Neutrophil extracellular traps in health and disease
DOI: https://doi.org/10.4414/smw.2016.14352
Paul
Hasler, Stavros
Giaglis, Sinuhe
Hahn
Summary
Polymorphonuclear neutrophil granulocytes are the first responders of the immune system to threats by invading microorganisms. In the traditional view, they combat the intruders by phagocytosis and externalisation of granules containing lytic and microbicidal factors. A dozen years ago, this concept was expanded by the observation that neutrophils may react to bacteria by extruding their nuclear chromosomal DNA with attached nuclear and cytoplasmic constituents to form extracellular reticular structures. Since they trapped and immobilised the microbes, they were designated neutrophil extracellular traps (NETs), and their ensuing cell death NETosis. Subsequently, the NETs were shown to act against different types of pathogens, including viruses, and an intricate interplay between the NETs and countermeasures of the pathogens became apparent.
The NETs were also found to induce inflammatory responses in the host that contributed to the pathophysiology of autoinflammatory and even autoimmune diseases. Of special interest is the direct link that NETs provide to infections that may initiate and maintain inflammation without the participation of adaptive immunity. In contrast, neutrophils seem capable of activating B cells to produce antibodies relevant to autoimmunity independently of T cell help. Further results imply NETs in the occurrence of thrombosis of the veins and recently also in the generation of arterial plaque.
Data from the studies on the defence against pathogens and the pathophysiology of inflammation and thrombosis have started to drive applications to modulate NET formation and its effects and may provide opportunities to optimise current diagnostic and therapeutic concepts.
Abbreviations
ACPA: anti-citrullinated peptide antibody
ANCA: anti-neutrophil cytoplasmic antibody
ATP: adenosine triphosphate
cAMP: cyclic adenosine monophosphate
CXCR2: C-X-C motif chemokine receptor 2
DNA: deoxyribonucleic acid
ERK: extracellular signal-regulated kinase
HIV: human immunodeficiency virus
HMGB1: High mobility group box 1 protein
IFN: interferon
Ig: immunoglobulin
IL: interleukin
LPS: lipopolysaccharide
MAP: mitogen-activated protein
MPO: myeloperoxidase
NADPH: nicotinamide adenine dinucleotide phosphate (reduced)
NE: neutrophil elastase
NET: neutrophil extracellular trap
PAD: peptidyl arginine deiminase
pDC: plasmacytoid dendritic cell
TLRs: polymorphonuclear neutrophil granulocyte Toll-like receptors
TNF-α: tumour necrosis factor-alpha
ROS: reactive oxygen species
RA: rheumatoid arthritis
SLE: systemic lupus erythematosus
Introduction
Leucocytes protect our bodies from the onslaught of infectious agents and aberrant cell proliferation. Each type of leucocyte comprises subtypes with different modes of dispatching threats. In humans, neutrophils (polymorphonuclear neutrophil granulocytes) are the dominant fraction of leucocytes in the peripheral blood. As the “first responders” of the immune system they act at the forefront against infectious agents invading across barriers into tissues, whether due to injury to the skin or to the mucous membranes in the respiratory or gastrointestinal tracts [1, 2]. They perform essential functions in host defence against a broad range of pathogens. Depending on the type of danger sensed, in the traditional view they fulfil their tasks mostly by phagocytosis or by subjecting pathogens to the lytic contents of cytoplasmic granules expelled into the extracellular space [2, 3]. Alternatively, they extrude their nuclear contents through the cytoplasm into the extracellular space in the form of neutrophil extracellular traps (NETs) [4]. NETs were originally described as a form of neutrophil response to bacteria and their cell wall components lipopolysaccharide (LPS) and peptidoglycan, which led to a newly identified form of cell death, termed NETosis, as opposed to necrosis or apoptosis. Apart from bacterial LPS and peptidoglycan, components of fungi, viruses and parasites may induce NET formation. In addition, microparticles, immune complexes, cytokines and chemokines, platelets, various cells of the immune system, endothelial cells and others can also provoke NETosis (fig. 1). Surface receptors of neutrophils that sense events leading to NETosis are Toll-like receptors (TLRs) 2 and 4, immunoglobulin (Ig) Fc receptors, intracellular TLRs 7 and 9 and others [5].
The signalling pathways leading to NETosis encompass calcium mobilisation, NADPH activation and generation of reactive oxygen species (ROS), the transfer of myeloperoxidase (MPO) and neutrophil elastase (NE) and peptidyl arginine deiminase IV (PAD4) to the nucleus. MPO and NE mediate nuclear delobulation and permeabilisation of the nuclear envelope, while PAD4 modifies chromatin via the citrullination of histones, a key requirement for chromatin decondensation. Chromosomal DNA with several attached nuclear contents (including those transferred to the nucleus during signalling) is subsequently extruded into the cytoplasm, where additional constituents are attached. Then the chromosomal filaments are expelled into the extracellular space and assume a lattice structure.
The destructive armamentarium of NETs and their adherent active components is not target specific, nor is their location restricted to the immediate environment of release. Although the activity of NETs is desirable to eliminate infection, the lack of specificity of action and localisation requires strict control of their formation and rapid removal to prevent injurious effects on adjacent tissues. Thus, both the inappropriate release and delay in inactivation and clearance of NETs may cause damage. Exposure of other elements of the immune system to the NETs themselves and constituents of tissues altered by NETs or their adherent components may induce inflammatory responses, which increase the original effects of NET release. Measures to counter the release of NETs exist not only at the level of inhibitory receptor signalling [6], but also to expedite their demise as outlined below.
Here we provide an outline of NET formation with a focus on factors mediating their release, and misguided NET formation, degradation and reactions to their presence.
Infectious agents
NETs adhere to and immobilise infectious agents, constraining them in immediate juxtaposition to the highly basic chromosomal histones, which, akin to the DNA, are inherently bactericidal [4]. This is reinforced by the action of NET-bound factors such as MPO, NE and cathepsins, and the antibacterial peptides calprotectin [7] and cathelicidin (LL37) [8]. The generation of ROS by NADPH oxidase in the process leading to NET formation, and activation of immediate-response reactants such as complement, further advance the potency of the NETs.
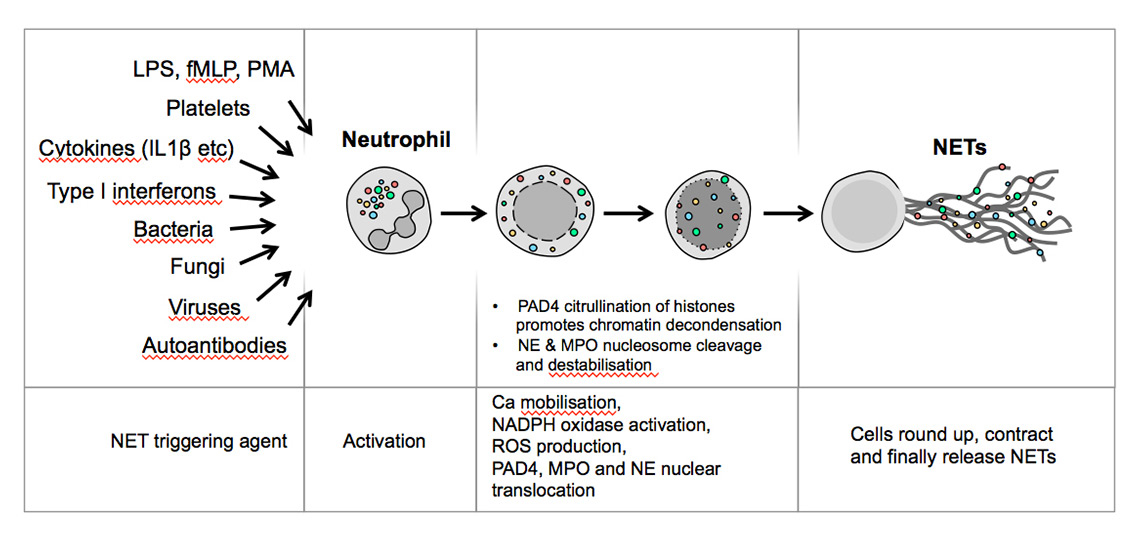
Figure 1
Various stimuli induce neutrophils to form neutrophil extracellular traps.
fMLP = N-formyl-methionyl-leucyl-phenylalanine; IL = interleukin; LPS = lipopolysaccharide; MPO = myeloperoxidase; NE = neutrophil elastase; NET = neutrophil extracellular trap; PAD = peptidyl arginine deiminase; PMA = phorbol myristate acetate; ROS = reactive oxygen species
NETs are effective against a wide range of bacteria, including Mycobacterium species, but also against fungi, viruses and parasites. The size of the pathogen has been suggested to influence the combat strategy embraced by the encroaching neutrophil, with larger organisms trapped by NETs, whereas smaller ones are more readily tackled by phagocytosis [9]. It is currently uncertain whether such a strategy is truly adopted in vivo, as recent evidence indicates that pathogens as small as viruses may be capable of inducing NETosis [10, 11]. In the following sections, we discuss the principal antimicrobial effects of NETs, the pathogens associated with NETosis and their strategies to foil the detrimental action of NETs.
Bacterial infection
In severe bacterial sepsis with endotoxaemia, neutrophils migrate to the liver sinusoids where they interact with platelets and release NETs. The NETs are capable of ensnaring bacteria from the blood and contributes to averting dissemination of the investigated Escherichia coli in a mouse model [12]. Further evidence for the contribution of NETs to countering infection was the decreased mortality in sepsis when signalling via phospholipase D2 was inhibited or abrogated in a mouse knockout model. Reduced phospholipase D2 signalling enhances the activity of C-X-C motif chemokine receptor 2 (CXCR2) activity and thereby NET formation, which is protective in potentially lethal sepsis [13]. High mobility group box 1 protein (HMGB1) contributes to enhancing NET formation via interaction with TLR4 [14]. Other strategies against infection are NET formation and phagocytosis mediated by lectin C type receptors that protected against Klebsiella pneumonia [15].
The exposure to host defences and the competition for survival have pressured target organisms to elaborate intricate countermeasures against the toxic effects of NETs. A question of longstanding interest has been why bacteria express endonucleases as virulence-enhancing factors. Since nucleases degrade DNA, they may have evolved to avoid killing by NETs. Staphylococcus aureus induces NETs that kill them primarily via the virulence factor Panton-Valentine leukocidin [16], but expresses a nuclease that degrades NETs, thereby reducing killing and enhancing infectivity of the bacteria in a mouse respiratory tract infection model [17]. Group A streptococcus deoxyribonuclease expression is related to virulence in an infection model of the peritoneum and skin [18]. Likewise, in Streptococcus pneumoniae infection, DNase enabled far higher bacterial reproduction in the lower airways and larger numbers in the circulation [19]. These and other strategies deployed by bacteria are summarised in table 1.
Bordetella pertussis virulence is contingent on the action of adenylate cyclase toxin. The toxin enters the neutrophil and drives the conversion of ATP to cAMP, resulting in supraphysiological cAMP levels and inhibition of NET formation [20].
In cystic fibrosis, CXCR2 signalling leading to NET formation is clinically relevant, since the extent of cell-free DNA in the lower airways is associated with obstruction. Indeed, antagonists of CXCR2 may alleviate the excessive release of DNA-rich mucus and relieve symptoms [22].
An example for the subtle regulation of the NET response is the lack of NET induction by Acinetobacter baumanii and its sparing from killing, whereas Pseudomonas aeruginosa, its pathogenic equivalent, induced NETs [23]. P. aeruginosa is susceptible to killing by the NETs, but excessive NETs may provide the basis for biofilm development that protects the pathogen from further attack. P. aeruginosa affects approximately 80% of cystic fibrosis patients. In comparison with recent infection, chronic infections with P. aeruginosa show decreased susceptibility to killing by NETs, which otherwise function normally in cystic fibrosis, implying an acquired resistance [24]. In addition to biofilm formation, a possible mechanism is that P. aeruginosa cloaks itself with host sialoglycoproteins, which induces interleukin (IL)-10 expression while impairing recognition by neutrophil receptors that induce NETs, thus reducing neutrophil activation and NET formation and allowing survival of the bacteria.
NETs induced by Mycobacterium tuberculosis induced high levels of IL-6, tumour necrosis factor-alpha (TNF-α), IL-1β, but also the immunosuppressive cytokine IL-10, which suppresses neutrophil activation and NETosis [25]. Though caught in the NETs, mycobacterial species survive, probably because of their cell wall structure. An extensive account of NET formation, cooperation between macrophages and neutrophils and other aspects of mycobacterial infection is provided elsewhere [26].
In sum, NETs are effective arms against bacteria and contribute to spatial containment and elimination. On the other hand, bacterial virulence ensues from successful inactivation of NETs by breaking up the DNA lattices, by reducing NET extrusion by interfering with signalling and by shifting the cytokine profile towards inhibition of immune cell function. As we will see in the next sections, other types of infectious agents deploy these and other strategies of evading the action of NETs, and infection by one may facilitate infection by a second microbe.
|
Table 1: Strategies of bacteria directed against neutrophil extracellular traps. |
|
Infectious organism
|
Mechanism of evasion
|
Effect
|
Reference
|
|
Staphylococcus aureus
|
Endonuclease |
Virulence and evasion of killing |
[17] |
| Group A streptococcus |
Endonuclease |
Virulence and evasion of killing |
[18] |
|
Neisseria gonorhoeae
|
Thermonuclease |
Virulence and evasion of killing |
[101] |
|
Neisseria meningitidis
|
Cathepsin G inhibition and bacterial outer membrane vesicle release |
Virulence and evasion of killing |
[102] |
|
Yersinia enterocolitica
|
Endonuclease |
Virulence and evasion of killing |
[103] |
|
Vibrio cholerae
|
Endonuclease |
Virulence and evasion of killing |
[104] |
|
Bordetella pertussis
|
Adenylate cyclase toxin |
Inhibition of NET formation |
[20] |
|
Leptospira species |
Endonuclease |
Virulence and evasion of killing |
[105] |
| Sepsis |
CXCR2 and phospholipase D2 activation |
Inhibition of NET formation |
[13, 21, 22] |
|
Mycobacterium tuberculosis
|
IL-10 adherent to NETs |
Reduced macrophage activity |
[25] |
| CXCR2 = C-X-C motif chemokine receptor type 2; IL = interleukin; NET = neutrophil extracellular trap |
Viral infection
Surprisingly, despite their minute size, several viruses have been found to provoke a NETotic response by neutrophils. Severe respiratory syncytial virus infection triggers large neutrophil numbers and copious DNA-rich mucus containing NET constituents in the lower airways [27]. Respiratory syncytial virus fusion protein, which acts via TLR4, activation of NADPH oxidase and the ERK and p38 MAP kinase pathways, mediates the release of the NETs (ERK: extracellular signal-regulated kinase; MAP: mitogen-activated protein). Based on their findings, Cortjens et al suggest targeting TLR4 and the kinase pathways to prevent harmful NET release into bronchiolar airways in severe cases of respiratory syncytial virus pneumonia [28].
Influenza virus often triggers pneumonitis with excessive neutrophil infiltration and NET formation that contribute to severe damage to the alveoli and endothelial cells, resulting in capillary leakage [11]. In addition, NETs induced by influenza virus fail to kill S. pneumonia, which may facilitate secondary S. pneumonia infection [29].
Pathogenic hantavirus was unexpectedly found to activate NET formation and to stimulate production of high levels of antinuclear and anti-double strand DNA antibodies. Whether these antibodies have an impact on viral replication or NET activity remains unresolved [30]. The pox virus may trigger NETs coupled with activation of the coagulation cascade. Trapped in the resultant clots, viruses may be effectively removed by macrophages [26].
The action of viruses may, however, be quite subversive, as was shown for human immunodeficiency virus (HIV), which triggers TLR-7 and -8 to generate NETs that competently eliminate it. However, at the same time HIV induces IL-10 production by dendritic cells, which counters the action of the neutrophil and promotes co-infection with other organisms [10]. Dengue virus provides a different viral strategy to counter the action of NETs. It impairs NET formation effectively by antagonising phorbol ester-related glucose uptake [31].
Fungal infection
Fungi also induce NETosis, and NETs kill both yeast and hyphal forms of Candida albicans with the aid of granular components attached to the NETs [32], especially calprotectin [7]. NETs are also effective at killing Aspergillus fumigatus [33], which seems to be a result of direct effects of NETs, since galactosaminogalactan, an A. fumigatus virulence factor, reduces the susceptibility to NET-mediated killing by directly inhibiting factors on the NETs rather than influencing intracellular ROS production and signalling leading to NET formation [34].
Parasitic infection
Of parasites reported to induce NETosis, Leishmania species have received most attention. L. infantum resistance to killing by NETs is mediated by nuclease activity under certain culture conditions [35], while L. amazonensis appears more susceptible to killing by NETs [36]. NETs have been observed to ensnare and immobilise the helminth Strongyloides stercoralis, facilitating killing by other immune cells [37].
Plasmodium falciparum infection has been associated with NET formation that evokes an autoimmune response that varies between children and adults. In the children, circulatory NETs adherent to parasites were associated with potentially damaging antinuclear antibodies against double-stranded DNA and may weaken the response to CpG oligonucleotide-based vaccination [38].
To recapitulate, NETs induced by viruses, fungi and parasites possess the function of immobilising and killing akin to those generated in response to bacteria. Similarly, the interactions between neutrophils and these pathogens have directed the adoption of survival mechanisms that target the integrity of the DNA backbone of the NETs, the activity of the NET adherent harmful components and the signalling devices that promote NETosis. The balance of these factors to the advantage of the host is a significant factor in the successful defense against virulence of pathogens and harmful infection.
Notably, the confrontation of pathogens by the neutrophils as components of innate immunity provokes the release of biologically active cytoplasmic and nuclear contents that profoundly alter microorganism and host. The impact for inflammation experienced by the host is the topic of the following sections.
Autoinflammatory and autoimmune disorders
Cell-free DNA – a common thread in autoinflammatory conditions and thrombosis?
An intriguing feature of the autoinflammatory conditions to be discussed next is that cell-free DNA, in particular that originating from neutrophils, may fundamentally contribute to their pathophysiology (fig. 2), as recently reviewed in depth [39, 40]. Initial evidence for such a mechanism was reported by the team of Leadbetter and Marshak-Rothstein, who determined that not only CpG DNA of microbial or mitochondrial origin, but also self-DNA complexes with IgG can trigger B cells [41]. The dual engagement of the B cell surface receptor specific for the Fc portion of IgG of rheumatoid factor specificity, and TLRs recognising DNA was sufficient to activate B cells independently of T cell help. Since DNA-IgG complexes are frequently observed in systemic lupus erythematosus (SLE), it was proposed that such a mechanism also be operative in inducing anti-DNA antibodies.
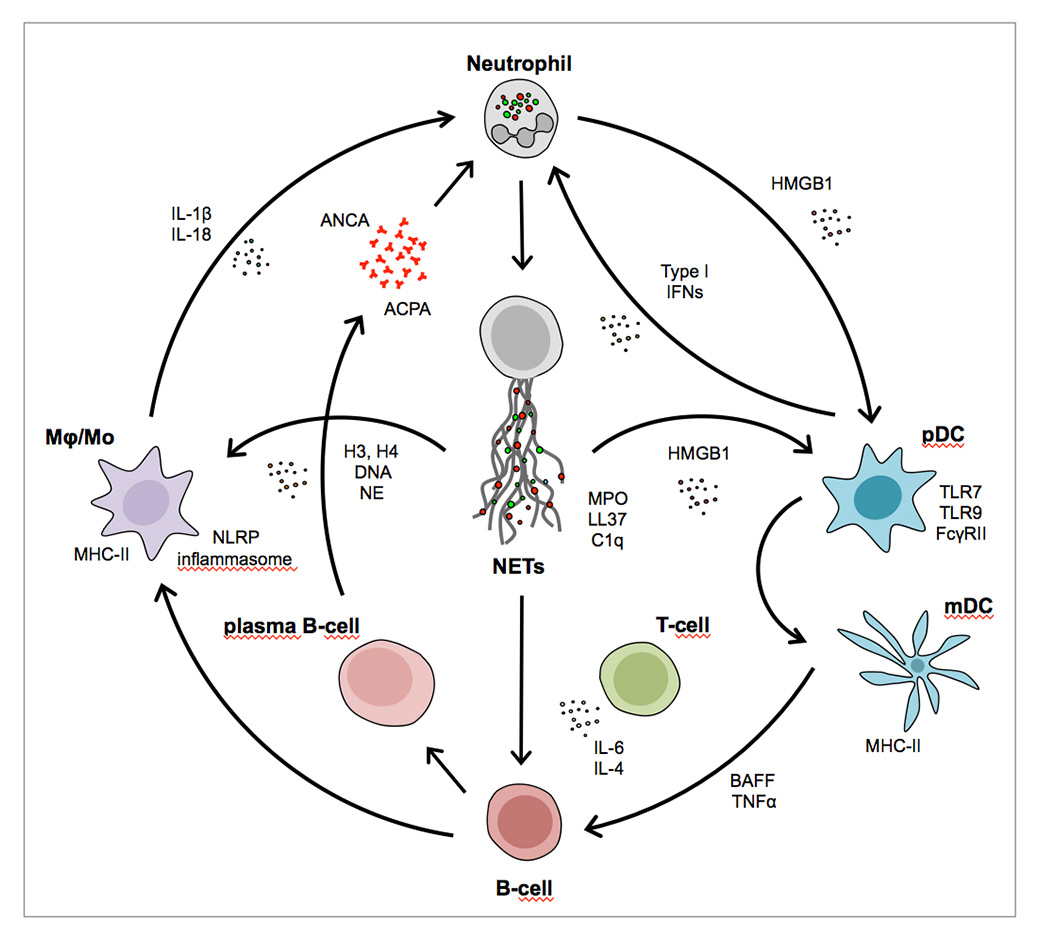
Figure 2
The interactions of pathways and innate and adaptive immune cell types connected with neutrophil extracellular trap formation.
ACPA = anti-citrullinated peptide antibody; ANCA = anti-neutrophil cytoplasmic antibody; BAFF = B cell activating factor; FcγRII = Fc gamma receptor II; H3, H4 = histones; HGMB1 = high mobility group box 1 protein; IFN = interferon; IL = interleukin; LL37 = cathelicidin; mDC = myeloid dendritic cell; MHC = major histocompatibility complex; Mϕ/Mo = monocyte subtypes; NE = neutrophil elastase; NET = neutrophil extracellular trap; pDC = plasmacytoid dendritic cells; TLR = Toll-like receptor; TNFα = tumour necrosis factor alpha
The group of Puga and Cerrutti subsequently detected convincing evidence for another mechanism of direct activation of B cells specific for NET intrinsic and NET-bound extraneous antigens. In the lymph node marginal zone, sinusoidal endothelial cells condition circulatory neutrophils to assume two subset phenotypes with the capacity to directly activate B cells, be it via cytokines or via NETs sporting trapped antigens of afferent lymphatic provenance [42]. Of special interest is that not only antigens of intestinal origin with different specificities, but also LPS, passed through the Peyer’s patches and the liver to gain access to the spleen and peripheral lymph nodes and provoke the neutrophil-mediated B cell activation. As in the system described by Leadbetter and Marshak-Rothstein, both the cytokine- and the NET-mediated B cell activation and antibody production occurred in the absence of T cell help [42].
A further key finding in this context was made by the group of Gilliet, who determined that self-DNA released by dying cells is taken up by plasmacytoid dendritic cells (pDCs) in a complex with the amphoteric peptide LL37 (cathelicidin), leading to their activation via TLR engagement [43]. Since LL37 is also present on NETs, such a mechanism may be operative under conditions of aberrant NETosis, as was later reported by this group in childhood SLE [44]. Likewise, in psoriasis, LL37 of capillary endothelial provenance forms complexes with DNA in the skin that are taken up by pDCs. These are activated via TLR9 to produce local interferon (IFN), setting off inflammation and epithelial hyperproliferation [45, 46].
The state of cell-free DNA or ribonucleoprotein immune complexes constitutes another key element in promoting a NET-dependent autoinflammatory state. This facet was recently reported on in detail, in that the oxidation of ribonucleoprotein immune complexes by neutrophil ROS promoted their NETotic capability. Furthermore, the extrusion of oxidised mitochondrial DNA during NETosis was found to be highly proinflammatory, triggering a type I interferon state in mice, which could be reduced by inhibition of ROS [47].
NETs may also contribute directly to the pathogenesis of rheumatoid arthritis (RA), in that these lattice structures are coated with myriad neutrophil-derived proteins including PAD4 and PAD2 [48] (fig. 3). These extracellular structures could function as nano-machines [49] that efficiently citrullinate arginine residues on a host of bystander proteins, thereby contributing significantly to the generation of new anti-citrullinated peptide (ACPA) species.
Inflammation is associated with procoagulatory states. In early studies NETs were shown to induce endothelial cell damage. Further work linked intravascular NETs to thrombosis, as we consider below in several inflammatory conditions.
Pregnancy disorders
In pregnancy, extensive adaptations of the innate and adaptive immune systems are required to ensure the survival of mother and fetus. Whereas the adaptive immune system is in general suppressed in normal gestation, the phagocytic activity and degranulation of neutrophils increase with its duration.
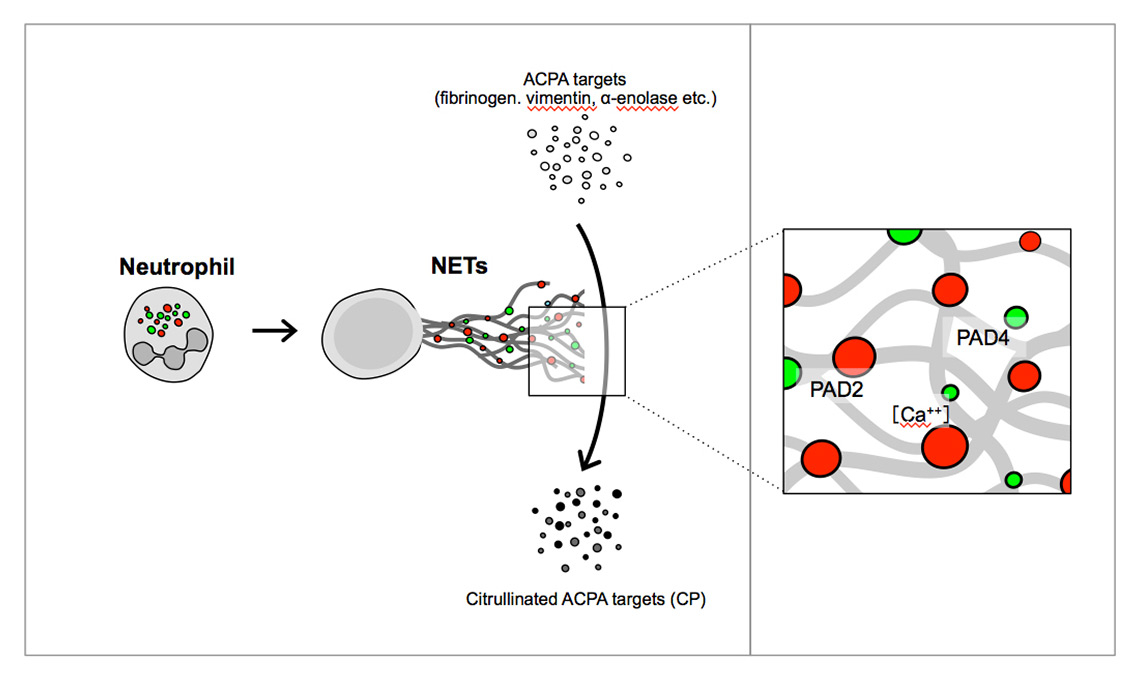
Figure 3
PAD2 and PAD4 as an example of active extracellular enzymes. PAD2 and PAD4 citrullinate arginine residues of neutrophil and bystander proteins to create ACPA targets.
ACPA = anti-citrullinated peptide antibody; NET = neutrophil extracellular trap; PAD = peptidyl arginine deiminase
Preeclampsia in pregnancy is characterised by inflammation in the context of hypertension, kidney failure and seizures. The only currently known causal treatment is delivery, which rapidly resolves the condition. A feature of preeclampsia is a significant elevation of cell-free DNA in the circulation.
While investigating the provenance of this circulatory DNA, we hypothesised that its source is inappropriate NET formation [50]. We were able to demonstrate that placental microparticles, which are released in elevated amounts in preeclampsia, efficiently induce NETs in isolated neutrophils in an IL-8-dependent manner. Immunohistochemistry of placental tissue showed greatly increased amounts of NETs directly in the intervillous space of affected placentae when compared with those from normal healthy deliveries [51]. In this way excessive NETosis can lead to placental tissue damage, frequently observed in patients with preeclampsia. We were further able to show that NETs can induce apoptosis of endothelial cells in co-culture experiments [52]. Thus, they may also contribute to systemic endothelial damage occurring in preeclampsia, augmenting that attributed to an imbalance in endothelial growth factors (placental soluble fms-like tyrosine kinase 1 [sFlt-1] and phosphatidylinositol-glycan biosynthesis class F protein [PIGF]).
The mechanism whereby placentally derived microparticles trigger NETosis is still unresolved, but may comprise activation of the TLR system, since these particles contain both RNA and DNA. In this manner, they could be viewed as viral analogues.
Another condition that impacts pregnancy is the antiphospholipid syndrome, in which anticardiolipin antibodies interact with neutrophils and the endothelium [53–55]. We initially proposed that this interaction could lead to the release of NETs via engagement with the C5a receptor [56]. A recent report has suggested that neutrophils from cases with antiphospholipid syndrome are more prone to undergo NETosis and that this may be triggered by antiphospholipid IgG antibodies [57].
Systemic lupus erythematosus
A prominent feature of the systemic, multiorgan inflammatory disease SLE is the generation of autoantibodies, a large proportion of which are directed against DNA and other nuclear components. SLE has been associated with ineffective clearance of apoptotic residues including nuclear contents, an increased pDC type 1 interferon response, affinity maturation of autoantibodies, and T and B cell abnormalities.
The presence of nuclear chromosomal DNA and attached nuclear and cytosolic components in the NETs provided the rationale for studies on the contribution of NETs to the disease process and as a source for neoantigens in SLE. The reduced clearance of NETs in a subset of SLE cases was attributed to decreased DNA degradation in the context of antibodies bound to NETs and DNase inhibitors. In these cases the increase in NETs was associated with nephritis [58]. Data from paediatric SLE patients indicated that primed neutrophils exposed to anti-ribonucleoprotein antibodies release NETs that provoke pDCs to produce large amounts of IFN-α, mediated by DNA and TLR9. The pDC response is subject to the uptake of DNA in conjunction with the antimicroblial neutrophil peptide LL37 (cathelicidin) [44]. A subset of neutrophils, low-density granulocytes, is especially prone to spontaneous, rapid NET formation and to generating large quantities of IFN-α [59]. NETs and their constituent LL37 were able to activate the NALP3 inflammasome in primed macrophages and to induce IL-1β and IL-18 production. This did not appear to differ between conventional neutrophils and low-density granulocytes, though the macrophages from SLE patients reacted more intensely [60]. NETs are degraded more slowly in SLE, and they could contribute to disease activity by activating complement [61].
Furthermore, NETS may damage blood vessels by modifying lipoproteins and activating metalloproteinases in SLE [62, 63]. This is supported by the reduction of vascular injury and organ damage by the inhibition of NET formation by preferential inhibition of PAD4 with chloramidine in a murine SLE model [64, 65].
Rheumatoid arthritis
RA is a chronic, destructive inflammatory disease of the joints. In roughly two thirds of patients ACPAs are detectable in the blood [66]. Since the progression of NETosis depends on the citrullination of histones mediated by PAD4, it has been attractive to hypothesise that NET formation plays a role in the pathogenesis of RA [67]. Indeed, neutrophils isolated from RA patients showed an increased propensity to undergo spontaneous NETosis as well as NETosis induced by ACPAs, immune complexes and other stimulants [48, 68], and in part by TNF-α and IL-17 [69]. The analysis of NET constituents for the diagnosis of RA showed remarkable results in that it yielded unexpectedly high receiver operator characteristic curve values. These were highest for nucleosomes, achieving an area under the curve of 0.97 and a sensitivity of 91% and specificity of 92% for differentiating RA cases from healthy controls. This applied to both ACPA positive and negative RA patients [48].
In a rat model of RA, collagen-induced arthritis, inhibition of PAD4 by chloramidine reduced inflammation and erosive changes, indicating that interfering with NET generation may be an effective therapeutic scheme [70]. It may be of value to target other signalling steps, including the G-protein coupled receptor, the translocation of NE and/or MPO to the nucleus and Ca2+/calcineurin signalling [71]. Another prospect is opened up by the inhibition of NET formation by monoclonal antibodies directed against citrullinated histones, which effectively reduced joint inflammation and erosion in the collagen-induced arthritis model of RA [72].
In Felty’s syndrome, a severe manifestation of rheumatoid arthritis with intense systemic inflammation, splenomegaly and neutropenia, autoantibodies that preferentially bind citrullinated histones have been detected [73]. The reactivity to citrullinated histones could be a marker for severe inflammation and the risk for severe disease progression.
The evidence suggests that the neutrophil plays a substantial role in the pathogenesis of RA. In this sense, neutrophils could provide a link between innate immune system activation and external factors contributing to disease initiation and flares, such as smoking and infections linked to the citrullination of histones. In addition, NETs may directly interact with T cells and lower their threshold for responding to specific antigens, even at suboptimal concentrations [74].
Psoriasis
Plasmacytoid dendritic cells have been implicated in the inflammation that predisposes the skin to react with hyperkeratosis in psoriasis. Though the pDCs sense microbial DNA via intracellular TLRs-7 and -9, they show practically no reactivity to self-DNA. Compartmentalisation of TLR9 in the endosomes prevents access of self-DNA to the intracellular location under normal circumstances. The inflammatory neutrophil peptide LL37 (see above) forms complexes with self-DNA, enabling the uptake into the pDCs, thus allowing access to TLR9 and a powerful IFN-α response. The source of LL37 in the case of psoriasis appears to be the endothelial cells in the skin vessels, which are prone to cytokine- and mechanical stress-mediated release of LL37 [43, 45, 46].
An intriguing observation is that the DNA can originate from neutrophils and mast cells undergoing extracellular trap formation in the skin. This occurs in conjunction with the release of IL-17, primarily by mast cells, but also by neutrophils, which are enriched in psoriatic skin lesions [75]. These observations led the authors to conclude that the effect of IL-17 blockade with specific antibodies in psoriasis is due to the inhibition of mast cell- and neutrophil-driven inflammation rather than that of T cells.
Vascular inflammation and thrombosis
ANCAs are a cornerstone of the diagnosis of small vessel vasculitis entities of granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis and microscopic polyangiitis. ANCAs can directly foster the respiratory burst of neutrophils. Neutrophils primed by TNF-α were found to undergo NET formation in vitro upon stimulation with ANCA. Furthermore, NETs with the coupled cytoplasmic granular myeloperoxidase and serine protease 3 were detected in the glomeruli of kidney biopsies from patients with active glomerulonephritis [76]. The quantity of NET products in the circulation correlates with activity of disease [77]. NETs and microparticles released from neutrophils in the circulation and bronchoalveolar lavage fluid in active ANCA-associated vasculitis contain tissue factor and could thus promote thrombosis in this condition [78]. The connection between S. aureus infection and granulomatosis with polyangiitis, which has been assessed in a recent systematic review [79], implies that NETosis triggered by S. aureus promotes increased activity of the disease.
To investigate whether NETs could be responsible for the vascular alterations in preeclampsia and other inflammatory conditions such as small vessel vasculitis, we conducted experiments on the interaction between neutrophils and endothelial cells. Endothelial cells activated with TNF-α- or phorbol ester-induced untreated neutrophils to undergo NETosis. In return, NETs caused damage to and death of endothelial cells, which depended on the structural integrity of the NETs and partially on IL-8, providing a mechanism for vessel wall damage in small vessel vasculitis, sepsis and preeclampsia [52]. Intravascular NETs also provide a scaffold to activate platelet aggregation, fibrin deposition and erythrocyte-rich thrombus formation [80]. By demonstrating the formation of thrombosis due to intravascular NETs, these findings suggested a link between increased thrombosis and inflammation associated with severe infection and other conditions. Another example is the contribution of NETs to transfusion-associated lung injury [81], which appears to be initiated by activated platelets [82]. Engelmann and Massberg coined the term immunothrombosis [83] to describe the concept of thrombosis induced by inflammation and NETosis (fig. 4) [83].
Recent reports substantiate a role for neutrophil inflammatory responses and NET formation in acute coronary syndrome. The amount of NETs in samples from coronary arteries involved in ST-elevation myocardial infarction was found to correlate with infarct size and extent of ST segment resolution [84]. In the infarct-related artery, NETs with bound tissue factor are released, which is associated with thrombosis downstream of the plaque. Plasma obtained from the myocardial infarct-related coronary artery contains elevated thrombin and was able to activate platelets, which in turn induced NET formation in vitro[85]. Of interest in the setting of chronic vessel wall disease is the recent finding that neutrophils exposed to cholesterol crystals released NETs. Macrophages in atherosclerotic plaques respond to the NETs by producing pro-IL-1β. A second stimulus, such as shear stress, can then induce the inflammatory cascade by the activation of caspase-1 and IL-1β and IL-18, which propagate the events leading to plaque formation and possibly also rupture, as in acute coronary syndrome [86].
Based on the implication of NETosis in vascular conditions, NET formation presents a target for intervention. DNase may reduce the activity of tissue factor in vitro, suggesting a possible therapeutic role in acute coronary ischaemia [85]. Early results from animal experiments found an encouraging reduction of vascular events by treatment with the PAD4 antagonist chloramidine, which has also been tested successfully in models of SLE-associated vascular disease [62, 63, 65] and RA [87] (see also above).
NETs in other conditions
NETosis has been observed in an extensive range of disease states with increased or decreased production of NETs. From the continually growing list, a brief selection of these conditions follows.
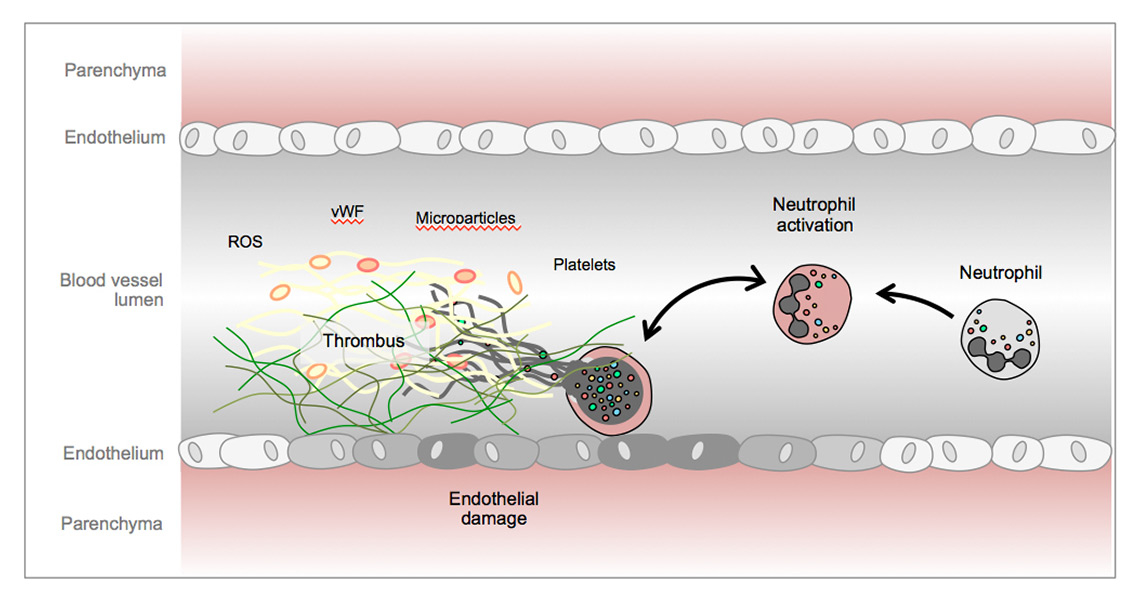
Figure 4
Endothelial cell damage, von Willebrand factor and activated platelets promote neutrophil extracellular trap formation and thrombosis in the vasculature.
ROS = reactive oxygen species; vWF = von Willebrand factor
Monosodium urate and calcium pyrophosphate dehydrate crystal-induced inflammation
Gout manifests as an inflammation of periarticular tissues and joints linked to the deposition of monosodium urate crystals. Monosodium urate crystals activate neutrophils to form NETs, as do serum and joint fluid from patients with acute gout [88]. NETs within tophi have been linked to the resolution of tophi and could offer a target to accelerate their resorption. Another mechanism mediating resolution of inflammation by NETs is proteolytic cleavage and inactivation of cytokines by attached enzymes [89, 90]. Calcium pyrophosphate dihydrate crystals promoted NET formation, which was susceptible to blocking of CXCR2, the principal neutrophil IL-8 receptor [91].
Diabetes mellitus
Hyperglycaemia is associated with the priming of neutrophils for oxidative burst and superoxide production. Further investigation showed that hyperglycaemia boosted NET formation and revealed that other signalling steps are also activated [92].
It is conceivable that the neutrophil responses in diabetes mellitus contribute to the damage to pancreatic islet cells and that the beneficial effect of IL-1 blockade in the treatment of diabetes mellitus type 2 could at least partially be attributed to its effects on neutrophils [93]. The direct damage to endothelial cells incited by NETs and the modification of high-density lipoprotein are mechanisms by which the vascular consequences of hyperglycaemia may be mediated.
Familial Mediterranean fever
Flares of familial Mediterranean fever are associated with an increase in neutrophils mediated by increased cleavage of pro-IL-1β to active IL-1β by caspase-9, which is itself activated by the NALP3 inflammasome in macrophages. A recent report found that in patients with familial Mediterranean fever neutrophils produce NETs with adherent IL-1β in association with attacks of familial Mediterranean fever [94].
Chronic granulomatous disease
Chronic granulomatous disease with multiple infections from birth entails a genetic deficiency of NADPH oxidase and vastly reduced NET formation. A. fumigatus in particular causes tenacious and invasive pulmonary infections. Genetic reconstitution of NADPH oxidase function in a patient with the disease restored ROS production, NET formation and rapidly cleared A. fumigatus [95].
Cancer
Both beneficial and adverse effects of neutrophils have been gaining attention [96]. In biopsy samples of two of eight cases of Ewing sarcoma, tumour-associated neutrophils were found to have produced NETs [97]. In vitro and in vivo animal studies of NETs showed that these entrap metastatic lung cancer cells and promote the formation of metastases in the liver. This was abrogated by treatment with DNase or neutrophil elastase [98]. The effects of cell-free DNA on increased coagulation in breast cancer were the subject of many studies before they were attributed to NET formation in the case of chemotherapy with epirubicin and doxorubicin [99]. Further evidence for the contribution of NET formation was found in models of chronic myelogenous leukaemia and breast and lung cancer. Increased numbers of blood neutrophils with an increased propensity to undergo NET formation coincided with thrombotic events, indicating a mechanism for the increased incidence of thrombosis and poorer outcomes of the neoplasia in these cases [100]. Neutrophil effects including NET formation in the setting of cancer may receive greater attention if these observations are corroborated.
Overview
From their first description in 2004, NETs have been a remarkable phenomenon as an addition to the neutrophil repertoire of phagocytosis and degranulation to combat infection. The powerful action of NETs against bacteria and fungi has gained widespread attention and insufficient NET formation may make the host susceptible to overwhelming infection. Equally impressive is their deployment and action against infection by viruses and parasites. NETs cannot, however, operate against their targets without vigorous opposition by several mechanisms to blunt or abrogate their effects.
NETs are composed of chromosomal DNA adorned with a host of lytic and catalytic enzymes and microbicidal components. Their release into the tissues and the bloodstream may initiate inflammation and thrombosis that damage the host. Components of the NETs are established antigens in inflammatory diseases, and they may provide the basis for systemic inflammation, organ damage and autoantibody generation independently of T cell help.
Modulating NET formation has shown promise for the treatment of infectious, inflammatory and thrombotic diseases. The introduction of NETs into therapeutic strategies and also diagnostics will hopefully continue to give exciting results.
Conclusion
The study of NETs has profoundly altered the perception of neutrophil biology and of the role of neutrophils in defence against pathogens and in the pathophysiology of inflammation that damages the body. The details of the mechanisms involved show mechanistic insights and have started to allow targeted interventions that promise novel therapeutic approaches in diseases from infections to vascular thrombotic events.
References
1 Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Extracellular neutrophil traps in periodontitis. J Periodontal Res. 2009;44(5):664–72.
2 Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218.
3 Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210(7):1283–99.
4 Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5.
5 Futosi K, Fodor S, Mocsai A. Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int Immunopharmacol. 2013;17(3):638–50.
6 Van Avondt K, van der Linden M, Naccache PH, Egan DA, Meyaard L. Signal Inhibitory Receptor on Leukocytes-1 Limits the Formation of Neutrophil Extracellular Traps, but Preserves Intracellular Bacterial Killing. J Immunol. 2016;196(9):3686–94.
7 Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639.
8 Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol. 2010;191(3):677–91.
9 Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15(11):1017–25.
10 Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe. 2012;12(1):109–16.
11 Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179(1):199–210.
12 McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324–33.
13 Lee SK, Kim SD, Kook M, Lee HY, Ghim J, Choi Y, et al. Phospholipase D2 drives mortality in sepsis by inhibiting neutrophil extracellular trap formation and down-regulating CXCR2. J Exp Med. 2015;212(9):1381–90.
14 Tadie JM, Bae HB, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. Am J Physiol Lung Cell Mol Physiol. 2013;304(5):L342–9.
15 Sharma A, Steichen AL, Jondle CN, Mishra BB, Sharma J. Protective role of Mincle in bacterial pneumonia by regulation of neutrophil mediated phagocytosis and extracellular trap formation. J Infect Dis. 2014;209(11):1837–46.
16 Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol. 2010;185(12):7413–25.
17 Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Kockritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2(6):576–86.
18 Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci U S A. 2005;102(5):1679–84.
19 Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16(4):401–7.
20 Eby JC, Gray MC, Hewlett EL. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infect Immun. 2014;82(12):5256–69.
21 [This reference was retracted and therefore deleted from the reference list in this article.]
22 Marcos V, Zhou-Suckow Z, Onder Yildirim A, Bohla A, Hector A, Vitkov L, et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediators Inflamm. 2015;2015:408935.
23 Kamoshida G, Kikuchi-Ueda T, Tansho-Nagakawa S, Nakano R, Nakano A, Kikuchi H, et al. Acinetobacter baumannii escape from neutrophil extracellular traps (NETs). J Infect Chemother. 2015;21(1):43–9.
24 Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6(9):e23637.
25 Braian C, Hogea V, Stendahl O. Mycobacterium tuberculosis- induced neutrophil extracellular traps activate human macrophages. J Innate Immun. 2013;5(6):591–602.
26 Hahn S, Giaglis S, Chowdury CS, Hosli I, Hasler P. Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. Semin Immunopathol. 2013;35(4):531.
27 Cortjens B, de Boer OJ, de Jong R, Antonis AF, Sabogal Pineros YS, Lutter R, et al. Neutrophil Extracellular Traps Cause Airway Obstruction During Respiratory Syncytial Virus Disease. J Pathol. 2016;238(3):401–11.
28 Funchal GA, Jaeger N, Czepielewski RS, Machado MS, Muraro SP, Stein RT, et al. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS One. 2015;10(4):e0124082.
29 Narayana Moorthy A, Narasaraju T, Rai P, Perumalsamy R, Tan KB, Wang S, et al. In vivo and in vitro studies on the roles of neutrophil extracellular traps during secondary pneumococcal pneumonia after primary pulmonary influenza infection. Front Immunol. 2013;4:56.
30 Raftery MJ, Lalwani P, Krautkrmer E, Peters T, Scharffetter-Kochanek K, Kruger R, et al. beta2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J Exp Med. 2014;211(7):1485–97.
31 Moreno-Altamirano MM, Rodriguez-Espinosa O, Rojas-Espinosa O, Pliego-Rivero B, Sanchez-Garcia FJ. Dengue Virus Serotype-2 Interferes with the Formation of Neutrophil Extracellular Traps. Intervirology. 2015;58(4):250–9.
32 Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8(4):668–76.
33 Bruns S, Kniemeyer O, Hasenberg M, Aimanianda V, Nietzsche S, Thywissen A, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6(4):e1000873.
34 Lee MJ, Liu H, Barker BM, Snarr BD, Gravelat FN, Al Abdallah Q, et al. The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps. PLoS Pathog. 2015;11(10):e1005187.
35 Guimaraes-Costa AB, DeSouza-Vieira TS, Paletta-Silva R, Freitas-Mesquita AL, Meyer-Fernandes JR, Saraiva EM. 3'-nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps. Infect Immun. 2014;82(4):1732–40.
36 Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A. 2009;106(16):6748–53.
37 Bonne-Annee S, Kerepesi LA, Hess JA, Wesolowski J, Paumet F, Lok JB, et al. Extracellular traps are associated with human and mouse neutrophil and macrophage mediated killing of larval Strongyloides stercoralis. Microbes Infect. 2014;16(6):502–11.
38 Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, et al. Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar J. 2008;7:41.
39 Barnado A, Crofford LJ, Oates JC: At the Bedside. Neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J Leukoc Biol. 2016;99(2):265–78.
40 Grayson PC, Carmona-Rivera C, Xu L, Lim N, Gao Z, Asare AL, et al. Neutrophil-Related Gene Expression and Low-Density Granulocytes Associated With Disease Activity and Response to Treatment in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2015;67(7):1922–32.
41 Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–7.
42 Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13(2):170–80.
43 Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009;206(9):1983–94.
44 Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3(73):73ra20.
45 Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–9.
46 Lande R, Chamilos G, Ganguly D, Demaria O, Frasca L, Durr S, et al. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur J Immunol. 2015;45(1):203–13.
47 Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nature Med. 2016;22(2):146–53.
48 Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16(3):R122.
49 Simmel FC. DNA-based assembly lines and nanofactories. Curr Opin Biotechnol. 2012;23(4):516–21.
50 Gupta A, Hasler P, Gebhardt S, Holzgreve W, Hahn S. Occurrence of neutrophil extracellular DNA traps (NETs) in pre-eclampsia: a link with elevated levels of cell-free DNA? Ann N Y Acad Sci. 2006;1075:118–22.
51 Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66(11):1146–54.
52 Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584(14):3193–7.
53 Chen Q, Guo F, Hensby-Bennett S, Stone P, Chamley L. Antiphospholipid antibodies prolong the activation of endothelial cells induced by necrotic trophoblastic debris: implications for the pathogenesis of preeclampsia. Placenta. 2012;33(10):810–15.
54 Ostensen M, Villiger PM, Forger F. Interaction of pregnancy and autoimmune rheumatic disease. Autoimmun Rev. 2012;11(6-7):A437–46.
55 Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update. 2005;11(4):411–23.
56 Hahn S, Giaglis S, Hoesli I, Hasler P. Neutrophil NETs in reproduction: from infertility to preeclampsia and the possibility of fetal loss. Front Immunol. 2012;3:362.
57 Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated With Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis Rheumatol. 2015;67(11):2990–3003.
58 Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A. 2010;107(21):9813–18.
59 Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–97.
60 Kahlenberg JM, Carmona-Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol. 2013;190(3):1217–26.
61 Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol. 2012;188(7):3522–31.
62 Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis. 2015;74(7):1417–24.
63 Smith CK, Vivekanandan-Giri A, Tang C, Knight JS, Mathew A, Padilla RL, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol. 2014;66(9):2532–44.
64 Knight JS, Subramanian V, O'Dell AA, Yalavarthi S, Zhao W, Smith CK, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74(12):2199–2206.
65 Knight JS, Zhao W, Luo W, Subramanian V, O'Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123(7):2981–93.
66 McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–19.
67 Zhong XY, von Muhlenen I, Li Y, Kang A, Gupta AK, Tyndall A, et al. Increased concentrations of antibody-bound circulatory cell-free DNA in rheumatoid arthritis. Clin Chem. 2007;53(9):1609–14.
68 Behnen M, Leschczyk C, Moller S, Batel T, Klinger M, Solbach W, et al. Immobilized immune complexes induce neutrophil extracellular trap release by human neutrophil granulocytes via FcgammaRIIIB and Mac-1. J Immunol. 2014;193(4):1954–65.
69 Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra140.
70 Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186(7):4396–404.
71 Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9(5):e97088.
72 Chirivi R. Anti-Citrullinated Protein Antibodies as Novel Therapeutic Drugs in Rheumatoid Arthritis. J Clin Cell Immunol. 2013;01(S6).
73 Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, et al. Felty's syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum. 2012;64(4):982–92.
74 Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188(7):3150–9.
75 Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500.
76 Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–5.
77 Soderberg D, Kurz T, Motamedi A, Hellmark T, Eriksson P, Segelmark M. Increased levels of neutrophil extracellular trap remnants in the circulation of patients with small vessel vasculitis, but an inverse correlation to anti-neutrophil cytoplasmic antibodies during remission. Rheumatology (Oxford). 2015;54(11):2085–94.
78 Kambas K, Chrysanthopoulou A, Vassilopoulos D, Apostolidou E, Skendros P, Girod A, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73(10):1854–63.
79 Kronbichler A, Kerschbaum J. The Influence and Role of Microbial Factors in Autoimmune Kidney Diseases: A Systematic Review. 2015;2015:858027.
80 Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr,. et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107(36):15880–5.
81 Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119(26):6335–43.
82 Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661–71.
83 Engelmann B, Massberg S: Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2012;13(1):34–45.
84 Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenbock A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. 2015;116(7):1182–92.
85 Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405–14.
86 Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–20.
87 Lopez-Longo FJ, Oliver-Minarro D, de la Torre I, Gonzalez-Diaz de Rabago E, Sanchez-Ramon S, Rodriguez-Mahou M, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(4):419–24.
88 Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, et al. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PLoS One. 2011;6(12):e29318.
89 Maueroder C, Kienhofer D, Hahn J, Schauer C, Manger B, Schett G, et al. How neutrophil extracellular traps orchestrate the local immune response in gout. J Mol Med (Berl). 2015;93(7):727–34.
90 Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20(5):511–7.
91 Pang L, Hayes CP, Buac K, Yoo DG, Rada B. Pseudogout-associated inflammatory calcium pyrophosphate dihydrate microcrystals induce formation of neutrophil extracellular traps. J Immunol. 2013;190(12):6488–500.
92 Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815–9.
93 Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–26.
94 Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, Chrysanthopoulou A, et al. Neutrophil extracellular traps regulate IL-1beta-mediated inflammation in familial Mediterranean fever. Ann Rheum Dis. 2016;75(1):269–77.
95 Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114(13):2619–22.
96 Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–31.
97 Berger-Achituv S, Brinkmann V, Abed UA, Kuhn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48.
98 Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;67484.
99 Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J Thromb Haemost. 2011;9(11):2313–21.
100 Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A. 2012;109(32):13076–81.
101 Juneau RA, Stevens JS, Apicella MA, Criss AK. A thermonuclease of Neisseria gonorrhoeae enhances bacterial escape from killing by neutrophil extracellular traps. J Infect Dis. 2015;212(2):316–24.
102 Lappann M, Danhof S, Guenther F, Olivares-Florez S, Mordhorst IL, Vogel U. In vitro resistance mechanisms of Neisseria meningitidis against neutrophil extracellular traps. Mol Microbiol. 2013;89(3):433–49.
103 Mollerherm H, Neumann A, Schilcher K, Blodkamp S, Zeitouni NE, Dersch P, et al. Yersinia enterocolitica-mediated degradation of neutrophil extracellular traps (NETs). FEMS Microbiol Lett. 2015;362(23):fnv192.
104 Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Leitner DR, et al. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 2013;9(9):e1003614.
105 Scharrig E, Carestia A, Ferrer MF, Cedola M, Pretre G, Drut R, et al. Neutrophil Extracellular Traps are Involved in the Innate Immune Response to Infection with Leptospira. PLoS Negl Trop Dis. 2015;9(7):e0003927.



