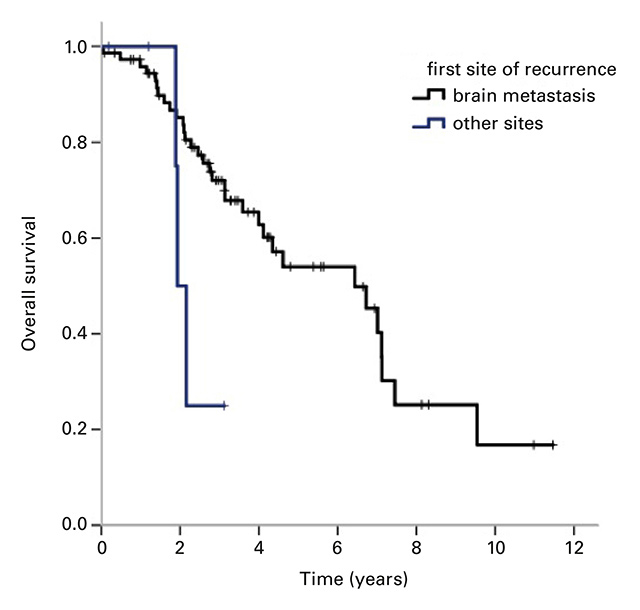
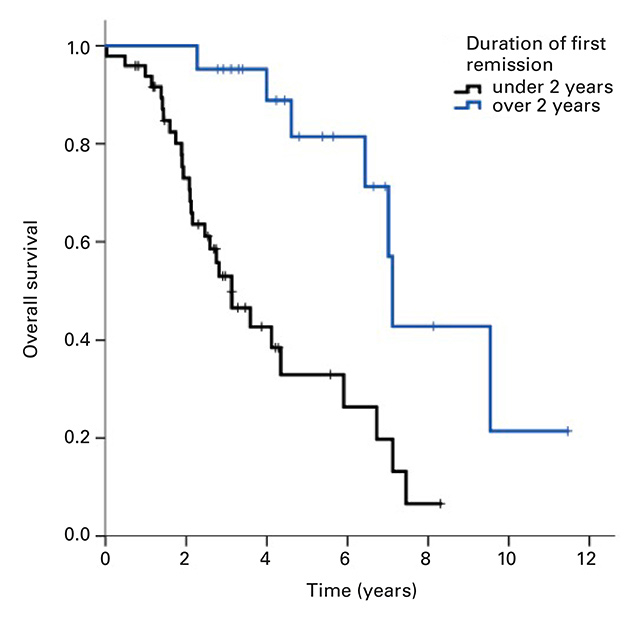
Figure 2
Kaplan-Meier curves of overall survival for (a) brain metastases as first site of recurrence vs other sites, p = 0.043 and (b) duration of first remission <2 years vs ≥2 years, p <0.001.
Left ventricular dysfunction during trastuzumab treatment occurred in four patients (4.9%). Three of them had grade 3 dysfunction and one grade 2 dysfunction. Three of these patients had received prior anthracycline therapy during adjuvant treatment.
The univariate analysis (table 4) showed no statistically significant difference in the OS by the time of initial diagnosis (before/after 2009), by hormone receptor status, or primary versus secondary disease. CNS metastases as first site of recurrence were significantly associated with a shorter median OS of 1.9 years (CI 1.7–2.2 years, p = 0.04) compared with patients with liver metastases (4.6 years, 95% CI 3.6–5.7) or bone metastases only (7.1 years, 95% CI 0.0–14.7) (Fig. 2a). In patients with first remission duration of ≥2 years, liver metastases were not associated with a longer survival, compared with other sites of metastasis.
The OS was significantly shorter in patients with first remission duration of <2 years (3.1 years, 95% CI 2.1–4.1) compared with ≥2 years (7.1 years, 95% CI 6.9–7.3; p = <0.001) (Fig. 2b).
| Table 2: Treatment of patients with brain metastases (n = 34). | |
| Treatment1 | Patients n (%) |
| Trastuzumab intrathecal2 | 3 (8.8%) |
| Lapatinib | 14 (41.2%) |
| Brain radiotherapy | 31 (91.2%) |
| Dexamethason | 7 (20.6%) |
| Brain surgery | 5 (6.2%) |
| No specific treatment | 2 (5.8%) |
| 1 Multiple treatments can apply 2 For leptomeningeal spread | |
| Table 3: Best overall response rate. | ||||||
| Therapy | n | CR | PR | SD | PD | ORR (CR+PR) |
| Trastuzumab + chemotherapy | 66 | 14 (21.2%) | 28 (34.6%) | 4 (4.9%) | 20 (24.7%) | 42 |
| Trastuzumab + endocrine (no chemotherapy) | 5 | 0 | 3 | 2 | 3 | |
| Trastuzumab only | 2 | 0 | 0 | 2 | 0 | |
| Trastuzumab + Pertuzumab | 3 | 1 | 2 | 0 | 3 | |
| Lapitinib + chemotherapy | 5 | 1 | 0 | 4 | 1 | |
| Total | 81 | 16 (19.8%) | 33 (40.8%) | 4 (4.9%) | 28 (34.5%) | 49 (60.5%) |
| CR = complete response; ORR = objective response rate; PD = progressive disease; PR = partial response, SD = stable disease | ||||||
Trastuzumab in combination with chemotherapy comprises the standard first-line treatment of HER2-positive metastatic breast cancer and has significantly improved prognosis of these patients over the last decade. Some patients have been shown to remain in remission for many years; however owing to limited published data on long-term remission, only few clinical predictive factors are known.
In this retrospective cohort study, the OS was 5.9 years, which is higher than in other comparable retrospective analyses (table 5). This difference might be explained by the fact that this analysis included patients treated up to 2014, who might have received newer anti-HER2 treatments and modern regimens of chemotherapy distribution. Newer HER2-targeted substances such as pertuzumab, trastuzumab-emtansin and lapatinib have influenced the outcome of HER2-positive metastatic breast cancer, as shown in various studies including the CLEOPATRA trial [22, 23].
The median PFS in this cohort was 13.6 months, which is slightly higher than in other studies. There was no difference between the OS of patients with primary metastases and OS of patients with secondary metastases, but the group with primary metastases had a longer PFS (25 vs 11.4 months, p = 0.006). An explanation could be that patients receiving chemotherapy and/or trastuzumab in the adjuvant setting may not respond as well to the first-line treatment of metastatic disease as chemotherapy-naive patients.
This study was able to show that patients with the brain as first site of recurrence have a significantly shorter median OS than patients with metastases elsewhere. Brain metastases in HER2-positive breast cancer are common and difficult to treat. The 10-year cumulative incidence for the development of CNS metastases is about 6.8% in HER2-positive and 3.5% in HER2-negative disease [24], which might be due to a biological predisposition [15] or the limited ability of trastuzumab to penetrate the blood-brain barrier as a result of its high molecular weight [11, 25, 26]. However, Dijkers et al. showed that trastuzumab can target brain lesions because of a disruption of the blood-brain barrier at the site of the metastasis [27]. The newer HER2-targeted oral tyrosine kinase inhibitor lapatinib is smaller and seems to have a better effect on brain metastases [28]. Lapatinib in combination with capecitabine has been shown to prolong time to progression and OS [29]. In our cohort, 42.4% of the patients with brain metastases had received lapatinib.
Other retrospective analyses have tried to find predictors of long-term outcome (table 5). Olson et al. reported that brain metastases and duration of first remission are prognostic factors [18]. Murthy et al. showed that hormone receptor status is a predictive factor [13].
Witzel et al. analysed patients who had non-progressive disease for at least 2 years [30]. They showed a positive association between longer time to progression and age at initiation of trastuzumab treatment (age less than 50 years), good performance status (Eastern Cooperative Oncology Group score 0) and initial response to trastuzumab treatment (complete remission). Furthermore, they demonstrated that therapy cessation or interruption should be avoided as it results in a shorter time to progression. Gene expression studies have shown that the HER2-enriched intrinsic subtype has a higher response rate than HER2 tumours of the luminal intrinsic subtype [31]. More data on the question of clinicopathological predictors or potential biomarkers for long-term outcome is necessary to improve treatment approaches. Based on the currently available evidence, therapy with trastuzumab should not be stopped.
The long-term use of trastuzumab raises concerns about adverse effects, most importantly its cardiotoxicity. Given as a single agent, trastzumab was associated with an incidence of cardiotoxicity of 1.4% over a follow-up period of 2 years [32]. In the Cochrane collaboration study of 2014, heart toxicity was found to be between three and four times more likely [7]. The risk of the cardiotoxicity seems to be multifactorial, with a higher risk when trastuzumab is administered with anthracycline, with pre-existing arterial hypertension or in older patients [33]. In our study, four patients (4.9%) with left ventricular dysfunction were reported.
There are some potential limitations to this study. First of all, the retrospective nature of the study leads to some potential biases. Over the last decade chemotherapy distribution, regimens and HER2-targeted treatments have changed in a way that may render our cohort nonhomogeneous and therefore limited the statistical power. Furthermore, we used a small cohort of 81 patients with HER2-positive metastatic breast cancer at the University Hospital of Zurich for our study. For some analyses, the number of patients might have been too small to allow a robust, statistically meaningful comparison between different parameters and patient outcome.
In conclusion, this retrospective cohort study was not able to identify reliable clinicopathological predictors for long-term outcome for patients under treatment with anti-HER2-targeted substances, but showed that the prognosis of HER2-positive metastatic breast cancer has improved and will probably continue to improve with the combination of treatments with newer anti-HER2 substances. Nevertheless, brain metastases remain a clinical problem [34] and are associated with a shorter overall survival.
| Table 4: Predictors of overall survival from first-line metastatic treatment to death or last follow-up for all patients in the cohort. | ||||
| n | Deaths | Univariate | ||
| Median OS (95% CI) | p-value | |||
| Overall | 81 | 37 | 5.90 (3.5–8.3) | |
| Age at diagnosis of MBC | 81 | 37 | Continuous variable | 0.98 |
| Menopausal status | 0.922 | |||
| Premenopausal | 29 | 10 | 5.9 (3.9–7.9) | |
| Postmenopausal | 42 | 22 | 4.6 (2.2–7.0) | |
| First diagnosis | 0.38 | |||
| Before 2009 | 39 | 22 | Median not reached | |
| After 2009 | 42 | 14 | 6.4 (4.0–8.9) | |
| ER and/or PR status | 0.24 | |||
| ER+/PR+ or ER+/PR− | 52 | 21 | 6.7 (5.6–7.9) | |
| ER−/PR− | 29 | 15 | 3.1 (1.4–4.9) | |
| Disease status | 0.175 | |||
| De novo MBC (primary) | 26 | 9 | 7.1 (2.0–12.2) | |
| Not de novo MBC (secondary) | 55 | 27 | 5.9 (3.0–8.7) | |
| Number of site(s) of initial recurrence | 0.151 | |||
| 1 | 42 | 18 | 7.1 (4.2–10.0) | |
| 2 | 25 | 10 | 4.0 (1.8–6.2) | |
| 3 | 12 | 6 | 4.6 (3.7–5.6) | |
| 4 | 2 | 2 | Median was reached | |
| Site(s) of initial recurrence | ||||
| CNS only | 6 | 4 | 1.9 (1.7–2.2) | 0.04 |
| Bone only | 12 | 4 | 7.1 (0.0–14.7) | 0.88 |
| Liver (10 only liver, 26 + other sites) | 36 | 16 | 4.6 (3.6–5.7) | 0.85 |
| Duration of first remission | <0.001 | |||
| <2 years | 49 | 29 | 3.1 (2.1–4.1) | |
| ≥2 years | 21 | 7 | 7.1 (6.9–7.3) | |
| Initial response to first-line treatment | 0.56 | |||
| Complete response | 17 | 3 | Median was reached | |
| Partial response | 31 | 14 | 5.9 (2.4–9.4) | |
| CI = confidence interval; CNS = central nervous system; ER = oestrogen receptor; MBC = metastatic breast cancer; OS = overall survival; PR = progesterone receptor | ||||
| Table 5: Summary of retrospective analyses of HER2-positive metastatic breast cancer. | ||||||||
| Study [Reference] | Location | Nr patients (study period) | Median OS (Range) | Median PFS (Range) | ORR | CNS metastases | Heart dysfunction | Predictive factors |
| Olson et al. [18] | Boston USA | 113 (1999–2005) | 3.5 years (3.0–4.4) | 8% as first site 55% in total | Brain metastases, number of sites of metastases, duration of first remission | |||
| Huober et al. [19] | Multicentre CH | 72 (2006–2007) | 2.7 years | 8.1 months | 43% total | – | ||
| Jackisch et al. [20] | Multicentre DE | 1843 (2000–2010) | 2.9 years (2.8–3.0) | 11.8 months (11.1–12.6) | 58% | 5% as first site | 2.3% | Age, organ involvement, primary vs secondary metastatic disease |
| Yeo et al. [21] | London UK | 215 (2001–2010) | 2.6 years (2.2–3.3) | 12 months (10.3–14.6) | 65% | 9% as first site | 13% | Performance status |
| Bringolf et al. | Zurich CH | 81 (2004–2014) | 5.9 years (3.5–8.3) | 13.6 months (9–18.3) | 61% | 7.4% as first site 42% in total | 4.9% | Brain metastases, duration of first remission |
| Murthy et al. [13] | Ann Arbor USA | 168 (1991–2015) | 3.9 years (3.4–5.2) | 15 months (13–17.22) | 4% as first site 39% in total | Hormone receptor status, age, extent of disease | ||
1 Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi:http://dx.doi.org/10.1056/NEJM200103153441101.
2 Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al.; Herceptin Adjuvant (HERA) Trial Study Team. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi:http://dx.doi.org/10.1056/NEJMoa052306.
3 Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–8. doi:http://dx.doi.org/10.1200/JCO.2008.19.9844.
4 Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi:http://dx.doi.org/10.1126/science.2470152.
5 Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9(3):1165–72. doi:http://dx.doi.org/10.1128/MCB.9.3.1165.
6 Gschwind A, Fischer OM, Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat Rev Cancer. 2004;4(5):361–70. doi:http://dx.doi.org/10.1038/nrc1360.
7 Balduzzi S, Mantarro S, Guarneri V, Tagliabue L, Pistotti V, Moja L, et al. Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. 2014;6(6):CD006242. doi:http://dx.doi.org/10.1002/14651858.CD006242.pub2.
8 Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat. 2013;141(3):507–14. doi:http://dx.doi.org/10.1007/s10549-013-2711-y.
9 Ihnenfeld Arciénega I, Imesch P, Fink D, Dedes KJ. Prolonged complete remission of metastatic HER2-positive breast cancer after continuous trastuzumab treatment: a case report and review of the literature. Target Oncol. 2015;10(2):297–301. doi:http://dx.doi.org/10.1007/s11523-014-0350-9.
10 Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, et al.; American Society of Clinical Oncology. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078–99. doi:http://dx.doi.org/10.1200/JCO.2013.54.0948.
11 Beda M, Basso U, Ghiotto C, Monfardini S; M. B. When should trastuzumab be stopped after achieving complete response in HER2-positive metastatic breast cancer patients? Tumori. 2007;93(5):491–2.
12 Syrios J, Dokou A, Tsavaris N. Sustained complete remission of human epidermal growth factor receptor 2-positive metastatic breast cancer in the liver during long-term trastuzumab (Herceptin) maintenance therapy in a woman: a case report. J Med Case Reports. 2010;4(1):401. doi:http://dx.doi.org/10.1186/1752-1947-4-401.
13 Murthy P, Kidwell KM, Schott AF, Merajver SD, Griggs JJ, Smerage JD, et al. Clinical predictors of long-term survival in HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2016;155(3):589–95. doi:http://dx.doi.org/10.1007/s10549-016-3705-3.
14 Tisman G. Inhibition of HER2/estrogen receptor cross-talk, probable relation to prolonged remission of stage IV breast cancer: a case report. Tumori. 2009;95(6):804–7.
15 Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–55. doi:http://dx.doi.org/10.1158/1078-0432.CCR-06-2478.
16 Gullo G, Zuradelli M, Sclafani F, Santoro A, Crown J. Durable complete response following chemotherapy and trastuzumab for metastatic HER2-positive breast cancer. Ann Oncol. 2012;23(8):2204–5. doi:http://dx.doi.org/10.1093/annonc/mds221.
17 Huober J, Fasching PA, Barsoum M, Petruzelka L, Wallwiener D, Thomssen C, et al. Higher efficacy of letrozole in combination with trastuzumab compared to letrozole monotherapy as first-line treatment in patients with HER2-positive, hormone-receptor-positive metastatic breast cancer – results of the eLEcTRA trial. Breast. 2012;21(1):27–33. doi:http://dx.doi.org/10.1016/j.breast.2011.07.006.
18 Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–31. doi:http://dx.doi.org/10.1016/j.breast.2012.12.006.
19 Huober J, Baumann M, Rochlitz C, Aebi S, Güth U, von Moos R, et al. Trastuzumab treatment beyond progression in advanced breast cancer: patterns of care in six Swiss breast cancer centers. Oncology. 2011;81(3-4):160–6. doi:http://dx.doi.org/10.1159/000333396.
20 Jackisch C, Schoenegg W, Reichert D, Welslau M, Selbach J, Harich HD, et al. Trastuzumab in advanced breast cancer--a decade of experience in Germany. BMC Cancer. 2014;14(1):924. doi:http://dx.doi.org/10.1186/1471-2407-14-924.
21 Yeo B, Kotsori K, Mohammed K, Walsh G, Smith IE. Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast. 2015;24(6):751–7. doi:http://dx.doi.org/10.1016/j.breast.2015.09.008.
22 Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, et al.; CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. doi:http://dx.doi.org/10.1056/NEJMoa1113216.
23 Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, et al.; CLEOPATRA Study Group. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–34. doi:http://dx.doi.org/10.1056/NEJMoa1413513.
24 Pestalozzi BC, Zahrieh D, Price KN, Holmberg SB, Lindtner J, Collins J, et al.; International Breast Cancer Study Group (IBCSG). Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol. 2006;17(6):935–44. doi:http://dx.doi.org/10.1093/annonc/mdl064.
25 Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18(11):2349–51. doi:http//dx.doi/10.1200/jco.2000.18.11.2349.
26 Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006;15(2):219–25. doi:http://dx.doi.org/10.1016/j.breast.2005.04.017.
27 Dijkers EC, Oude Munnink TH, Kosterink JG, Brouwers AH, Jager PL, de Jong JR, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–92. doi:http://dx.doi.org/10.1038/clpt.2010.12.
28 Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100(15):1092–103. doi:http://dx.doi.org/10.1093/jnci/djn216.
29 Kaplan MA, Isikdogan A, Koca D, Kucukoner M, Gumusay O, Yildiz R, et al. Clinical outcomes in patients who received lapatinib plus capecitabine combination therapy for HER2-positive breast cancer with brain metastasis and a comparison of survival with those who received trastuzumab-based therapy: a study by the Anatolian Society of Medical Oncology. Breast Cancer. 2014;21(6):677–83. doi:http://dx.doi.org/10.1007/s12282-013-0441-y.
30 Witzel I, Müller V, Abenhardt W, Kaufmann M, Schoenegg W, Schneeweis A, et al. Long-term tumor remission under trastuzumab treatment for HER2 positive metastatic breast cancer – results from the HER-OS patient registry. BMC Cancer. 2014;14(1):806. doi:http://dx.doi.org/10.1186/1471-2407-14-806.
31 Montemurro F, Prat A, Rossi V, Valabrega G, Sperinde J, Peraldo-Neia C, et al. Potential biomarkers of long-term benefit from single-agent trastuzumab or lapatinib in HER2-positive metastatic breast cancer. Mol Oncol. 2014;8(1):20–6. doi:http://dx.doi.org/10.1016/j.molonc.2013.08.013.
32 Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi:http://dx.doi.org/10.1200/JCO.20.3.719.
33 Baumann C, Castiglione-Gertsch M. Trastuzumab. Schweiz Med Forum. 2007;7:879–83.
34 Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–43. doi:http://dx.doi.org/10.1158/1078-0432.CCR-10-2962.
Disclosure statement: KJD received honoraria for consultancies and advisory boards from Roche Pharmaceuticals Switzerland. No other potential conflict of interest relevant to this article was reported.