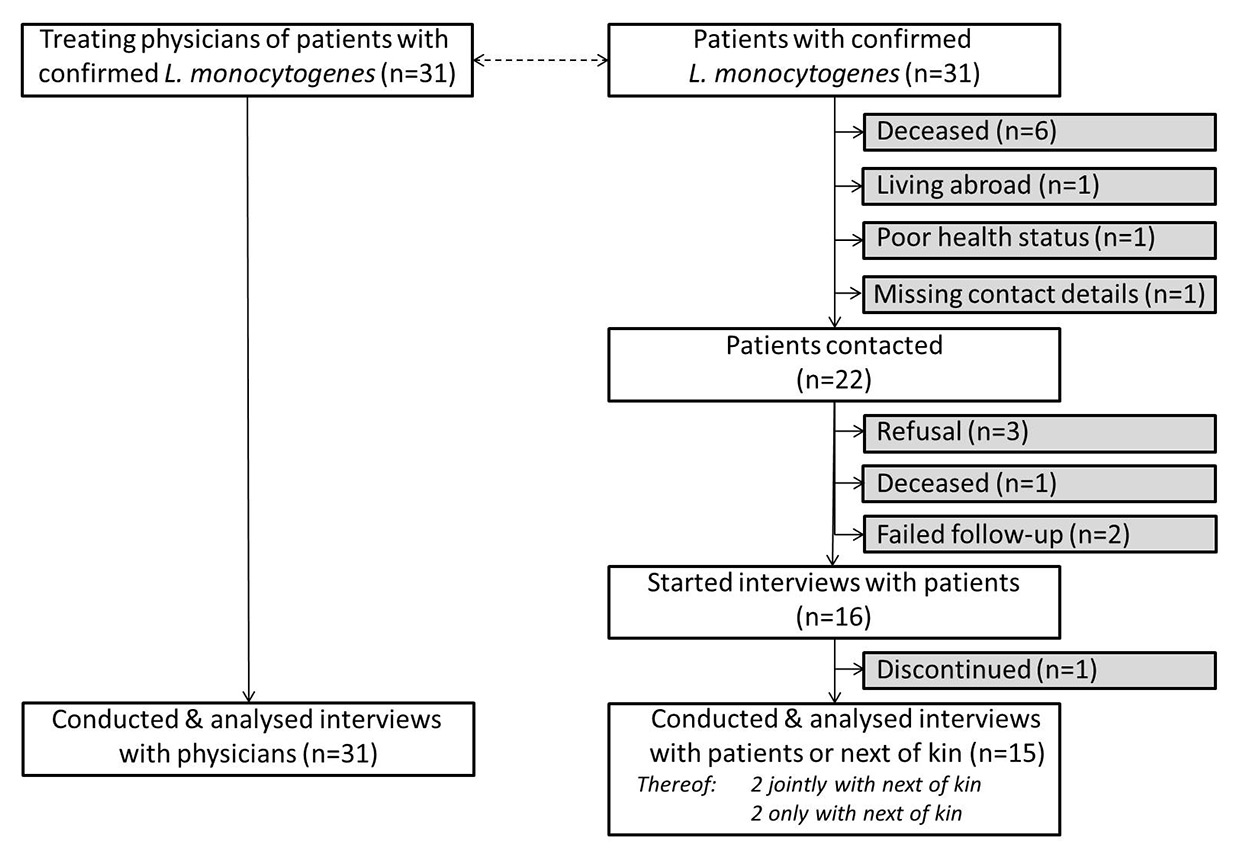
Figure 1
Participation of patients with confirmed L. monocytogenes infection and their treating physicians in the epidemiological outbreak investigation, Switzerland, 30 January to 11 May 2014.
DOI: https://doi.org/10.4414/smw.2016.14366
Epidemiological investigations of foodborne outbreaks require rapid, valid, specific and complete information on the affected population and their food consumption history. Typically, this information is collected directly from patients or their next of kin. In outbreaks that are characterised by severe infections, critical health status or high mortalities, such as listeriosis [1–3], this can be a challenge. In such outbreaks the exploration of additional information sources is indicated [1, 4].
Listeria monocytogenes is a facultative anaerobic, Gram-positive bacillus that can cause listeriosis, a severe human disease, especially in patients with an impaired immune system [5]. The bacteria can contaminate a range of foods, such as meat and vegetables, unpasteurised milk products and cooked or processed food, including certain soft cheeses or ready-to-eat meats, and raw or smoked seafood [3, 6–8]. In 2002, a laboratory analysis of ready-to-eat foods in Switzerland identified the pathogen in 67 items, most commonly in raw meat cured sausages, smoked fishes and semi-hard cheeses [9]. L. monocytogenes is robust to low and high temperatures, making contamination after cooking and before packaging a common problem.
Immunocompetent people may be symptom-free or experience acute febrile gastroenteritis after infection with L. monocytogenes. At highest risk of listeriosis are immunocompromised individuals, the elderly, pregnant women and their unborn babies, and neonates. In those vulnerable individuals, listeriosis can cause severe to life-threatening conditions, including sepsis, meningitis or encephalitis. Pregnant women may experience spontaneous abortions, stillbirths or preterm births [3, 10, 11]. Most symptomatic listeriosis patients are hospitalised and the case-fatality rate is high (averaging 20–30%) compared with other foodborne illnesses [5, 12]. Incubation periods vary depending on the clinical manifestations [13] and range between 3 and 70 days, with a typical onset of symptoms after 2–3 weeks [5].
Between 29 October 2013 and 23 April 2014 the Federal Office of Public Health (FOPH) in Switzerland registered a nationwide outbreak of L. monocytogenes serotype 4b, belonging to a single distinct pulsed-field gel electrophoresis (PFGE) pulsotype [14]. There was no hypothesis on the outbreak source.
The FOPH initiated an epidemiological investigation with the aim to identify the outbreak source. The epidemiological outbreak investigation identified pre-cut, ready-to-eat green salads, purchased from one retail chain in Switzerland, as the likely source. In parallel, self-reporting of a wholesale, salad-producing company with the cantonal authority led to a laboratory investigation and the analysis identified the same product as the source for the observed increase in listeriosis cases [14].
Here, we use the data collected during the epidemiological investigation of the Swiss listeriosis outbreak for a complementary analysis to describe the importance of the quality and timeliness of obtained information, and conformities and differences between information given by the treating physicians and patient-reported data. Further, we discuss the added value of physician interviews, lessons learnt and the implications for future outbreak investigations.
A multi-method outbreak investigation was launched in January 2014 entailing (1) laboratory investigations, comprising microbiological analysis of product and environmental samples from food production companies and (2) an epidemiological investigation including interviews with treating physicians and patients and/or their next of kin. Detailed findings on the outbreak source have been published [14].

Figure 1
Participation of patients with confirmed L. monocytogenes infection and their treating physicians in the epidemiological outbreak investigation, Switzerland, 30 January to 11 May 2014.
The epidemiological outbreak investigation was designed as a prospective case-case comparison of laboratory confirmed L. monocytogenes cases.The samples were then classified as outbreak-cases if confirmed with the outbreak pulsotype, or as control cases, if the pulsotype differed from the outbreak strain. The latter analysis was done by the National Centre for Enteropathogenic Bacteria and Listeria (NENT) using pulsed-field gel electrophoresis.
Patients reported to the National Notification System for Infectious Diseases of the FOPH were enrolled. The investigation included all patients with confirmed L. monocytogenes reported between 30 January and 11 May 2014, and their treating physicians.
The FOPH informed the treating physicians by letter about the outbreak and the on-going epidemiological investigation. A physician of the outbreak investigation team then contacted the treating physicians and carried out a telephone interview of approximately 15 minutes. The interview was based on a structured questionnaire covering the following topics: underlying diseases and risk factors, regular medication, symptoms and the suspected source of the L. monocytogenes infection.
Patients were informed by letter of the FOPH and afterwards contacted by the outbreak investigation team by telephone, whereby consent for participation was sought. Upon consent, patients were visited for face-to-face interviews using a structured questionnaire. Interviews were conducted with the patient or, if not possible, carried out as proxy interviews with the next of kin. Questions on the disease, signs and symptoms, activities, consumer behaviours, eating habits and food history in the 4 weeks prior to illness were asked. Questions on food history were structured in “food groups” (e.g. “cheeses”), sub-groups (e.g. “soft cheeses”), and finally individual “foods” (e.g. “camembert”), with respective skip patterns. Patients indicated their food consumption in categories of certainty and regularity for the past 4 weeks (i.e. either they specifically remembered or they eat the food regularly). Patients who denied consuming the food or who were unsure when replying, were explicitly asked if they ‘never’ consume or ‘don’t know’ the food item. (The full questionnaire can be requested from the corresponding author.) Interviews were predominantly conducted in hospitals because of the hospitalisation of most patients and lasted between 40 minutes and almost 2 hours (median 1:25 hours). Throughout the investigation period, patients, interviewers and investigators had no hypothesis or knowledge about the outbreak source.
For the initial source identification we conducted a case-case comparison of outbreak and control cases [14]. For the purpose of the current complementary analysis, we compared the information obtained from patients with that of their treating physicians. We analysed which information source provided specific and complete information in a rapid manner on the affected population and their food consumption history and described which information would not be available if treating physicians had not been asked. As a result of the small case numbers, statistics are mainly descriptive.
The Swiss Epidemics Act (SR 818.101 EpG) and the Reporting Ordinance (SR 818.141.1) provide the ethical and legal basis for this outbreak investigation. Article 9 of the Reporting Ordinance obliges physicians to provide information to the outbreak investigation team, though we emphasised that participation was voluntary. Patients were asked for their oral informed consent before conducting the interview.
The sample consisted of 31 patients with confirmed L. monocytogenes. As shown in table 1, 52% of patients were female. The median age was 67 years (range 7–94 years), with only one patient being a child of 7 years, 29% being between 18 and 59 years of age, another 32% being between 60 and 79 years and 36% being 80 years or older.
All treating physicians of the 31 included listeriosis patients could be contacted and were available for interviews. With 14 physicians we carried out interviews at first contact. In other cases, we agreed on an interview date, usually on the same day or within 24 hours after initial contact. Only one interview was conducted 4 days after the initial contact. All physicians completed the entire questionnaire.
We contacted 22 of the 31 listeriosis patients or their next of kin. Reasons for non-contacts with patients or their next of kin were: patients deceased or lived abroad, their health status did not allow interviewing or their contact details were unavailable.
On average, 2.5 contacts were necessary to reach the respective person or their next of kin. Of those contacted, 16 agreed to an interview. Reasons for not receiving information from patients after initial contact included refusals, failure of follow-up or death. The median time between initial contact and interviews was 2 days (range 1–7 days). Fifteen of the 16 patients or their next of kin completed the entire interview. One interview was discontinued because of exhaustion of the patient. In two situations the patient and their next of kin were interviewed jointly. For two patients only the next of kin could be interviewed. The 52% of patients who were unable to participate, refused participation or discontinued the interview were slightly older than those participating and affected by more severe and often multiple underlying conditions (table 1).
The information gained from physicians revealed that 71% (n = 22) of patients were affected by at least one immunosuppressing disease or were pregnant. Patients were most often affected by tumours (e.g. prostate carcinoma, lung tumours; 39%, n = 12), diabetes (type 1 or 2; 16%, n = 5) and autoimmune disorders (e.g. of rheumatological, renal, dermatological, neurological nature; 13%, n = 4).
Of the 31 patients, 29% (n = 9) were not diagnosed with an immunosuppressing condition, though we consider that 77% (n = 7) of these patients had an impaired immune system because of their age (≥60 years). Hence, accounting for disease, pregnancy and age, we assume that at the time of infection, 94% (n = 29) listeriosis patients were immunocompromised.
All except one patient were hospitalised, 57% (n = 17) because of listeriosis, 23% (n = 7) because of listeriosis and an underlying disease or pregnancy, and 20% (n = 6) only because of the underlying disease. On a 1–10 rating scale, physicians rated the general health status of patients at admission into hospital or first consultation with a median rank of 5 (range: 2–9), where a rating of 10 indicates a very good health status.
Information from treating physicians on the total sample revealed that fever/chills were followed by abdominal pain/cramps and limited vigilance and by vomiting and other neurological symptoms (table 2). One of the 31 patients had no symptoms and the infection was only coincidently detected during routine tests.
In the sub-sample of the 15 interviewed patients, physicians mentioned also fever/chills as the most frequently occurring symptoms followed by headaches, other neurological symptoms and other organ manifestations. In comparison, interviewed patients most frequently reported fever/chills, neurological symptoms (e.g. anisocoria, aphasia, and facial nerve paralysis), vomiting, diarrhoea and other symptoms (e.g. pleural effusion, tachycardia, ascites, sepsis).
Treating physicians focused on cheeses and raw milk products when asking their patients about the potential source of infection. Other foods or habits that were mentioned included: “fresh foods”, fondue chinoise, salami, broad-leaved garlic pesto and sausages, seafood, smoked fish, vegetables and bean salad. Overall the physicians’ questioning on food consumption appeared unstructured. For 12 (39%) patients, physicians could not extract information about a potential exposure source, either because: (1) physicians were unable to interact with patients owing to the patient’s health status, (2) physicians did not (yet) conduct food history taking, or (3) no risk food items or exposures could be identified.
Food history taking with patients by face-to-face interviews led to a more comprehensive and detailed picture of food consumption than the interviews with physicians, mainly because we applied a structured and detailed questionnaire. Despite the long recall period, patients remembered their food consumption and habits in the past 4 weeks before onset of symptoms well, which we attribute to (1) the use of “anchors” or “individual landmark events” with the help of the patient’s calendar [15] and (2) patients ability to classify foods according to categories of “consumption likelihood”. Patients could identify items that they “never” consume or items that they “regularly” consume and, thus, were also likely to have been consumed in the past 4 weeks. Also items that had not been consumed could be identified by patients with high confidence. The remaining items defined the grey zone for which the respondent could not with certainty confirm the consumption. Next of kin interviews were helpful if done with individuals who lived with the patient in the same household or were carers.
The ratings of consumption likelihood in the questionnaire enabled us to narrow down possible sources by (1) identifying food groups or food items that were consumed by almost all outbreak cases; (2) excluding food groups or food items that at least one outbreak case never consumes; and (3) comparing the consumption patterns between outbreak and control cases. All of the five outbreak cases interviewed ate pre-cut, ready-to-eat green salads compared with only 4 of the 10 interviewed control cases [16]. This difference was statistically significant (p <0.05). We gained even greater confidence in this result as all outbreak cases reported purchasing the pre-cut, ready-to-eat green salads from the same retail chain.
| Table 1: Sociodemographics and comorbidities of patients with confirmed L. monocytogenes infection as stratified by their status of participation, Switzerland, 30 January – 11 May 2014. | |||
| Participants (n = 15) | Drop-outs* (n = 16) | Total (n = 31) | |
| Gender | |||
| Female | 5 (33%) | 11 (69%) | 16 (52%) |
| Age categories (years) | |||
| <18 | 1 (7%) | 0 (0%) | 1 (3%) |
| 18–<60 | 6 (40%) | 3 (19%) | 9 (29%) |
| 60–<80 | 3 (20%) | 7 (44%) | 10 (32%) |
| 80+ | 5 (33%) | 6 (38%) | 11 (36%) |
| Comorbidities | |||
| Tumours | 5 (33%) | 7 (44%) | 12 (39%) |
| Cirrhosis of the liver | 1 (7%) | 2 (13%) | 3 (10%) |
| Alcoholism | 0 (0%) | 1 (6%) | 1 (3%) |
| Diabetes (type 1 or 2) | 2 (13%) | 3 (19%) | 5 (16%) |
| Chronic hepatitis | 0 (0%) | 2 (13%) | 2 (6%) |
| Organ transplantation | 1 (7%) | 0 (0%) | 1 (3%) |
| Chronic intestinal inflammation (e.g. Crohn’s disease, ulcerative colitis) | 1 (7%) | 0 (0%) | 1 (3%) |
| Autoimmune disorder (rheumatological, renal, dermatological, neurological) | 3 (20%) | 1 (6%) | 4 (13%) |
| * Including non-contacts, refusals, failure of follow-up, discontinued interviews, death | |||
| Table 2: Signs and symptoms of patients with confirmed L. monocytogenes infection as reported by patients and their treating physicians (multiple answers possible), Switzerland, 30 January – 11 May 2014. | |||
| Patient interviews (n = 15) | Treating physician interviews | ||
| Subsample (patients interviewed) (n = 15) | Total sample (n = 31) | ||
| Headaches | 3 (20%) | 4 (27%) | 5 (16%) |
| Impaired vigilance | 2 (13%) | 3 (20%) | 9 (29%) |
| Sensitivity to light | 1 (7%) | 1 (7%) | 1 (3%) |
| Vertigo | 1 (7%) | 1 (7%) | 1 (3%) |
| Meningism | 1 (7%) | 2 (13%) | 3 (10%) |
| Other neurological symptoms | 5 (33%) | 4 (27%) | 7 (23%) |
| Abdominal pain/cramps | – | 1 (7%) | 10 (32%) |
| Nausea | 1 (7%) | – | 4 (13%) |
| Vomiting | 4 (27%) | 1 (7%) | 7 (23%) |
| Diarrhoea | 4 (27%) | 2 (13%) | 6 (19%) |
| Fever/chills | 9 (60%) | 12 (80%) | 20 (65%) |
| Muscle pain / joint pain | 1 (7%) | 3 (20%) | 4 (13%) |
| Lower back pain | 2 (13%) | 1 (7%) | 2 (6%) |
| Other | 8 (53%) | 3 (20%) | 11 (35%) |
| Confirmed meningitis* | NA | 2 (13%) | 4 (13%) |
| Meningoencephalitis* | NA | – | 2 (6%) |
| Organ involvement* | NA | 4 (27%) | 4 (13%) |
| NA = not applicable * Patients were not asked for these clinical categories | |||
We used the epidemiological investigation of a nationwide listeriosis outbreak in Switzerland for a complementary analysis comparing information on clinical aspects and the food history between patients and their treating physicians.
Fifteen out of a total of 31 listeriosis patients were interviewed in this outbreak investigation. The non-response of 16 cases limited our ability to describe the affected population and their food consumption patterns, and thus we tried to identify additional information sources, i.e. the treating physician or a next of kin. The Swiss Epidemics Act obliges physicians to contribute in outbreak investigations and we identified them as an informative, easy-to-access and highly collaborative source of information, which can fill information gaps on signs and symptoms and underlying health conditions. An additional positive effect of physician interviews was that physicians could inform patients about the ongoing investigation and facilitate collaboration between patients and the investigation team. We observed that the direct interaction between the treating physician and the patient gave additional credibility to the investigation and had a positive influence on the patients’ willingness to participate. The information from the physician also provided valuable insight for the briefing of interviewers about the patients’ health status and possible associated challenges for the interview. If a next of kin had to be interviewed the physician was able to provide guidance on who could be contacted.
The importance of understanding underlying conditions and the clinical situation of listeriosis patients has recently been demonstrated by Maertens De Noordhout et al. [17]. Initial attempts to obtain information on underlying diseases during patient interviews in this listeriosis outbreak were impaired by (1) the patients’ non-medical descriptions, (2) the lack of precise differentiation between signs and symptoms of the underlying condition and of listeriosis and (3) ethical considerations, as most patients were severely ill. Consequently, treating physicians provided the only comprehensive overview on the health situation of the patient and underlying conditions.
We found that patients described their signs and symptoms comprehensively but structured questioning was challenging. Patients described mostly gastrointestinal and other symptoms such as pneumonia or loss of appetite, but reported neurological symptoms less often than the treating physician. Comprehensive information was obtained from physicians but we observed differences between records from patients and physicians. The latter focused more on the severe neurological symptoms, possibly also related to the patient’s underlying condition, and may have interpreted the gastrointestinal symptoms as less important. Further, signs and symptoms must be attributed to listeriosis with caution as many could also have been due to the underlying disease.
Physicians’ main focus was the clinical treatment of the patient and not the source identification. Thus, they had very limited information on the food consumption history of the 31 patients and could not contribute to identifying the source of infection. If done, information on food consumption history obtained by physicians was not systematically collected, incomplete or unspecific. At times, a food history was not taken owing to: (1) L. monocytogenes being often an incidental diagnosis, overlaid by serious comorbidities and underlying conditions that set the physicians’ focus on treatment rather than on the identification of the source of infection; (2) insufficient knowledge about foods relevant to L. monocytogenes infections; and (3) lack of time.
As shown previously, use of a detailed and structured questionnaire, with patients or their next of kin, is expedient in providing detailed information on food habits and consumption patterns prior to onset of symptoms and in providing a clue towards the outbreak source [14, 18]. Small numbers of conducted interviews remain a concern for the identification of the outbreak source. However, in our case information from patients was sufficient to confirm pre-cut, ready-to-eat green salads as the most likely outbreak source. The microbiological investigation, which was performed in parallel, identified the same product.
Table 3 summarises our experience with the different information sources.
| Table 3: Information obtained from treating physicians and patients with confirmed L. monocytogenes infection. | ||
| Physicians | Patients | |
| Underlying conditions and clinical assessment | +++ | + and not applicable |
| Signs and symptoms of listeriosis | +++ | ++ |
| Food consumption | + | +++ |
| +++ detailed information; ++ reasonable detailed information; + insufficiently detailed information; not applicable: questions were not asked | ||
Because most listeriosis outbreak investigations report on the identification of the outbreak source, there is limited evidence on the added value of information sources beyond the affected persons and laboratory investigations. In our outbreak investigation, we narrowed the information gap by including treating physicians in the epidemiological investigation. Our experience shows that physicians are a valuable information source that can be tapped in foodborne outbreak investigations, especially for diseases where patients suffer from severe illness or die. Furthermore, the involvement of the treating physicians helped us to establish and mediate necessary contacts with patients or their next of kin and added to the credibility of the investigation for them. This was of particular importance as the severity of the health conditions were a high psychological burden to the patient and their family members. Situations where this might be applicable are outbreaks of e.g. listeriosis or when mainly children are affected (e.g. haemolytic-uraemic syndrome).
Firstly, comparisons between data obtained from physicians and patients were not inherently built into the design of the outbreak investigation. Hence, the comparison does not rely on identical sets of questionnaires and comparisons were only selectively possible. Secondly, the small sample size limited the possibility and usefulness of statistical comparisons. Also, the internal and external validity of our study results may be impeded by the small sample size and the specificities of the outbreak situation or country context [16]. Thirdly, the relatively high proportion of patients who were unable to participate may have led to a selection bias. Fourthly, patients did not maintain a food diary as this was a prospective outbreak investigation. Hence, we relied solely on the respondents’ memory and cannot compare the indicated to true consumption.
Foodborne outbreak investigations may be impeded by a lack of information from severely ill patients owing to either their underlying health conditions, the severity of the foodborne infection or both. Extending the information base during this outbreak to the treating physician was shown to be an effective tool to ease access to patients for food history taking and, thus, the rapid identification of the infection source. In addition, physicians are a reliable source of specific and complete information on the clinical situation of each patient and their underlying conditions. However, patients are vital to provide detailed information on the food consumption history. In conclusion, an outbreak investigation of Listeria monocytogenes ideally entails both patient and physician interviews.
Acknowledgement:The authors are grateful to all physicians, patients and respective relatives participating in the outbreak investigation. We are particularly thankful to our interviewers and data entry persons, Meike Zuske, Dr. Damiano Urbinello, Alexandra Nicola, Wendelin Moser and Sophie Haeser, for their professional commitment. We very much appreciate the contributions of Dr. med. Esther Künzli who has facilitated the development of the questionnaires and Dr. Thomas Fürst, who assisted with the data analysis. We also like to thank the colleagues from the Cantonal laboratories, the Federal Food Safety and Veterinary Office and the National Reference Laboratory for their contributions in identifying the outbreak source.
1 Haechler H, Marti G, Giannini P, Lehner A, Jost M, Beck J, et al. Outbreak of listerosis due to imported cooked ham, Switzerland 2011. Euro Surveill. 2013;18(18):20469.
2 Maertens de Noordhout C, Devleesschauwer B, Angulo FJ, Verbeke G, Haagsma J, Kirk M, et al. The global burden of listeriosis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(11):1073–82.
3 Hernandez-Milian A, Payeras-Cifre A. What is new in listeriosis? Biomed Res Int. 2014:358051.
4 Gaulin C, Gravel G, Bekal S, Currie A, Ramsay D, Roy S. Challenges in listeriosis cluster and outbreak investigations, Province of Quebec, 1997–2011. Foodborne Pathog Dis. 2014;11(1):1–7.
5 Heymann DL, editor. Control of Communicable Diseases Manual. 20th ed. Washington: American Public Health Association; 2015.
6 Schlech WF, 3rd, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, et al. Epidemic listeriosis – evidence for transmission by food. N Engl J Med. 1983;308(4):203–6.
7 Hof H, Szabo K, Becker B. Epidemiology of listeriosis in Germany: a changing but ignored pattern. Dtsch Med Wochenschr. 2007;132(24):1343–8.
8 Cartwright EJ, Jackson KA, Johnson SD, Graves LM, Silk BJ, Mahon BE. Listeriosis outbreaks and associated food vehicles, United States, 1998–2008. Emerg Infect Dis. 2013;19(1):1–9.
9 Baumgartner A, Schmid H. Listeria monocytogenes in genussfertigen Lebensmitteln: eine Auswertung der amtlichen Untersuchungen in der Schweiz der Jahre 2006–2008. Journal für Verbraucherschutz und Lebensmittelsicherheit. 2013;8:109–17.
10 Schuchat A, Swaminathan B, Broome CV. Epidemiology of human listeriosis. Clin Microbiol Rev. 1991;4(2):169–83.
11 Swaminathan B, Gerner-Smidt P. The epidemiology of human listeriosis. Microb Infect. 2007;9(10):1236–43.
12 Fry AMB, Christopher R, Griffin Patricia M, Hughes James M. Foodborne Disease. In: Mandell GLB, John E.; Dolin, Raphael, editor. Principles and Practice of Infectious Diseases 1. Philadelphia: Elsevier; 2005. p. 1286–301.
13 Goulet V, King LA, Vaillant V, de Valk H. What is the incubation period for listeriosis? BMC Infect Dis. 2013;13:11.
14 Stephan R, Althaus D, Kiefer S, Lehner A, Hatz C, Schmutz C, et al. Foodborne transmission of Listeria monocytogenes via ready-to-eat salad: A nationwide outbreak in Switzerland, 2013–2014. Food Control. 2015;57:14–7.
15 Glasner T, van der Vaart W. Applications of calendar instruments in social surveys: a review. Qual Quant. 2009;43(3):333–49.
16 Mann C. Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J. 2003;20:54–60.
17 Maertens De Noordhout C, Devleesschauwer B, Maertens De Noordhout A, Blocher J, Haagsma JA, Havelaar AH, et al. Comorbidities and factors associated with central nervous system infections and death in non-perinatal listeriosis: a clinical case series BMC Infect Dis. 2016;16:256.
18 Knoblauch A, Bratschi M, Zuske M, Althaus D, Stephan R, Hächler H, et al. Cross-border outbreak of Salmonella enterica ssp.entericaserovar Bovismorbificans: multiple approaches for an outbreak investigation in Germany and Switzerland. Swiss Med Wkly. 2015;145:w14182.
Disclosure statement:The epidemiological outbreak investigation was initiated and financed by the Federal Office of Public Health. Funding: No specific funding has been received for the writing of the manuscript. The authors declare no conflict of interest.