Figure 1
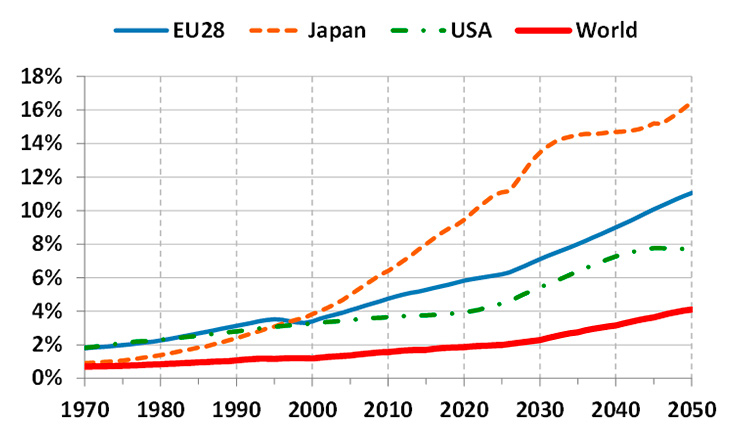
Percentage of the population over 80 years old (based on data from [1]).
DOI: https://doi.org/10.4414/smw.2016.14375
The provision of appropriate healthcare to the population is unquestionably a major worldwide societal challenge. This is a critical issue even for the richest and most developed countries, which are facing a daunting forecast for the future.

Figure 1
Percentage of the population over 80 years old (based on data from [1]).
A main driver behind the global healthcare crisis is population ageing, a demographic phenomenon that is quickly progressing across the globe. According to the Organization for Economic Co-operation and Development (OECD), the percentage of the worldwide population over 80 years old is currently around 2%, but this number is expected to reach 4% by 2050 [1]. The situation is more severe in developed countries, where life expectancy is longer. For example, the population over 80 years old in Europe is currently around 5% and is expected to reach 11% by 2050 (fig. 1).
Population ageing is significantly impacting healthcare systems in at least two ways: (1) the number of patients that need to be cared for is steadily increasing; (2) the percentage of healthcare workers relative to the percentage of elderly is decreasing. The increasing number of patients comes from the well-known correlation between age and the need for healthcare, i.e., the older a person gets, the more she/he needs care. This is clearly demonstrated, for example, by the public healthcare spending data from Canada presented in figure 2, which shows a sharp rise in healthcare costs in the senior years [3].
On the other hand, the relative decrease in the proportion of healthcare workers is increasing the demands on the active workforce, which is itself ageing in many countries. Data from the World Health Organization (WHO) indicates that the average age of nurses employed today is between 41 and 45 years old in several European countries, including Denmark, France, Iceland, Norway and Sweden [4]. Furthermore, the WHO states that the global health worker shortfall is already over 4.2 million.
This unnerving situation is further complicated by ever increasing healthcare costs, mainly driven by increasing hospital charges, the increasing cost of professional services, and the increasing price of drugs and medical devices [5]. It is estimated that the per capita health spending across the OECD countries has grown, in real terms, by an average of 4.1% annually over the 10-year period between 1997 and 2007. “By comparison, average economic growth over this period was 2.6%, resulting in an increasing share of the economy devoted to health in most countries” [6].
With the growing demands on health systems, it is inevitable that the future of healthcare will be linked to robotics. Although robots represent a significant cost, manufacturing has demonstrated that the use of robots can also offer significant savings and hence can contribute towards the establishment of high quality, sustainable, and affordable healthcare systems [7]. Important application domains that could benefit include medical training, rehabilitation, prosthetics, surgery, diagnosis, and physical and social assistance to disabled and elderly people [8, 9].
In addition to the benefits it can bring to patients, health workers and the overall healthcare system, the development of healthcare robotics has the potential to have a significant impact on industrial and commercial activity. The reason, according to a report from the Economist Intelligence Unit [10], is quite simple: the huge healthcare societal need is also a huge business opportunity. The global medical robotics market is expected to reach USD 11.4 billion by 2020 from USD 4.2 billion in 2015, registering a compound annual growth rate (CAGR) of 22.2% during this period [11].
Within the overarching healthcare sector, surgery is a major area for robotics. Surgical robotics is of growing economic importance as new systems demonstrate they can revolutionise surgical operations, raising precision, safety and efficiency to previously unimaginable levels. Globally, surgical robotics is expected to grow from a USD 3.3 billion market in 2014 to USD 6.4 billion by 2020, registering a CAGR of 10.2% over the period [12].
Microsurgery can be truly described as a highly skilled “art”, integrating years of medical knowledge and experience with complex, highly precise and dexterous manual operations. The term “microsurgery” was traditionally used to classify delicate surgical procedures requiring the use of an operating microscope. However, the recent advent of high resolution imaging sensors now allows microsurgery to be performed also in a minimally invasive fashion through the use of endoscopes. In any case, irrespective of the viewing technique, the vast majority of microsurgerical procedures are still performed directly by “the dexterous hands of highly capable clinicians, who go through extensive training periods to acquire the specialized skills necessary for realizing successful micro-operations” [13].
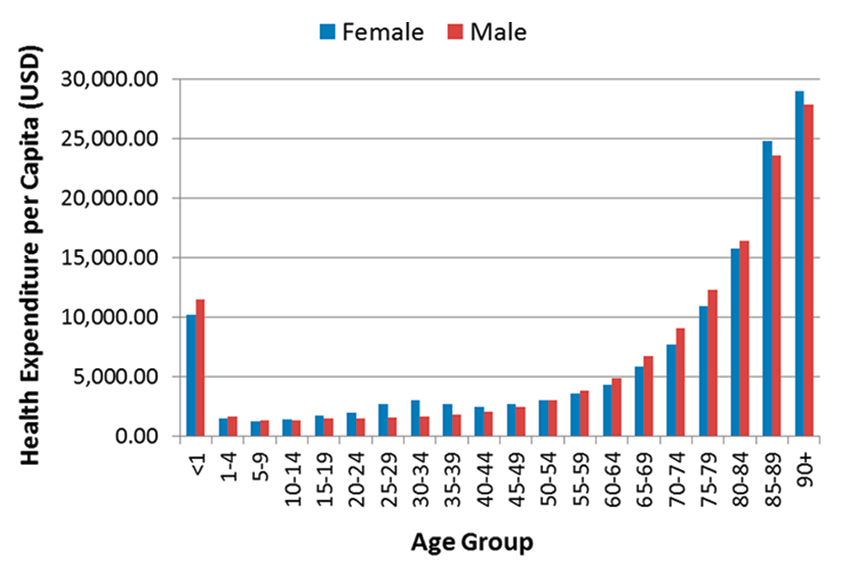
Figure 2
Canada’s public healthcare expenditure per capita per age group in 2013 (based on data from [2]).
Given the minuscule dimensions of the surgical site and the stringent precision requirements, microsurgery is a prime area for the application of robots. Even more than for regular, larger scale operations, robot-assisted systems can have a deep impact in microsurgery, providing significantly increased dexterity, controllability and precision to surgeons, allowing the execution of more precise and safer operations, or even the pioneering of previously impossible procedures. All of these benefits can directly impact the work and productivity of microsurgeons in several ways, including: (1) reduced physically induced stress, allowing surgeons to perform more operations per day without fatigue or decrease in performance; and (2) extended professional career, allowing surgeons to reach expert levels earlier in their careers and to maintain high levels of precision and dexterity for longer.
Applications of precision medicine based on microsurgery techniques already include a large range of surgical specialties, but this number is expected to increase as technologies allowing the early detection of diseases continue to improve. Currently, the anastomosis of small blood vessels and nerves is one of the most common microsurgery procedures, used chiefly in plastic and reconstructive operations. However, other treatments are starting to make growing use of precise excision of tissue from delicate organs, typically to treat benign or malignant lesions. For example, microsurgery techniques are regularly used in paediatric and fetal surgery, ophthalmology, otolaryngology, and urology. Table 1 presents examples of such applications with their respective estimated accuracy requirements and currently used imaging and tissue manipulation methods.
Another important and growing application area for microsurgery (and thus for microsurgery robots) is oncology. On one hand, the annual incidence of cancer worldwide is expected to quickly increase in the near future, largely as a result of global ageing, reaching 17 million by 2020 and 27 million by 2030 [27]. This projection is corroborated by other studies, including that of Yancik [28], which highlights the disproportionality with which cancer affects senior citizens (people over 65 years old) and how this is expected to impact cancer incidence in the aging society. On the other hand, the establishment of new routine examinations based on better imaging technologies and diagnosis systems, such as immunosignaturing [29], are continuously enhancing the detection of cancers at early stages. This is not only an important factor for increasing the chances of survival (fig. 3) but also allows less extensive surgery [31], potentially limiting interventions to the microsurgery of small tissue volumes.
Robotic microsurgery of early tumours offers the promise of treatment with minimal collateral damage, both in terms of the functionality of organs operated upon, and of the patient’s postoperative quality of life. It also has the potential to allow faster and safer surgical procedures, leading to faster patient recovery and reduced burden on the healthcare system. Nonetheless, to deliver these benefits on a large scale, robotic microsurgery systems still have to overcome some key challenges, as described in the next section.
| Table 1: Examples of clinical specialties based on microsurgery | ||||
| Clinical specialty (example) | Estimated accuracy requirement | Imaging method | Tissue manipulation method | References |
| Fetal surgery (twin-twin transfusion syndrome) | 250 µm | Endoscope | Hand-held laparoscopy instruments | [14, 15] |
| Ophthalmology (retinal vein cannulation) | 100 µm | Microscope | Hand-held instruments | [16, 17] |
| Otology (hearing aid implantation) | 400 µm | Microscope | Hand-held instruments | [18, 19] |
| Laryngology (vocal cord cordectomy) | 50 µm | Microscope | Hand-held instruments; laser micromanipulator | [20–22] |
| Reconstructive plastic surgery (microvascular anastomosis) | 50 µm | Microscope | Hand-held instruments | [23, 24] |
| Urology (vasectomy reversal) | 50 µm | Microscope; endoscope | Hand-held instruments; surgical robot | [25, 26] |
Mechatronics forms the first major challenge for the development of microsurgical robots for a number of reasons, including the need for dexterous miniaturised tools and end-effectors that are compatible with the surgical applications. The need for miniaturisation is, and will continue to be, important in all areas of surgery, but especially in minimally invasive surgical procedures, which have already lead to reduced lengths of hospitalisation, surgical complications and postoperative pain compared with traditional open procedures. This is particularly significant for older patients, who tend to be less tolerant of major surgery [32].
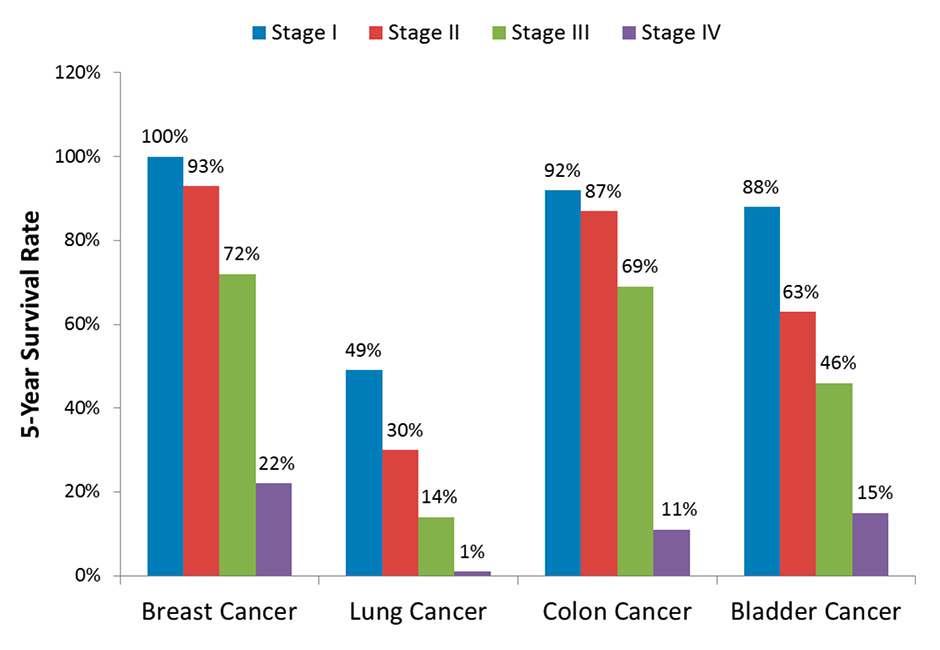
Figure 3
Five-year survival rate for common cancer types by stage at diagnosis (based on data from [30]).
Microelectromechanical systems (MEMS) technology has the potential to play a significant role in reducing the size and increasing the functionality of microsurgical devices [33], although limitations related to actuation power, dexterity and robustness of the MEMS devices continue to be open issues for practical surgical applications [34].
Microsurgery also presents stringent demands for the precision and speed of surgical tools. Tooling and interfaces should be fast enough to follow surgeon commands in real time and also provide microscopic precision to allow proper control during delicate procedures. Therefore, another significant challenge for microsurgical robots will be overcoming hardware, actuation and control problems to reconcile these competing objectives.
Finally, flexibility is an important requirement and a significant challenge for microsurgical robots designed to operate inside the human body. Flexibility increases the capacity of the system to reach difficult parts of the anatomy with minimal or no collateral damage to other organs, tissue or structures. However, achieving dexterity and precise manipulation with a flexible system is a nontrivial and currently unresolved problem. Possible solutions under research include miniature continuum robots [35, 36] and selectively compliant/stiffening mechanisms [37, 38].
Perception challenges for microsurgery robots are mainly related to the need for magnified stereoscopic visualisation of the surgical site, and the capacity to sense the small interaction forces between surgical tools and tissue in intracorporeal minimally invasive applications. Continued progress on the miniaturisation and quality of imaging sensors, largely driven by the cell-phone industry, is quickly reaching a point where small, high-resolution, chip-on-the-tip stereoscopic imaging sensors will allow flexible 3D microendoscopy [39]. On the other hand, sensing the interactions forces to allow safer tissue manipulation and palpation during microsurgery remains an active research topic, within which opto-electronic sensors are becoming the preferred solution due to their sensitivity, safety and potential to be integrated into small flexible tools [40–42].
Another perception challenge for future microsurgery systems relates to the capability to detect cancer tissue intraoperatively with high sensitivity and specificity. The creation of systems providing such capability will lead to more effective and higher quality microsurgery, allowing surgeons to define precise surgical margins that ensure total tumour eradication with minimal damage to healthy tissue. Progress in this direction uses a range of technologies, including narrow-band imaging (NBI) [43], fluorescence [44], autofluorescence [45], optical coherence tomography (OCT) [46] and mass spectroscopy [47]. The next challenge arising in this domain will be to miniaturise and integrate the most effective technologies into microsurgical robotic systems to fully exploit the benefits of real-time cancer detection.
Microsurgery robots allow surgeons to operate beyond the typical direct perceptual and manual dexterity limits of humans, but this in itself creates challenges related to the design and use of technologies to mediate actions such as surgical site visualisation, control of surgical tools and management of multisensory feedback derived from robot-patient interactions [13]. Consequently, the surgeon-robot interface must match the skills, limits and needs of the surgeon to increase surgical performance and safety.
The main issue to be solved here is the creation of a visualisation and control interface to maximise the ergonomics, intuitiveness of robot control and overall usability of the system. Current commercially available visualisation technologies are certainly sufficient to provide the surgeon with a high quality real-time stereoscopic view of the surgical area, so the challenge lies in the overall interface design and selection of the most appropriate visualisation technology for the application. Customised solutions include direct visualisation with traditional stereo-optical microscopes [48–50] and indirect methods based on stereoscopic and head-mounted displays [51, 52].
In future applications, indirect visualisation will probably be preferred both because it is required for non-line-of-sight surgery, and because it facilitates the use of augmented reality (AR) techniques to enhance the surgeon’s visual perception and awareness. AR allows relevant information to be added directly to the surgeon’s field of view. It can be used, for example, to highlight cancerous tissue [53, 54], provide intuitive feedback on intraoperative measurements [55], or to define and visualise surgical plans [56]. However, AR is yet another significant challenge in this area and a number of issues still have to be solved before it can be reliably used in surgical applications. These include the development of robust real-time algorithms to perceive the three-dimensional structure of the surgical scene and to estimate tissue motion and deformation through vision.
Finally, additional challenges in surgeon-robot interfaces are related to the intuitiveness and the capabilities of control devices and associated robot control software. Microsurgical robots can be, in fact, hand-held, co-manipulated or teleoperated, but the goal of the control interface is always to enable intuitive interaction to facilitate improvements to surgical precision, accuracy and safety. In this respect, haptic feedback is seen as a major feature to improve microsurgical performance, enabling the surgeon to touch and feel the surgical site and to control interaction forces to avoid damage to delicate anatomical structures. Haptic feedback can also enhance surgical precision and safety through guidance features [57] and active constraints [58]. In addition, haptics is an effective channel for sensory substitution, which can be used to provide critical intraoperative information to the surgeon without disrupting or overloading his/her visual channel [59, 60].
Microsurgery robotics is an active and growing research area, which is progressively overcoming critical challenges to deliver clinically applicable systems. As discussed by Marcus et al., these included economic-, clinical-, and research-related factors that act as barriers to the translation of laboratory prototypes into commercial systems [23].
Most of the microsurgery robot prototypes created to date focus on ophthalmology and ear, nose and throat (ENT) applications. In addition, as with almost all robotic systems, they are almost exclusively designed to be teleoperated or co-manipulated, which helps to avoid operational, ethical and legal barriers related to the automation of surgical tasks [61]. Still, at the present time there is no commercial microsurgical robot available in the market.
The only robot currently in clinical use for microsurgery is the Intuitive Surgical’s da Vinci robot [52]. Although this system was not created for such applications, and does not offer proper microsurgical tools, it is widely available in hospitals around the globe. This has prompted clinical research into its possible use for microsurgery, and the application is steadily growing [62]. The results of such efforts are not only contributing to establishing clinical evidence of the benefits of robotics in microsurgery, but also increasing the interest of microsurgeons in robotic tools. This in turn is generating a positive feedback for the expansion of research into a wider range of microsurgical applications. Table 2 provides an overview of recent microsurgery robots found in the literature.
| Table 2: Examples of microsurgery robots currently under development. | ||||
| Robot name | Main clinical application | Characteristics | Institution | Ref. |
| Micron | Ophthalmology | Hand-held actively stabilised tool that actively reduces unintentional motions to increase microsurgery precision | Carnegie Mellon University, USA | [63] |
| EyeRobot2.1 | Ophthalmology | Co-manipulation robot based on a symmetric mechanical RCM mechanism and capable of 3 DOF force sensing at the tool tip for vitreoretinal eye surgery | Johns Hopkins University, USA | [49] |
| Eye-RHAS | Ophthalmology | Teleoperated robot based on a mechanical remote RCM mechanism for vitreoretinal eye surgery | Eindhoven University of Technology, The Netherlands | [64] |
| Robotic Retinal Surgery | Ophthalmology | Teleoperated or co-manipulated RCM robot for retinal surgery with force sensing capabilities | University of Leuven, Belgium | [40] |
| Smart Surgical Drill | Otology | Hand-held autonomous smart drill for cochleostomy capable of controlling drill bit protrusion beyond the medial surface to within 0.02 mm of the ideal position | Brunel University, UK | [65] |
| Miniature Robot | Otology | Image-guided 5 DOF parallel robot for precise bone drillings for hearing aid implantation | University of Bern, Switzerland | [66] |
| Bone-Attached Robot | Otology | Image-guided 4 DOF milling robot that is fixed to the patient’s skull using a rigid positioning frame screwed into the surface of the skull | Vanderbilt University, USA | [67] |
| RALP | Laryngology | Teleoperated 2 DOF laser micromanipulator robot for vocal cord laser microsurgery controlled through a tablet interface | Istituto Italiano di Tecnologia, Italy | [68] |
| µRALP | Laryngology | Teleoperated flexible robotic endoscope for transoral laser microsurgery featuring stereo vision and micromechatronic laser manipulator controlled through a tablet interface | µRALP research consortium, Europe | [69] |
| MicroSure | Reconstructive surgery | Teleoperated 7 DOF robot with force sensing and haptic feedback for reconstructive microsurgery | Eindhoven University of Technology, The Netherlands | [50] |
| DOF = degrees of freedom; RCM = remote centre-of-motion | ||||
Laser phonomicrosurgery is a challenging medical procedure used to treat abnormalities on the vocal cords through a minimally invasive transoral approach. The current state-of-the-art surgical setup is illustrated in figure 4. This type of operation typically involves the precise excision of tissue mass associated with benign or malignant lesions. The surgery is performed under a microscope using a surgical laser beam and other specialised surgical instruments such as long microsurgical forceps. The procedure is extremely delicate, not simply because of the small dimensions of the surgical site (often in the order of few millimetres), but also because a major clinical goal involves minimising trauma to surrounding healthy tissue, which directly impacts the organ functionality and the patient’s postoperative voice and quality of life.
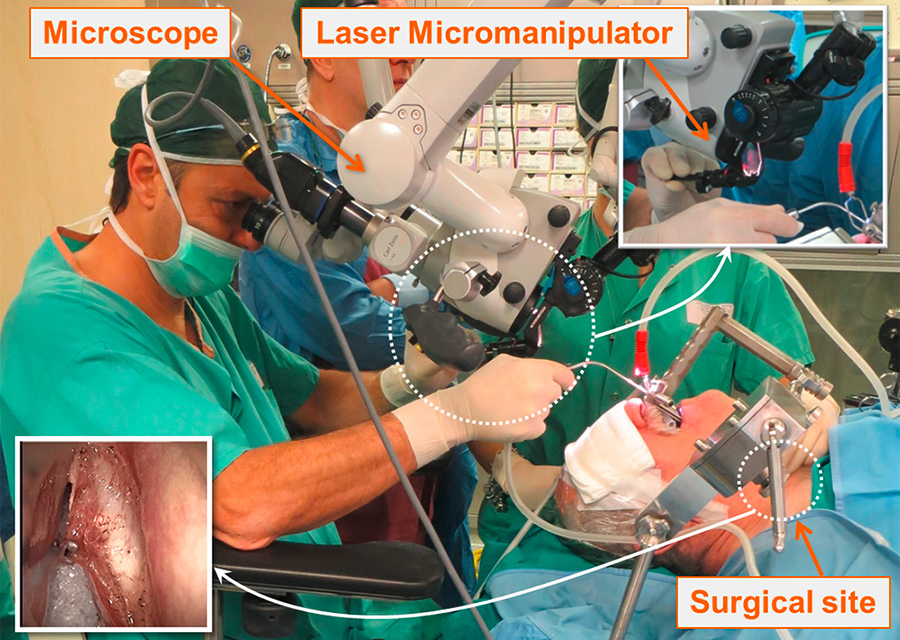
Figure 4
Current laser phonomicrosurgery setup imposes severe challenges on the surgeon in terms of surgical precision, laser controllability and ergonomics.
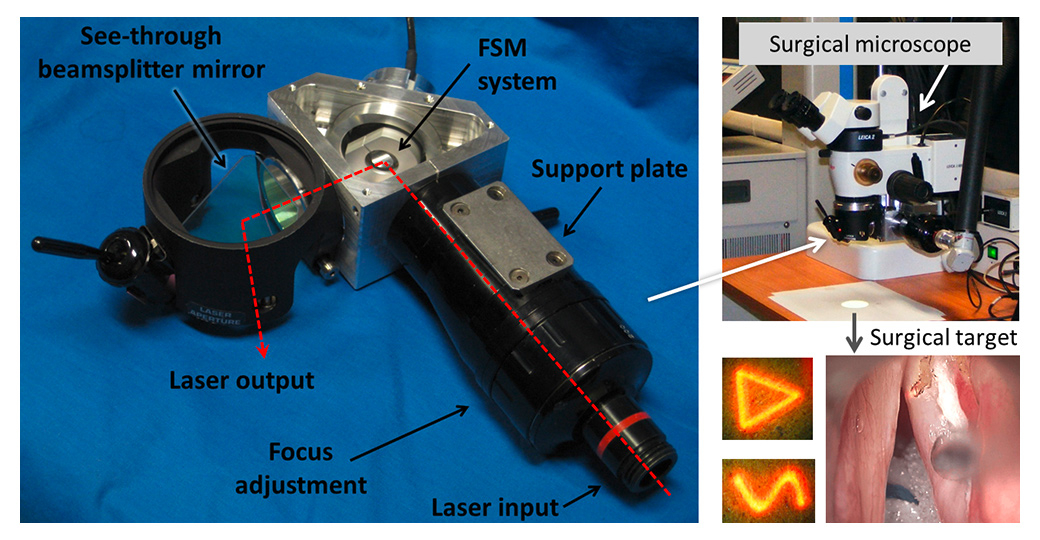
Figure 5
The IIT laser micromanipulator for robot-assisted laser microsurgery. The device can accurately follow surgeon commands in real-time, including the generation of customised laser scan patterns that significantly enhance the quality of laser ablations and allow preview of surgical actions.
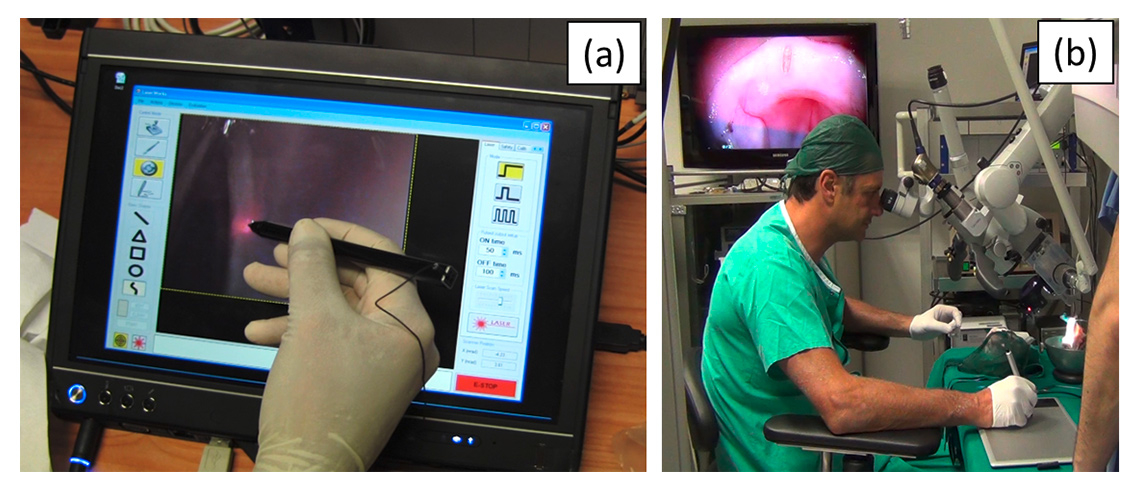
Figure 6
Robot-assisted laser control interfaces. The Virtual Scalpel system enables intuitive and accurate control of the surgical laser using a stylus pen and a touchscreen interface (a) or a graphics tablet (b).
Given the challenges and limitations associated with this surgical procedure, laser phonomicrosurgery is a prime application area for robotics [68]. This is an area where new technologies can significantly impact surgical capabilities and outcomes. For this reason, multidisciplinary collaborative research between the Istituto Italiano di Tecnologia (IIT, Genoa, Italy) and the San Martino Hospital (UNIGE, Genoa, Italy) has focused on the development of innovative robot-assisted systems for this specific application. The research has resulted in the creation of a motorised and fully programmable laser micromanipulator device (fig. 5) that eliminates a major limitation of current laser microsurgery systems: the unassisted manual control (using a mechanical joystick) of the laser beam. The IIT laser micromanipulator is based on a fast steering mirror, offering rapid and highly accurate robotic control of the laser beam motions with precision and repeatability of 4 µm at the typical operating distance of 400 mm [70]. These specifications allow the system to accurately scan the laser over trajectories defined in real time by the surgeon, greatly enhancing the quality of laser-tissue interactions and the precision and safety of the surgical operations [56].
In addition to the mechatronics advancements of the robotic laser micromanipulator, the research produced results in surgeon-robot interface design for laser control, using a system called Virtual Scalpel [71]. This control interface is based on a tablet computer that controls the surgical laser directly from a live video of the surgical area via a stylus pen (fig. 6a). By touching the tablet, the surgeon commands the robotic system to automatically aim the laser at that exact point and start the ablation process. This allows the stylus to work as a “conventional” scalpel with real effect on the surgical target. Comparative experiments performed with surgeons and medical students showed that this system is highly precise and intuitive to use, resulting in a twofold improvement in surgical accuracy [68]. These results are illustrated in figure 7 and demonstrate another important assistive benefit of microsurgical robotic systems: the capability to facilitate operations and enhance surgical performance through the design of innovative control interfaces.
Preclinical trials with the Virtual Scalpel also led to the creation of an alternative interface concept that promises to bring the system to real clinical application in a shorter period of time. This alternative interface is shown in figure 6b. It imposes minimal modifications to the current surgical setup by decoupling the surgical site visualisation from the tablet-based laser control. This allows the surgeon to continue to use the traditional stereo surgical microscope while benefiting from the tablet interface and the advances brought by the new robotic technologies [48].
Additional benefits of the IIT RALP system include assistive/augmentation functions created to further enhance surgical precision, efficiency and safety. For example, cognitive models of laser-tissue interactions have been developed to provide real-time feedback on the laser incision depth [72], allowing significantly improved control over this third dimension of the laser ablation process (fig. 7), and the full automation of laser incisions with high accuracy [73]. Other examples include functions for the automatic vaporisation of tissue within a surgeon-defined area and to a specific depth, and the definition of virtual fixtures (no-go areas), which offer the possibility to protect delicate areas of the surgical field from accidental laser damage [51].
The RALP system described above provides a significant step change with respect to the current state of the art in laser phonomicrosurgery equipment. Nevertheless, a number of limitations related to the surgical setup remain unaddressed, namely: (1) the need to use a microscope, which requires a direct line of sight to the surgical field; (2) the use of a free-beam surgical laser, which also requires unobstructed direct line of sight to operate; and (3) the need to use long microsurgical forceps to manipulate the delicate laryngeal tissue, which requires high dexterity and surgical skills only obtained through extensive practice.
To address the first two limitations, an international multidisciplinary research consortium funded by the European Commission and led by IIT developed the endoscopic laser phonomicrosurgery system µRALP [69, 74]. The system, presented in figure 8, miniaturises the traditional surgical setup and places the imaging and laser actuation devices on the tip of a flexible endoscope that can be inserted into the throat of the patient, thus eliminating current requirements for a direct line of sight. Stereoscopic imaging is provided by two miniature cameras that allow augmented-reality visualization of the surgical field through a virtual microscope interface [51, 55]. The surgical laser beam is delivered through a flexible optical fibre to the tip of the endoscope, where it is controlled by a micromechatronic laser manipulator [75] using commands directed by the surgeon through the tablet interface.
The µRALP surgical system was evaluated through extensive experimentation involving expert ENT surgeons from San Martino Hospital (Genoa, Italy) and from the University Hospital of Besançon (France). Trials included cadaveric studies and demonstrated that the system has real potential to become the next standard in precision microsurgery both in the upper airways and other difficult-to-reach parts of the human body. Nonetheless, to achieve full clinical acceptance further research, development and innovation actions are required to increase the robustness, further miniaturise the system, and add tissue manipulation capabilities. This will eliminate the last remaining limitations.
Healthcare is a major societal challenge with daunting forecasts for the future, especially given the population ageing trend observed around the globe. The increasing number of patients, decreasing proportion of healthcare workers, and the increasing costs of care provide no lack of reasons to be concerned about the future. On the other hand, technological progress and the rise of medical robotics offer hope for the establishment of sustainable, affordable and high-quality healthcare systems.
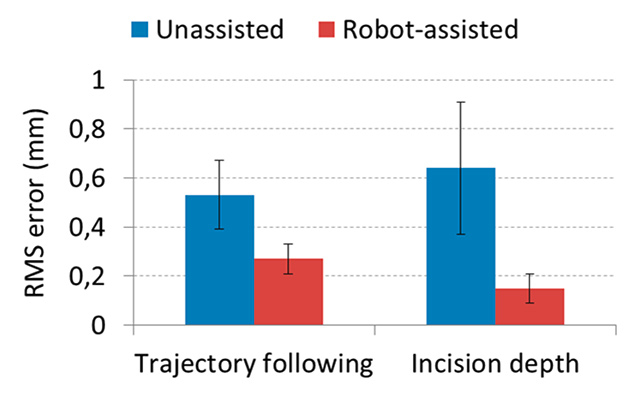
Figure 7
Experimental results demonstrating accuracy improvements in laser control provided by the IIT’s RALP system. (a) Root mean square (RMS) error on trajectory following experiments [68]. (b) RMS error on laser incision depth control [60].
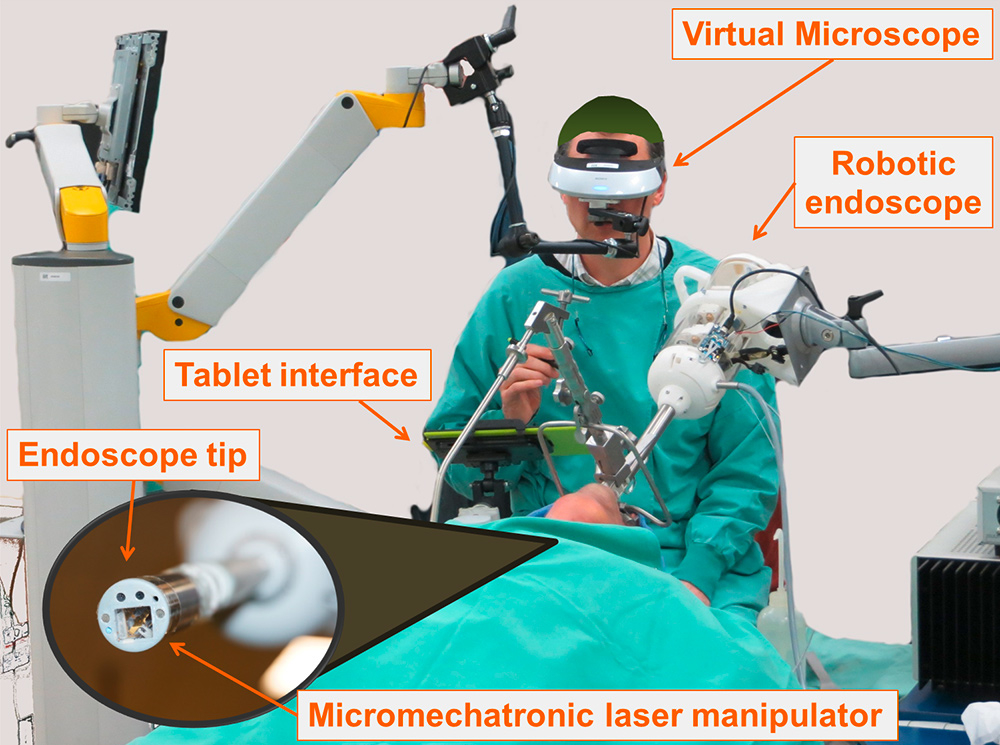
Figure 8
The µRALP surgical system for endoscopic laser phonomicrosurgery during a cadaver trial.
Robotics can offer significant contributions to the training of medical personnel, improving the skill levels of trainees and lengthening the effective career of experienced surgeons while enhancing their capacity and efficiency in providing care. This is especially significant in microsurgery, which requires a specialised set of skills and capabilities only acquired through extensive training. Microsurgery is, in fact, expected to gain importance in a growing range of surgical specialties as a result of progress in disease detection, diagnosis and treatment at early stages. In this scenario, robotic microsurgery will be at the forefront of surgical treatment.
Microsurgery robotics offers the promise of enhanced precision, safety, efficiency and quality to highly delicate and demanding operations by augmenting the surgeon’s sensing and actuation capabilities. However, several challenges have yet to be overcome in terms of mechatronics, perception and surgeon-robot interfaces before microsurgical robots and surgeons can achieve their full potential in operating rooms. Nonetheless, research is quickly progressing and a number of microsurgery robots are coming out of laboratories and demonstrating significant clinical benefits in challenging applications such as reconstructive plastic surgery, ophthalmology, otology and laryngology. These are reassuring results offering hope for a brighter healthcare future.
Disclosure statement: No financial support and no potential conflict of interest relevant to this article were reported.
1 Historical Population Data and Projections (1950–2050) [Internet]. Organisation for Economic Co-operation and Development (OECD). [cited 2016 Aug 1]. Available from https://stats.oecd.org/
2 Canadian Institute for Health Information. National Health Expenditure Trends, 1975 to 2015. Ottawa: CIHI; 2015.
3 Deraspe R. Canada’s Aging Population and Public Policy – The Effects on Health Care. Ottawa, Canada. Parliamentary Information and Research Service, Social Affairs Division; 2011 Oct. Publication No.: 2011-122-E.
4 World Health Organization. Features: 10 facts on health workforce crisis [Internet]. c2008 – [cited 2016 Aug 1]. Available from: http://www.who.int/features/factfiles/health_workforce/en/
5 Moses H, 3rd, Matheson DH, Dorsey ER, George BP, Sadoff D, Yoshimura S. The anatomy of health care in the United States. JAMA. 2013;310(18):1947–63. doi:http://dx.doi.org/10.1001/jama.2013.281425.
6 Organisation for Economic Co-operation and Development. Health at a Glance 2009: OECD Indicators. Paris: OECD Publishing; 2009.
7 Butter M, Rensma A, van Boxsel J, Kalisingh S, Schoone M, Leis M, et al. Robotics for Healthcare: Final Report. Brussels: DG Information Society, European Commission; 2008.
8 Datteri E, Tamburrini G. Ethical Reflections on Health Care Robotics. In: Capurro R, Nagenborg M, editors. Ethics and Robotics. Amsterdam: IOS Press; 2009. p. 35–48.
9 Broekens J, Heerink M, Rosendal H. Assistive Social Robots in Elderly Care: A Review. Gerontechnology (Valkenswaard). 2009;8(2):94–103. doi:http://dx.doi.org/10.4017/gt.2009.08.02.002.00.
10 Kielstra P, Scott I. (The Economist Intelligence Unit Limited, London). Healthcare Strategies for an Ageing Society. Philips; 2009.
11 Markets and Markets. Medical Robots Market [Internet]. c2015 – [cited 2016 Aug 1]. Available from: http://www.marketsandmarkets.com/PressReleases/medical-robotic-systems.asp
12 Allied Market Research. World Surgical Robotics Market – Opportunities and Forecast, 2014 – 2020 [Internet]. c2016 Jan – [cited 2016 Aug 1]. Available from: https://www.alliedmarketresearch.com/surgical-robotics-market
13 Mattos LS, Pardo D, Olivieri E, Barresi G, Ortiz J, Fichera L, et al. Microsurgery Systems. In: Eren H, Webster JG, editors. The E-Medicine, E-Heath, M-Health, Telemedicine, and Telehealth Handbook, vol. II – Telehealth and Mobile Health. Boca Raton (FL): CRC Press, Taylor & Francis Group; 2015. p. 61–89.
14 Vrecenak JD, Flake AW. Fetal surgical intervention: progress and perspectives. Pediatr Surg Int. 2013;29(5):407–17. doi:http://dx.doi.org/10.1007/s00383-013-3304-x.
15 Behrendt N, Galan HL. Twin-twin transfusion and laser therapy. Curr Opin Obstet Gynecol. 2016;28(2):79–85. doi:http://dx.doi.org/10.1097/GCO.0000000000000247.
16 Singhy SPN, Riviere CN. Physiological tremor amplitude during retinal microsurgery. In: Bioengineering Conference. Proceedings of the IEEE 28th Annual Northeast Bioengineering Conference; 2002 April 20–21; Philadelphia, USA. IEEE; 2002. p. 171–2.
17 Gijbels A, Vander Poorten EB, Gorissen B, Devreker A, Stalmans P, Reynaerts D. Experimental validation of a robotic comanipulation and telemanipulation system for retinal surgery. In: Biomedical Robotics and Biomechatronics. Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics; 2014 Aug 12–15; Sao Paulo, Brazil. IEEE; 2014. p. 144–50.
18 Warren FM, Balachandran R, Fitzpatrick JM, Labadie RF. Percutaneous cochlear access using bone-mounted, customized drill guides: demonstration of concept in vitro. Otol Neurotol. 2007;28(3):325–9. doi:http://dx.doi.org/10.1097/01.mao.0000253287.86737.2e.
19 Coulson CJ, Reid AP, Proops DW, Brett PN. ENT challenges at the small scale. Int J Med Robot. 2007;3(2):91–6. doi:http://dx.doi.org/10.1002/rcs.132.
20 Steiner W, Ambrosch P. Endoscopic Laser Surgery of the Upper Aerodigestive Tract: With Special Emphasis on Cancer Surgery. Stuttgart: Thieme; 2000.
21 Mattos LS, Caldwell DG, Dellepiane M, Grant E. Design and control of a robotic system for assistive laser phonomicrosurgery. In: Engineering in Medicine and Biology. Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology; 2010 Aug 31 – Sep 4; Buenos Aires, Argentina. IEEE; 2010. p. 5411–5.
22 Olds KC, Chalasani P, Pacheco-Lopez P, Iordachita I, Akst LM, Taylor RH. Preliminary evaluation of a new microsurgical robotic system for head and neck surgery. In: Intelligent Robots and Systems. Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems; 2014 Sept 14–18; Chicago, USA. IEEE; 2014. p. 1276–81.
23 Hamahata A, Kubo K, Matsumoto H, Yamaki T, Sakurai H. Usefulness of stabilizer for microanastomoses of internal thoracic vessels during diep flap breast reconstruction. J Reconstr Microsurg Open. 2016;1(1):26–8. doi:http://dx.doi.org/10.1055/s-0036-1571845.
24 Matsumura N, Hayashi N, Kamiyama H, Kubo M, Shibata T, Okamoto S, et al. Microvascular anastomosis at 30-50× magnifications (super-microvascular anastomosis) in neurosurgery. Surg Neurol Int. 2011;2(1):6. doi:http://dx.doi.org/10.4103/2152-7806.76143.
25 Patel AP, Smith RP. Vasectomy reversal: a clinical update. Asian J Androl. 2016;18(3):365–71. doi:http://dx.doi.org/10.4103/1008-682X.175091.
26 Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl. 2013;15(1):67–74. doi:http://dx.doi.org/10.1038/aja.2012.131.
27 World Health Organization, National Institute on Aging (USA), National Institutes of Health (USA). Global Health and Aging. Betheseda, Maryland: NIH Publications; 2011.
28 Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–41. doi:http://dx.doi.org/10.1097/00130404-200511000-00002.
29 Stafford P, Cichacz Z, Woodbury NW, Johnston SA. Immunosignature system for diagnosis of cancer. Proc Natl Acad Sci USA. 2014;111(30):E3072–80. doi:http://dx.doi.org/10.1073/pnas.1409432111.
30 American Cancer Society. Key statistics about breast cancer [Internet]. c2016 – [cited 2016 May 5]. Available from: http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-survival-by-stage/
31 Cancer Research UK. Saving lives and averting costs? The case for earlier diagnosis just got stronger [Internet]. c2014 – [cited 2016 Aug 1]. Available from: http://scienceblog.cancerresearchuk.org/2014/09/22/saving-lives-and-averting-costs-the-case-for-earlier-diagnosis-just-got-stronger/
32 Marcus H, Nandi D, Darzi A, Yang GZ. Surgical robotics through a keyhole: from today’s translational barriers to tomorrow’s “disappearing” robots. IEEE Trans Biomed Eng. 2013;60(3):674–81. doi:http://dx.doi.org/10.1109/TBME.2013.2243731.
33 Rebello KJ. Applications of MEMS in surgery. Proc IEEE. 2004;92(1):43–55. doi:http://dx.doi.org/10.1109/JPROC.2003.820536.
34 Pedrocchi A, Hoen S, Ferrigno G, Pedotti A. Perspectives on MEMS in bioengineering: a novel capacitive position microsensor [and laser surgery and drug delivery applications]. IEEE Trans Biomed Eng. 2000;47(1):8–11. doi:http://dx.doi.org/10.1109/10.817612.
35 Bajo A, Dharamsi LM, Netterville JL, Garrett CG, Simaan N. Robotic-assisted micro-surgery of the throat: The trans-nasal approach. In: Robotics and Automation. Proceedings of the 2013 IEEE International Conference on Robotics and Automation; 2013 May 6-10; Karlsruhe, Germany. IEEE; 2013. p. 232–8.
36 Bergeles C, Gosline AH, Vasilyev NV, Codd PJ, Del Nido PJ, Dupont PE. Concentric tube robot design and optimization based on task and anatomical constraints. IEEE Trans Robot. 2015;31(1):67–84. doi:http://dx.doi.org/10.1109/TRO.2014.2378431.
37 Hadi Sadati SM, Noh Y, Elnaz Naghibi S, Kaspar A, Nanayakkara T. Stiffness control of soft robotic manipulator for minimally invasive surgery (mis) using scale jamming. In: Intelligent Robotics and Applications. Proceedings of the 8th International Conference on Intelligent Robotics and Applications; 2015 Aug 24-27; Portsmouth, UK. Springer International Publishing; 2015. p. 141–51.
38 Shiva A, Stilli A, Noh Y, Faragasso A, Falco ID, Gerboni G, et al. Tendon-based stiffening for a pneumatically actuated soft manipulator. IEEE Robot Autom Lett. 2016;1(2):632–7. doi:http://dx.doi.org/10.1109/LRA.2016.2523120.
39 Charalampaki P, Igressa A, Mahvash M, Pechlivanis I, Schick B. Optimal invasive key-hole neurosurgery with a miniaturized 3D chip on the tip: Microendoscopic device. Asian J Neurosurg. 2013;8(3):125–31. doi:http://dx.doi.org/10.4103/1793-5482.121681.
40 Gijbels A. Development and evaluation of robotic technology for safe and successful retinal vein cannulation [dissertation]. Leuven (Belgium): KU Leuven; 2015.
41 He X, Handa J, Gehlbach P, Taylor R, Iordachita I. A submillimetric 3-DOF force sensing instrument with integrated fiber Bragg grating for retinal microsurgery. IEEE Trans Biomed Eng. 2014;61(2):522–34. doi:http://dx.doi.org/10.1109/TBME.2013.2283501.
42 Noh Y, Sareh S, Würdemann H, Liu H, Back J, Housden J, et al. Three-axis fiber-optic body force sensor for flexible manipulators. IEEE Sens J. 2016;16(6):1641–51. doi:http://dx.doi.org/10.1109/JSEN.2015.2488099.
43 Barbalata C, Mattos LS. Laryngeal tumor detection and classification in endoscopic video. IEEE J Biomed Health Inform. 2016;20(1):322–32. doi:http://dx.doi.org/10.1109/JBHI.2014.2374975.
44 Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation – a new cutting edge. Nat Rev Cancer. 2013;13(9):653–62. doi:http://dx.doi.org/10.1038/nrc3566.
45 Žargi M, Fajdiga I, Šmid L. Autofluorescence imaging in the diagnosis of laryngeal cancer. Eur Arch Otorhinolaryngol. 2000;257(1):17–23. doi:http://dx.doi.org/10.1007/PL00007506.
46 Yu H, Shen JH, Shah RJ, Simaan N, Joos KM. Evaluation of microsurgical tasks with OCT-guided and/or robot-assisted ophthalmic forceps. Biomed Opt Express. 2015;6(2):457–72. doi:http://dx.doi.org/10.1364/BOE.6.000457.
47 Balog J, Sasi-Szabó L, Kinross J, Lewis MR, Muirhead LJ, Veselkov K, et al. Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med. 2013;5(194):194ra93. doi:http://dx.doi.org/10.1126/scitranslmed.3005623.
48 Mattos LS, Guastini L, Mora F, Caldwell DG, Peretti G. Robot-assisted system for free-beam transoral laser microsurgery. In: Medical and Biological Engineering and Computing. Proceedings of the XIV Mediterranean Conference on Medical and Biological Engineering and Computing; 2016 Mar 31 – Apr 2; Paphos, Cyprus. Springer International Publishing; 2016. p. 714–9.
49 He X, Roppenecker D, Gierlach D, Balicki M, Olds K, Gehlbach P, et al. Toward clinically applicable steady-hand eye robot for vitreoretinal surgery. In: Proceedings of the International Mechanical Engineering Congress and Exposition, vol. 2: Biomedical and Biotechnology; 2012 Nov 9-15; Houston, USA. ASME; 2012. p. 145–53.
50 Cau R. Design and realization of a master-slave system for reconstructive microsurgery [dissertation]. Eindhoven, (Netherlands): Technische Universiteit Eindhoven; 2014.
51 Deshpande N, Ortiz J, Caldwell DG, Mattos LS. Enhanced computer-assisted laser microsurgeries with a virtual microscope based surgical system. In: Robotics and Automation. Proceedings of the 2014 IEEE International Conference on Robotics and Automation; 2014 May 31 – June 7; Hong Kong, China. IEEE; 2014. p. 4194–9.
52 Intuitive Surgical Inc. da Vinci Surgical System [Internet]. c2016 – [cited 2016 Aug 3]. Available from: http://www.intuitivesurgical.com/products/davinci_surgical_system/
53 Sridhar AN, Hughes-Hallett A, Mayer EK, Pratt PJ, Edwards PJ, Yang GZ, et al. Image-guided robotic interventions for prostate cancer. Nat Rev Urol. 2013;10(8):452–62. doi:http://dx.doi.org/10.1038/nrurol.2013.129.
54 Reaungamornrat S, Liu WP, Wang AS, Otake Y, Nithiananthan S, Uneri A, et al. Deformable image registration for cone-beam CT guided transoral robotic base-of-tongue surgery. Phys Med Biol. 2013;58(14):4951–79. doi:http://dx.doi.org/10.1088/0031-9155/58/14/4951.
55 Schoob A, Kundrat D, Lekon S, Kahrs LA, Ortmaier T. Color-encoded distance for interactive focus positioning in laser microsurgery. Opt Lasers Eng. 2016;83:71–9. doi:http://dx.doi.org/10.1016/j.optlaseng.2016.03.002.
56 Mattos LS, Caldwell DG. Safe teleoperation based on flexible intraoperative planning for robot-assisted laser microsurgery. In: Medicine and Biology. Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2012 Aug 28 – Sept 1; San Diego, USA. IEEE; 2012. p. 174–8.
57 Bowyer SA, Rodriguez y Baena F. Dissipative control for physical human-robot interaction. IEEE Trans Robot. 2015;31(6):1281–93. doi:http://dx.doi.org/10.1109/TRO.2015.2477956.
58 Bowyer SA, Rodriguez Y Baena F. Deformation invariant bounding spheres for dynamic active constraints in surgery. Proc Inst Mech Eng H. 2014;228(4):350–61. doi:http://dx.doi.org/10.1177/0954411914527440.
59 Meli L, Pacchierotti C, Prattichizzo D. Sensory subtraction in robot-assisted surgery: fingertip skin deformation feedback to ensure safety and improve transparency in bimanual haptic interaction. IEEE Trans Biomed Eng. 2014;61(4):1318–27. doi:http://dx.doi.org/10.1109/TBME.2014.2303052.
60 Fichera L, Pacchierotti C, Olivieri E, Prattichizzo D, Mattos LS. Kinesthetic and vibrotactile haptic feedback improves the performance of laser microsurgery. In: Proceedings of the IEEE Haptics Symposium; 2016 April 8-11; Philadelphia, USA. IEEE; 2016. p. 59-64.
61 Stanberry B. Telemedicine: barriers and opportunities in the 21st century. J Intern Med. 2000;247(6):615–28. doi:http://dx.doi.org/10.1046/j.1365-2796.2000.00699.x.
62 Liverneaux P, Berner S, Bednar M, Parekattil S, Mantovani G, Selber J, eds. Telemicrosurgery – Robot Assisted Microsurgery. Paris: Springer-Verlag France; 2013.
63 Maclachlan RA, Becker BC, Tabarés JC, Podnar GW, Lobes LA, Jr, Riviere CN. Micron: An actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012;28(1):195–212. doi:http://dx.doi.org/10.1109/TRO.2011.2169634.
64 Abedloo E, Gholami S, Taghirad HD. Eye-rhas manipulator: From kinematics to trajectory control. In: Robotics and Mechatronics. Proceedings of the 2015 3rd RSI International Conference on Robotics and Mechatronics; 2015 Oct 7-9; Tehran, Iran. IEEE; 2015. p. 61–6.
65 Brett PN, Baker DA, Reyes L, Blanshard J. An automatic technique for micro-drilling a stapedotomy in the flexible stapes footplate. Proc Inst Mech Eng H. 1995;209(4):255–62. doi:http://dx.doi.org/10.1243/PIME_PROC_1995_209_352_02.
66 Salzmann J, Zheng G, Gerber N, Stieger C, Arnold A, Rohrer U, et al. Development of a miniature robot for hearing aid implantation. In: Intelligent Robots and Systems. Proceedings of the 2009 IEEE/RSJ International Conference on Intelligent Robots and Systems; 2009 Oct 10-15; St. Louis, USA. IEEE; 2009. p. 2149–54.
67 Dillon NP, Balachandran R, Fitzpatrick JM, Siebold MA, Labadie RF, Wanna GB, et al. A compact, bone-attached robot for mastoidectomy. J Med Device. 2015;9(3):0310031–7. doi:http://dx.doi.org/10.1115/1.4030083.
68 Mattos LS, Deshpande N, Barresi G, Guastini L, Peretti G. A novel computerized surgeon-machine interface for robot-assisted laser phonomicrosurgery. Laryngoscope. 2014;124(8):1887–94. doi:http://dx.doi.org/10.1002/lary.24566.
69 µRALP European Project Consortium. µRALP Project Summary [Internet]. c2015 – [cited 2016 Aug 1]. Available from: http://www.microralp.eu/
70 Mattos LS, Dellepiane M, Caldwell DG. Next-generation micromanipulator for computer-assisted laser phonomicrosurgery. In: Medicine and Biology. Proceedings of the 33rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2011 Aug 30 – Sep 3; Boston, USA. IEEE; 2011. p. 4555–9.
71 Mattos LS, Dagnino G, Becattini G, Dellepiane M, Caldwell DG. A virtual scalpel system for computer-assisted laser microsurgery. In: Intelligent Robots and Systems. Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems; 2011 Sep 25-30; San Francisco, USA. IEEE; 2011. p. 1359–65.
72 Fichera L, Pardo D, Illiano P, Ortiz J, Caldwell DG, Mattos LS. Online estimation of laser incision depth for transoral microsurgery: approach and preliminary evaluation. Int J Med Robot. 2016;12(1):53–61. doi:http://dx.doi.org/10.1002/rcs.1656.
73 Fichera L, Pardo D, Illiano P, Caldwell DG, Mattos LS. Feed forward incision control for laser microsurgery of soft tissue. In: Robotics and Automation. Proceedings of the IEEE International Conference on Robotics and Automation; 2015 May 26-30; Seattle, USA. IEEE; 2015. p. 1235–40.
74 µRALP European Project Consortium. The µralp project: Micro-technologies and systems for robot-assisted laser phonomicrosurgery [Internet]. c2015 – [cited 2016 Aug 1]. Available from: http://www.microralp.eu/
75 Rabenorosoa K, Tasca B, Zerbib A, Rougeot P, Andreff N, Pengwang TE. Squipabot: A mesoscale parallel robot for a laser phonosurgery. Int J Optomechatronics. 2015;9(4):310–24. doi:http://dx.doi.org/10.1080/15599612.2015.1059534.