Cardiorespiratory hospitalisation and mortality reductions after smoking bans in Switzerland
DOI: https://doi.org/10.4414/smw.2016.14381
Ana M
Vicedo-Cabrera, Martin
Röösli, Dragana
Radovanovic, Leticia
Grize, Fabienne
Witassek, Christian
Schindler, Laura
Perez
Summary
INTRODUCTION: Smoking bans are considered one of the most effective policies to reduce population exposure to tobacco smoke and prevent adverse health outcomes. However, evidence on the effect of contextual variables on the effectiveness of smoking bans is still lacking.
AIMS: The patchwork of cantonal smoke-free laws in Switzerland was used as a quasi-experimental setting to assess changes after their introduction in: hospitalisations and mortality due to cardiorespiratory diseases in adults; total hospitalisations and hospitalisations due to respiratory disorders in children; and the modifying effects of contextual factors and the effectiveness of the laws.
METHODS: Using hospital and mortality registry data for residents in Switzerland (2005–2012), we conducted canton-specific interrupted time-series analyses followed by random effects meta-analyses to obtain nationwide smoking ban estimates by subgroups of age, sex and causes of hospitalisation or death. Heterogeneity of the impact caused by strictness of the ban and other smoking-related characteristics of the cantons was explored through meta-regression.
RESULTS: Total hospitalisation rates due to cardiovascular and respiratory diseases did not significantly change after the introduction of the ban. Post-ban changes were detected in ischaemic heart disease hospitalisations, with a 2.5% reduction (95% confidence interval [CI)] −6.2 to 1.3%) for all ages and 5.5% (95% CI −10.8 to −0.2%) in adults 35–64 years old. Total mortality due to respiratory diseases decreased by 8.2% (95% CI −15.2 to −0.6%) over all ages, and chronic obstructive pulmonary disease mortality decreased by 14.0% (95% CI −22.3 to −4.5%) in adults ≥65 years old. Cardiovascular mortality did not change after the introduction of the ban, but there was an indication of post-ban reductions in mortality due to hypertensive disorders (−5.4%, 95% CI −12.6 to 2.3%), and congestive heart failure (−6.0%, 95% CI −14.5 to 3.4%). No benefits were observed for hospitalisations due to respiratory diseases in children or for infant mortality. The type of smoking ban implemented explained the heterogeneity of benefits across cantons for some outcomes.
CONCLUSION: Smoking bans in Switzerland were associated with overall reductions in cardiovascular and respiratory hospitalisation and mortality in adults.
Introduction
Together with increased tobacco prices, smoking ban are one of the most effective policies to reduce exposure to tobacco smoke and prevent related adverse health outcomes [1]. There is now a large body of evidence showing that the implementation of smoking bans may considerably reduce cardiovascular hospitalisations, especially those due to acute myocardial infarction, worldwide [2–5]. Although less explored, smoking bans have also been shown to particularly benefit vulnerable populations such as children, pregnant women and the elderly [6–9]. Recent evidence suggests that smoking bans protect nonsmokers from the harm of passive smoking, with a more pronounced effect from more comprehensive laws [10, 11]. Smoking bans have also been associated with active-smoking reduction and cessation [12, 13]. Despite this evidence, 18% of the world population, including residents in only eight European countries, live in regions with comprehensive national smoke-free laws. Most implemented partial laws do not fully protect the population from passive smoke exposure in public places (i.e., allow smoking in restricted areas in hospitality venues with service of a specific size) [14]. A better understanding of vulnerabilities of the population and other contextual factors (i.e. chronic health conditions, socioeconomic profile, smoking behaviours) influencing the effectiveness of the laws in different contexts is needed to better support worldwide implementation of smoking bans and further strengthen antitobacco laws.
In Switzerland, smoking in public places and workplaces is regulated at both the national and cantonal level (cantons are the largest administrative units of the Swiss Federal state). In May 2010, the Swiss parliament approved a nationwide federal smoking ban covering indoor public places and workplaces, but with several exceptions in the hospitality sector (authorised smoking establishments <80 m2 and dedicated smoking rooms in larger establishments). When the federal ban was enacted, 12 out of 26 cantons had previously introduced their own laws as early as 2007, some with a higher level of protection for hospitality workers (fig. 1; fig. S1 in appendix 1: supplementary figures and tables). This patchwork of regulation is an ideal natural experiment to explore the health impact of contextual aspects of the regulations. Except for a nationwide study on pregnancy outcomes [15], only a few small canton-specific studies on the health effects of the smoking ban exist in Switzerland. These past studies evaluated principally changes in hospital admissions for acute myocardial infarction after the laws came into force, and rarely focused on other outcomes, such as respiratory diseases [16–19]. In addition, none could address other questions related to mechanisms influencing the effectiveness of the smoking ban.
This study aimed at providing, for Switzerland, a comprehensive picture of the changes in mortality and hospitalisations due to cardiorespiratory diseases in adults and children achieved after the introduction of the smoking bans. We also aimed to give more evidence on the potential mechanisms explaining the variable effectiveness of the laws according to characteristics of the population.
Methods
Population and outcomes
We evaluated the cantonal and nationwide impact of smoke-free laws in Switzerland using hospital admissions and mortality data for Switzerland between 2005 and 2012 from the Health Registry of the Swiss Federal Statistical Office (Bundesamt für Statistik, BFS). All cases among Swiss residents were aggregated into monthly counts in each study year (according to the date of death or hospitalisation) by canton, gender, age group (children if ≤15 years old, adults if ≥35 years old) and specific causes. People aged between 16 and 34 years (inclusive) were not included in the adult category because of their different physiology and behaviour towards tobacco. Only emergency hospital admissions were considered.
The cardiovascular and respiratory outcomes considered in adults (≥35 years old), categorised according to the International Classification of Diseases Tenth Revision (ICD-10), were: ischaemic heart disease (IHD) (I20–I25), cerebrovascular diseases (I60–I69), hypertensive disorders (I10–I15), arrhythmia (I47–I49), congestive heart failure (I50), chronic obstructive respiratory disease (COPD) (J40–J44, J47), asthma (J45–J46) and pneumonia (J12–J18). We conducted subanalyses for total myocardial infarction cases (I21), with ST-elevation (STEMI) (I210–I213) and without ST-elevation (NSTEMI) (I214). Stratified analyses in adults per subcategory of age (adults between 35 and 64 years of age, and ≥65 years old) and gender were performed.
We restricted the analysis in children (≤15 years old) to hospital admissions due to respiratory infections (J00–22), and total infant mortality (<1 year of age) and mortality due to perinatal conditions (P00–96). We did not consider other causes of death or hospitalisation in this age group because the number of cases per month and canton was too small.
The causes of death or hospitalisation, definitions and age groups included in our study are presented in table S1 (appendix 1). The selection of these groups of causes was based on previous smoking-ban studies and in order to cover a wider spectrum of diseases, population subgroups, mechanisms and degrees of vulnerability.
Analysis of overall effects
We performed canton-specific interrupted time-series analyses to estimate the changes in mortality and hospital admissions after the introduction of the smoking ban in each canton, by age group and main cause category. Quasi-Poisson regression models were applied, and the smoking ban impact was modelled with a step function (by assigning a value of one to the months of the ban period and zero to pre-ban months, according to the specific date of introduction in each canton) following the usual approach of interrupted time-series design. We controlled for long-term trends (by a linear function of calendar time, consisting in a continuous series from first day of the pre-ban period to the last day of the smoking ban period in each canton-specific model), seasonality (with a four-level categorical variable, representing the different seasons), influenza peaks (by linear function of the monthly mean consultation rates of influenza-like illnesses for each canton, provided by the BAG Bundesamt für Gesundheit), and number of days of the month (as logarithm). Age- and gender-specific annual population for each canton was introduced as offset. Because some cantons introduced their own smoke-free laws at different timepoints prior to the federal legislation, the main analysis was restricted to periods of 2 years before and 2 years after the date of implementation of the law in the corresponding canton (fig. S1, appendix 1). This assumption was relaxed in a sensitivity analysis (see below). We also tested serial autocorrelation of residuals across 24 lags for each of the cantonal time series models, using the partial autocorrelation function.
We performed random-effect meta-analyses of the canton-specific estimates to obtain the nationwide percent change in mortality and hospitalisation rates after the implementation of the smoking bans. The heterogeneity between the canton-specific results was assessed with I2 statistics and the test of heterogeneity. Through visual inspection of the temporal trends, we identified and excluded from each cause-specific analysis those cantons showing inconsistent or unexplainable changes through time.
Analysis of effect modification
We used meta-regression to explore potential modifications of impacts related to the extent and severity of smoking bans: that is, we differentiated between cantons following only the federal smoking ban (“federal law”) and those with more prohibitive bans and additional occupational exposure restrictions (“federal law + restrictions”) (fig. 1). We also examined whether impacts of the smoking ban were related to changes in cantonal levels of active and passive smoking after the bans by applying the same statistical procedure. These data were obtained from two different rounds of the Swiss Health Survey, in 2007 and 2012 (http://www.bfs.admin.ch/bfs/portal/de/index/infothek/erhebungen__quellen/blank/blank/ess/04.html). This is a population-based survey performed every 5 years that collects information on health status and habits from a representative sample of Swiss residents aged 15 years or over. Cantons whose prevalence of reported active smoking had decreased between the two survey years were compared with cantons that did not show a decrease. Given that all cantons experienced a decrease in passive smoking during the study period, we compared groups of cantons according to whether this decrease in passive smoking was above or below the overall median value. All meta-regression analyses were adjusted by the socioeconomic and health profile of the population in each canton, because previous studies reported socioeconomic disparities in smoking ban effectiveness or level of coverage [20, 21]. Methods have been described elsewhere [15]. Briefly, two independent scores were developed by principal component analysis of several socioeconomic and health indicators collected at cantonal level and jointly included in the meta-regression models as linear functions.
Sensitivity analysis
We performed several sensitivity analyses to check the robustness of our results: (1) we introduced the canton-specific monthly count of a “non-smoking-ban related outcome” (see definition below) as explanatory variable to additionally capture the baseline trends in hospitalization and mortality rates in each canton, (2) we did not restrict the post- and pre-ban study periods to 2 years, (3) no canton was excluded from the meta-analysis, (4) we simulated a “fake” smoking ban preceding the true one by 1 year in each canton and we restricted the study period to 1 year before and 1 year after this to exclude the true smoking ban date from this analysis, (5) we estimated the smoking ban impact on a control or “non-smoking-ban related” outcome, which we assumed not to be associated with the ban. For this purpose, we selected deaths due to external causes (V01–Y98) and hospital admission due to injuries (S00–S98). To avoid multiple comparisons, meta-regression and sensitivity analyses were conducted only on hospitalisations and mortality due to IHD and COPD as the two main outcomes representative of the smoking ban impact on health in adults, as shown in several previous studies [22–24].
All analyses were conducted with statistical software R (Team R Development Core) and STATA (StataCorp, College Station. TX).
Results
All supplementary figures and tables are presented in appendix 1.
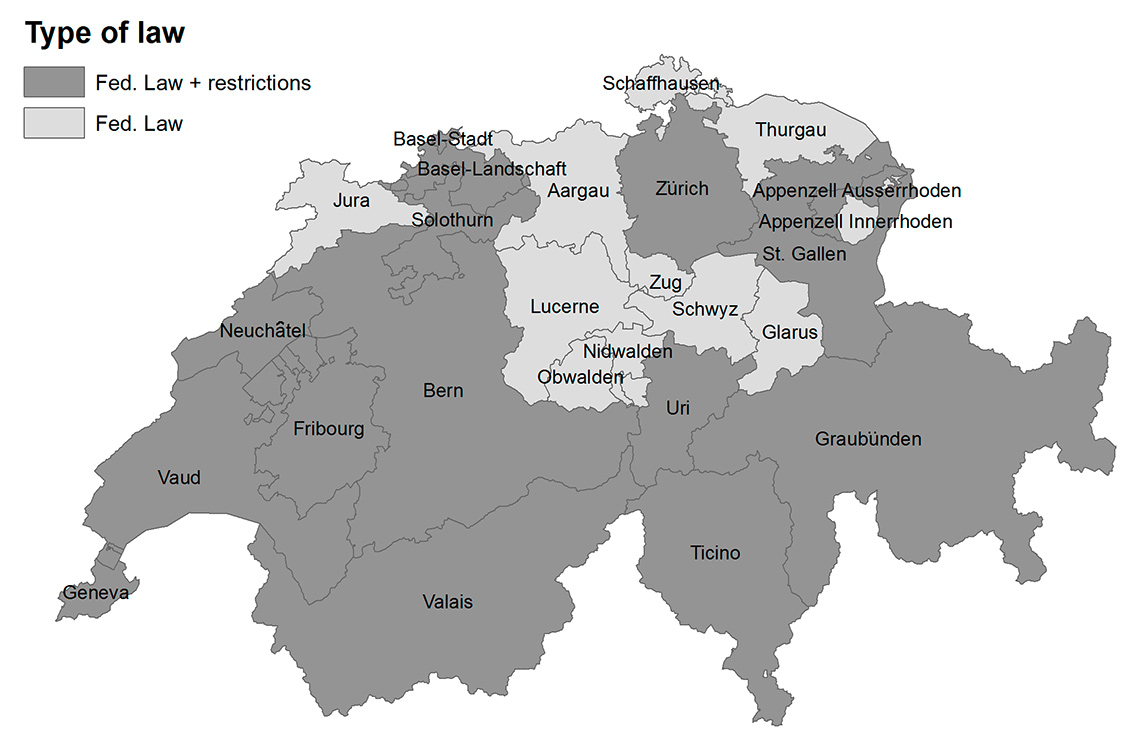
Figure 1
Geographical distribution of the two types of smoking ban implemented in each canton in Switzerland. Fed. Law (light grey): cantons which followed only the Federal Smoking Ban; Fed. Law + restrictions (dark grey): cantons which applied additional restrictions to the Federal Smoking Ban.
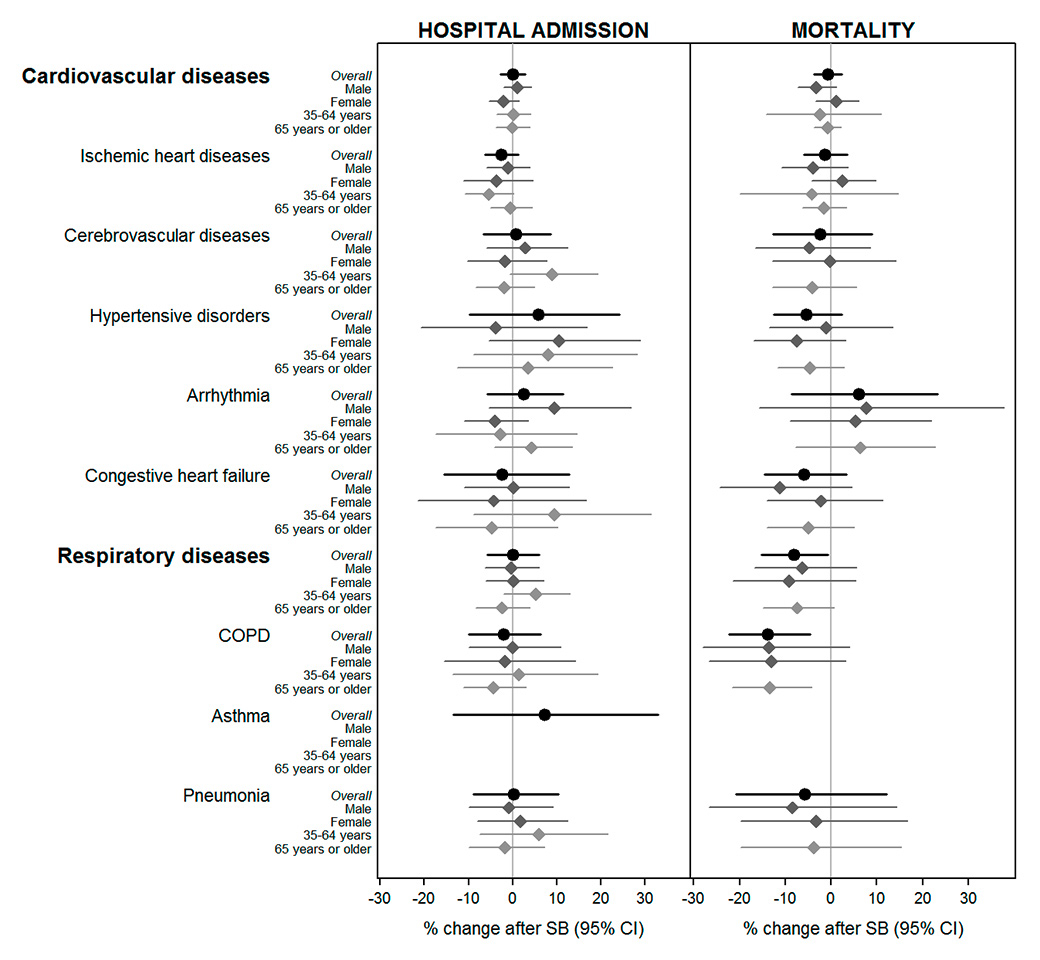
Figure 2
Gender- and age-specific estimates of percentage (and 95% confidence intervals, CIs) change in hospital admissions and mortality in adults (≥35 years old) by the main groups of causes after the introduction of the smoking ban (data restricted to the periods 2 years before and 2 years after the introduction of the smoking ban in each canton).
COPD = chronic obstructive pulmonary disease; SB = smoking ban
Hospital admissions: Canton Aargau was excluded from the meta-analysis of cardiovascular diseases, ischaemic heart disease, cerebrovascular diseases; Canton Bern was excluded from the meta-analysis of hypertensive disorders, arrhythmia, congestive heart failure.
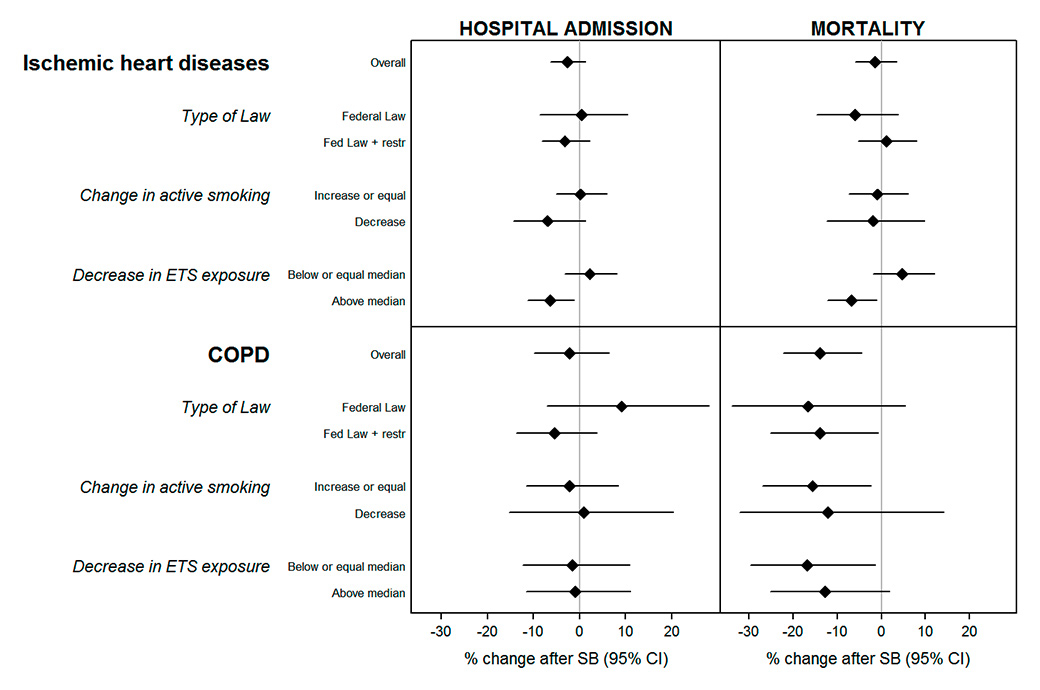
Figure 3
Change (and 95% confidence interval, CI) in hospital admissions and mortality due to ischaemic heart disease and chronic obstructive pulmonary disease (COPD) in adults (≥35 years old) after the introduction of the smoking ban, by type of law and smoking profile of the canton (adjusted for the socioeconomic and health profile of the population).
SB = smoking ban.
Type of law: Fed. Law = cantons which followed only the federal smoking ban, Fed. Law + restr = cantons which applied additional restrictions to the federal smoking ban.
Change in active smoking: difference between the reported smoking prevalence in the Swiss Health Surveys of 2007 and 2012. Stratified by non-decrease vs decrease in prevalence.
Decrease in environmental tobacco smoke (ETS) exposure: difference between the reported ETS exposure in the Swiss Health Surveys of 2007 and 2012. Since there was always a decrease, cantons were classified according to whether the decrease was above or below the median value.
During the restricted period of 2 years before to 2 years after the introduction of the smoking ban in each canton in Switzerland, there were a total of 365 283 hospitalisations due to cardiovascular diseases and 111 043 due to respiratory diseases in adults (≥35 years old), with a monthly average of 208 and 87 cases per canton, respectively (table 1). Hospitalisations due to IHD, COPD and pneumonia were the most frequent, representing 26%, 20% and 45%, respectively, of total hospitalisations due to cardiovascular or respiratory diseases. Cardiovascular mortality amounted to 87 833 cases (69 cases per month and canton) and mortality due to respiratory diseases to 15 142 (12 cases per month and canton). We found that hospitalisation and mortality rates were in general higher for males (except for asthma hospital admissions) and in the older age category (≥65 years old) with over 90% of the deaths occurring in this age group (table S2).
Nationwide smoking ban estimates for outcome groups in adults (≥35 years) are reported in table 1. Before graphical display of the data, we excluded the cantons of Aargau and Bern from the meta-analysis for hospital admissions for some specific cardiovascular causes because of sudden unexplained changes in trends (detailed list in a footnote to table 1, monthly time trends of the cantonal outcomes excluded from meta-analysis in figure S2). Whereas canton-specific mortality estimates seemed to be homogeneous, large variability was found for hospitalisations, with statistically significant heterogeneity for most of the outcomes (except IHD, arrhythmia, COPD and asthma) (table S3, fig. S3).
Overall, hospitalisation rates for the main cardiovascular and respiratory causes did not significantly change after the introduction of the ban. However, we found a reduction by −2.5% (95% confidence interval [CI] −6.2 to 1.3%) in IHD hospital admissions over all ages, with a stronger decrease detected in adults between 35 and 65 years old (−5.5%, 95% CI −10.8 to 0.2%) (fig. 2, numerical data in table S4). This was confirmed with consistent reductions in hospitalisations due to STEMI (−7.3%, 95% CI −13.2 to −1.0%) in 35- to 65-year-old adults (table S5).
Respiratory mortality decreased by −8.2% (95% CI −15.2 to −0.6%) following the introduction of the bans, with larger reductions for COPD of up to −13.9% (95% CI −22.3 to −4.5%) in all age groups, and −13.4% (95% CI −21.7 to −4.18%) in adults ≥65 years old (fig. 2). Smaller, nonsignificant reductions in cardiovascular mortality were observed, with some indication of an effect on mortality for hypertensive disorders in females (−7.5%, 95% CI −17.0 to 3.1%, p = 0.195), and congestive heart failure in males (−11.1%, 95% CI −24.4 to 4.5%, p = 0.192).
Results for infant mortality and respiratory hospital admissions in children were inconsistent, with large confidence intervals (table S6).
We found some heterogeneity of effects by type of smoking ban implemented. There were greater reductions in hospitalisations due to IHD and COPD in cantons applying more restrictive smoking bans. We found a reduction in hospitalisation for IHD of 3.1% (95% CI −8.2 to 2.2%) for cantons with federal law + restrictions vs a null effect of 0.5% (95% CI −8.6 to 10.5%) for cantons with federal law only. In hospitalisation for COPD, we found a 5.3% (95% CI −13.7 to 3.8%) reduction in cantons with federal law + restrictions, but there was no effect for cantons with federal law only (9.1%, 95% CI −7.1 to 18.2%). In both cases, however, differences between categories of type of ban were not statistically significant (fig. 3, numerical results in table S7).
For IHD, similar patterns were observed when changes in smoking-related exposure characteristics of the cantons after the ban were used in the meta-regression instead of type of ban implemented. For example, the benefits of the ban were limited to cantons reporting a reduction in active smokers during the period when smoking bans were implemented, and cantons with a larger decrease in passive smoking exposure showed stronger post-ban reductions in hospitalisations (IHD hospitalisation: −6.3%, 95% CI −11.2 to −1.2% in cantons with higher than median decrease vs 2.3%, 95% CI −3.3 to 8.2% in cantons with decreases equal to or lower than the median change, p-value for the interaction 0.04). In addition, we found some heterogeneity of effects for IHD mortality. IHD mortality decreased by 6.8% (95% CI −12.2 to 1.1%) in cantons with a higher than median decrease in passive smoking exposure, but there was no effect in cantons with decreases equal to or lower than the median change (4.8%, 95% CI −1.9 to 12.0%, p value for the interaction 0.02). Compared with IHD, the effect of the smoking ban on COPD varied little when using smoking-related cantonal characteristics other than type of ban.
The results of sensitivity analysis are reported in table S8 for the two selected representative outcomes. Smoking ban estimates did not vary significantly when applying additional control for time trends (sensitivity analysis [SA]1). Except for IHD mortality, there was a decrease in the effects of the smoking ban when the study period was extended from 2005 to 2012 for all cantons (SA2). Similarly, the smoking ban estimate for IHD hospitalisations became smaller and more uncertain when all cantons were included in the model (SA3). Overall, significant changes were not observed when the date of the introduction of the smoking ban in each canton was displaced by 1 year (SA4), or when the analysis was conducted with control outcomes (SA5). We only found a significant reduction in COPD hospitalisations in SA4, which was mostly due to a very strong decrease in the biggest canton, Zurich (data not shown).
|
Table 1:Percent change in hospital admissions and mortality in adults (≥35 years) due to the main groups of causes after the introduction of the smoking ban (95% confidence interval) (data restricted to the period including 2 years before and 2 years after the introduction of the smoking ban in each canton). |
|
Cause of hospitalisation or death
(ICD-10 codes in parenthesis)
|
Hospital admissions
|
Mortality
|
|
N (%)*
|
% change
(95% CI)†
|
p-value
|
N (%)*
|
% change
(95% CI)
|
p-value
|
|
Cardiovascular diseases (ICD-10: I00–I99)
|
265 283 |
0.02 (−2.71 to 2.82) |
0.990 |
87 833 |
−0.72 (−3.67 to 2.31) |
0.623 |
|
Ischaemic heart diseases (I20–I25)
|
69 829 (26.3) |
−2.54 (−6.23 to 1.30) |
0.183 |
33 880 (38.6) |
−1.38 (−5.98 to 3.45) |
0.555 |
|
Cerebrovascular diseases (I60–I69)
|
48 420 (18.3) |
0.78 (−6.55 to 8.69) |
0.834 |
15 578 (17.7) |
−2.48 (−12.7 to 8.92) |
0.644 |
|
Hypertensive disorders (I10–I15)
|
20 049 (7.6) |
5.86 (−9.78 to 24.2) |
0.469 |
10 679 (12.2) |
−5.43 (−12.6 to 2.27) |
0.154 |
|
Arrhythmia (I47–I49)
|
25 096 (9.5) |
2.46 (−5.72 to 11.4) |
0.552 |
2 873 (3.3) |
6.09 (−8.62 to 23.2) |
0.422 |
|
Congestive heart failure (I50)
|
37 663 (14.2) |
−2.41 (−15.6 to 12.8) |
0.731 |
7 061(8.0) |
−5.98 (−14.5 to 3.37) |
0.192 |
|
Respiratory diseases (ICD-10: J00–J99)
|
111 043 |
0.03 (−5.67 to 6.06) |
0.993 |
15 142 |
−8.20 (−15.2 to −0.64)
|
0.035 |
|
Chronic obstructive pulmonary disease (J40–J44, J47)
|
22 251 (20.0) |
−2.08 (−9.85 to 6.35) |
0.604 |
7 308 (48.3) |
−13.9 (−22.3 to −4.54)
|
0.006 |
|
Asthma (J45–J46)
|
3 285 (3.0) |
7.26 (−13.4 to 32.9) |
0.506 |
406 (2.7) |
|
|
|
Pneumonia (J12–J18)
|
50 377 (45.4) |
0.22 (−8.92 to 10.3) |
0.962 |
4 911 (32.4) |
−5.71 (−20.7 to 12.2) |
0.492 |
| CI = confidence interval; N = total number of cases
* Percentage of cases due to the specific cause within the respective main disease category. N = total number of cases.
† Canton of Aargau was excluded from the meta-analysis of cardiovascular diseases, ischaemic heart disease, cerebrovascular diseases; Canton of Bern was excluded from the meta-analysis of hypertensive disorders, arrhythmia, congestive heart failure.
Statistically significant results (p <0.05) are in bold type. |
Discussion
Main findings
This is the first comprehensive nationwide study on the cardiorespiratory effects of smoking bans in Switzerland. We found that the smoking bans were associated with nationwide reductions of −5.5% in hospital admissions for IHD of adults between 35 and 65 years old, and −8% in respiratory mortality and −14% in COPD mortality in the oldest adults. No benefit of the laws could be inferred for children in terms of hospitalisations due to respiratory diseases or infant mortality. The type of smoking ban implemented explained the heterogeneity of benefits across cantons for some outcomes.
The health benefits of smoking bans on cardiorespiratory outcomes in Switzerland were previously assessed in small studies performed in only 3 out of the 26 Swiss cantons (Geneva, Ticino and Graubünden), which were among those applying more comprehensive laws [16–19, 25]. Overall, our results confirm a nationwide benefit of smoking bans in Switzerland for IHD hospitalisation, as found in some of these previous local Swiss studies, although comparison of magnitude of effects is difficult given the different methodology and modelling approaches applied between our study and the local studies. Our overall estimate is consistent with results from past studies, particularly a recent review that found a reduction of 8% in hospitalisations for acute coronary events from studies performed in locations with only partial bans [2, 17–19, 22, 26, 27].
It is hypothesised that changes in active or passive tobacco exposure induce physiological changes that increase the risk of ischaemia and arrhythmias in the short term [28]. In adults, the rapid benefits on IHD outcomes observed after the smoking bans are explained by a rapid reduction in tobacco exposure, which quickly removes potential triggers for acute cardiovascular outcomes [2]. We found that benefits for IHD hospitalisations materialised in adults between 35 and 65 years old, an effect reported in only a few previous studies [29]. In Switzerland, these people might be considered to be a vulnerable group at increased risk of an acute coronary event because they would not be under pharmacological treatment or have healthier lifestyle choices. This may explain in part why our study found rapid benefits for this age group after implementation of smoking bans.
Fewer studies have evaluated the association between respiratory outcomes in adults and smoking ban. Some studies reported consistent reductions in respiratory admissions in adults, but others, like us, did not [29]. However, our results are within the range of the reduction in COPD mortality of −38% in older adults found in a recent Irish study [9]. We found reductions in respiratory mortality from COPD independently of the type of smoking ban implemented and the magnitude of change in active and passive smoking at population level. These individuals are probably at a very vulnerable stage of their disease and are altogether more susceptible to any small change in tobacco smoke exposure [30]. We found some evidence of a reduction in mortality from hypertensive disorders and congestive heart failure. The major causes of death for patients with COPD or chronic cardiovascular disorders include acute myocardial infarction, heart failure, pulmonary embolism and cardiac arrhythmia [31]. Although these deaths may be coded on the basis of the main diagnosis, individuals with some underlying chronic cardiorespiratory pathologies may be more likely to die from related acute coronary outcomes which could explain our findings. Our results point to a potential underestimation of the benefits of the law on high-risk, chronically ill patients; further investigation using registries that can better identify these individuals is needed.
Although we previously found strong evidence of the effects of the smoking ban on birth outcomes in Switzerland [15], no impact of the bans on respiratory hospital admissions of children and infant mortality could be detected in this study. A recent meta-analysis reported a 10% reduction in asthma admissions in children [6], whereas another study not included in this review found inconsistent associations [32]. Our null findings might be due to the low number of cases in each canton, and the use of other data sources such as routine doctor registry data might have been more suitable in order to capture not only the more severe cases. It could also be possible that smoking bans changed the behaviour of smokers in private places (home, car, etc.) where children spend most of their time. However, evidence for such a shift is still not totally consistent and is unavailable for Switzerland [33–35].
Indication of larger impacts on IHD and COPD hospitalisations was found in cantons that applied additional restrictions to the federal smoke-free law. These results suggest consistent benefits for groups, such as those who are more socially active or at employment age, experiencing a more noticeable beneficial change in tobacco exposure in restaurants and bars. It might be the case that hospitalised populations are a priori less severe health state than those at a more advanced stage of disease. Indeed, a differential pattern of effect by smoking ban was not observed in mortality estimates, which were based almost entirely on adults of retirement age (more than 90% of the total number of deaths) and who probably do not frequent hospitality venues regularly. Our results confirm that only more restrictive smoking bans can reduce direct or indirect tobacco exposure to sufficiently protect the population at large.
Strengths and limitations of the study
We believe our models are robust for several reasons. First, it is unlikely that they are sensitive to potential changes coinciding with the smoking bans but unrelated to them because we capitalised on a quasi-experimental setting that minimises this risk (smoking bans were implemented at different time-points across cantons). The null findings from the “fake” smoking ban analysis for most of the outcomes and in the regular analysis of outcomes not related to the smoking ban reinforce this assumption (sensitivity analyses SA4 and SA5). We further considered regional differences in health and socioeconomic profiles, with the inclusion of the corresponding scores in the meta-regression analysis. To prevent potential selection bias, we used routinely collected registry-based data, with nearly complete coverage of the population in all cantons. If there were any bias, our estimates would be an underestimation rather than an overestimation of the ban effect. The implementation of smoking bans in Switzerland entailed a long legislative process, with the introduction in some cantons of partial anti-tobacco initiatives preceding the federal smoke-free law or the more restrictive cantonal bans. To simplify our analysis, a unique date of smoking ban introduction, which corresponded to the time when the most comprehensive smoke-free law was implemented, was assigned for each canton. However, we believe that the potential effect of pre-ban initiatives on mortality and hospitalisations would have been captured in our estimates as well, given that most of them were introduced during the 2 years before the ban, which was considered in our analysis as control time. Further, exposure misclassification of place of residence and mobility between cantons (commuting due to work), probably produce an underestimation of effect. Similar underestimation is expected from nondifferential misclassification of diseases.
Of note, estimates for IHD were slightly sensitive to the inclusion of a nonlinear function of time. Barr et al. previously raised the issue of the necessity to adjust for potential nonlinear trends in order to prevent biased smoking ban estimates [36]. However, we consider the use of a linear time trend to be suitable given the short pre- and post-ban periods (2 years) used, as others have suggested [3]. Moreover, as we stratified analyses by canton, the use of nonlinear functions of time might have absorbed some of the effects of the smoking ban. We found significant heterogeneity among canton-specific smoking ban estimates for most of the causes of hospital admissions, although not for mortality. This different pattern might be related to coding differences associated with the health system of each canton. We acknowledge that our findings would be generalisable only to other countries with similar population characteristics, smoking habits and smoke-free laws. And finally, based on our findings, we cannot differentiate between the role of environmental tobacco smoke exposure at home and in public spaces, and further studies using tobacco monitoring data should be performed to better assess the contribution of each of the two main environmental tobacco smoke sources to the overall impact of the smoking ban.
Conclusions
Smoking bans in Switzerland were associated with reductions in cardiovascular and respiratory hospitalisation and mortality in adults. Our results also suggest that reduction in passive and active smoking is one of the most relevant predictors of the health impact of smoking bans, mainly for IHD. The enforcement of even more comprehensive bans at national level could contribute to reductions in passive and active tobacco exposure sufficient to benefit the whole population. Given the unique situation of smoking ban implementation in Switzerland, we were able to provide a more complete picture of the benefits, and simultaneously address several research questions by exploring the regional heterogeneity of the effects according to the degree of protection by the law and vulnerability profiles.
Appendix 1: Supplementary tables and figures
|
Table S1: Causes of hospitalisation or death (according to ICD-10). |
|
Population
Age
|
Main group
|
ICD-10 code
|
Subcategory
|
ICD-10 Code
|
| Adults ≥35 years |
Cardiovascular |
I |
Ischaemic heart diseases |
I20–I25 |
| Acute myocardial infarction |
I21 |
| STEMI |
I210–I213 |
| NSTEMI |
I214 |
| Unstable angina |
I20 |
| Chronic ischaemic heart disease |
I25 |
| Congestive heart failure |
I50 |
| Cerebrovascular |
I60–I69 |
| Hypertensive disorders |
I10–I15 |
| Respiratory |
J |
Chronic lower respiratory diseases |
J40–J47 |
| Chronic obstructive pulmonary disease |
J40–J44, J47 |
| Asthma |
J45–J46 |
| Pneumonia |
J12–J18 |
| External* |
V01–Y98 |
|
|
| Injuries* |
S00–S98 |
|
|
| Infants <1 year |
Total |
All |
Conditions originating in the perinatal period |
P00–P96 |
| Children
≤15 years |
Respiratory |
J |
Respiratory infections |
J00–J22 |
| ICD-10 = International Classification of Diseases Tenth Revision; NSTEMI = non-ST-elevation myocardial infarction; STEMI = ST-elevation myocardial infarction
* Outcomes not related to the smoking ban |
|
Table S2: Description of the gender- and age-specific series of mortality and hospital admissions in adults (≥35 years old) for the main groups of causes (data restricted to the period including 2 years before and 2 years after the introduction of the smoking ban in each canton). |
|
Cause of hospitalisation or death(ICD-10 codes in parenthesis)
|
Hospital admission
|
Mortality
|
|
N / %*
|
Monthly mean N
†
(SD)
|
N / %*
|
Monthly mean N
†
(SD)
|
|
Cardiovasculardiseases (I)
|
Total |
265 283 |
208.2 (214.0) |
87 833 |
68.9 (73.5) |
| Male |
55.7% |
115.9 (117.9) |
45.0% |
31.0 (33.0) |
| ≥65 years |
72.3% |
57.6 (58.7) |
92.6% |
63.8 (68.3) |
|
Ischaemic heart diseases (I20–I25)
|
Total |
69 829 |
54.8 (55.8) |
33 880 |
26.6 (30.2) |
| Male |
68.3% |
37.4 (38.2) |
52.6% |
14.0 (15.8) |
| ≥65 years |
60.7% |
33.2 (34.5) |
90.8% |
24.2 (27.7) |
|
Cerebrovascular diseases (I60–I69)
|
Total |
48 420 |
38.0 (41.0) |
15 578 |
12.2 (13.6) |
| Male |
53.2% |
20.2 (21.8) |
39.0% |
4.8 (5.5) |
| ≥65 years |
75.0% |
28.5 (31.2) |
94.4% |
11.5 (13.0) |
|
Hypertensive disorders (I10–I15)
|
Total |
20 049 |
15.7 (19.9) |
10 679 |
8.4 (9.4) |
| Male |
36.5% |
5.7 (7.5) |
33.2% |
2.8 (3.3) |
| ≥65 years |
78.9% |
12.4 (16.3) |
96.2% |
8.1 (9.1) |
|
Arrhythmia (I47–I49)
|
Total |
25 096 |
19.7 (21.1) |
2 873 |
2.3 (2.8) |
| Male |
50.8% |
10.0 (11.0) |
43.0% |
1.0 (1.4) |
| ≥65 years |
73.3% |
14.4 (15.7) |
92.8% |
2.1 (2.6) |
|
Congestive heart failure (I50)
|
Total |
37 663 |
29.6 (31.2) |
7 061 |
5.5 (6.0) |
| Male |
51.4% |
15.2 (15.9) |
35.9% |
2.0 (2.4) |
| ≥65 years |
90.1% |
26.6 (28.2) |
97.8% |
5.4 (5.9) |
|
Respiratory (J)
|
Total |
111 043 |
87.2 (95.8) |
15 142 |
11.9 (12.8) |
| Male |
55.4% |
48.3 (52.1) |
53.3% |
6.3 (7.0) |
| ≥65 years |
69.0% |
60.1 (66.8) |
92.7% |
11.0 (11.9) |
|
COPD (J40–J44, J47)
|
Total |
22 251 |
17.5 (20.5) |
7 308 |
5.7 (6.5) |
| Male |
57.4% |
10.0 (11.9) |
59.5% |
3.4 (4.1) |
| ≥65 years |
75.9% |
13.2 (15.7) |
91.9% |
5.3 (6.0) |
|
Asthma (J45–J46)
|
Total |
3 285 |
2.6 (3.5) |
406 |
0.3 (0.7) |
| Male |
31.3% |
0.8 (1.3) |
25.9% |
0.1 (0.3) |
| ≥65 years |
45.8% |
1.2 (1.8) |
88.4% |
0.3 (0.6) |
|
Pneumonia (J12–J18)
|
Total |
50 377 |
39.5 (43.7) |
4 911 |
3.9 (4.6) |
| Male |
56.8% |
22.5 (24.3) |
45.7% |
1.8 (2.3) |
| ≥65 years |
73.6% |
29.1 (32.3) |
95.1% |
37. (4.4) |
| COPD = chronic obstructive pulmonary disease; ICD-10 = International Classification of Diseases Tenth Revision; N = total number of cases; SD = standard deviation
* Relative frequency related to the total number of cases due each specific cause
† Mean monthly number of cases in each canton |
|
Table S3: Assessment of heterogeneity within canton-specific results included in the meta-analysis. |
|
Cause of hospitalisation or death(ICD-10 codes in parenthesis)
|
Hospital admission*
|
Mortality
|
|
I2
|
Test for heterogeneity
p-value
|
I2
|
Test for heterogeneity
p-value
|
|
Cardiovascular diseases (I)
|
13.5% |
0.04 |
0.0% |
0.79 |
|
Ischaemic heart diseases (I20–I25)
|
8.8% |
0.68 |
7.6% |
0.59 |
|
Cerebrovascular diseases (I60–I69)
|
45.1% |
<0.01 |
40.5% |
0.01 |
|
Hypertensive disorders (I10–I15)
|
63.1% |
<0.01 |
0.0% |
0.73 |
|
Arrhythmia (I47–I49)
|
26.5% |
0.08 |
0.0% |
0.67 |
|
Congestive heart failure (I50)
|
79.9% |
<0.01 |
0.0% |
0.78 |
|
Respiratory diseases (J)
|
42.0% |
0.02 |
0.0% |
0.58 |
|
COPD (J40–J44, J47)
|
22.2% |
0.29 |
0.0% |
0.63 |
|
Asthma (J45–J46)
|
14.0% |
0.06 |
– |
– |
|
Pneumonia (J12–J18)
|
53.1% |
<0.01 |
21.2% |
0.13 |
| COPD = chronic obstructive pulmonary disease; ICD-10 = International Classification of Diseases Tenth Revision
* Canton Aargau was excluded from the meta-analysis of cardiovascular diseases, ischaemic heart disease, cerebrovascular diseases; Canton Bern was excluded from the meta-analysis of hypertensive disorders, arrhythmia, congestive heart failure. |
|
Table S4: Gender- and age-specific percent estimates (and 95% confidence interval) in change in hospital admissions and mortality in adults (≥35 years old) due to the main groups of causes after the introduction of the smoking ban (data restricted to the period including 2 years before and 2 years after the introduction of the smoking ban in each canton). Results are displayed in figure 2.
|
|
Cause of hospitalisation or death
(ICD-10 codes in parenthesis)
|
Hospital admission*
|
Mortality
|
|
% change (95% CI)
|
p-value
|
p-value int†
|
% change (95% CI)
|
p-value
|
p-value int†
|
|
Cardiovascular diseases (I)
|
Total |
0.02
(−2.71 to 2.82) |
0.990 |
|
−0.72
(−3.67 to 2.31) |
0.623 |
|
| Male |
1.00
(−2.10 to 4.20) |
0.517 |
0.205 |
−3.22
(−7.32 to 1.07) |
0.133 |
0.171 |
| Female |
−2.03
(−5.43 to 1.49) |
0.242 |
|
1.19
(−3.43 to 6.03) |
0.606 |
|
| 35–64 years old |
0.14
(−3.56 to 3.99) |
0.938 |
0.940 |
−2.44
(−14.17 to 10.89) |
0.695 |
0.803 |
| ≥65 years old |
−0.06
(−3.81 to 3.84) |
0.974 |
|
−0.79
(−3.73 to 2.24) |
0.593 |
|
|
Ischaemic heart diseases (I20–I25)
|
Total |
−2.54
(−6.23 to 1.30) |
0.183 |
|
−1.38
(−5.98 to 3.45) |
0.555 |
|
| Male |
−1.09 (−5.84 to 3.91) |
0.651 |
0.588 |
−3.86
(−10.78 to 3.61) |
0.289 |
0.213 |
| Female |
−3.65
(−11.17 to 4.51) |
0.355 |
|
2.55
(−4.25 to 9.83) |
0.457 |
|
| 35–64 years old |
−5.46
(−10.76 to 0.16) |
0.056 |
0.173 |
−4.17
(−19.92 to 14.68) |
0.629 |
0.782 |
| ≥65 years old |
−0.44
(−5.00 to 4.35) |
0.848 |
|
−1.62
(−6.29 to 3.29) |
0.497 |
|
|
Cerebrovascular diseases (I60–I69)
|
Total |
0.78
(−6.55 to 8.69) |
0.834 |
|
−2.48
(−12.69 to 8.92) |
0.644 |
|
| Male |
2.83
(−5.94 to 12.41) |
0.525 |
0.489 |
−4.81
(−16.49 to 8.51) |
0.446 |
0.624 |
| Female |
−1.72
(−10.36 to 7.76) |
0.701 |
|
−0.22
(−12.79 to 14.16) |
0.973 |
|
| 35–64 years old |
8.87
(−0.60 to 19.24) |
0.066 |
0.071 |
|
|
|
| ≥65 years old |
−1.97
(−8.44 to 4.97) |
0.555 |
|
−4.14
(−12.89 to 5.49) |
0.372 |
|
|
Hypertensive disorders (I10–I15)
|
Total |
5.86
(−9.78 to 24.22) |
0.469 |
|
−5.43
(−12.55 to 2.27) |
0.154 |
|
| Male |
−3.83
(−20.79 to 16.77) |
0.682 |
0.273 |
−1.02
(−13.59 to 13.38) |
0.878 |
0.447 |
| Female |
10.49
(−5.33 to 28.94) |
0.195 |
|
−7.46
(−16.95 to 3.11) |
0.153 |
|
| 35–64 years old |
8.03
(−8.91 to 28.12) |
0.359 |
0.729 |
|
0.504 |
|
| ≥65 years old |
3.53
(−12.60 to 22.64) |
0.676 |
|
−4.65
(−11.63 to 2.87) |
0.208 |
|
|
Arrhythmia (I47–I49)
|
Total |
2.46
(−5.72 to 11.36) |
0.552 |
|
6.09
(−8.62 to 23.17) |
0.422 |
|
| Male |
9.54
(−5.40 to 26.84) |
0.212 |
0.116 |
7.73
(−15.73 to 37.71) |
0.538 |
0.877 |
| Female |
−4.03
(−11.03 to 3.51) |
0.273 |
|
5.33
(−9.04 to 21.97) |
0.473 |
|
| 35–64 years old |
−2.75
(−17.39 to 14.47) |
0.727 |
0.456 |
|
|
|
| ≥65 years old |
4.28
(−4.19 to 13.51) |
0.317 |
|
6.37
(−7.88 to 22.82) |
0.385 |
|
|
Congestive heart failure (I50)
|
Total |
−2.41
(−15.57 to 12.81) |
0.731 |
|
−5.98
(−14.47 to 3.37) |
0.192 |
|
| Male |
0.23
(−10.94 to 12.81) |
0.968 |
0.695 |
−11.13
(−24.40 to 4.47) |
0.145 |
0.366 |
| Female |
−4.27
(−21.42 to 16.62) |
0.652 |
|
−2.23
(−14.10 to 11.27) |
0.722 |
|
| 35–64 years old |
9.40
(−8.89 to 31.35) |
0.321 |
0.247 |
|
|
|
| ≥65 years old |
−4.67
(−17.49 to 10.13) |
0.501 |
|
−4.96
(−14.01 to 5.04) |
0.305 |
|
|
Respiratory diseases (J)
|
Total |
0.03
(−5.67 to 6.06) |
0.993 |
|
−8.20
(−15.18 to −0.64)
|
0.035
|
|
| Male |
−0.37
(−6.27 to 5.90) |
0.901 |
0.894 |
−6.31
(−16.82 to 5.53) |
0.270 |
0.753 |
| Female |
0.24
(−6.16 to 7.08) |
0.941 |
|
−9.12
(−21.54 to 5.28) |
0.193 |
|
| 35–64 years old |
5.19
(−2.02 to 12.93) |
0.154 |
0.119 |
|
0.334 |
|
| ≥65 years old |
−2.47
(−8.43 to 3.89) |
0.423 |
|
−7.38
(−14.81 to 0.69) |
0.070 |
|
|
COPD (J40–J44, J47)
|
Total |
−2.08
(−9.85 to 6.35) |
0.604 |
|
−13.86
(−22.27 to −4.54)
|
0.006
|
|
| Male |
−0.02
(−9.89 to 10.92) |
0.996 |
0.853 |
−13.50
(−28.03 to 3.96) |
0.117 |
0.966 |
| Female |
−1.74
(−15.50 to 14.26) |
0.812 |
|
−13.02
(−26.62 to 3.10) |
0.103 |
|
| 35–64 years old |
1.44
(−13.68 to 19.20) |
0.857 |
0.518 |
|
|
|
| ≥65 years old |
−4.34
(−11.17 to 3.02) |
0.229 |
|
−13.39
(−21.71 to −4.18)
|
0.007
|
|
|
Asthma (J45–J46)
|
Total |
7.26
(−13.42 to 32.87) |
0.506 |
|
|
|
|
| Male |
|
|
|
|
|
|
| Female |
12.31
(−13.68 to 46.12) |
0.372 |
|
|
|
|
| 35–64 years old |
|
|
|
|
|
|
| ≥65 years old |
|
|
|
|
|
|
|
Pneumonia (J12–J18)
|
Total |
0.22
(−8.92 to 10.28) |
0.962 |
|
−5.71
(−20.71 to 12.15) |
0.492 |
|
| Male |
−0.88
(−9.94 to 9.08) |
0.851 |
0.709 |
−8.41
(−26.62 to 14.31) |
0.422 |
0.710 |
| Female |
1.77
(−7.93 to 12.49) |
0.721 |
|
−3.22
(−19.77 to 16.74) |
0.722 |
|
| 35–64 years old |
6.00
(−7.50 to 21.47) |
0.386 |
0.358 |
|
|
|
| ≥65 years old |
−1.74
(−9.91 to 7.18) |
0.681 |
|
−3.79
(−19.78 to 15.38) |
0.665 |
|
| COPD = chronic obstructive pulmonary disease; ICD-10 = International Classification of Diseases Tenth Revision
* Canton Aargau was excluded from the meta-analysis of cardiovascular diseases, ischaemic heart disease, cerebrovascular diseases; Canton Bern was excluded from the meta-analysis of hypertensive disorders, arrhythmia, congestive heart failure.
† Test of interaction between the estimates of each gender- and age-category (z-score test).
Statistically significant results (p <0.05) are in bold type. |
|
Table S5: Gender- and age-specific estimates in change in risk (and 95% confidence interval) of hospital admission in adults (≥35 years old) due to acute myocardial infarction (total, with ST-elevation, without ST-elevation) after the introduction of the smoking ban (data restricted to period of 2 years before and 2 years after the introduction of the smoking ban in each canton). |
|
Cause of hospital admission
(ICD-10 codes in parentheses)
|
|
% change (95% CI)
|
p-value
|
|
Acute myocardial infarction (I21)
|
Overall |
−2.55 (−9.30 to 4.70) |
0.47 |
| Male |
−1.32 (−9.31 to 7.38) |
0.75 |
| Female |
−2.27 (−12.4 to 9.06) |
0.67 |
| 35–64 years |
−4.84 (−13.0 to 4.11) |
0.27 |
| ≥65 years |
−0.89 (−8.53 to 7.40) |
0.82 |
|
AMI STEMI (I210–I213)
|
Overall |
−4.76 (−11.2 to 2.15) |
0.16 |
| Male |
−7.25 (−13.2 to −0.95) |
0.03 |
| Female |
2.80 (−13. 6 to 22.3) |
0.75 |
| 35–64 years |
−6.14 (−15.1 to 3.73) |
0.20 |
| ≥65 years |
−2.05 (−10.2 to 6.85) |
0.63 |
|
AMI NSTEMI (I214)
|
Overall |
2.23 (−9.36 to 15.3) |
0.71 |
| Male |
8.26 (−7.81 to 27.1) |
0.32 |
| Female |
−5.74 (−19.6 to 10.5) |
0.45 |
| 35–64 years |
0.83 (−14.9 to 19.5) |
0.92 |
| ≥65 years |
1.92 (−9.66 to 15.0) |
0.75 |
| AMI = acute myocardial infarction; ICD-10 = International Classification of Diseases Tenth Revision; NSTEMI = non-ST-elevation myocardial infarction; STEMI = ST-elevation myocardial infarction
Canton Aargau was excluded from the meta-analysis. |
|
Table S6: Description of the children included and estimates of change in risk after the introduction of the smoking ban in infant mortality (<1 year of age) and respiratory hospital admission in children (≤15 years) (series restricted to period of 2 years before and 2 years after the introduction of the smoking ban in each canton). |
|
Cause of hospitalisation or death
(ICD-10 codes in parenthesis)
|
N / %*
|
Monthly mean N
†
(SD)
|
% change
(95% CI)
|
p-value
|
|
Infant mortality (all)
|
1 160 |
1.4 (1.9) |
−16.6
(−42.5 to 21.1) |
0.32 |
| Due to perinatal conditions (P00–P96) |
57.5% |
0.8 (1.3) |
−6.7
(−39.2 to 43.4) |
0.74 |
|
Children respiratory hospital admission (J)
|
35 271 |
27.7 (36.7) |
3.6
(−7.2 to 15.5) |
0.52 |
| Respiratory infections (J00–J22) |
83.2% |
23.1 (31.7) |
2.7
(−9.7 to 16.7) |
0.68 |
| CI: confidence interval; ICD-10 (International Classification of Diseases Tenth Revision) SD: standard deviation,
† Relative frequency related to the total number of cases due to each specific cause
* Mean monthly number of cases in each canton
Infant mortality: excluded cantons (AI, AR, GL, JU, NW, OW, SH, TG, UR, ZG) because mean monthly count below 0.5. Remaining cases accounting for 93.5% in total, 95.5% in only due to perinatal conditions. |
|
Table S7: Change (and 95% confidence interval) in hospital admissions and mortality due to ischaemic heart diseases and chronic obstructive respiratory diseases in adults (≥35 years old) after the introduction of the smoking ban, by type of law and smoking profile of the canton (adjusted for the socioeconomic and health profile of the population). Results shown in figure 3. |
|
Hospital admissions
|
Mortality
|
|
% change
(95% CI)
|
p-value int*
|
% change
(95% CI)
|
p-value int*
|
|
Ischaemic heart diseases
|
Overall |
−2.54
(−6.23 to 1.30) |
0.18 |
−1.38
(−5.98 to 3.45) |
0.56 |
| Type of law |
Federal law |
0.49
(−8.60 to 10.5) |
0.54 |
−5.94
(−14.72 to 3.75) |
0.26 |
| Fed law + restr |
−3.13
(−8.19 to 2.21) |
|
1.19
(−5.23 to 8.06) |
|
| Change in active smoking |
Increase or equal |
0.26 (−5.11 to 5.93) |
0.20 |
−0.92
(−7.37 to 5.98) |
0.89 |
| Decrease |
−6.90
(−14.4 to 1.23) |
|
−1.92
(12.34 to 9.74) |
|
| Decrease in ETS exposure |
Below or equal median |
2.30
(−3.26 to 8.18) |
0.04 |
4.81
(−1.91 to 12.0) |
0.02 |
| Above median |
−6.34
(−11.2 to −1.21) |
|
−6.81
(−12.20 to −1.08) |
|
|
COPD
|
Overall |
−2.08
(−9.85 to 6.35) |
0.60 |
−13.9
(−22.3 to −4.54) |
<0.01 |
| Type of Law |
Federal law |
9.14
(−7.05 to 28.2) |
0.17 |
−16.6
(−34.1 to 5.45) |
0.82 |
| Fed law + restr |
−5.34
(−13.7 to 3.83) |
|
−13.8
(25.2 to −0.70) |
|
| Change in active smoking |
Increase or equal |
−2.05
(−11.5 to 8.41) |
0.79 |
−15.6
(−27.1 to −2.30) |
0.81 |
| Decrease |
0.95
(−15.3 to 20.4) |
|
−12.1
(−32.3 to 14.1) |
|
| Decrease in ETS exposure |
Below or equal median |
−1.43
(−12.4 to 10.9) |
0.95 |
−16.8 (−29.8 to −1.43) |
0.69 |
| Above median |
−0.92
(−11.6 to 11.1) |
|
−12.8
(−25.3 to 1.87) |
|
| CI = confidence interval; COPD = chronic obstructive pulmonary disease; ETS = environmental tobacco smoke
Type of smoking ban: Federal law = cantons which followed only the federal smoking Ban; Fed. law + restr = cantons which applied additional restrictions to the federal smoking ban.
Change in active smoking: difference between the reported smoking prevalence in the Swiss Health Surveys of 2007 and 2012 (active smoker if positive answer to the question: “Do you usually smoke?”). Stratified by non-decrease vs decrease in prevalence.
Decrease in ETS exposure: difference between the reported ETS exposure in the Swiss Health Surveys of 2007 and 2012 (exposed to ETS if answering at least 1 hour to the question “How much time are you exposed to smoke everyday?”). Since there was always a decrease, cantons were classified according to whether the decrease was above or below the median value.
*Test of interaction (z-score) p-value. |
|
Table S8: Sensitivity analysis. |
|
Hospital admissions*
|
Mortality
|
|
% change
(95% CI)
|
p-value
|
% change
(95% CI)
|
p-value
|
|
Ischaemic heart disease
|
| Main |
−2.54
(−6.23 to 1.30) |
0.18 |
−1.38
(−5.98 to 3.45) |
0.55 |
| SA1 Control trend with control outcome |
−3.06
(−6.89 to 0.92) |
0.12 |
−0.20
(−5.08 to 4.93) |
0.94 |
| SA2 Study period 2005–2012 |
0.85
(−5.67 to 7.82) |
0.80 |
−1.86
(−4.98 to 1.36) |
0.24 |
| SA3 All cantons |
−1.14
(−5.93 to 3.89) |
0.64 |
na |
|
| SA4 SB 1 year before |
0.06
(−9.96 to 11.2) |
0.99 |
−4.66
(−10.3 to 1.28) |
0.12 |
|
COPD
|
| Main |
−2.08
(−9.85 to 6.35) |
0.60 |
−13.9
(−22.3 to −4.54) |
0.01 |
| SA1 Control trend with control outcome |
−1.39
(−9.01 to 6.85) |
0.72 |
−14.4
(−22.5 to −5.41) |
<0.01 |
| SA2 Study period 2005–2012 |
1.00
(−7.76 to 10.6) |
0.82 |
−5.78
(−10.9 to −0.38) |
0.04 |
| SA3 All cantons |
na |
|
na |
|
| SA4 SB 1 year before |
−19.8
(−32.8 to −4.28) |
0.02 |
−1.29 (−22.1 to 25.2) |
0.91 |
| SA5 Outcome not smoking-ban related†
|
1.29
(−2.14 to 4.84) |
0.45 |
−1.71
(−7.45 to 4.39) |
0.56 |
| CI = confidence interval; COPD = chronic obstructive pulmonary disease; na = not applicable; SB = smoking ban
SA1: include monthly count of control outcome to additionally adjust time trend; SA2: study period 2005–2012 (not restricted to 2 years before and after the introduction of the smoking ban); SA3: all cantons (canton Argau not excluded); SA4: Smoking ban introduced 1 year before (excluded canton of Ticino) and restricted to the period of 1 year before and 1 year after introduction of the smoking ban.
* Canton Aargau excluded for ischaemic heart disease
† Outcomes not smoking-ban related: deaths due to external causes (V01–Y98), hospital admission due to injuries (S00–S98). |
Figure S3
Forest plots of the overall and canton-specific results for ischaemic heart disease (IHD) and chronic obstructive pulmonary disease (COPD) hospitalisations and mortality in adults ≥35 years old after the introduction of the smoking ban. (Canton Argau was excluded from IHD hospital admission analysis.)
Figure S3
Forest plots of the overall and canton-specific results for ischaemic heart disease (IHD) and chronic obstructive pulmonary disease (COPD) hospitalisations and mortality in adults ≥35 years old after the introduction of the smoking ban. (Canton Argau was excluded from IHD hospital admission analysis.)
Figure S3
Forest plots of the overall and canton-specific results for ischaemic heart disease (IHD) and chronic obstructive pulmonary disease (COPD) hospitalisations and mortality in adults ≥35 years old after the introduction of the smoking ban. (Canton Argau was excluded from IHD hospital admission analysis.)
References
1 Hoffman SJ, Tan C. Overview of systematic reviews on the health-related effects of government tobacco control policies. BMC Public Health. 2015;15(1):744. doi:http://dx.doi.org/10.1186/s12889-015-2041-6.
2 Jones MR, Barnoya J, Stranges S, Losonczy L, Navas-Acien A. Cardiovascular Events Following Smoke-Free Legislations: An Updated Systematic Review and Meta-Analysis. Curr Environ Health Rep. 2014;1(3):239–49. doi:http://dx.doi.org/10.1007/s40572-014-0020-1.
3 Lee PN, Fry JS, Forey BA. A review of the evidence on smoking bans and incidence of heart disease. Regul Toxicol Pharmacol. 2014;70(1):7–23. doi:http://dx.doi.org/10.1016/j.yrtph.2014.06.014.
4 Mackay DF, Irfan MO, Haw S, Pell JP. Meta-analysis of the effect of comprehensive smoke-free legislation on acute coronary events. Heart. 2010;96(19):1525–30. doi:http://dx.doi.org/10.1136/hrt.2010.199026.
5 Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126(18):2177–83. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.112.121301.
6 Been JV, Nurmatov UB, Cox B, Nawrot TS, van Schayck CP, Sheikh A. Effect of smoke-free legislation on perinatal and child health: a systematic review and meta-analysis. Lancet. 2014;383(9928):1549–60. doi:http://dx.doi.org/10.1016/S0140-6736(14)60082-9.
7 Cox B, Vangronsveld J, Nawrot TS. Impact of stepwise introduction of smoke-free legislation on population rates of acute myocardial infarction deaths in Flanders, Belgium. Heart. 2014;100(18):1430–5. doi:http://dx.doi.org/10.1136/heartjnl-2014-305613.
8 Mackay D, Haw S, Ayres JG, Fischbacher C, Pell JP. Smoke-free legislation and hospitalizations for childhood asthma. N Engl J Med. 2010;363(12):1139–45. doi:http://dx.doi.org/10.1056/NEJMoa1002861.
9 Stallings-Smith S, Zeka A, Goodman P, Kabir Z, Clancy L. Reductions in cardiovascular, cerebrovascular, and respiratory mortality following the national irish smoking ban: interrupted time-series analysis. PLoS One. 2013;8(4):e62063. doi:http://dx.doi.org/10.1371/journal.pone.0062063.
10 Ye X, Yao Z, Gao Y, Xu Y, Xu Y, Zhu Z, et al. Second-hand smoke exposure in different types of venues: before and after the implementation of smoke-free legislation in Guangzhou, China. BMJ Open. 2014;4(2):e004273. doi:http://dx.doi.org/10.1136/bmjopen-2013-004273.
11 Tabuchi T, Hoshino T, Nakayama T. Are Partial Workplace Smoking Bans as Effective as Complete Smoking Bans? A National Population-Based Study of Smoke-Free Policy Among Japanese Employees. Nicotine Tob Res. 2016;18(5):1265–73. doi:http://dx.doi.org/10.1093/ntr/ntv115.
12 Zablocki RW, Edland SD, Myers MG, Strong DR, Hofstetter CR, Al-Delaimy WK. Smoking ban policies and their influence on smoking behaviors among current California smokers: a population-based study. Prev Med. 2014;59:73–8. doi:http://dx.doi.org/10.1016/j.ypmed.2013.11.018.
13 Troelstra SA, Bosdriesz JR, de Boer MR, Kunst AE. Effect of Tobacco Control Policies on Information Seeking for Smoking Cessation in the Netherlands: A Google Trends Study. PLoS One. 2016;11(2):e0148489. doi:http://dx.doi.org/10.1371/journal.pone.0148489.
14 WHO report on the global tobacco epidemic, 2015: raising taxes on tobacco. Geneva; World Health Organization: 2015.
15 Vicedo-Cabrera AM, Schindler C, Radovanovic D, Grize L, Witassek F, Dratva J, et al. Benefits of smoking bans on preterm and early-term births: a natural experimental design in Switzerland. Tob Control. 2016 Apr 26;pii:tobaccocontrol-2015-052739. doi: 10.1136/tobaccocontrol-2015-052739.
16 Dusemund F, Baty F, Brutsche MH. Significant reduction of AECOPD hospitalisations after implementation of a public smoking ban in Graubünden, Switzerland. Tob Control. 2015;24(4):404–7. doi:http://dx.doi.org/10.1136/tobaccocontrol-2013-051290.
17 Bonetti PO, Trachsel LD, Kuhn MU, Schulzki T, Erne P, Radovanovic D, et al. Incidence of acute myocardial infarction after implementation of a public smoking ban in Graubünden, Switzerland: two year follow-up. Swiss Med Wkly. 2011;141:w13206.
18 Trachsel LD, Kuhn MU, Reinhart WH, Schulzki T, Bonetti PO. Reduced incidence of acute myocardial infarction in the first year after implementation of a public smoking ban in Graubuenden, Switzerland. Swiss Med Wkly. 2010;140(9-10):133–8.
19 Di Valentino M, Muzzarelli S, Limoni C, Porretta AP, Rigoli A, Barazzoni F, et al. Reduction of ST-elevation myocardial infarction in Canton Ticino (Switzerland) after smoking bans in enclosed public places – No Smoke Pub Study. Eur J Public Health. 2015;25(2):195–9. doi:http://dx.doi.org/10.1093/eurpub/cku067.
20 Huang J, King BA, Babb SD, Xu X, Hallett C, Hopkins M. Sociodemographic Disparities in Local Smoke-Free Law Coverage in 10 States. Am J Public Health. 2015;105(9):1806–13. doi:http://dx.doi.org/10.2105/AJPH.2015.302655.
21 Stallings-Smith S, Goodman P, Kabir Z, Clancy L, Zeka A. Socioeconomic differentials in the immediate mortality effects of the national Irish smoking ban. PLoS One. 2014;9(6):e98617. doi:http://dx.doi.org/10.1371/journal.pone.0098617.
22 Khuder SA, Milz S, Jordan T, Price J, Silvestri K, Butler P. The impact of a smoking ban on hospital admissions for coronary heart disease. Prev Med. 2007;45(1):3–8. doi:http://dx.doi.org/10.1016/j.ypmed.2007.03.011.
23 Eisner MD, Iribarren C, Yelin EH, Sidney S, Katz PP, Sanchez G, et al. The impact of SHS exposure on health status and exacerbations among patients with COPD. Int J Chron Obstruct Pulmon Dis. 2009;4:169–76. doi:http://dx.doi.org/10.2147/COPD.S4681.
24 Cesaroni G, Forastiere F, Agabiti N, Valente P, Zuccaro P, Perucci CA. Effect of the Italian smoking ban on population rates of acute coronary events. Circulation. 2008;117(9):1183–8. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.107.729889.
25 Humair J-P, Garin N, Gerstel E, Carballo S, Carballo D, Keller P-F, et al. Acute respiratory and cardiovascular admissions after a public smoking ban in Geneva, Switzerland. PLoS One. 2014;9(3):e90417. doi:http://dx.doi.org/10.1371/journal.pone.0090417.
26 Agüero F, Dégano IR, Subirana I, Grau M, Zamora A, Sala J, et al. Impact of a partial smoke-free legislation on myocardial infarction incidence, mortality and case-fatality in a population-based registry: the REGICOR Study. PLoS One. 2013;8(1):e53722. doi:http://dx.doi.org/10.1371/journal.pone.0053722.
27 Christensen TM, Møller L, Jørgensen T, Pisinger C. The impact of the Danish smoking ban on hospital admissions for acute myocardial infarction. Eur J Prev Cardiol. 2014;21(1):65–73. doi:http://dx.doi.org/10.1177/2047487312460213.
28 Rajkumar S, Stolz D, Hammer J, Moeller A, Bauer GF, Huynh CK, et al. Effect of a smoking ban on respiratory health in nonsmoking hospitality workers: a prospective cohort study. J Occup Environ Med. 2014;56(10):e86–91. doi:http://dx.doi.org/10.1097/JOM.0000000000000262.
29 Frazer K, Callinan JE, McHugh J, van Baarsel S, Clarke A, Doherty K, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2016 [cited 2016 Mar 14]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005992.pub3/abstract
30 Stallings-Smith S, Zeka A, Goodman P, Kabir Z, Clancy L. Reductions in cardiovascular, cerebrovascular, and respiratory mortality following the national irish smoking ban: interrupted time-series analysis. PLoS One. 2013;8(4):e62063. doi:http://dx.doi.org/10.1371/journal.pone.0062063.
31 Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J. 2003;22(5):809–14. doi:http://dx.doi.org/10.1183/09031936.03.00031403.
32 Been JV, Millett C, Lee JT, van Schayck CP, Sheikh A. Smoke-free legislation and childhood hospitalisations for respiratory tract infections. Eur Respir J. 2015;46(3):697–706. doi:http://dx.doi.org/10.1183/09031936.00014615.
33 Fernández MF, Artacho-Cordón F, Freire C, Pérez-Lobato R, Calvente I, Ramos R, et al. Trends in children’s exposure to second-hand smoke in the INMA-Granada cohort: an evaluation of the Spanish anti-smoking law. Environ Res. 2015;138:461–8. doi:http://dx.doi.org/10.1016/j.envres.2015.03.002.
34 Kairouz S, Lasnier B, Mihaylova T, Montreuil A, Cohen JE. Smoking restrictions in homes after implementation of a smoking ban in public places. Nicotine Tob Res. 2015;17(1):41–7. doi:http://dx.doi.org/10.1093/ntr/ntu125.
35 Mons U, Nagelhout GE, Allwright S, Guignard R, van den Putte B, Willemsen MC, et al. Impact of national smoke-free legislation on home smoking bans: findings from the International Tobacco Control Policy Evaluation Project Europe Surveys. Tob Control. 2013;22(e1):e2–9. doi:http://dx.doi.org/10.1136/tobaccocontrol-2011-050131.
36 Barr CD, Diez DM, Wang Y, Dominici F, Samet JM. Comprehensive smoking bans and acute myocardial infarction among Medicare enrollees in 387 US counties: 1999-2008. Am J Epidemiol. 2012;176(7):642–8. doi:http://dx.doi.org/10.1093/aje/kws267.


