Interdisciplinary decision-making and treatment of intracranial aneurysms in the era of complementary microsurgical and endovascular techniques
DOI: https://doi.org/10.4414/smw.2016.14372
Serge
Marbacher, Michael
Diepers, Timo
Kahles, Krassen
Nedeltchev, Luca
Remonda, Javier
Fandino
Summary
Rupture of an intracranial aneurysm is a life-threatening event. Only one third of intracranial aneurysms rupture during a patient’s lifetime. Accurate markers that predict which intracranial aneurysms rupture and which do not are currently lacking in routine clinical practice. Therefore, the treatment decision is a careful balance between the natural history of the intracranial aneurysm and the risk of intervention based on aneurysm- and patient-specific risk factors. Many of these risk factors are also used to determine the modality of intervention. In this review, the authors discuss the interdisciplinary decision-making process and treatment approach in the era of complementary techniques for intracranial aneurysm obliteration.
Introduction
Rupture of an intracranial aneurysm (IA) causing subarachnoid haemorrhage (SAH) is a devastating event that is still associated with a 50% case fatality rate, despite major improvements in surgical techniques, diagnosis and interventional treatment [1]. In Switzerland, an estimated 250 000 patients are harbouring IAs and about 600 Swiss patients present with SAH every year [2, 3]. Although the incidence of SAH has remained stable over the past decade [4], general practitioners and internal medicine specialists, as well as highly informed individuals such as neurologists, interventional neuroradiologists and neurosurgeons, are likewise challenged by a rising number of newly detected unruptured IAs (UIAs) as a result of an increase in cranial imaging [5].
The decision to treat a UIA represents a clinical dilemma. The decision-making process underpinning whether or not to treat is a difficult balance of risk assessment between treatment on the one hand and risk of stroke, permanent neurological damage and death caused by spontaneous rupture on the other hand. This review provides an updated overview of the literature that supports decision-making in daily clinical practice and highlights the general trend towards multidisciplinary and complementary approaches to treat patients with IA.
Assessment of intracranial aneurysm rupture risk
Risk factors for IA rupture can be categorised into patient- and aneurysm-specific risk factors. Case-control studies for estimation of the natural history of UIA have determined robust patient-specific risk factors, including patient’s age, cigarette smoking, history of hypertension, female sex, and previous SAH. Aneurysm-specific risk factors are based on IA size, location, and morphological characteristics – such as irregularities, growth on serial imaging, size and aspect ratio (aneurysm height/neck width).
All currently available studies on the natural history of UIA demonstrate that the risk of IA rupture increases with increasing IA size and suggest that small UIAs have a low risk of rupture. In the prospective arm of the International Study of Unruptured Intracranial Aneurysms (ISUIA), a 5-year cumulative rupture rate of 0% for patients without prior SAH and anterior circulation aneurysms of less than 7 mm in size was demonstrated [6]. The risk of rupture for aneurysms smaller than 5 mm presented in the Unruptured Cerebral Aneurysm Study of Japan (UCAS) was 0.36% per year [7], which was in line with another Japanese prospective study specially designed to study the risk of small UIA (Small Unruptured Intracranial Aneurysm Verification study [SUAVe]; 0.34% per year for single IA) [8].
Based on these figures, preventive treatment is rarely justified and some patients might not be referred to a multidisciplinary stroke centre for risk analysis. However, the ISUIA and UCAS data contrast with other series [9–13], as well as clinical experience, which demonstrated that many aneurysms below a threshold of 7 mm do rupture more frequently. The incidence of de-novo IA found in routine follow-up screening is low (4.4%), but the rupture risk (14.5% over a 5-year period) is much higher than the risk of rupture of small-sized IA reported in ISUIA [6, 11]. Reasons for the discrepancy between these reports and the above-mentioned UIA cohort studies may be the generally high prevalence of small UIA [14] (up to 85–90% of all SAH are caused by small IA <10 mm [14–17]) and the partially biased selection process of UIA studies. In ISUIA, UCAS and SUAVE, the decision to treat an UIA was left to the discretion of the physician. Therefore, patients with a presumably high risk of rupture did not enter the observation cohort. Furthermore, a substantial number of patients received treatment (crossover after the UIA became symptomatic or asymptomatic growth occurred). Therefore, patients with aneurysms with a high risk of rupture were removed from the observation cohort. In addition, the rupture risk prediction holds true only for the first 5 years after diagnosis (owing to limited follow-up data) and cannot directly be projected to an anticipated lifetime risk. Short (5-year) observation periods may not accurately take account of the hypothesis that the risk of IA rupture over time is a nonlinear, discontinuous function of time with periods with high and low risks of rupture [18–21].
Predictive and scoring models for rupture risk estimation and management recommendation
In order to facilitate clinical decision-making in the treatment of IA systems for IA, risk assessment and management recommendations were developed. The PHASES score is a predictive model that provides absolute 5-year IA rupture risks based on six parameters (population, hypertension, age, size, location, and previous SAH) extracted from prospectively collected data from six cohort studies on UIA natural history (table 1) [22]. In a multicentre cohort of >500 patients with UIA, the PHASES risk score demonstrated its ability to identify aneurysms with a high relative risk of growth [23]. Further studies on the validation of the PHASES score are needed. In contrast to the PHASES score, the UIA treatment score (UIATS) is only indirectly based on published data. The UIATS derives from consensus on contemporary clinical practice of UIA treatment among an international expert panel consisting of neurologists, neurosurgeons, interventional neuroradiologists, and clinical epidemiologists. The score not only includes and rates patient- and aneurysm-related UIA risk factors, but also weights treatment-related factors that should be combined to ultimately reach a management recommendation (table 2) [24]. Since the UIATS model has as yet been tested only in a study with 30 selected UIA cases [24], prospective studies are necessary to test its applicability and validity in daily clinical routine.
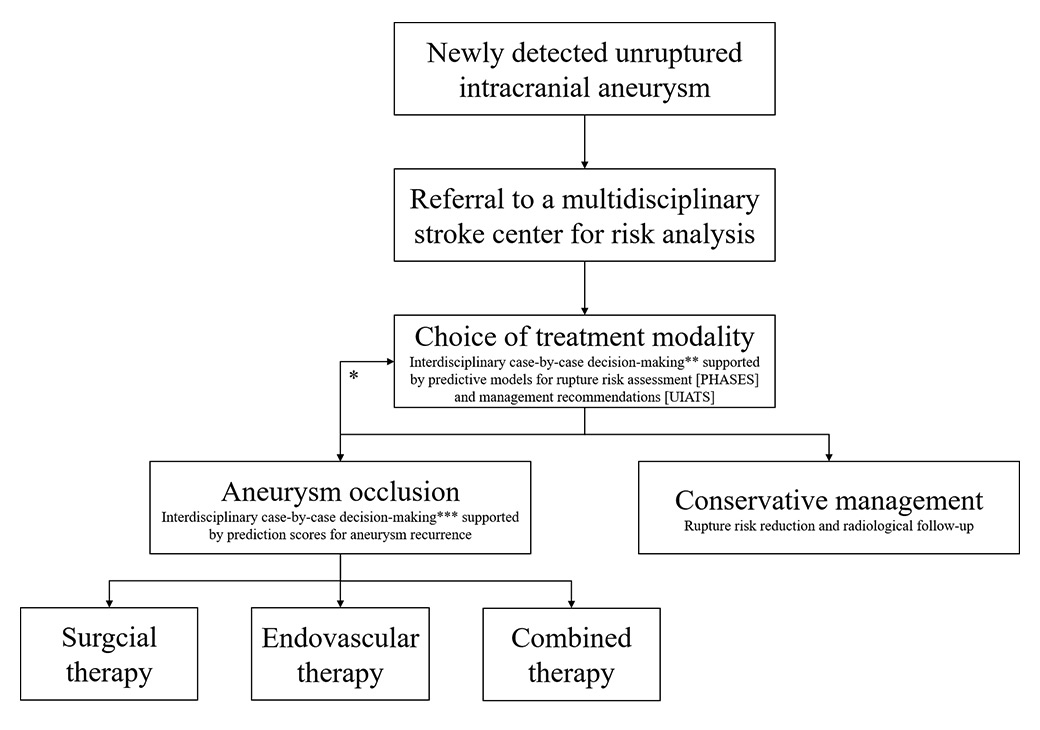
Figure 1
Proposed algorithm for the management of unruptured intracranial aneurysm.
* Intervention-related risks may influence treatment indication as such.
** Trade-off between the risk of rupture based on individual patient- and aneurysm-specific risk factors versus the individual treatment risk based on the surgeon’s or interventionalist’s experience, treatment modality, and aneurysm angioarchitecture.
*** Trade-off between interventional risk and potential long-term stability based on the patient’s condition, local resources, and aneurysm complexity and location.
Despite these recently developed scoring systems that summarise the cumulative effect of identified risk factors for IA rupture, the decision whether or not to treat remains difficult. Although robust data on risk rates of prophylactic surgical and endovascular treatment exist (morbidity and mortality rates of 4.7–6.7% and 1.7–1.8%, respectively) [25, 26], the trade-off between the risk of natural history and the risk associated with invasive therapy is complicated by the unequal comparison of an estimated risk over a period of time versus the risk of a single event [27, 28]. This equation is further complicated by the fact that the risk of intervention depends very much on the skill and experience of attending physicians and their annual case load (it is recommended that endovascular and surgical treatment of UIAs be performed at high-volume centres) [29]. In summary, decision making may be best based on interdisciplinary case-by-case discussion of clinical and radiological findings by highly informed individuals.
Conservative management of a UIA is recommended when the risk of aneurysm repair outweighs the risk of rupture. Current conservative management options for UIA include risk reduction for IA rupture (control of hypertension and cessation of lifestyle risk factors [smoking, alcohol and drug consumption]), and clinical and radiological follow-up (monitor of IA growth). A flow chart of the decision-making process for a newly detected UIA is given in figure 1.
|
Table 1: PHASES score for UIA rupture risk estimation [22]. |
|
Factors
|
Points
|
| (P) Population |
|
| North American, European (other than Finnish) |
|
| Japanese |
3 |
| Finnish |
5 |
| (H) Hypertension |
|
| No |
|
| Yes |
1 |
| (A) Age |
|
| <70 years |
|
| ≥70 years |
1 |
| (S) Size of aneurysm |
|
| <7.0 mm |
|
| 7.0 mm – 9.9 mm |
3 |
| 10.0 mm – 19.9 mm |
6 |
| ≥20 mm |
10 |
| (E) Earlier SAH from another aneurysm |
|
| No |
|
| Yes |
1 |
| (S) Site of aneurysm |
|
| ICA |
|
| MCA |
2 |
| ACA/ Pcom/ posterior |
4 |
| PHASES risk score (cumulated points) |
5-year risk of IA rupture (%) |
| ≤2 |
0.4 |
| 3 |
0.7 |
| 4 |
0.9 |
| 5 |
1.3 |
| 6 |
1.7 |
| 7 |
2.4 |
| 8 |
3.2 |
| 9 |
4.3 |
| 10 |
5.3 |
| 11 |
7.2 |
| ≥12 |
17.8 |
| To calculate the PHASES risk score for an individual, the number of points associated with each indicator can be added up to obtain the total risk score. ACA = anterior cerebral artery; AI = intracranial aneurysm; ICA = internal carotid artery; MCA = middle cerebral artery; PComA = posterior communicating artery; SAH = subarachnoid haemorrhage; UIA = unruptured intracranial aneurysm.
|
Interdisciplinary treatment planning
For almost a century, surgical IA repair was the only possible treatment method to isolate an IA from the circulation. Since the early 1980s, controlled deployment of coils using the Guglielmi detachable coil system paved the way for the widespread use of endovascular approaches as therapy for IA obliteration. In contrast to the invasiveness of the extravascular approach (craniotomy and brain retraction), which provides excellent durability of IA obliteration (3–7% recurrence) [30–33], the physiological approach using the intravascular space as natural route (endovascular) results in inferior IA repair (20–30% recurrence) [34–38].
Although the rebleeding rate from IA recurrence after endovascular treatment is very low, ongoing follow-up and a high rate of retreatment (approximately half of reopened IAs) [34] cause significant clinical problems. Aneurysm-specific (size, rupture status and presence of intraluminal thrombus) and treatment-related factors (method and initial occlusion rate) affecting IA recanalisation rates provide the basis for the IA Recanalization Stratification Scale to predict retreatment after endovascular therapy (table 3) [41]. The score was externally validated in independent cohorts from four centres and proved to be a valid prognostic index for quantitatively predicting retreatment risk after endovascular therapy [39]. Therefore, the score helps to predict long-term durability and acts as one of the factors influencing treatment planning.
Over the past few decades, both surgical and endovascular techniques have progressed significantly. With the exception of one trial comparing coiling with conservative treatment [42] that was stopped after 3 years because of poor recruitment, there are no randomised trials comparing occlusion techniques for UIA. Observational data from UIA repair by clipping or coiling suggests similar long-term outcomes for both modalities [43]. Until now, data from the available four randomised controlled trials in ruptured IA suggest that neither treatment modality is clearly and consistently superior in terms of safety and efficacy [32, 44–46]. Long-term follow-up data suggested equipoise in functional outcome for surgical and endovascular treatment of ruptured intracranial aneurysms and diminished the debate over superiority of one over the other treatment modality [47–49].
There is a growing body of evidence that specific patient subgroups may benefit from one or other of the two treatment modalities. Middle cerebral artery aneurysms (often superficially located at the bi/trifurcation [>80%], and with an unfavourable neck diameter and dome size ratio for coiling [50]), as well as patients presenting with a significant intraparenchymal haematoma [51] (>50 ml) or acute subdural haematoma [52], are believed to be ideal candidates for surgery [53]. On the other hand, older individuals [54, 55], poor grade patients and those with confirmed cerebral vasospasm [56], and posterior circulation aneurysms (especially basilar apex [57]) seem to be better candidates for coiling. Factors that suspected to favour one treatment over the other are summarised in table 4.
The determination as to whether surgical or endovascular intervention, or both, is required to offer the most durable and minimally invasive patient- and aneurysm-specific therapy should be based on a multidisciplinary consensus. Periodical meetings of an institutional cerebrovascular board in order to review cases and guidelines are indispensable to enhance the quality and transparency of the treatment. Similar to the decision-finding on management of UIA, the choice of best interventional therapy is achieved by case-by-case discussion weighing all relevant factors that may influence the interventional risks and potential long-term stability of the treatment plan. Factors to be assessed are IA angioarchitecture (size, shape, neck configuration, relation to parent arteries, local collateral circulation, intraluminal thrombosis, wall calcifications), IA location, patient’s condition (age, comorbidities, presence of space-occupying intracranial haematoma, vascular status, collaterals) and resources (expertise, technical skills, availability of personnel and facilities) for either surgical, endovascular or combined therapy.
|
Table 2: UIA treatment score for UIA management recommendation [24]. |
| |
|
Favours UIA repair
|
Favours UIA conservative management
|
|
Patient
|
| Age (single) |
<40 years |
4 |
|
| 40–60 years |
3 |
|
| 61–70 years |
2 |
|
| 71–80 years |
1 |
|
| >80 years |
|
|
| Risk factor incidence (multiple) |
Previous SAH from a different aneurysm |
4 |
|
| Familial intracranial aneurysms or SAH |
3 |
|
| Japanese, Finnish, Inuit ethnicity |
2 |
|
| Current cigarette smoking |
3 |
|
| Hypertension (systolic BP >140 mm Hg) |
2 |
|
| Autosomal-polycystic kidney disease |
2 |
|
| Current drug abuse (cocaine, amphetamine) |
2 |
|
| Current alcohol abuse |
1 |
|
| Clinical symptoms related to UIA (multiple) |
Cranial nerve deficit |
4 |
|
| Clinical or radiological mass effect |
4 |
|
| Thromboembolic events from the aneurysm |
3 |
|
| Epilepsy |
1 |
|
| Other (multiple) |
Reduced quality of life due to fear of rupture |
2 |
|
| Aneurysm multiplicity |
1 |
|
| Life expectancy due to chronic and/or malignant Diseases (single) |
<5 years |
|
4 |
| 5–10 years |
|
3 |
| >10 years |
|
1 |
| Comorbid disease (multiple) |
Neurocognitive disorder |
|
3 |
| Coagulopathies, thrombophilic diseases |
|
2 |
| Psychiatric disorder |
|
2 |
|
Aneurysm
|
| Maximum diameter |
≤3.9 mm |
|
|
| 4.0–6.9 mm |
1 |
|
| 7.0–12.9 mm |
2 |
|
| 13–24.9 mm |
3 |
|
| ≥25 mm |
4 |
|
| Morphology (multiple) |
Irregularity or lobulation |
3 |
|
| Size ratio >3 or aspect ratio >1.6 |
1 |
|
| Location (single) |
BA bifurcation |
5 |
|
| Vertebral/basilar artery |
4 |
|
| AcomA or PcomA |
2 |
|
| Other (multiple) |
Aneurysm growth on serial imaging |
4 |
|
| Aneurysm de novo formation on serial imaging |
3 |
|
| Contralateral stenoocclusive vessel disease |
1 |
|
|
Treatment
|
| Age-related risk (single) |
<40 years |
|
|
| 40–60 years |
|
1 |
| 61–70 years |
|
3 |
| 71–80 years |
|
4 |
| >80 years |
|
5 |
| Aneurysm size-related risk (single) |
<6.0 mm |
|
|
| 6.0–10.0 mm |
|
1 |
| 10.1–20.0 mm |
|
3 |
| >20 mm |
|
5 |
| Aneurysm complexitiy-related risk (complexity) |
High |
|
3 |
| Low |
|
|
| Intervention-related risk |
Constant* |
| To calculate a management recommendation, points in each column (favour UIA repair or UIA conservative management) are added up. This results in two numerical values – one favouring UIA repair and the other favouring conservative treatment. A difference of 3 points or more indicates an individual treatment recommendation for the column with the higher score. In the event of similar scores (± 2 point difference or less) either management approach could be supported.AcomA = anterior communicating artery; APKD = autosomal-polycystic kidney disease; AR = aspect ratio (ratio of aneurysm dome dimension and neck width); BA = basilar artery; BP = blood pressure; IA = intracranial aneurysm; PcomA = posterior communicating artery; QoL = quality of life; SAH = subarachnoid haemorrhage; SR = size ratio (largest aneurysm diameter divided by parent artery diameter); UIA = unruptured intracranial aneurysm; VA = vertebral artery.
* Minimal intervention related risk is always added as a constant factor (5 points). |
|
Table 3: Prediction score for recanalisation of an intracranial aneurysm after endovascular therapy [39]. |
|
|
Points Assigned
|
|
Aneurysm-specific factors
|
|
| Size, mm |
|
| >10 |
+2 |
| ≤10 |
|
| Ruptured |
+2 |
| Unruptured |
|
| Thrombus |
+2 |
| No thrombus |
|
|
Treatment-related factors
|
|
| Coils only |
|
| Stent-assistance |
–1 |
| Flow diversion |
–2 |
| Initial treatment result |
|
| Raymond Roy 1 |
|
| Raymond Roy 2 |
+1 |
| Raymond Roy 3 |
+2 |
|
Recanalisation prediction score (cumulated points)
|
Probability of retreatment (%)
|
| –2 |
4.9 |
| –1 |
5.7 |
| 0 |
5.8 |
| 1 |
13.1 |
| 2 |
19.2 |
| 3 |
34.9 |
| 4 |
32.7 |
| 5 |
73.2 |
| 6 |
89.5 |
| 7 |
100 |
| Raymond Roy = Raymond Roy occlusion classification (RROC) [40] for the assessment of initial treatment success by means of aneurysm occlusion, assessed by digital subtraction angiography as follows:
Class 1: complete obliteration (no contrast filling of the dome, body, or neck of the aneurysm)
Class 2: residual neck (neck filling without opacification of the aneurysmal sac)
Class 3: residual aneurysm (contrast agent in the dome of the aneurysm)
Flow diverters are uniformly categorised as RROC class 1. |
|
Table 4: Summary of factors either in favour of surgical or endovascular intervention. |
|
Surgical treatment
|
Endovascular treatment
|
| Younger age (<50 years) |
Older age (>70 years) |
| Middle cerebral artery and pericallosal aneurysms |
Posterior circulation aneurysms (especially BA aneurysms) |
| Branches arising from neck or sac |
Poor grade SAH patients |
| Presence of space occupying haematoma |
Confirmed DCVS |
| Wide aneurysm neck |
Small aneurysm neck, unilobular shape |
| Unfavourable angioarchitecture for coiling |
Significant medical comorbidities |
| BA = basilar artery; DCVS = delayed cerebral vasospasm; SAH = subarachnoid haemorrhage |
Combined microsurgical and endovascular treatment approaches
Disadvantages of both treatment modalities can be compensated by combining microsurgical and endovascular techniques [58]. While patient subgroups may clearly qualify for either surgical or endovascular treatment some patients could benefit from multimodal therapy [53, 57, 58]. Especially in case of IAs with a complex angioarchitecture of the IA per se, but also of its parent arteries successful treatment may requires a combined endovascular and microsurgical treatment approach. Apart from combined staged endovascular and open microsurgical procedures, a hybrid operating room offers single-stage combined treatment options (fig. 2) [58–60].
Figure 2
Treatment of an intracranial aneurysm in the hybrid operating room.
Combined endovascular and microsurgical techniques for the treatment of intracranial aneurysm in the hybrid operating room (Neurocentre, Kantonsspital Aarau, Switzerland).
Future perspectives for IA rupture risk estimation and prediction of treatment success
There is a lack of clinical evidence for the manifold clinical scenarios of an individual patient suffering from an UIA. Despite known general patient- and aneurysm-related IA rupture risk factors, rupture risk prediction models, and management recommendation scores the choice of management of a distinct UIA remains challenging [61]. In addition to the six factors (population, hypertension, age, size, location, and previous SAH) currently used to predict the rupture risk in the PHASES score, radiologically detectable risk factors (such as wall inflammation, irregular morphology, and wall shear stress) should be considered to achieve a more individualized risk assessment. There is also an urgent need to improve the estimation of benefits, effectivness, and risks of specific surgical and endovascular treatment modalities (especially for new endovascular devices).
Understanding of IA wall biology hold much promise to help in UIA decision-making. Histopathology of human IA samples have long indicated an association between the grade of IA wall degeneration and rupture status [62, 63] and preclinical animal studies confirmed that aneurysms with degenerated walls are prone to growth, inflammation and rupture [64, 65]. It is likely that in the foreseeable future, improved knowledge and visualisation of biological processes in IA walls will not only aid in better determination of the IA’s natural history, but will be advantageous in choosing the best treatment approach by means of better prediction of long-term durability of the chosen therapy.
Conclusions
The decision how to manage UIA (preventive repair by surgical or endovascular methods versus conservative treatment by risk reduction and radiological follow-up) remains a dilemma for both patient and clinician. Scoring models for rupture risk estimation (PHASES) and interdisciplinary management recommendation (UIATS) form a good basis for discussion to weigh up treatment options in daily clinical practice. Nevertheless, final decision-making is still optimised when based on interdisciplinary case-by-case discussion of clinical and radiological findings by highly informed individuals. In the future, the addition of pathobiological characterisation of IAs is likely to improve rupture risk assessment and estimation of treatment success. The controversy concerning the best IA occlusion modality may be replaced by an era of complementary approaches to achieve optimal long-term durability at the lowest interventional risk, but further evidence by large clinical trials are needed.
References
1 Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–42. doi:http://dx.doi.org/10.1016/S1474-4422(09)70126-7. PubMed
2 Hoffmann E, Marbacher S, Jakob S, Takala J, Remonda L, Fandino J. Incidence of vasospasm, outcome, and quality of life after endovascular and surgical treatment of ruptured intracranial aneurysms: Results of a single-center prospective study in Switzerland. Int Sch Res Notices. 2011;782568. doi:10.5402/2011/782568
3 Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–36. doi:http://dx.doi.org/10.1016/S1474-4422(11)70109-0. PubMed
4 Rincon F, Rossenwasser RH, Dumont A. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2013;73(2):217–22, discussion 212–3. doi:http://dx.doi.org/10.1227/01.neu.0000430290.93304.33. PubMed
5 Gabriel RA, Kim H, Sidney S, McCulloch CE, Singh V, Johnston SC, et al. Ten-year detection rate of brain arteriovenous malformations in a large, multiethnic, defined population. Stroke. 2010;41(1):21–6. doi:http://dx.doi.org/10.1161/STROKEAHA.109.566018. PubMed
6 Wiebers DO, Whisnant JP, Huston J, 3rd, Meissner I, Brown RD, Jr, Piepgras DG, et al.; International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10. doi:http://dx.doi.org/10.1016/S0140-6736(03)13860-3. PubMed
7 Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al., UCAS Japan Investigators. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. 2012;366(26):2474–82. doi:http://dx.doi.org/10.1056/NEJMoa1113260. PubMed
8 Sonobe M, Yamazaki T, Yonekura M, Kikuchi H. Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke. 2010;41(9):1969–77. doi:http://dx.doi.org/10.1161/STROKEAHA.110.585059. PubMed
9 Crompton MR. Mechanism of growth and rupture in cerebral berry aneurysms. BMJ. 1966;1(5496):1138–42. doi:http://dx.doi.org/10.1136/bmj.1.5496.1138. PubMed
10 Beck J, Rohde S, Berkefeld J, Seifert V, Raabe A. Size and location of ruptured and unruptured intracranial aneurysms measured by 3-dimensional rotational angiography. Surg Neurol. 2006;65(1):18–25, discussion 25–7. doi:http://dx.doi.org/10.1016/j.surneu.2005.05.019. PubMed
11 Kemp WJ, 3rd, Fulkerson DH, Payner TD, Leipzig TJ, Horner TG, Palmer EL, et al. Risk of hemorrhage from de novo cerebral aneurysms. J Neurosurg. 2013;118(1):58–62. doi:http://dx.doi.org/10.3171/2012.9.JNS111512. PubMed
12 Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J, et al. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269(1):258–65. doi:http://dx.doi.org/10.1148/radiol.13121188. PubMed
13 Güresir E, Vatter H, Schuss P, Platz J, Konczalla J, de Rochement RM, et al. Natural history of small unruptured anterior circulation aneurysms: a prospective cohort study. Stroke. 2013;44(11):3027–31. doi:http://dx.doi.org/10.1161/STROKEAHA.113.001107. PubMed
14 Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29(1):251–6. doi:http://dx.doi.org/10.1161/01.STR.29.1.251. PubMed
15 Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis. 2002;11(6):304–14. doi:http://dx.doi.org/10.1053/jscd.2002.130390. PubMed
16 Molyneux AJ, Kerr RSC, Yu L-M, Clarke M, Sneade M, Yarnold JA, et al.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–17. doi:http://dx.doi.org/10.1016/S0140-6736(05)67214-5. PubMed
17 Forget TR, Jr, Benitez R, Veznedaroglu E, Sharan A, Mitchell W, Silva M, et al. A review of size and location of ruptured intracranial aneurysms. Neurosurgery. 2001;49(6):1322–5, discussion 1325–6. doi:http://dx.doi.org/10.1097/00006123-200112000-00006. PubMed
18 Chang HS. Simulation of the natural history of cerebral aneurysms based on data from the International Study of Unruptured Intracranial Aneurysms. J Neurosurg. 2006;104(2):188–94. doi:http://dx.doi.org/10.3171/jns.2006.104.2.188. PubMed
19 Koffijberg H, Buskens E, Algra A, Wermer MJ, Rinkel GJ. Growth rates of intracranial aneurysms: exploring constancy. J Neurosurg. 2008;109(2):176–85. doi:http://dx.doi.org/10.3171/JNS/2008/109/8/0176. PubMed
20 Ferns SP, Sprengers ME, van Rooij WJ, van den Berg R, Velthuis BK, de Kort GA, et al. De novo aneurysm formation and growth of untreated aneurysms: a 5-year MRA follow-up in a large cohort of patients with coiled aneurysms and review of the literature. Stroke. 2011;42(2):313–8. doi:http://dx.doi.org/10.1161/STROKEAHA.110.591594. PubMed
21 Chmayssani M, Rebeiz JG, Rebeiz TJ, Batjer HH, Bendok BR. Relationship of growth to aneurysm rupture in asymptomatic aneurysms 7 mm: a systematic analysis of the literature. Neurosurgery. 2011;68(5):1164–71, discussion 1171. PubMed
22 Greving JP, Wermer MJ, Brown RD, Jr, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13(1):59–66. doi:http://dx.doi.org/10.1016/S1474-4422(13)70263-1. PubMed
23 Backes D, Vergouwen MD, Tiel Groenestege AT, Bor AS, Velthuis BK, Greving JP, et al. Phases score for prediction of intracranial aneurysm growth. Stroke. 2015;46(5):1221–6. doi:http://dx.doi.org/10.1161/STROKEAHA.114.008198. PubMed
24 Etminan N, Brown RD, Jr, Beseoglu K, Juvela S, Raymond J, Morita A, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology. 2015;85(10):881–9. doi:http://dx.doi.org/10.1212/WNL.0000000000001891. PubMed
25 Naggara ON, Lecler A, Oppenheim C, Meder JF, Raymond J. Endovascular treatment of intracranial unruptured aneurysms: a systematic review of the literature on safety with emphasis on subgroup analyses. Radiology. 2012;263(3):828–35. doi:http://dx.doi.org/10.1148/radiol.12112114. PubMed
26 Kotowski M, Naggara O, Darsaut TE, Nolet S, Gevry G, Kouznetsov E, et al. Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry. 2013;84(1):42–8. doi:http://dx.doi.org/10.1136/jnnp-2011-302068. PubMed
27 Mayer TE, Etminan N, Morita A, Juvela S. The unruptured intracranial aneurysm treatment score: A multidisciplinary consensus. Neurology. 2016;86(8):792–3. doi:http://dx.doi.org/10.1212/01.wnl.0000481228.68055.71. PubMed
28 Raymond J. Incidental intracranial aneurysms: rationale for treatment. Curr Opin Neurol. 2009;22(1):96–102. doi:http://dx.doi.org/10.1097/WCO.0b013e32831fee91. PubMed
29 Thompson BG, Brown RD, Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, Jr, et al.; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association; American Stroke Association. Guidelines for the management of patients with unruptured intracranial aneurysms: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(8):2368–400. doi:http://dx.doi.org/10.1161/STR.0000000000000070. PubMed
30 David CA, Vishteh AG, Spetzler RF, Lemole M, Lawton MT, Partovi S. Late angiographic follow-up review of surgically treated aneurysms. J Neurosurg. 1999;91(3):396–401. doi:http://dx.doi.org/10.3171/jns.1999.91.3.0396. PubMed
31 Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke. 2001;32(5):1191–4. doi:http://dx.doi.org/10.1161/01.STR.32.5.1191. PubMed
32 Koivisto T, Vanninen R, Hurskainen H, Saari T, Hernesniemi J, Vapalahti M. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms. A prospective randomized study. Stroke. 2000;31(10):2369–77. doi:http://dx.doi.org/10.1161/01.STR.31.10.2369. PubMed
33 Owen CM, Montemurro N, Lawton MT. Microsurgical management of residual and recurrent aneurysms after coiling and clipping: An experience with 97 patients. Neurosurgery. 2015;62(Suppl 1):92–102. doi:http://dx.doi.org/10.1227/NEU.0000000000000791. PubMed
34 Ferns SP, Sprengers MES, van Rooij WJ, Rinkel GJE, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009;40(8):e523–9. doi:http://dx.doi.org/10.1161/STROKEAHA.109.553099. PubMed
35 Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years’ experience. J Neurosurg. 2003;98(5):959–66. doi:http://dx.doi.org/10.3171/jns.2003.98.5.0959. PubMed
36 Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34(6):1398–403. doi:http://dx.doi.org/10.1161/01.STR.0000073841.88563.E9. PubMed
37 Dorfer C, Gruber A, Standhardt H, Bavinzski G, Knosp E. Management of residual and recurrent aneurysms after initial endovascular treatment. Neurosurgery. 2012;70(3):537–53, discussion 553–4. doi:http://dx.doi.org/10.1227/NEU.0b013e3182350da5. PubMed
38 Crobeddu E, Lanzino G, Kallmes DF, Cloft HJ. Review of 2 decades of aneurysm-recurrence literature, part 2: Managing recurrence after endovascular coiling. AJNR Am J Neuroradiol. 2013;34(3):481–5. doi:http://dx.doi.org/10.3174/ajnr.A2958. PubMed
39 Ogilvy CS, Chua MH, Fusco MR, Griessenauer CJ, Harrigan MR, Sonig A, et al. Validation of a system to predict recanalization after endovascular treatment of intracranial aneurysms. Neurosurgery. 2015;77(2):168–73, discussion 173–4. doi:http://dx.doi.org/10.1227/NEU.0000000000000744. PubMed
40 Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32(9):1998–2004. doi:http://dx.doi.org/10.1161/hs0901.095600. PubMed
41 Ogilvy CS, Chua MH, Fusco MR, Reddy AS, Thomas AJ. Stratification of recanalization for patients with endovascular treatment of intracranial aneurysms. Neurosurgery. 2015;76(4):390–5, discussion 395. doi:http://dx.doi.org/10.1227/NEU.0000000000000651. PubMed
42 Raymond J, Darsaut TE, Molyneux AJ; TEAM collaborative Group. A trial on unruptured intracranial aneurysms (the TEAM trial): results, lessons from a failure and the necessity for clinical care trials. Trials. 2011;12(1):64. doi:http://dx.doi.org/10.1186/1745-6215-12-64. PubMed
43 Gonda DD, Khalessi AA, McCutcheon BA, Marcus LP, Noorbakhsh A, Chen CC, et al. Long-term follow-up of unruptured intracranial aneurysms repaired in California. J Neurosurg. 2014;120(6):1349–57. doi:http://dx.doi.org/10.3171/2014.3.JNS131159. PubMed
44 McDougall CG, Spetzler RF, Zabramski JM, Partovi S, Hills NK, Nakaji P, et al. The barrow ruptured aneurysm trial. J Neurosurg. 2012;116(1):135–44. doi:http://dx.doi.org/10.3171/2011.8.JNS101767. PubMed
45 Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al.; International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–74. doi:http://dx.doi.org/10.1016/S0140-6736(02)11314-6. PubMed
46 Li ZQ, Wang QH, Chen G, Quan Z. Outcomes of endovascular coiling versus surgical clipping in the treatment of ruptured intracranial aneurysms. J Int Med Res. 2012;40(6):2145–51. doi:http://dx.doi.org/10.1177/030006051204000612. PubMed
47 Korja M. ISAT: end of the debate on coiling versus clipping? Lancet. 2015;385(9984):2250–1. doi:http://dx.doi.org/10.1016/S0140-6736(15)61059-5. PubMed
48 Thomas AJ, Ogilvy CS. ISAT: equipoise in treatment of ruptured cerebral aneurysms? Lancet. 2015;385(9969):666–8. doi:http://dx.doi.org/10.1016/S0140-6736(14)61736-0. PubMed
49 Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015;385(9969):691–7. doi:http://dx.doi.org/10.1016/S0140-6736(14)60975-2. PubMed
50 Hernesniemi J, Dashti R, Niemelä M, Romani R, Rinne J, Jääskeläinen JE. Microsurgical and angiographic anatomy of middle cerebral artery aneurysm. Neurosurgery. 2010;66(5):E1030. doi:http://dx.doi.org/10.1227/NEU.0B013E3181D8CCAB. PubMed
51 Rinne J, Hernesniemi J, Niskanen M, Vapalahti M. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38(1):2–11. doi:http://dx.doi.org/10.1097/00006123-199601000-00002. PubMed
52 Marbacher S, Fandino J, Lukes A. Acute subdural hematoma from ruptured cerebral aneurysm. Acta Neurochir (Wien). 2010;152(3):501–7. doi:http://dx.doi.org/10.1007/s00701-009-0521-0. PubMed
53 Regli L, Dehdashti AR, Uske A, de Tribolet N. Endovascular coiling compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: an update. Acta Neurochir Suppl (Wien). 2002;82:41–6. PubMed
54 Sturiale CL, Brinjikji W, Murad MH, Lanzino G. Endovascular treatment of intracranial aneurysms in elderly patients: a systematic review and meta-analysis. Stroke. 2013;44(7):1897–902. doi:http://dx.doi.org/10.1161/STROKEAHA.113.001524. PubMed
55 Lubicz B, Leclerc X, Gauvrit JY, Lejeune JP, Pruvo JP. Endovascular treatment of ruptured intracranial aneurysms in elderly people. AJNR Am J Neuroradiol. 2004;25(4):592–5. PubMed
56 Kremer C, Groden C, Hansen HC, Grzyska U, Zeumer H. Outcome after endovascular treatment of Hunt and Hess grade IV or V aneurysms: comparison of anterior versus posterior circulation. Stroke. 1999;30(12):2617–22. doi:http://dx.doi.org/10.1161/01.STR.30.12.2617. PubMed
57 Lusseveld E, Brilstra EH, Nijssen PC, van Rooij WJ, Sluzewski M, Tulleken CA, et al. Endovascular coiling versus neurosurgical clipping in patients with a ruptured basilar tip aneurysm. J Neurol Neurosurg Psychiatry. 2002;73(5):591–3. doi:http://dx.doi.org/10.1136/jnnp.73.5.591. PubMed
58 Choudhri O, Mukerji N, Steinberg GK. Combined endovascular and microsurgical management of complex cerebral aneurysms. Front Neurol. 2013;4:108. doi:http://dx.doi.org/10.3389/fneur.2013.00108. PubMed
59 Fandino J, Taussky P, Marbacher S, Muroi C, Diepers M, Fathi AR, et al. The concept of a hybrid operating room: applications in cerebrovascular surgery. Acta Neurochir Suppl (Wien). 2013;115:113–7. PubMed
60 Murayama Y, Arakawa H, Ishibashi T, Kawamura D, Ebara M, Irie K, et al. Combined surgical and endovascular treatment of complex cerebrovascular diseases in the hybrid operating room. J Neurointerv Surg. 2013;5(5):489–93. doi:http://dx.doi.org/10.1136/neurintsurg-2012-010382. PubMed
61 Etminan N, Beseoglu K, Barrow DL, Bederson J, Brown RD, Jr, Connolly ES, Jr, et al. Multidisciplinary consensus on assessment of unruptured intracranial aneurysms: proposal of an international research group. Stroke. 2014;45(5):1523–30. doi:http://dx.doi.org/10.1161/STROKEAHA.114.004519. PubMed
62 Kataoka K, Taneda M, Asai T, Kinoshita A, Ito M, Kuroda R. Structural fragility and inflammatory response of ruptured cerebral aneurysms. A comparative study between ruptured and unruptured cerebral aneurysms. Stroke. 1999;30(7):1396–401. doi:http://dx.doi.org/10.1161/01.STR.30.7.1396. PubMed
63 Frösen J, Piippo A, Paetau A, Kangasniemi M, Niemelä M, Hernesniemi J, et al. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35(10):2287–93. doi:http://dx.doi.org/10.1161/01.STR.0000140636.30204.da. PubMed
64 Marbacher S, Frösén J, Marjamaa J, Anisimov A, Honkanen P, von Gunten M, et al. Intraluminal cell transplantation prevents growth and rupture in a model of rupture-prone saccular aneurysms. Stroke. 2014;45(12):3684–90. doi:http://dx.doi.org/10.1161/STROKEAHA.114.006600. PubMed
65 Marbacher S, Marjamaa J, Bradacova K, von Gunten M, Honkanen P, Abo-Ramadan U, et al. Loss of mural cells leads to wall degeneration, aneurysm growth, and eventual rupture in a rat aneurysm model. Stroke. 2014;45(1):248–54. doi:http://dx.doi.org/10.1161/STROKEAHA.113.002745. PubMed

