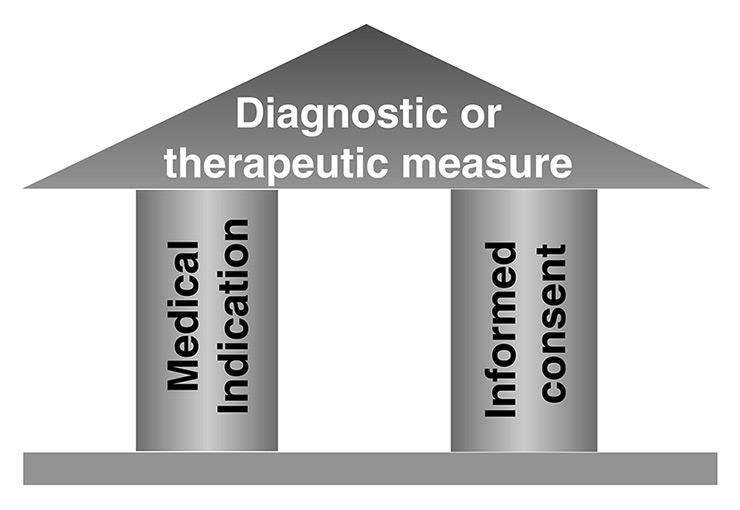
Figure 1
Prerequisites for any ethically justified medical action.
DOI: https://doi.org/10.4414/smw.2016.14369
The current discussion on how to avoid the risks of overtreatment is extremely relevant for patients with chronic and advanced disease. Initiatives such as “Choosing wisely®” [1, 2], launched by the American Board of Internal Medicine in 2012, have started, albeit timidly, to expose some of the most obvious instances of frequent overdiagnosis and overtreatment in the medical system. A plethora of medical associations have collaborated to draw up a list of commonly used treatment measures that are, according to evidence-based standards, not beneficial and sometimes even harmful. The resulting lists of negative recommendations (recommendations not to use certain treatment measures) have been shown to have an impact on clinical care [3].
In the meantime, other countries have taken up this idea, including Switzerland [43]. In 2014, the Swiss Society of General Internal Medicine listed five treatments and tests that are commonly prescribed in ambulatory general internal medicine although the evidence shows that they carry more harm than benefit (“Smarter Medicine” initiative) [4]. The Swiss Academy of Medical Sciences advised and actively supports medical associations in the pursuit of such initiatives [5]. The German Society of Internal Medicine has broadened the concept by formulating not only a list of negative recommendations based on overtreatment, but also a list of positive recommendations where undertreatment is the problem [6].
This discussion has to be welcomed from the point of view of palliative and end-of-life care. There is widespread clinical experience that overtreatment is particularly prevalent at the end of life. One reason for this is obvious: our fear of death and the entrenched traditions of life-saving, activist medicine pushes us to act rather than withhold, and to continue a non-beneficial treatment rather than stop it and accept the impending death [7]. In addition, the supposed menace of judicial proceedings prompts a “defensive medicine” with diagnostic and therapeutic measures solely carried out to protect the clinician from potential (and often improbable) lawsuits [6]. Ultimately, palliative care is still a relatively new specialty; therefore it largely lacks the evidence base of methodologically sound studies, and even where such evidence exists [8], it has not yet percolated to every health care professional to influence the way they perform end-of-life care.
The discussion on overtreatment also highlights that there is a lack of awareness of the proper decision-making process governing medical action. We would like to draw attention to the old and somewhat neglected concept of medical indication as a prerequisite for all medical action [9]. Where a treatment lacks medical indication, the term “medical futility” is often used. However, this concept has many downsides, since (1) it is negative rather than positive, (2) it carries pejorative connotations, and (3) there is no consensus definition of medical futility [7, 10].
When we ask medical students about the indispensable prerequisites for any form of medical action, be it diagnostic or therapeutic, almost all students are quick to answer: the patient’s informed consent. But very few students are able to identify the second, but logically prior and at least equally important prerequisite: the medical indication (fig. 1).

Figure 1
Prerequisites for any ethically justified medical action.
Although this notion is widely used in the medical literature and in clinical practice, there has been surprisingly little discussion in the literature on its conceptualisation and operationalisation. For the purpose of this article, and based on accepted standards in clinical medicine and medical ethics, we would like to define medical indication as “the appropriateness of a specific diagnostic or therapeutic measure in the patient’s concrete clinical situation, in light of the best available evidence concerning its expected positive and negative effects for the patient”. Thus, in order to ascertain the presence or absence of medical indication, three simple questions need to be answered:
1. What is the goal of the proposed measure?
2. Is this goal realistically achievable?
3. Does this goal entail a net benefit for the patient?
Question 1 may seem trivial, but it is not. In a retrospective study looking at palliative care consultations on end-of-life decisions in intensive care units (ICUs), in over half of the consultations the decision was clarified by this question alone [7]. The number of situations in acute care hospitals in which clinical measures continue without a clear and explicit goal of care is grossly underestimated in our clinical experience. Moreover, clarifying the goal of care may reveal that the involved healthcare professionals have quite divergent and often incompatible goals that they each pursue.
Question 2 is trickier: what does it mean to “realistically” achieve the desired goal of care? Obviously, there is a certain element of judgement in this term because it involves prognostication. In modern medicine there is a growing body of evidence, documented in well-conducted clinical studies, systematic literature reviews and rigorous clinical guidelines, showing the ineffectiveness of certain clinical measures to achieve the intended goals. It is the physician’s role to become familiar with this evidence and apply it to the clinical situation at hand using clinical judgement and experience. Below we will present two poignant examples of measures that fail to achieve their goals: artificial hydration and oxygen in the dying phase, and enteral nutrition in advanced dementia.
Question 3 represents the intuition that not every treatment that is possible and effective is also beneficial to the patient and thus meaningful. Some treatment measures may do more harm than good to the patient, or bear disproportionately more risks than benefits. To answer this question, it is necessary to apply the intended goal of care to the concrete situation of the individual patient and to consider both the probability of success (see question 2) and the foreseeable impact of the treatment on the patient’s quality of life. Both the ethical duties of beneficence and non-maleficence require clinicians to act in a way that the patient is better off through their action than without it [11]. An example of such problematic treatment measures is the continued use of cardiac pacemakers and implantable cardioverter defibrillators in the dying phase (see below).
These three questions disentangle the decision making process on medical indication and show that it is, like many clinical decisions, a compound decision, based on both a factual, evidence-based judgement and a value judgement. When it comes to value judgements, the patient’s values are paramount; therefore, shared decision making (involving patients/surrogates, physicians and other team members) is required whenever possible. This process requires skilled communication and can be facilitated by appropriate, evidence-based tools, including visual decision aids [12].
There are two instances, however, where unilateral decision by the physician is both permitted and necessary:
‒ When the patient’s opinion cannot be obtained in time, such as in emergency situations, and there is no surrogate decision maker available, it is up to the physicians to make the decision based on their estimation of the patient’s best interests, whenever possible in collaboration with other health care professionals.
‒ When a treatment or diagnostic measure is contraindicated, i.e. it is ineffective (in relation to the chosen treatment goal) and possibly harmful, the physician’s duty is to not even mention this treatment to the patient, and to refuse to perform it if the patient or the family asks for it.
No physician or nurse would want their patients to die of suffocation or suffering thirst. This is a meaningful, beneficial goal of care. If this attitude is combined with a lack of knowledge, however, it leads to a reflex behaviour that can be observed almost everywhere patients die in a medicalised setting, including nursing homes (hospitals and nursing homes accounting for approx. 75–80% of deaths in high-income countries).
The reflex behaviour goes as follows: once a patient is identified as “dying” (which usually implies non-responsiveness and inability to eat or drink), doctors and nurses automatically start intravenous hydration (to prevent thirst) and oxygen administration via nasal cannula (to prevent shortness of breath). Unfortunately, though born of good intentions, these measures are both useless and harmful [13, 14]. They are useless because the sensation of thirst at the end of life is not responsive to the parenteral infusion of fluids, but depends on the degree of dryness of the oral mucosa. Similarly, the shallow respiration of the dying is physiological and not a sign of distress. If dyspnoea occurs, it is usually not due to hypoxaemia, but to ventilation problems. Both treatment measures are also harmful because nasal oxygen administration in dying patients, who typically breathe through the open mouth, aggravates oral dryness and thirst sensation. Conversely, the intravenous fluids cannot be excreted properly owing to terminal renal insufficiency, which may lead to lung oedema and exacerbate the dyspnoea. Thus, these widespread and seemingly humane measures actually produce or aggravate each other and the very symptoms that they are meant to prevent or treat.
In the final stage of dementia, patients are often unable to feed themselves or even to be fed orally because of dysphagia [15]. The conscious initiation of the swallowing process is lost as a result of progressive cortical degeneration. This usually signals the beginning of the terminal phase, which can last for days or even weeks, depending on the patient’s nutritional status and comorbidities [16]. In several high-income countries, this situation prompts the placement of a percutaneous endoscopic gastrostomy (PEG) tube to start artificial nutrition and hydration [17]. Potential goals of this therapeutic measure include:
‒ Prolonged survival
‒ Improved quality of life
‒ Improved nutritional status
‒ Faster healing of pressure ulcers
‒ Diminished aspiration risk
Although the theoretical rationale for this treatment measure may seem plausible at first sight, several systematic literature reviews, including a Cochrane review, show that none of these goals are attained with a PEG placement in advanced dementia [18–20]. On the contrary, the risk of aspiration and infection is actually increased [21, 22], which may in fact shorten survival and worsens quality of life. Moreover, patients with dementia may react aggressively to PEG tubes necessitating the use of restraints with grave consequences for the patients’ quality of life. A further major drawback of PEG tubes in this population is the risk of losing social contacts and human attention by nurses and others that are commonly associated with hand feeding and oral food ingestion.
This imbalance of risks and benefits justifies the recommendation that artificial nutrition and hydration, as a general rule, should not be initiated in patients with advanced dementia, in particular via PEG tubes [18, 23–25]. In Switzerland, this practice appears to be less prevalent than in other high-income countries, owing in part to quality assurance guidelines that require justification for PEG tube placements in dying nursing home patients.
In aging societies, an increasing number of patients have cardiac pacemakers or implantable cardioverter defibrillators (ICDs) implanted because of chronic heart disease with arrhythmia. These technologies represent a major progress in medicine that saves the lives of numerous people and enables them to live more safely and with a higher quality of life. But when these patients enter the dying phase (from whatever cause), these technologies may prolong the dying process and cause considerable pain and suffering [26]. A treatment that merely prolongs the dying process and adds more suffering to dying is not medically indicated. Nonetheless, one third to two thirds of ICD patients receive defibrillation therapy during the final days of their lives [26]. It is therefore not surprising that the American Academy of Hospice and Palliative Medicine has listed the use of cardiac devices in the dying phase as one of five examples of overtreatment [25]. There are various means to deactivate or block the function of these devices in the dying process, but this has to be planned and effectively implemented.
Importantly, at the end of life, as in any other medical situation, causative treatment, wherever possible, is preferable to symptomatic treatment. For example, a 92-year old patient with dyspnoea due to aortic valve stenosis is not automatically a candidate for morphine treatment, as she might benefit more from transcatheter aortic valve replacement [27]. Thus, decisions on medical indication always need to be made on an individual, case-by case basis, taking into account all available options.
The examples depicted above concern situations which are far from infrequent at the end of life. They can be solved by using the same principles of evidence-based medicine that we strive to apply to the rest of medicine. Unfortunately, the closer we come to the point of our patients’ deaths, the more irrational our behaviour as physicians seems to become. One reason for this may lie in the unacknowledged fear of our own demise that these situations evoke.
For sure, there are other medical interventions at the end of life for which we do not have the same solid evidence base as for the ones illustrated. In those cases, we have to be content with the best available evidence, which may in fact be expert opinion and our own clinical experience. And we are ethically obliged to enable and encourage high-quality research studies in order to close as many knowledge gaps as possible in end-of-life care.
If there is uncertainty or controversy regarding a medical indication, it may help to consider the inverse relation between, on one hand, the size of the net benefit that the goal of care would bring to the patient (factoring in the potential decreases in quality of life associated with the treatment) and, on the other hand, the probability of achieving the intended goal of care. The more a patient stands to benefit from an intervention, the lower the chances of success we are ready to accept. Conversely, when the expected net benefit for the patient is very small, the probability of achieving this marginal benefit would have to be very high in order to justify the intervention.
How do we communicate with patients in the many cases where the medical indication is doubtful, i.e. where the data concerning the proposed treatment or diagnostic measure are absent, scant or contradictory, or the expected effect is of questionable clinical value? Examples of this situation are cholinesterase inhibitors for the treatment of Alzheimer dementias, mammography screening programmes [28, 29] or, in palliative care, the use of antidepressants for demoralisation at the end of life or pain treatment for chronic disorders of consciousness [30, 31]. To answer this question, we must bear in mind that almost all patients start from the implicit assumption that a doctor would never suggest something ineffective or harmful to them. In other words, patients trust that their physicians make reasonable judgements regarding medical indication. Therefore, the onus of truthfulness lies with us.
From this follows a very simple rule for this kind of conversation: be honest. If you have doubts about the treatment, express them. If you have uncertainties, admit them. If you feel that the benefit-risk ratio of the treatment is unfavourable, say so. But remember that patients, especially with life-threatening illnesses, tend to grab every tiny hope we offer to them. Use language that is easily comprehensible, personal and empathetic. Patients do not expect their doctor to be omniscient. Instead, trust builds by being human, by sharing doubts, by reflecting and deliberating together. Being honest and encouraging hope is no contradiction: there are numerous ways to embody optimism, reinforce confidence and strengthen decision-making capacity in the patient while sticking to the truth.
When the medical indication is doubtful, patient autonomy becomes even more important. It is perfectly acceptable that patients, following their own value judgement, may choose to temporarily lower their quality of life (e.g., by subjecting themselves to the side effects of chemotherapy) in exchange for a chance to meaningfully prolong it. The trouble lies in the word “meaningfully”: it may bias the patients’ judgement when drugs are officially released on the market, but offer only a statistical survival advantage of a couple of weeks while having severe side effects (and horrific costs). If you feel that you have a moral obligation to inform patients of the existence of these drugs, then you need to tell them in detail what they can realistically expect from them – and what they cannot. Even patients without decision-making capacity should be involved as much as possible in the decision process by the physician and the surrogate decision maker [32].
Furthermore, sometimes treatments are proposed for which there is no scientific evidence at all, but just anecdotal, sometimes second-hand, reports. These acts of therapeutic desperation are often prompted by patient and family insistence, coupled with physicians’ unwillingness to admit that there is no more beneficial disease-modifying treatment available – a situation, by the way, which is sure to happen at some point in the lives of all our patients as well as in our own. So if there is a treatment that you yourself would never undergo, and from which you would protect your own family members in a similar situation, then you should refrain from offering it to your patient.
The frequent doubts, controversies and errors regarding medical indication, and the recent rise of patient autonomy and advance directives are two sides of the same coin. It is exactly because physicians are so often unsure about medically indicated treatment at the end of life that patients feel the urge to document their treatment preferences in advance. Yet, the traditional form of advance directives, the isolated living will as an individual act of the future patient, has been shown to have significant shortcomings and be only marginally effective [33, 34]. Instead, the medical and ethical literature now advocates a more comprehensive, long-term, professionally facilitated communication process between the (future) patients, their families, the physicians and other health care professionals termed “advance care planning” (ACP) [35, 36]. There is abundant evidence showing that these comprehensive ACP programmes are more effective than advance directives alone in realising an end-of-life treatment that is concordant with the patient’s preferences [37, 38].
Although ACP is a major step towards making good decisions at the end of life, it by no means renders the concept of medical indication obsolete or dispensable – quite the contrary. As the range of therapeutic options at the end of life continually increases (with palliative care research, new drugs and new interventions), it is hardly possible to anticipate all potential clinical situations and the range of therapeutic options that will be medically indicated in these situations. There is an unalterable asymmetry between clinical knowledge at the time of decision making and the limited knowledge at the time of anticipated treatment planning. Advance directives that express consent to or refusal of specific treatment measures at the end of life will hence often be too narrow and inapplicable to the clinical situation that has arisen.
To avoid this inapplicability, ACP should focus more on goals of care than on specific interventions (such as resuscitation, artificial ventilation, antibiotics). If patients have anticipatorily expressed what goal(s) of care they prioritise for themselves, the physician can select from the range of options those that best realise the chosen goals.
We remember a dying patient with advanced bladder cancer and cystitis who was suffering from excruciating pain that could not be controlled even by the strongest analgesics. Although he wanted to avoid antibiotics because of their life-prolonging effect, it was only when an antibiotic was administered that the pain subsided. Similarly, noninvasive ventilation may be very helpful in palliative care of neurodegenerative disorders even when no life prolongation is wanted [39].
In general, the appropriateness of a specific treatment measure in a given clinical situation rests primarily on the data available on their effectiveness with regard to the patient’s goals. For example, for a patient’s whose greatest wish is to survive until the birth of a grandchild, invasive life-sustaining measures would need to be judged differently than for a patient who absolutely wants to stay at home and die there, no matter what it takes. This is why ACP should be centred on identifying and documenting preferred goals of care for likely future situations [40].
The correct way of translating the patients’ goals of care into appropriate medical action (or its important counterpart, which might be termed “benevolent abstention”) is our responsibility as physicians, since we have the expertise required to do so. If we ignore our core task to evaluate the medical indication and defer the entire clinical decision making to the patients or their surrogates (instead of engaging in shared decision making), we do not provide the best care for our patients and place an unbearable burden on them and their families [41]. In order to live up to these professional responsibilities, we need to engage in a continuous communication process where the patients provide the lead and we provide guidance and support as needed.
In our opinion, it would be helpful for everybody if physicians became more aware of their role, their competencies and their responsibilities (not only) in end-of-life care. This includes the duty to analyse a clinical situation based on best available evidence first, and to then communicate the results of this analysis to patients and their families in an appropriate manner. This will allow for proper shared decision making when needed, and for emotional relief of the families when not.
Concentrating on the patients’ and families’ priorities and goals of care rather than on technical decisions, which are the domain of physicians’ expertise, may help to establish a relationship of trust that heeds to the wise words of the Danish philosopher Søren Kierkegaard – words which can provide guidance for the entire medical profession: “If we want to help somebody, we must first find out where he stands. This is the secret of all caring. Those who cannot do this are stuck with an illusion if they think they can help others. In order to really be able to help somebody, I must understand more than he does – but first and foremost I must understand what he understands. If I do not, then my greater understanding won‘t help him at all”[42].
1 Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely – the politics and economics of labeling low-value services. N Engl J Med. 2014;370(7):589–92. doi:http://dx.doi.org/10.1056/NEJMp1314965.
2 Wolfson D, Santa J, Slass L. Engaging physicians and consumers in conversations about treatment overuse and waste: a short history of the choosing wisely campaign. Acad Med. 2014;89(7):990–5. doi:http://dx.doi.org/10.1097/ACM.0000000000000270.
3 Rosenberg A, Agiro A, Gottlieb M, Barron J, Brady P, Liu Y, et al. Early Trends Among Seven Recommendations From the Choosing Wisely Campaign. JAMA Intern Med. 2015;175(12):1913–20. doi:http://dx.doi.org/10.1001/jamainternmed.2015.5441.
4 Gaspoz JM. Smarter medicine: do physicians need political pressure to eliminate useless interventions? Swiss Med Wkly. 2015;145:w14125.
5 Swiss Academy of Medical Sciences. Sustainable medicine. Position paper. Basel: SAMW; 2012.
6 Hasenfuss G, Märker-Herrmann E, Hallek M, Fölsch UR. Initiative «Klug entscheiden». Gegen Unter- und Überversorgung. Dtsch Arztebl. 2016;113:A600. German.
7 Jox RJ, Schaider A, Marckmann G, Borasio GD. Medical futility at the end of life: the perspectives of intensive care and palliative care clinicians. J Med Ethics. 2012;38(9):540–5. doi:http://dx.doi.org/10.1136/medethics-2011-100479.
8 Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–42. doi:http://dx.doi.org/10.1056/NEJMoa1000678.
9 Dörries A, Lipp V. Medizinische Indikation. Ärztliche, ethische und rechtliche Perspektiven. Grundlagen und Praxis. Stuttgart: Kohlhammer; 2015. German.
10 Schneiderman LJ. Defining Medical Futility and Improving Medical Care. J Bioeth Inq. 2011;8(2):123–31. doi:http://dx.doi.org/10.1007/s11673-011-9293-3.
11 Beauchamp TL, Childress JF. Principles of Biomedical Ethics. 7th ed. New York Oxford: Oxford University Press; 2013.
12 Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213–21. doi:http://dx.doi.org/10.1001/jamainternmed.2015.1679.
13 Morita T, Hyodo I, Yoshimi T, Ikenaga M, Tamura Y, Yoshizawa A, et al.; Japan Palliative Oncology Study Group. Association between hydration volume and symptoms in terminally ill cancer patients with abdominal malignancies. Ann Oncol. 2005;16(4):640–7. doi:http://dx.doi.org/10.1093/annonc/mdi121.
14 Hui D, Dev R, Bruera E. The last days of life: symptom burden and impact on nutrition and hydration in cancer patients. Curr Opin Support Palliat Care. 2015;9(4):346–54. doi:http://dx.doi.org/10.1097/SPC.0000000000000171.
15 Rösler A, Pfeil S, Lessmann H, Höder J, Befahr A, von Renteln-Kruse W. Dysphagia in Dementia: Influence of Dementia Severity and Food Texture on the Prevalence of Aspiration and Latency to Swallow in Hospitalized Geriatric Patients. J Am Med Dir Assoc. 2015;16(8):697–701. doi:http://dx.doi.org/10.1016/j.jamda.2015.03.020.
16 Volicer L. Dementias. In: Voltz R, Bernat JL, Borasio GD, editors. Palliative Care in Neurology. Oxford: Oxford University Press; 2004.
17 Mitchell SL, Teno JM, Kiely DK, Shaffer ML, Jones RN, Prigerson HG, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–38. doi:http://dx.doi.org/10.1056/NEJMoa0902234.
18 Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review. Clin Interv Aging. 2014;9:1733–9. doi:http://dx.doi.org/10.2147/CIA.S53153.
19 Cervo FA, Bryan L, Farber S. To PEG or not to PEG: a review of evidence for placing feeding tubes in advanced dementia and the decision-making process. Geriatrics. 2006;61(6):30–5.
20 Sampson EL, Candy B, Jones L. Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst Rev. 2009;(2):CD007209.
21 Fox KA, Mularski RA, Sarfati MR, Brooks ME, Warneke JA, Hunter GC, et al. Aspiration pneumonia following surgically placed feeding tubes. Am J Surg. 1995;170(6):564–7, discussion 566–7. doi:http://dx.doi.org/10.1016/S0002-9610(99)80016-6.
22 Bliss DZ, Johnson S, Savik K, Clabots CR, Willard K, Gerding DN. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129(12):1012–9. doi:http://dx.doi.org/10.7326/0003-4819-129-12-199812150-00004.
23 Moran C, O’Mahony S. When is feeding via a percutaneous endoscopic gastrostomy indicated? Curr Opin Gastroenterol. 2015;31(2):137–42. doi:http://dx.doi.org/10.1097/MOG.0000000000000152.
24 American Geriatrics Society Ethics Committee and Clinical Practice and Models of Care Committee. American Geriatrics Society feeding tubes in advanced dementia position statement. J Am Geriatr Soc. 2014;62(8):1590–3. doi:http://dx.doi.org/10.1111/jgs.12924.
25 Fischberg D, Bull J, Casarett D, Hanson LC, Klein SM, Rotella J, et al.; HPM Choosing Wisely Task Force. Five things physicians and patients should question in hospice and palliative medicine. J Pain Symptom Manage. 2013;45(3):595–605. doi:http://dx.doi.org/10.1016/j.jpainsymman.2012.12.002.
26 Carlsson J, Paul NW, Dann M, Neuzner J, Pfeiffer D. The deactivation of implantable cardioverter-defibrillators: medical, ethical, practical, and legal considerations. Dtsch Arztebl Int. 2012;109(33-34):535–41.
27 Siqueira D, Abizaid A, Arrais M, Sousa JE. Transcatheter aortic valve replacement in elderly patients. J Geriatr Cardiol. 2012;9(2):78–82. doi:http://dx.doi.org/10.3724/SP.J.1263.2011.12291.
28 Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348(feb11 9):g366. doi:http://dx.doi.org/10.1136/bmj.g366.
29 Hogan DB. Long-term efficacy and toxicity of cholinesterase inhibitors in the treatment of Alzheimer disease. Can J Psychiatry. 2014;59(12):618–23.
30 Robinson S, Kissane DW, Brooker J, Burney S. A Review of the Construct of Demoralization: History, Definitions, and Future Directions for Palliative Care. Am J Hosp Palliat Care. 2016;33(1):93–101. doi:http://dx.doi.org/10.1177/1049909114553461.
31 Pistoia F, Sacco S, Sarà M, Carolei A. The perception of pain and its management in disorders of consciousness. Curr Pain Headache Rep. 2013;17(11):374. doi:http://dx.doi.org/10.1007/s11916-013-0374-3.
32 Jox RJ, Michalowski S, Lorenz J, Schildmann J. Substitute decision making in medicine: comparative analysis of the ethico-legal discourse in England and Germany. Med Health Care Philos. 2008;11(2):153–63. doi:http://dx.doi.org/10.1007/s11019-007-9112-0.
33 Fagerlin A, Schneider CE. Enough. The failure of the living will. Hastings Cent Rep. 2004;34(2):30–42. doi:http://dx.doi.org/10.2307/3527683.
34 Jox RJ, In der Schmitten J, Marckmann G. Ethische Grenzen und Defizite der Patientenverfügung. In: Coors M, Jox RJ, In der Schmitten J, editors. Advance Care Planning Von der Patientenverfügung zur gesundheitlichen Vorausplanung. Stuttgart: Kohlhammer; 2015. German.
35 Hammes BJ, Rooney BL. Death and end-of-life planning in one midwestern community. Arch Intern Med. 1998;158(4):383–90. doi:http://dx.doi.org/10.1001/archinte.158.4.383.
36 In der Schmitten J, Lex K, Mellert C, Rothärmel S, Wegscheider K, Marckmann G. Implementing an advance care planning program in German nursing homes: results of an inter-regionally controlled intervention trial. Dtsch Arztebl Int. 2014;111(4):50–7.
37 Houben CH, Spruit MA, Groenen MT, Wouters EF, Janssen DJ. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–89. doi:http://dx.doi.org/10.1016/j.jamda.2014.01.008.
38 Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000–25. doi:http://dx.doi.org/10.1177/0269216314526272.
39 Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–41. doi:http://dx.doi.org/10.1016/S0140-6736(07)60944-1.
40 Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003. doi:http://dx.doi.org/10.1001/jamainternmed.2014.5271.
41 Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336–46. doi:http://dx.doi.org/10.7326/0003-4819-154-5-201103010-00008.
42 Kierkegaard S. Synspunkter for min Forfatter Virksomhet (Der Gesichtspunkt für meine Wirksamkeit als Schriftsteller) 1859. In: Kierkegaard S, ed. Die Schriften über sich selbst. Regensburg: Eugen Diederichs Verlag; 1951:38–9. German.
43 Levinson W, Kallewaard M, Bhatia RS, Wolfson D, Shortt S, Kerr EA; Choosing Wisely International Working Group. "Choosing Wisely": a growing international campaign. BMJ Qual Saf. 2015;24(2):167-74. doi: 10.1136/bmjqs-2014-003821.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.