New concepts in the management of dyslipidaemia
DOI: https://doi.org/10.4414/smw.2016.14378
Baris
Gencer, Nicolas
Rodondi, Francois
Mach
Summary
Recently, the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) published a consensus paper giving guidance on the definition and management of statin-associated muscle symptoms (SAMS), as well as the use of proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors in very high-risk patients. The occurrence of SAMS can have a major negative impact on treatment adherence and, consequently, on the prognosis of cardiovascular diseases. In addition, both the ESC guidelines on the prevention of cardiovascular disease (CVD) in clinical practice with sections addressing global strategies to minimise the burden of CVD at population and individual levels, and the 2016 ESC/EAS guideline for the management of dyslipidaemias, focus on evaluation and treatment of SAMS. The release of these guidelines was a source of great interest to clinicians, as new emergent therapies, such as the PCSK9 inhibitors, have been approved for the treatment of dyslipidaemias: recently, both the US Food and Drugs Administration (FDA) and the European Medicines Agency (EMA) approved the use of PCSK9 inhibitors as add-ons for the treatment of hypercholesterolaemia in cases where low-density lipoprotein cholesterol (LDL-C) target levels could not be reached with maximum tolerated statin doses alone, or instead of statins in the event of SAMS. Because of the relatively high cost of these new therapies, physicians need to justify the use of PCSK9 inhibitors by demonstrating that their high-risk patients’ LDL-C levels have remained high (1) despite a well-conducted, but insufficiently effective high-intensity statin therapy (e.g. rosuvastatin 10–20 mg or atorvastatin 40–80 mg), or (2) in the event of the patient developing side effects, in particular severe SAMS, during treatment with at least three statins. In addition to SAMS, the use of PCSK9 inhibitors may be considered in patients with documented atherosclerotic cardiovascular disease or in patients with familial hypercholesterolaemia and poorly controlled LDL-C under the combination of maximum tolerated stain and ezetimibe.
Introduction
Statins are one of the most widely prescribed medications and the recommended first-line pharmacological therapy for the treatment of hypercholesterolaemia [1]. Statin therapy decreases low-density lipoprotein cholesterol (LDL-C) levels by up to 50%, and, subsequently, the incidence of cardiovascular events [2]. Large meta-analyses have shown that for each 1 mmol/l decrease in LDL-C levels, the relative risk of cardiovascular events is reduced by 20%, but with a larger absolute risk reduction among high-risk patients [3]. Therefore, guidelines recommend the use of appropriate clinical scores to identify patients who could most benefit from statin therapy, especially those at high risk of cardiovascular events [2, 4]. Although statins demonstrate long-term efficacy in reducing LDL-C levels, some concerns still remain regarding their safety. Although no significant differences in adverse event rates (including muscle symptoms) have been highlighted between statin and placebo arms in randomised controlled trials [5], in real-life data sources, such as registries, between 7 and 29% of patients complain of SAMS [6–8]. These are usually benign with normal or slightly increased creatine kinase (CK) concentrations; less than 1% (1 per 1000 to 1 per 10 000 per year) develop a serious adverse event in the form of myositis and CK elevation >10× the upper limit of normal (ULN) [9]. The advent of SAMS also depends on statin dosage and the presence of other risk factors [10].
Recently, the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) published a consensus paper giving guidance on the definition and management of SAMS [11]. In addition, both the ESC guidelines on the prevention of cardiovascular disease (CVD) in clinical practice with sections addressing global strategies to minimise the burden of CVD at population and individual levels [12], and the 2016 ESC/ EAS guideline for the management of dyslipidaemias, focus on evaluation and treatment of SAMS [13]. The release of these guidelines was a source of great interest to clinicians, as new emergent therapies, such as proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors, have been approved for the treatment of dyslipidaemias [14]. The publication of guidelines is especially important for clinicians faced with controversial situations, such as the use of statin therapy in primary prevention, but they are also meant to help to provide more consistent care based on available evidence.
Evidence on the management of lipid disorders is currently developing fast after recent findings with nonstatin agents, such as jejunal NPC1L1 protein blockers (ezetimibe) [15] or the novel PCSK9 inhibitors [16]. Nonstatin agents may come to play an increasingly important role as add-ons to statins to further decrease LDL-C levels, or even as substitutes for statins in the case of statin intolerance [14]. This is especially true in secondary prevention after acute coronary syndrome (ACS), where the recommended LDL-C target is very stringent (≤1.8 mmol/l; ≤70 mg/dl), or in patients with SAMS [17]. In the light of these new developments, this review aims to provide an update for clinicians about the main issues surrounding SAMS and their impact on the choice and usage of current and future therapies.
Description of statin-associated muscle symptoms
SAMS include a wide range of heterogeneous clinical presentations (myalgia, myopathy and rhabdomyolysis), reflected by the variety of definitions in the literature and the absence of a “gold standard” diagnostic test (table 1) [11]. Symptoms such as muscle pain or aching, stiffness, tenderness, weakness or cramp (also referred to as myalgia), possibly associated with statin use, occur mainly without an elevation of CK and are usually symmetrical [18, 19]. In addition, the occurrence of subclinical muscle disturbances (asymptomatic) has been described with the use of statins in observational studies, but the clinical long-term consequence remains still unclear [20–22]. The absence of a dedicated questionnaire in randomised controlled trials and of placebo controls in observational studies represent methodological limitations that still make it difficult to determine any causal inference between statin use and muscle symptoms.
In clinical practice, the temporal association of SAMS with statin initiation, discontinuation and rechallenge can help to make a clinical diagnosis. The large majority of patients who present SAMS are able to tolerate another statin for at least 12 months after switching, suggesting that the causes of SAMS may not be only due, or generalisable, to statins [6]. However, the occurrence of prolonged muscular damage might prevail, limiting the possibility of statin re-exposure. In addition, elderly patients have other comorbidities including osteoarticular disorders that can mimic the musculoskeletal symptoms observed with statins [23]. Serious adverse events, such as SAMS associated with CK elevations >10-fold, are referred to as myopathy and their incidence is approximately 1 per 10 000 patients per year with a standard statin dose (e.g., simvastatin 40 mg daily) [9]. The risk, however, varies among statins, and increases with higher statin doses and relative to patient characteristics (age >80 years, female, low body mass index) [10]. Rhabdomyolysis, occurring in about 1 in 100 000 statin-treated patients per year, is a severe form of muscle damage associated with very high CK levels, myoglobinaemia and/or myoglobinuria, and a concomitantly increased risk of renal failure [9].
|
Table 1:Classification of statin-associated muscle symptoms proposed by the EAS/ESC Consensus Panel [11]. |
|
Group
|
Symptoms
|
Biomarker
|
Comment
|
Association with statin
|
| Myalgia |
None |
CK <4 × ULN |
Raised CK found incidentally |
May be related to statin therapy, check thyroid function or if exercise-related. |
| None |
CK >4 × ULN |
Small asymptomatic rises in CK have been observed. |
Clinical significance is unclear. |
| Muscle symptoms |
Normal CK |
Often called “myalgia”. |
May be related to statin therapy. Causality is uncertain. |
| Muscle symptoms |
CK <10 × ULN |
Minor elevations of CK in the context of muscle symptoms are commonly due to increased exercise or physical activity. |
May be statin related or indicate an increased risk for severe underlying muscle problems. |
| Myopathy |
Muscle symptoms |
CK >10 × ULN |
Often called “myopathy” by regulatory agencies. Pain is typically generalised and proximal with muscle tenderness and weakness. |
An excess with usual statin dose about 1 per 10 000 per year. May be associated with underlying muscle disease. |
| Rhabdomyolysis |
Muscle symptoms |
CK >40 × ULN |
Rhabdomyolysis when associated with renal impairment and/or myoglobinuria. |
Statin therapy should not be reintroduced. |
| CK = creatinine kinase; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; ULN = upper limit of normal. |
Impact of statin-associated muscle symptoms on current therapy
In 2013, the American Heart Association (AHA) / American College of Cardiology (ACC) recommended tailoring statin treatment intensity with lower doses in patients at risk of side effects, such as elderly patients or those taking drugs that might interact with statins (e.g., patients with human immunodeficiency virus or immunosuppressant therapies) [2]. In patients taking immunosuppressants, such as after transplantation, or in patients with chronic kidney disease, the risk of developing dyslipidaemia is increased, but the evidence for the benefit of statins is limited, given the lack of data. SAMS could have a huge impact on the continuation of therapy, as it remains a frequent reason (up to 75%) for patients or physicians to opt for treatment discontinuation within 2 years of statin therapy initiation [24, 25]. Such nonadherence is associated with poorer clinical outcomes, as suggested by higher mortality in secondary prevention [26]. However, the discontinuation of statin therapy is a source of concern mostly for the group of patients at low risk (e.g., primary prevention) or those presenting a benign condition, such as myalgia without CK elevation. The fear of side effects, a negative “conviction” or image of statin therapy among the general population, or even physicians, can all contribute to statin therapy discontinuation and are probably the most common factors that impact current statin therapy decisions [27]. For most cases a rechallenge with another statin at a lower dose is well tolerated [6]. Given the absence of alternative lipid-lowering agents with comparable efficacy, the occurrence of SAMS could be having a major negative impact on the treatment of hypercholesterolaemia, and thereby on the risk of developing CVD [11]. In this regard, the physician’s role is especially important to best manage SAMS and encourage, where possible, the use of this efficacious and well-documented therapy for the prevention and treatment of CVD.
Management of statin-associated muscle symptoms
Recently, the ESC and EAS published a consensus paper providing guidance on the definition and management of SAMS [11]. In the event of SAMS, a critical approach is needed to exclude secondary causes (hypothyroidism and other common myopathies, such as polymyalgia rheumatic or intensive physical activity). Patients should be screened for other agents associated with muscle side effects, drug-drug interactions or polymedication. After excluding secondary causes or predisposing factors for SAMS, a careful assessment of the reason why a statin was prescribed, including its risk/benefit ratio, is recommended (fig. 1). Patients in secondary prevention clearly benefit from statin therapy and in this case priority should be given to statin continuation/reinitiation strategies to overcome muscle symptoms.
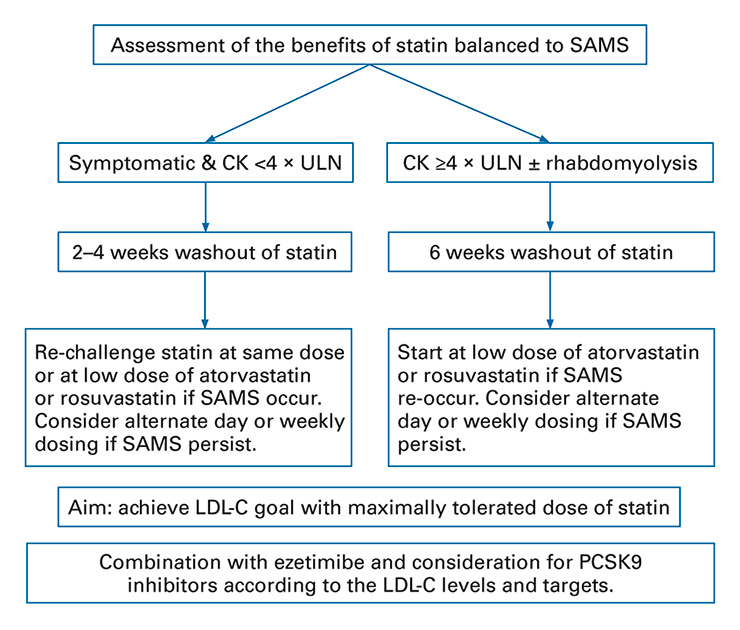
Figure 1
Clinical management of statin-associated muscle symptoms based on the EAS/ESC consensus panel guidelines.
CK = creatinine kinase; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin kexin 9; SAMS = statin-associated muscle symptoms; ULN = upper limit of normal. Algorithm based on the EAS/ESC consensus document [11].
All statins have a common mechanism of action, but each has a specific chemical structure, pharmacokinetic properties and lipid-lowering efficacy. Atorvastatin, simvastatin and fluvastatin are relatively lipophilic, whereas pravastatin and rosuvastatin are more hydrophilic [28]. Hydrophilic statins have greater hepatoselectivity with respect to hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, an important property given that the majority of cholesterol is produced in the liver [28]. Statins are predominantly metabolised by the cytochrome P450 (CYP450) family of enzymes, including the CYP3A4 isoenzyme that metabolises the greatest number of drugs in humans [29]. Lipophilic drugs are known to be much more susceptible to oxidative metabolism by the CYP450 system and are more likely to produce muscle toxicity because of the risk of interactions with many drugs that inhibit CYP450 [28]. In cases where SAMS are associated with CK levels <4 × ULN, withdrawal followed by one or more rechallenges can help determine SAMS causality. Rechallenge strategies include the use of an alternative statin (up to at least three different types of statin), a statin at a lower dose, or low doses of rosuvastatin or atorvastatin on alternate days. If the LDL-C goal is not achieved with the maximum tolerated statin dose (despite rechallenge with at least three types of statin), the addition of ezetimibe or PCSK9 inhibitors may be considered.
For SAMS associated with CK >4 × ULN, statin therapy should be interrupted but rechallenge strategies, as described above, should be attempted in high-risk patients with concomitant monitoring, unless the CK concentration is >10 × ULN. In this case, statin treatment should be stopped to prevent any risk of rhabdomyolysis, and renal damage and secondary causes should be sought. In the absence of rhabdomyolysis, rechallenge with lower doses of a potent statin (atorvastatin or rosuvastatin) can be considered after normalisation of CK. In the event of rhabdomyolysis, intravenous hydration and urine alkalisation are recommended, and statin therapy should be stopped. If the LDL-C goal is not achieved with the maximum tolerated statin dosage, the addition of ezetimibe or PCSK9 inhibitors should be considered [15, 17]. Patients who present SAMS recurrence after the use of at least three different statins should be referred to a specialist. Of note, red yeast rice (Monascus purpureus) is a fermented product that has been shown to reduce LDL-C levels by 20% [30]. However, the evidence is poor on the reduction of cardiovascular events and well-designed randomised controlled trials are needed, especially regarding SAMS, as red yeast rice has a statin-like effect.
Impact of statin-associated muscle symptoms on the use of PCSK9 inhibitors
PCSK9 inhibitors have been intensively studied over recent years in phase III clinical studies showing dramatic decreases in LDL-C levels in patients intolerant to statins, or on top of the maximum tolerated dose of statin [14, 31]. The development of new lipid-lowering therapies was urgently needed for patients at high risk of cardiovascular events or those with familial hypercholesterolaemia, as these patients frequently present poorly controlled LDL-C levels despite treatment, mainly high-intensity statin therapy [32, 33]. The US Food and Drugs Administration (FDA) and European Medicines Agency (EMA) have approved the use of PCSK9 inhibitors in the treatment of hypercholesterolaemia if target LDL-C levels could not be reached despite a maximum tolerated statin dose or in the case of intolerance to statins [34]. This decision was based on two trials showing that PCSK9 inhibitors added to maximum tolerated statin doses can further decrease LDL-C by up to 60%; in both trials, the impact of LDL-C reduction appeared to have a statistical impact on clinical events, although neither of these trials was designed to measure this [35, 36]. In advance of large outcome randomised controlled trials, a recent meta-analysis reviewing the effects of PCSK9 in adults with hypercholesterolaemia reported a reduction in cardiovascular mortality by 55% (odds ratio 0.45, 95% confidence interval 0.23–0.86, p = 0.015), although currently the number of reported cardiovascular deaths is small [37]. PCSK9 clinical trials have mainly enrolled patients who had poorly controlled LDL-C despite maximum tolerated statin doses [14]. In Switzerland, the use of evolocumab (Repatha®) has been recently approved by the Swiss Agency for Therpeutic Products (Swissmedic), with specific criteria defining the patients who would need aggressive lipid-lowering therapy. The costs of these agents will be a critical issue for payers and clinicians, as well as the need to focus them on high-risk patients with poor LDL-C control [38]. It is estimated that the number neede to treat to save one cardiovascular event over 5 years is 28, with average treatment costs expected to be between 7000 and 8000 Euro/year [38]. As observed with some expensive emergent agents in the field of oncology, physicians will need to justify the use of PCSK9 inhibitors according to the LDL-C levels of their high-risk patients, as well as documentation of (1) well-conducted statin therapy of high intensity (e.g., rosuvastatin 10–20 mg or atorvastatin 40–80 mg), or (2) the impossibility to prescribe recommended statin therapy due to side effects, especially SAMS [39]. However, it has been estimated that at least 50% of patients considered as “statin intolerant” were in fact tolerant of lower or intermittent dosing strategies with statin rechallenge. Therefore, the ESC panel recommended monitoring the true incidence of statin intolerance to detect a possible risk if overdiagnosis of SAMS.
|
Table 2:Very high-risk patient groups for whom PCSK9 inhibitors may be considered, according to the ESC/EAS panel. |
|
Patient group
|
Previous treatment
|
Criteria for consideration of PCSK9 inhibition
|
| ASCVD, diabetes with target organ damage or major risk factor (smoking, FH or marked hypertension) |
Maximum tolerated efficacious statin (atorvastatin or rosuvastatin) + ezetimibe |
• LDL-C >3.6 mmol/l (140 mg/dl).
• Rapid progression of ASCVD within 5 years and LDL-C >2.6 mmol/l (100 mg/dl). |
| Severe FH without ASCCVD |
Maximum tolerated efficacious statin (atorvastatin or rosuvastatin) + ezetimibe |
• LDL-C >5.0 mmol/l (200 mg/dl)
• ≥1 additional risk factor indicative of very high risk (elevated Lp(a), marked hypertension, diabetes and premature ASCVD) and LDL-C >4.5 mmol/l. |
| Statin intolerant |
Ezetimibe |
• See above. |
| ASCVD = atherosclerotic cardiovascular disease; EAS = European Atherosclerosis Society; ESC = European Society of Cardiology; FH = familial hypercholesterolaemia; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase kexin subtilisin 9 |
New guidance for the rational use of PCSK9 inhibitors
Recently, a joint consensus statement from the EAS and ESC provided practical guidance for the use of PCSK9 inhibitors in very high cardiovascular risk patients, such as those with atherosclerotic cardiovascular disease, familial hypercholesterolaemia and statin intolerance. This statement takes an initial position to define criteria for consideration of PCSK9 inhibition pointing out the lack of strong evidence regarding the impact on clinical outcomes and the uncertainties regarding cost-effectiveness.
The aim of the ESC/EAS consensus statement was to ensure appropriate patient pretreatment before consideration of PCSK9 inhibition (table 2). The proposed algorithms recommend identifying very high-risk patients who would probably benefit from PCSK9 inhibition via an approach lowering LDL-C by at least 50% (e.g., from >3.6 mmol/l in secondary prevention or from >5.0 mmol/l in severe familial hypercholesterolaemia) and consequently reducing significantly absolute risk, while also taking into account the costs of these innovative treatments and financial restraints on healthcare budgets. This document defined very high-risk patients as those with atherosclerotic cardiovascular disease (ASCVD), accelerated ASCVD, or famial hypercholesterolaemia without ASCVD, who are likely to have an absolute risk reduction of more than 2%/year. These estimates are, of course, based on limited data and the recommendations will need to be updated once the effects on clinical outcomes are clarified in ongoing clinical trials. The task force also recognised that in ASCVD patients with additional high absolute risk, the use of PCSK9 inhibitors may be considered with lower LDL-C concentrations of between 2.6 and 3.4 mmol/l on an individual basis and the clinician’s judgement of the absolute cardiovascular risk.
However, there are several gaps in the current evidence for the use of PCKS9 monoclonal antibody therapy: (1) impact on regression versus progression of atherosclerotic plaque; (2) impact on cardiovascular outcome; (3) long-term safety, including neurocognitive and immunogenic effects; (4) lower and upper age limiss for treatment; (5) cost-effectiveness in patients populations at different levels of cardiovascular risk (e.g., coronary disease with comorbidities such as moderate to severe chronic kidney disease).
Ezetimibe inhibits intestinal uptake of dietary and biliary cholesterol and has the effect of lowering LDL-C levels by 20% in monotherapy or in addition to statin. In the IMPROVE-IT trial, (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) the addition of ezetimibe to standard treatment with simvastatin among 18 144 patients after an acute coronary syndrome (ACS) reduced the occurrence of cardiovascular events after 5 years (32.7% vs 34.7%, p = 0.016), as well as the LDL-C levels (1.8 mmol/l vs 1.4 mmol/l) [40]. The findings from the IMPROVE-IT trial support the use of ezetimibe as a second-line therapy if LDL-C target levels are not reached with maximum tolerated statin therapy, and also as a necessary therapy before the use of PCSK9 inhibitors is considered. Therefore, a patient at very high risk with poorly controlled LDL-C can be treated with three different pharmacological agents (statins, ezetimibe and PCSK9 inhibitors) to reach the recommended LDL-C targets.
Table 3 summarises LDL-C targets according to risk group.
|
Table 3:Recommended treatment goals for low-density lipoprotein cholesterol. |
| Risk categories |
LDL-C goal |
| Very high risk (>10% SCORE risk) |
<1.8 mmol/l or a reduction of ≥50% if baseline is between 1.8 and 3.5 mmol/l. |
| High risk (5–10% SCORE risk) |
<2.5 mmol/l or a reduction of ≥50% if baseline is between 2.5 and 4.9 mmol/l. |
| Low (<1% SCORE risk) or moderate (1–5% SCORE risk) |
<3.0 mmo/l |
| LDL-C = low-density lipoprotein cholesterol; SCORE = Systematic COronary Risk Evaluation |
Situation in Switzerland in secondary prevention
Recent data on patients in Switzerland who presented ACS suggested that the prevalence of familial hypercholesterolaemia was high (about 20%), especially in patients with premature ACS fo whom it was up to 50% [41, 42]. The achievement of lipid targets was poor, as only one third of patients with ACS had an LDL-C level <1.8 mmol/l and even fewer patients with familial hypercholesterolemia (less than 10%), although 70% were treated with high-intensity statin therapy. Based on the available data, 10–20% of patients could have an indication for a PCSK9 inhibitor after a coronary event. Identifying patients with familial hypercholesterolaemia with the use of a clinical score, such as the Dutch classification, can help physicians to select the patients who will need aggressive lipid-lowering therapies [43].
Conclusion
The occurrence of SAMS can affect adherence to statin therapy and, consequently, the prognosis of CVD. The EAS/ESC consensus panel has recently published a consensus paper on the definition and management of SAMS, as summarised in this review, as well as guidance for the use of PCSK9 inhibitors in very high-risk patients. Treatment of lipid disorders is entering a new area with the recent approval of evolocumab. PCSK9 inhibitors provide a novel therapeutic option for patients who present SAMS and uncontrolled LDL-C levels. More data will be needed to clarify the impact of new treatment opportunities on adherence and achievement of lipid targets in real life, including the eligibility of patients for PCSK9 inhibitors. It will be very important to identify which patients with SAMS can really benefit from PCSK9 inhibitors, and in which cases the switch to another statin may be sufficient to resolve SAMS.
Acknowledgement: Special gratitude is expressed to Aliki Buhayer (Prism Scientific Sàrl) for medical writing support.
References
1 Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al.; European Association for Cardiovascular Prevention & Rehabilitation; ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–818. doi:http://dx.doi.org/10.1093/eurheartj/ehr158. PubMed
2 Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25, Suppl 2):S1–45. doi:http://dx.doi.org/10.1161/01.cir.0000437738.63853.7a. PubMed
3 Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, et al., Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385(9976):1397–405. doi:http://dx.doi.org/10.1016/S0140-6736(14)61368-4. PubMed
4 Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al.; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33(13):1635–701. doi:http://dx.doi.org/10.1093/eurheartj/ehs092. PubMed
5 Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al.; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi:http://dx.doi.org/10.1056/NEJMoa0807646. PubMed
6 Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–34. doi:http://dx.doi.org/10.7326/0003-4819-158-7-201304020-00004. PubMed
7 Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients – the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–14. doi:http://dx.doi.org/10.1007/s10557-005-5686-z. PubMed
8 Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB, Mittleman MA. Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med. 2012;125(2):176–82. doi:http://dx.doi.org/10.1016/j.amjmed.2011.08.007. PubMed
9 Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97(8A):52C–60C. doi:http://dx.doi.org/10.1016/j.amjcard.2005.12.010. PubMed
10 Mancini GB, Tashakkor AY, Baker S, Bergeron J, Fitchett D, Frohlich J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Cardiol. 2013;29(12):1553–68. doi:http://dx.doi.org/10.1016/j.cjca.2013.09.023. PubMed
11 Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al.; European Atherosclerosis Society Consensus Panel. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012–22. doi:http://dx.doi.org/10.1093/eurheartj/ehv043. PubMed
12 Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al.; Authors/Task Force Members. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81. doi:http://dx.doi.org/10.1093/eurheartj/ehw106. PubMed
13 Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al.; Authors/Task Force Members; Additional Contributor. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS)Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016 August 27. [Epub ahead of print] PubMed
14 Gencer B, Lambert G, Mach F. PCSK9 inhibitors. Swiss Med Wkly. 2015;145:w14094. PubMed
15 Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al.; IMPROVE-IT Investigators. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387–97. doi:http://dx.doi.org/10.1056/NEJMoa1410489. PubMed
16 Shimada YJ, Cannon CP. PCSK9 (Proprotein convertase subtilisin/kexin type 9) inhibitors: past, present, and the future. Eur Heart J. 2015;36(36):2415–24. doi:http://dx.doi.org/10.1093/eurheartj/ehv174. PubMed
17 Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al.; Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. doi:http://dx.doi.org/10.1093/eurheartj/ehv320. PubMed
18 Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168(1):6–15. doi:http://dx.doi.org/10.1016/j.ahj.2014.03.019. PubMed
19 Parker BA, Capizzi JA, Grimaldi AS, Clarkson PM, Cole SM, Keadle J, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127(1):96–103. doi:http://dx.doi.org/10.1161/CIRCULATIONAHA.112.136101. PubMed
20 Draeger A, Monastyrskaya K, Mohaupt M, Hoppeler H, Savolainen H, Allemann C, et al. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. J Pathol. 2006;210(1):94–102. doi:http://dx.doi.org/10.1002/path.2018. PubMed
21 Mohaupt MG, Karas RH, Babiychuk EB, Sanchez-Freire V, Monastyrskaya K, Iyer L, et al. Association between statin-associated myopathy and skeletal muscle damage. Can Med Assoc J. 2009;181(1–2):E11–18.
22 Draeger A, Sanchez-Freire V, Monastyrskaya K, Hoppeler H, Mueller M, Breil F, et al. Statin therapy and the expression of genes that regulate calcium homeostasis and membrane repair in skeletal muscle. Am J Pathol. 2010;177(1):291–9. doi:http://dx.doi.org/10.2353/ajpath.2010.091140. PubMed
23 Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA; The National Lipid Association’s Muscle Safety Expert Panel. An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8(3, Suppl):S58–71. doi:http://dx.doi.org/10.1016/j.jacl.2014.03.004. PubMed
24 Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–7. doi:http://dx.doi.org/10.1001/jama.288.4.462. PubMed
25 Gencer B, Rodondi N, Auer R, Räber L, Klingenberg R, Nanchen D, et al. Reasons for discontinuation of recommended therapies according to the patients after acute coronary syndromes. Eur J Intern Med. 2015;26(1):56–62. doi:http://dx.doi.org/10.1016/j.ejim.2014.12.014. PubMed
26 Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–8. doi:http://dx.doi.org/10.1093/eurheartj/eht295. PubMed
27 Finegold JA, Manisty CH, Goldacre B, Barron AJ, Francis DP. What proportion of symptomatic side effects in patients taking statins are genuinely caused by the drug? Systematic review of randomized placebo-controlled trials to aid individual patient choice. Eur J Prev Cardiol. 2014;21(4):464–74. doi:http://dx.doi.org/10.1177/2047487314525531. PubMed
28 Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–25. doi:http://dx.doi.org/10.1111/j.1472-8206.2004.00299.x. PubMed
29 Bottorff M, Hansten P. Long-term safety of hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors: the role of metabolism-monograph for physicians. Arch Intern Med. 2000;160(15):2273–80. doi:http://dx.doi.org/10.1001/archinte.160.15.2273. PubMed
30 Mannarino MR, Ministrini S, Pirro M. Nutraceuticals for the treatment of hypercholesterolemia. Eur J Intern Med. 2014;25(7):592–9. doi:http://dx.doi.org/10.1016/j.ejim.2014.06.008. PubMed
31 Gencer B, Laaksonen R, Buhayer A, Mach F. Use and role of monoclonal antibodies and other biologics in preventive cardiology. Swiss Med Wkly. 2015;145:w14179. PubMed
32 Gencer B, Mach F. Sweetless’n low LDL-C targets for PCSK9 treatment. Eur Heart J. 2015;36(19):1146–8. doi:http://dx.doi.org/10.1093/eurheartj/ehv056. PubMed
33 Santos RD, Watts GF. Familial hypercholesterolaemia: PCSK9 inhibitors are coming. Lancet. 2015;385(9965):307–10. doi:http://dx.doi.org/10.1016/S0140-6736(14)61702-5. PubMed
34 Everett BM, Smith RJ, Hiatt WR. Reducing LDL with PCSK9 Inhibitors – The Clinical Benefit of Lipid Drugs. N Engl J Med. 2015;373(17):1588–91. doi:http://dx.doi.org/10.1056/NEJMp1508120. PubMed
35 Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al.; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9. doi:http://dx.doi.org/10.1056/NEJMoa1500858. PubMed
36 Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al.; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. doi:http://dx.doi.org/10.1056/NEJMoa1501031. PubMed
37 Navarese EP, Kolodziejczak M, Schulze V, Gurbel PA, Tantry U, Lin Y, et al. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(1):40–51. doi:http://dx.doi.org/10.7326/M14-2957. PubMed
38 Tice JA, Kazi DS, Pearson SD. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors for Treatment of High Cholesterol Levels: Effectiveness and Value. JAMA Intern Med. 2016;176(1):107–8. doi:http://dx.doi.org/10.1001/jamainternmed.2015.7248. PubMed
39 Shrank WH, Barlow JF, Brennan TA. New Therapies in the Treatment of High Cholesterol: An Argument to Return to Goal-Based Lipid Guidelines. JAMA. 2015;314(14):1443–4. doi:http://dx.doi.org/10.1001/jama.2015.10017. PubMed
40 Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al.; IMPROVE-IT Investigators. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387–97. doi:http://dx.doi.org/10.1056/NEJMoa1410489. PubMed
41 Nanchen D, Gencer B, Auer R, Räber L, Stefanini GG, Klingenberg R, et al. Prevalence and management of familial hypercholesterolaemia in patients with acute coronary syndromes. Eur Heart J. 2015;36(36):2438–45. doi:http://dx.doi.org/10.1093/eurheartj/ehv289. PubMed
42 Gencer B, Auer R, Nanchen D, Räber L, Klingenberg R, Carballo D, et al. Expected impact of applying new 2013 AHA/ACC cholesterol guidelines criteria on the recommended lipid target achievement after acute coronary syndromes. Atherosclerosis. 2015;239(1):118–24. doi:http://dx.doi.org/10.1016/j.atherosclerosis.2014.12.049. PubMed
43 Gencer B, Nanchen D. Identifying familial hypercholesterolemia in acute coronary syndrome. Curr Opin Lipidol. 2016;27(4):375–81. doi:http://dx.doi.org/10.1097/MOL.0000000000000311. PubMed
