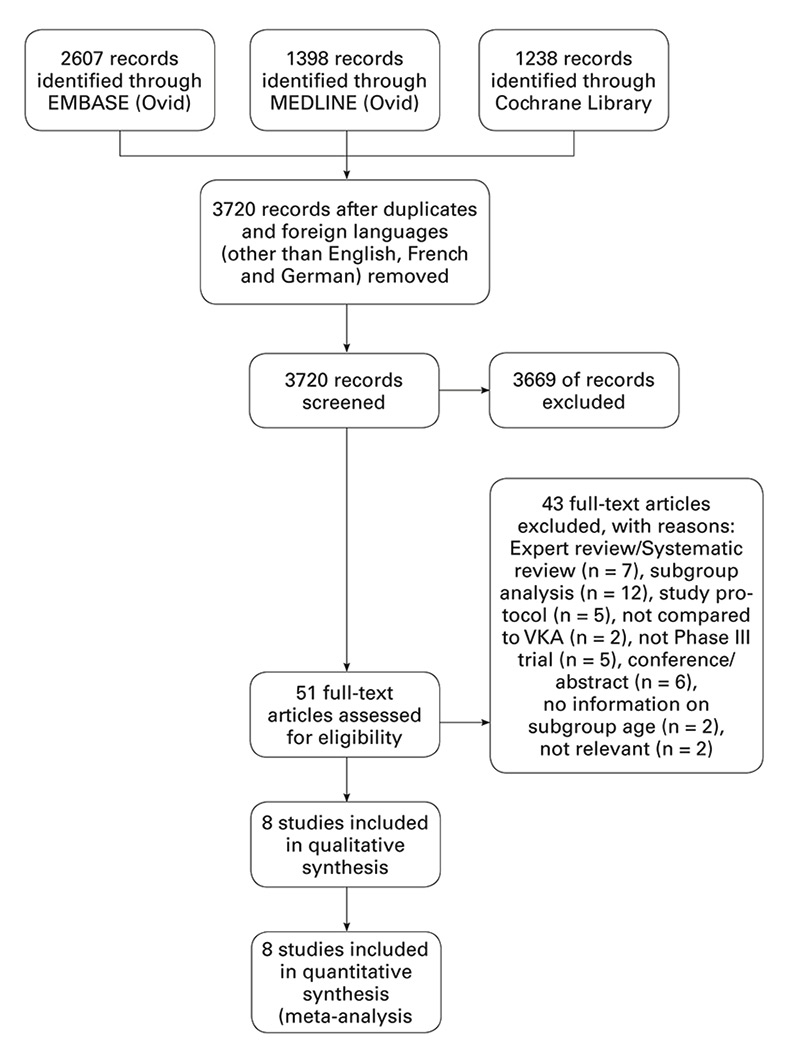
Figure 1
Study flow chart.
DOI: https://doi.org/10.4414/smw.2016.14356
Atrial fibrillation is the most frequent cardiac arrhythmia, with a prevalence of approximately 1.5 to 2% in the general population in developed countries. This disorder is associated with a high mortality, morbidity and impairment of quality of life [1]. The CHA2DS2-VASc score (congestive heart failure / left ventricular dysfunction, hypertension, age ≥75 years, diabetes mellitus, prior stroke / transient ischaemic attack / systemic embolism, vascular disease, aged 65 to 74 years, gender category) has been validated to stratify the stroke risk of patients with atrial fibrillation. Oral anticoagulant therapy should be considered for patients with a score of 1, and is recommended for patients with a score of ≥2 unless contraindicated [2]. Next to atrial fibrillation, venous thromboembolism (VTE) is a common indication for anticoagulation therapy. Pulmonary embolism and deep venous thrombosis are manifestations of VTE. The incidence of VTE is approximately 1 per 1000 annually in the adult population [3]. It is a common condition, which is associated with acute morbidity and mortality [4]. Upon diagnosis of VTE, antithrombotic agents are administered to prevent the progression of deep venous thrombosis and pulmonary embolism, and to relieve the acute symptoms associated with these conditions.
Since 2009 four products have been approved by the US Food and Drug Administration (FDA) and by the European Medicines Agency (EMA) and are available to prescribers: rivaroxaban (Xarelto®, Bayer), apixaban (Eliquis®, Pfizer), dabigatran (Pradaxa®, Boehringer-Ingelheim) and edoxaban (Lixiana®/Savaysa®, Daiichi Sankyo). Their efficacy and safety have been assessed in large phase III trials. Compared with vitamin K antagonists (VKAs), DOACs have shown a favourable risk-benefit profile in patients with atrial fibrillation or VTE [5–12].
With the ageing of the population, atrial fibrillation is becoming a growing global public health problem. The prevalence of atrial fibrillation among elderly (>75 years) is 10% [13]. The lifetime risk of suffering a stroke increases with age [14]. It is estimated that in 2050, the annual economic burden of atrial fibrillation is expected to be $30 billion in the USA [15]. Age is also considered to be a major risk factor for VTE and is associated with a rise in incidence of pulmonary embolus and deep venous thrombosis. Patients aged 70 years or older have an 18- to 28-fold increase in risk for deep venous thrombosis or pulmonary embolism compared with patients aged 20–29 years [16]. Although VKAs are considered the gold standard of therapy for stroke prevention in atrial fibrillation and VTE, VKAs remain underused because of concerns about VKA-related bleeding [17]. In patients aged 75 years or older, the incidence of VKA-related bleeding rises up to 5% per year [18]. VKAs are among the greatest medication-related risk factors for adverse drug reactions in the elderly [19]. As a consequence, studies have shown that only 50 to 60% of patients with atrial fibrillation eligible for anticoagulation are under oral anticoagulant therapy [20–24]. Furthermore, the complexity and inconvenience associated with VKA therapy favour nonadherence [25]. Among other risk factors, poor cognitive functions have been shown to favour VKA nonadherence [26]. Since their introduction on the market, DOACs are prescribed more and more for elderly populations [27]. Although all landmark studies comparing VKAs with DOACs included patients aged 65 years or more, concerns on the external validity of the results in this age group, and particularly among the frail elderly, have been raised [28, 29].
In 2014, a meta-analysis comparing rivaroxaban, apixaban and dabigatran with VKAs showed that DOACs were associated with equal or greater efficacy than VKAs in the prevention of stroke and systemic embolism in elderly patients with nonvalvular atrial fibrillation. Similar results were found for the prevention of VTE or VTE-related death. Interestingly, no higher bleeding risks were reported for this age group [30]. Recently, these results were confirmed by another meta-analysis including edoxaban in patients with nonvalvular atrial fibrillation as well as patients with acute VTE and extended treatment of VTE [31].
Direct head-to-head comparisons of DOACs have not yet been published. Previous meta-analyses of direct and indirect comparisons have provided some interesting insights regarding the safety and efficacy of DOACs when compared with each other [32, 33]. To our knowledge, no study so far has conducted indirect comparisons between DOACs in the particular subgroup of patients aged 75 years or more.
The aim of this article was to: (a) undertake a meta-analysis on the efficacy and safety of rivaroxaban, apixaban, edoxaban and dabigatran compared with VKAs in patients aged 75 years or more with nonvalvular atrial fibrillation or acute venous thromboembolism, (b) conduct indirect comparisons between the different DOACs. This will set the frame for a discussion on the use of DOAC in the elderly and particularly in the frail elderly.
Phase III trials that compared direct oral anticoagulants (dabigatran, apixaban, rivaroxaban and edoxaban) with vitamin K antagonists or a consecutive regimen of low molecular weight heparin (LMWH) and VKAs were included. Studies were included if the indication was acute venous thromboembolism or atrial fibrillation. We decided to exclude studies if the control arm was placebo, if the treatment indication was thromboprophylaxis or if they were assessing the efficacy and safety of DOACs with antiplatelet therapies. We also excluded studies assessing extended use of anticoagulation therapy, i.e., therapy extended beyond the initial period of treatment, as bleeding complications are more frequent in the early months after initiation of the therapy [34, 35].
The outcomes of interest were the efficacy and safety of the DOAC compared with VKAs. The primary efficacy endpoint for patients with VTE was a composite of DVT, nonfatal and fatal pulmonary embolus. In studies assessing the use of DOACs in nonvalvular atrial fibrillation, the primary efficacy endpoint was a composite of stroke and systemic embolic events. The primary efficacy endpoints were similar across the included studies with some minor differences (appendix 1).
The primary safety outcome was either major bleeding or a composite of major bleeding and clinically relevant non-major bleeding. In contrast to a previous meta-analysis conducted in the same age group, we decided that clinically relevant non-major bleeding should be considered in our primary composite safety outcome [31]. Indeed, we consider that the impact of clinically relevant non-major bleeding in the elderly in terms of morbidity might be important enough to be considered as primary safety endpoint, hence the choice of a composite safety endpoint of major bleeding and clinically relevant non-major bleeding. Additionally, previous studies have highlighted that a previous history of bleeding (independent of the severity) in elderly patients impacts on the prescriber’s decision about anticoagulation therapy [36]. All the included studies defined major bleeding according to the definition from the International Society on Thrombosis and Haemostasis [37]. The definition of clinically relevant non-major bleeding varied somewhat between included trials (appendix 2).
After defining the scope of our systematic review, we conducted an electronic search of the following databases from inception to June 2015: the Cochrane Library (CENTRAL), Medline (through Ovid) and Embase (through Ovid). We did not apply any language restrictions. The search strategy can be found in the appendix 3.
Two reviewers independently screened the titles and abstracts obtained in the search process. If one study was included by one reviewer only, the full article was assessed for eligibility by the other reviewer. Disagreements and doubts were resolved by consensus. The two reviewers independently extracted the data of included studies by using an electronic data extraction form. We developed our own data extraction form using the Cochrane Collaboration data collection form for randomised controlled trials as reference [38]. The data items extracted were: study design, intervention, indication, patients’ characteristics, follow-up time, type of statistical analysis, primary endpoint definitions and data on primary and efficacy outcome by age subgroup. Data were doubled-checked by the authors before they were entered into the analysis software.
All analyses were conducted with the R Statistical Software (Foundation for Statistical Computing, Vienna, Austria). We first conducted a meta-analysis comparing DOACs to VKAs by using the package “meta”. Indirect comparisons between DOACs were undertaken using “netmeta” [39, 40]. For each included trial, dichotomous data was summarised as odds ratios (ORs), and their corresponding 95% confidence intervals (CIs) were generated and pooled in fixed effect models with inverse-variance weighting. Statistical significance was set for a two tailed alpha level of 0.05.
We tested for heterogeneity between the included trials with the Higgins I2 statistic. I2 values of 25% represented a low amount of heterogeneity, and I2 values of 50% and 75%, moderate to high amount of heterogeneity, respectively. We intended to assess publication bias through funnel plot asymmetry and by using the Egger’s regression test only if at least 10 studies were included owing to the lack of power of this test when the number of studies included in the analysis is 10 or less [41, 42]. Sensitivity analysis was conducted by excluding open-label trials. For the studies where two different dosages were compared with the same control group, we decided to undertake separate pooled analysis including either the low dose or the high dose.
To assess the risk of bias for included studies we used the Cochrane Collaboration’s tool for assessing risk of bias including sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, handling of incomplete outcome data, and selective outcome reporting [42].
We used PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) as a reference for reporting [43].
A total of 3720 publications were identified according to our predefined inclusion criteria. The process of title and abstract screening left 51 articles on which we conducted a full text review (fig. 1). In total, we identified eight randomised controlled trials: four studies on atrial fibrillation and four studies on venous thromboembolism. For two of the included trials (ARISTOTLE, RE-LY) we included data from additional reports publishing subgroup analyses [44, 45]. We identified several potential sources of heterogeneity among our included studies: study design (five double-blind randomised controlled trials, three prospective randomised open-label blinded endpoint [PROBE] studies), follow-up period (3 months to 2.8 years), primary analysis and safety endpoint definition for clinically relevant non-major bleedings. Characteristics of the included trials can be found in table 1.

Figure 1
Study flow chart.
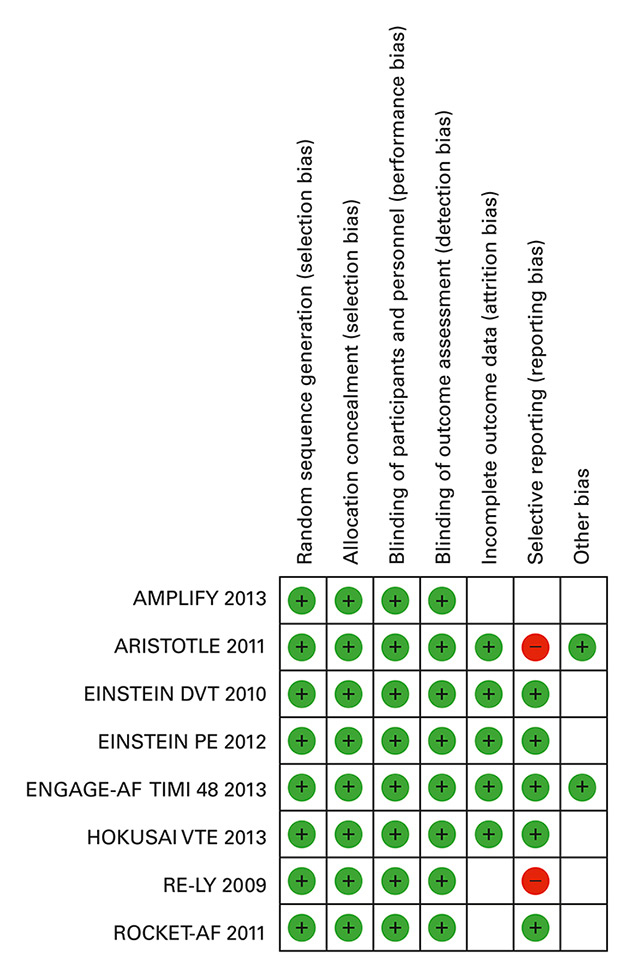
Figure 2
Risk of bias summary.
Green: low risk, red: high risk, white: unclear risk.
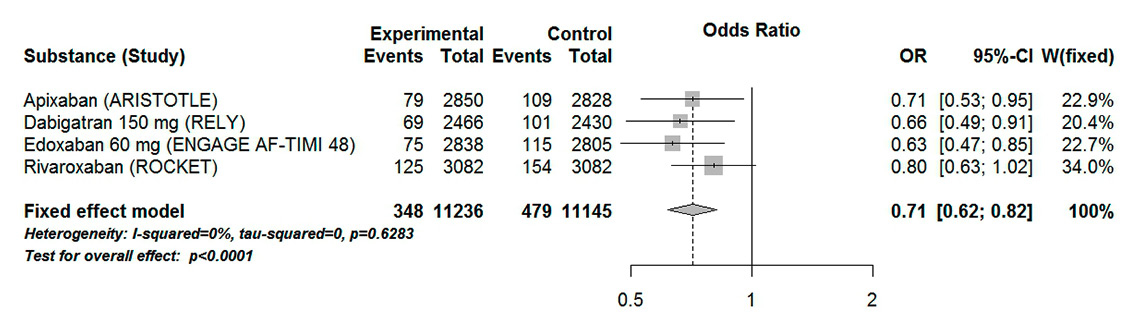
Figure 3
Forest plot of stroke or systemic embolism in patients with atrial fibrillation aged 75 years or over for analysis including high-dose dabigatran and edoxaban.
CI = confidence interval; experimental = direct oral anticoagulants; control = vitamin K antagonists
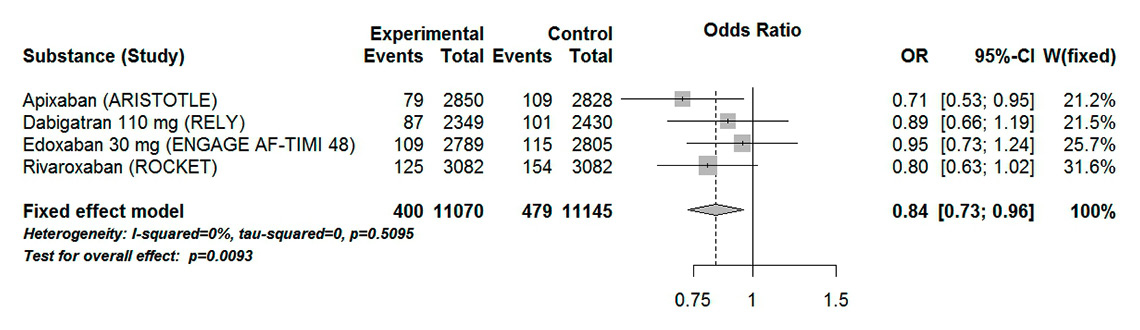
Figure 4
Forest plot of stroke or systemic embolism in patients with atrial fibrillation aged 75 years or over for analysis including low-dose dabigatran and edoxaban.
CI = confidence interval; experimental = direct oral anticoagulants; control = vitamin K antagonists
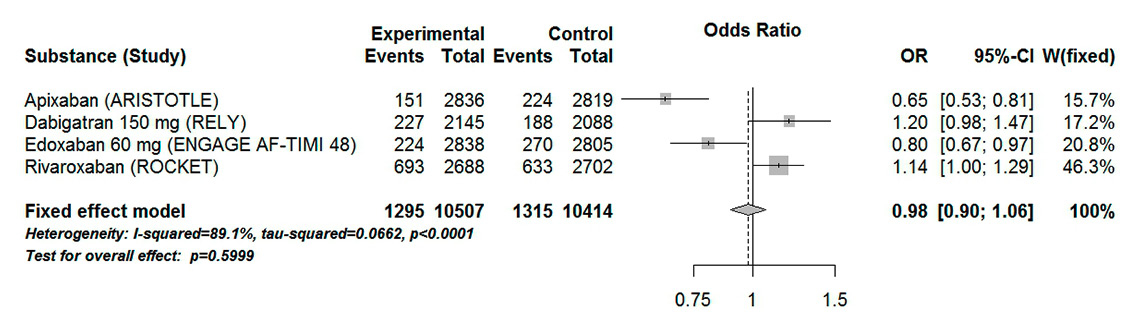
Figure 5
Forest plot of composite major and clinically relevant non-major bleeding in patients with atrial fibrillation aged 75 years or over for analysis including high-dose dabigatran and edoxaban.
CI = confidence interval; experimental = direct oral anticoagulants; control = vitamin K antagonists
We included a total of 30 655 patients aged 75 years or older (table 2). Limited information was included in the published data on the baseline characteristics (body mass index, weight, renal clearance) of this subgroup.
When assessing the risk of bias in our included trials, we found that most of the studies were associated with low risk of bias (fig. 2). In particular, for the three PROBE trials, we considered the performance bias and detection bias to be low risk. Indeed, the potential for bias was limited because an independent committee blinded to the treatment assignment adjudicated the suspected events in the three trials [46, 47]. Concerning the safety outcome, we considered the risk of bias as low to moderate. Although observer bias cannot be excluded in PROBE trials, recent surveys have shown that when robust endpoints are used, there is no statistical difference between outcomes in double-blind trials and PROBE trials, which can be attributed to the design alone [48, 49]. Reporting bias was common for bleeding outcomes in the elderly subgroup. Limited information was available regarding the profile of patients experiencing bleeding events. We therefore considered reporting bias to be a high risk of bias for the trials concerned.
We estimated that the number of included trials was too low to give enough power to assess publication bias through a funnel plot or more advanced regression tests.
The composite endpoint stroke or systemic embolism was reported in four of the included trials. When high-dose dabigatran (150 mg) and edoxaban (60 mg) were considered, DOACs were associated with a significant 29% odds reduction in stroke or systemic embolism (OR 0.71, 95% CI 0.62–0.82). There was a low heterogeneity associated with these results (I20%, Pheterogeneity 0.63) (fig. 3). Similar results were found when low-dose dabigatran (110 mg) and edoxaban (30 mg) were considered (OR 0.84, 95% CI 0.73–0.96, I20%, Pheterogeneity 0.51) (fig. 4). However, this effect is mainly driven by the ARISTOTLE and ROCKET trials. When considered separately, both dabigatran and edoxaban in reduced dose did not show a statistically significant difference compared with VKAs (OR 0.89, 95% CI 0.66–1.19 and OR 0.95, 95% CI 0.73–1.24, respectively).
In our analysis including high-dose dabigatran and edoxaban, there was no statistical difference between DOACs and VKAs in the major bleeding outcome (OR 0.98, 95% CI 0.90–1.06, p = 0.60). The result showed a high heterogeneity (I289%, Pheterogeneity <0.0001) (fig. 5). High-dose edoxaban and apixaban were the two DOACs that showed a statistically significant difference compared with VKAs. Interestingly, when low-dose edoxaban and dabigatran were included in the analysis, a statistically significant 12% odds reduction was observed (OR 0.88, 95% CI 0.80–0.96, p = 0.004) (fig. 6). However, these results were associated with a high heterogeneity (I295%, Pheterogeneity <0.0001).
In order to explore the heterogeneity, we performed a sensitivity analysis excluding the RE-LY trial, which was an open-label trial. The sensitivity analysis showed a statistically significant reduction in the number of major bleeding or clinically non-relevant bleeding events compared with warfarin in the analysis including low-dose edoxaban (OR 0.85, CI 95% 0.77-0.94, p = 0.001) but not high-dose edoxaban (OR 0.94, CI 95% 0.85–1.03, p = 0.17). However, both analyses were associated with statistically significant high heterogeneity (I296%, Pheterogeneity <0.0001 for analysis including edoxaban 60 mg and I291%, Pheterogeneity <0.0001 for analysis including edoxaban 30 mg). We did not have baseline characteristics of the participants aged ≥75 years old in these trials (body mass index, weight, concurrent medication, aspirin use), which could have helped us to explain the remaining heterogeneity.
Treatment with DOACs significantly reduced the number of recurrent VTE events or VTE-related deaths (OR 0.54, 95% CI 0.35–0.82). The results showed low heterogeneity (I20%, Pheterogeneity 0.79) (fig. 7). There was a statistical difference between DOACs and VKAs in the major or clinically relevant non-major bleeding events (OR 0.63, 95% CI 0.46–0.85, p = 0.003) (fig. 8). There was a statistically significant moderate heterogeneity in the results (I273%, Pheterogeneity 0.01). The results were mainly driven by the HOKUSAI-VTE study. We considered that heterogeneity might be explained by the fact that the safety outcome in AMPLIFY (2013) concerned major bleeding events only, whereas the EINSTEIN DVT (2010), EINSTEIN PE (2012) and HOKUSAI VTE (2013) defined the primary safety outcome as major and clinically relevant non-major bleeding. We saw a statistical difference when the open-label trials were excluded (OR 0.72, 95% CI 0.52–1.00); the results showed a high heterogeneity (I279%, Pheterogeneity 0.03).
Indirect comparison of DOACs in the prevention of stroke or systemic embolism in patients aged 75 years or more with nonvalvular atrial fibrillation did not reveal any statistical difference between apixaban, rivaroxaban, and high-dose as well as low-dose dabigatran and edoxaban (supplementary fig. S1, appendix 4). Interestingly however, when major or clinically relevant non-major bleeding were considered, apixaban did show a statistically significant odds reduction compared with dabigatran 150 mg (OR 0.54, 95% CI 0.41–0.73), dabigatran 110 mg (OR 0.63, 95% CI 0.47–0.86) and rivaroxaban (OR 0.57, 95 CI 0.45–0.73). The latter was associated with higher odds ratios for bleeding compared with both edoxaban doses, although low-dose edoxaban showed the highest odds reduction (OR 0.41, 95% CI 0.32–0.53 for edoxaban 30 mg vs OR 0.71, 95% CI 0.57–0.89). Finally, both doses of dabigatran were associated with increased odds ratios for bleeding compared with apixaban (dabigatran 150 mg: OR 1.84, 95% CI 1.37–2.47; dabigatran 110 mg: OR 1.58, 95% CI 1.17–2.13), low-dose edoxaban (dabigatran 110 mg: OR 2.19, 95% CI 1.62–2.96) and high-dose edoxaban (dabigatran 150 mg: OR 1.49, 95% CI 1.13–1.96) (fig. S2, appendix 4).
Indirect comparison of DOACs for the composite endpoint recurrent VTE or VTE-related death did not show any statistical difference. However, edoxaban showed a statistically significant higher odds ratio for bleeding when compared with apixaban (OR 3.58, 95% CI 1.13–11.40) and rivaroxaban (OR 2.94, 95% CI 1.22–7.08). As we did not have access to the number of events in the two dose groups of edoxaban in the HOKUSAI trial, we could not assess whether this effect was confirmed with a reduced dose of edoxaban (fig. S3, appendix 4).
| Table 1:Characteristics of included trials. | |||||||||
| Trial name (year) | Trial design | Indications | Intervention drug and dosage | Control drug (INR target) | Follow up | Type of analysis for primary endpoints | Primary endpoint definition | ||
| Efficacy | Safety | Efficacy | Safety | ||||||
| AMPLIFY (2013) [9] | Randomised double-blind triple-dummy | VTE | Apixaban 10 mg b.i.d. for 7 days then 5 mg b.i.d. for 6 months | LMWH+VKA (INR 2.0–3.0) | 6 months | ITT PP | OT approach: Randomised subjects who received ≥1 dose of drug (1) | Composite of recurrent VTE and VTE-related death during 6 months of therapy | Major bleeding |
| ARISTOTLE (2011) [10] | Randomised double-blind double-dummy | NVAF | Apixaban 5 mg b.i.d. or 2.5 mg b.i.d. (2) | VKA | 1.8 years (median) | ITT | OT approach: Randomised subjects who received ≥1 dose of drug (1) | Composite of stroke and systemic embolism | Major bleeding |
| EINSTEIN DVT (2010) [8] | Randomised open-label (PROBE) | DVT | Rivaroxaban 15 mg b.i.d. for 3 weeks, then 20 mg q.d. | LWMH + VKA (INR 2.0–3.0) | 3, 6 or 12 months | ITT PP | Valid-for-safety population (3) ITT (4) | Composite of recurrent VTE or VTE-related death | Composite of major bleeding and CRNMB |
| EINSTEIN PE (2012) [12] | PE | ||||||||
| ROCKET AF (2011) [14] | Randomised double-blind double-dummy | NVAF | Rivaroxaban 20 mg q.d. or 15 mg q.d. (5) | VKA | 707 days (median) | PP ITT (6) | OT approach: Randomised subjects who received ≥1 dose of drug (1) | Composite of stroke and systemic embolism | Composite of major bleeding and CRNMB |
| ENGAGE AF-TIMI 48 (2013) [13] | Randomised double-blind double-dummy | NVAF | Edoxaban High-exposure group: 60 mg q.d. (9) Low-exposure group:30 mg q.d. (9) | VKA | 2.8 years (median) | mITT/OT (7) ITT (8) | OT approach: Randomised subjects who received ≥1 dose of drug (1) | Composite of stroke and systemic embolism | Major bleeding |
| HOKUSAI-VTE (2013) [11] | Randomised open-label double dummy | VTE | Edoxaban 60 mg q.d. (1) | VKA | 8.2 months | mITT (10) | OT approach: Randomised subjects who received ≥1 dose of drug (1) | Composite or recurrent VTE and VTE-related death | Composite of major bleeding and CRNMB |
| RE-LY (2009) [64] | Randomised nonblinded for VKA (PROBE) | NVAF | Dabigatran 150 b.i.d 110 b.i.d | VKA | 2.0 years (median) | ITT | ITT | Composite of stroke and systemic embolism | Major bleeding |
| CRNMB = clinically relevant non-major bleeding; DVT = deep venous thromboembolism; INR = prothrombin time international normalised ratio; ITT = intention to treat; LMWH = low molecular weight heparin; NVAF = nonvalvular atrial fibrillation; OT = on treatment; PE = pulmonary embolism; PP = per protocol; PROBE = prospective randomised open blinded endpoint; VTE = venous thromboembolism, (1) Participants were categorised in the group to which they were randomised unless they received incorrect study treatment during the study. In this case, subjects were categorised according to the treatment received. (2) Dose reduction if included subjects fulfilled at least 2 of the following criteria: age >80 years, weight <60 kg, serum creatinine ≥1.5 mg/dl (3) Analysis of bleeding events that occurred during treatment or within 2 days after end of treatment (4) Analysis of bleeding events and mortality (5) Dose reduction if creatinine clearance 30–49 ml/min (inclusive) (6) ITT population analysis if noninferiority proved in the PP population (7) All randomised participants who received at least one dose of randomised study drug. OT analysis method used for the randomised treatment even if a subject inadvertently received the incorrect drug or dosage or had his/her dose reduced during the study (8) If non inferiority was proven (9) Subjects with one or more of the following criteria had a 50% dose reduction: creatinine clearancel ≥30 ml/min and ≤50 ml/min, concomitant use of verapamil and quinidine (10) All randomised subjects who received at least one dose of randomised study drug. Analyses based on the randomised treatment even if a subject inadvertently received the incorrect study drug. | |||||||||
| Table 2:Group characteristics of included trials. | |||||||
| Trial Name (Year) | Age (years) (1) | Patients per arm (n) | Patients ≥75 years per arm (n) | Patients ≥75 years included in trial (%) | |||
| DOAC | Control (2) | DOAC | Control (2) | DOAC | Control (2) | ||
| AMPLIFY (2013) | 57.2 ± 16.0 | 56.7 ± 16.0 | 2691 | 2704 | 389 | 360 | 13.88 |
| ARISTOTLE (2011) | 70 | 70 | 9120 | 9081 | 2850 | 2828 | 31.20 |
| ROCKET AF (2011) | 73 | 73 | 7131 | 7133 | 3082 | 3082 | 43.21 |
| EINSTEIN DVT (2010) | 55.8 ± 16.4 | 56.4 ± 16.3 | 1731 | 1718 | 215 | 225 | 12.76 |
| EINSTEIN PE (2012) | 57.9 ± 7.3 | 57.5 ± 7.2 | 2419 | 2413 | 441 | 402 | 17.45 |
| ENGAGE AF-TIMI 48 (2013) | E60: 72 E30: 72 | 72 | E60 N = 7035, E30 N = 7034 | 7036 | E60: 2838 E30: 2789 | 2805 | 39.95 |
| HOKUSAI VTE (2013) | 55.7 ± 16.3 | 55.9 ± 16.2 | 4118 | 4122 | 560 | 544 | 13.40 |
| RE-LY (2009) | D110: 71.4 ± 8.6 D150: 71.5 ± 8.8 | 71.6 ± 8.6 | D110 N = 6015, D150 N = 6076 | 6022 | D110: 2349 D150: 2466 | 2430 | 28.14 |
| DVT = deep venous thrombosis; LMWH = low molecular weight heparin; DOAC = direct oral anticoagulants; PE = pulmonary embolism; VKA = vitamin K antagonists; VTE = venous thromboembolism (1) expressed as median or mean ± standard deviation (2) VKA in ARISTOTLE, ROCKET AF, ENGAGE AF-TIMI 48, RE-LY, LMWH + VKA in AMPLIFY, EINSTEIN DVT, EINSTEIN PE, HOKUSAI VTE | |||||||
The purpose of this review was to assess the safety and efficacy of apixaban, dabigatran, edoxaban and rivaroxaban in the elderly participants with nonvalvular atrial fibrillation or acute symptomatic VTE. After comparing DOACs with VKAs we conducted indirect comparisons between DOACs in order to address current lack of evidence regarding head to head comparisons between these substances in the elderly.
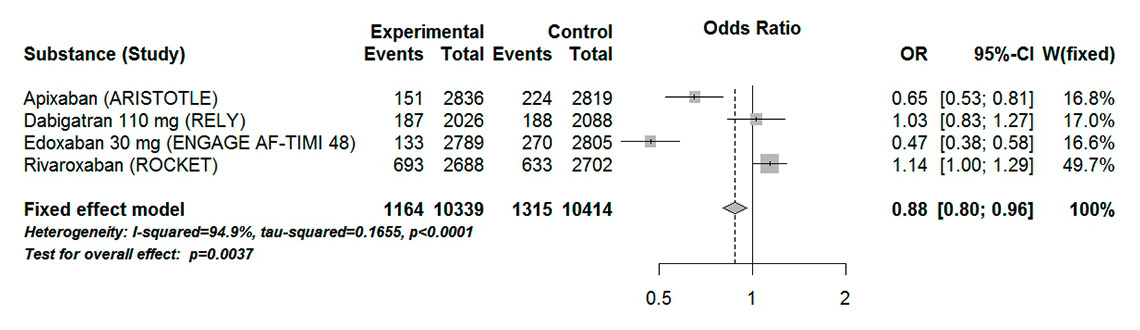
Figure 6
Forest plot of composite major and clinically relevant non-major bleeding in patients with atrial fibrillation aged 75 years or over for analysis including low dose dabigatran and edoxaban.
CI = confidence interval; experimental = direct oral anticoagulants; control = vitamin K antagonists
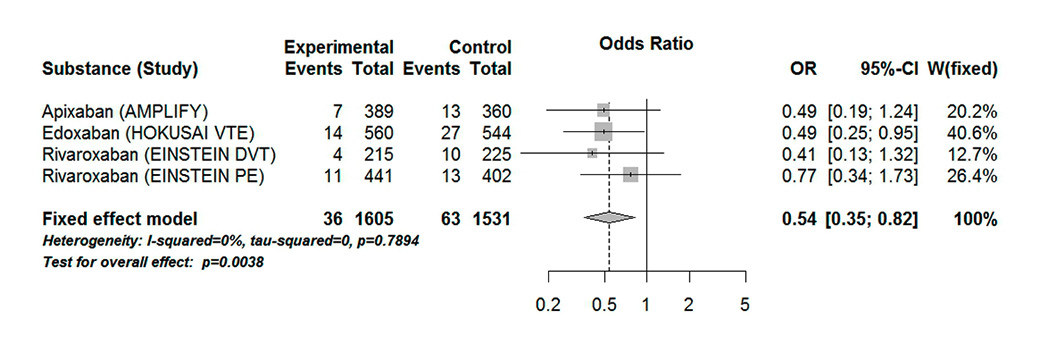
Figure 7
Forest plot of composite endpoint recurrent VTE or VTE-related death in patients with VTE aged 75 years or over.
CI = confidence interval; experimental = direct oral anticoagulants; control = vitamin K antagonists; DVT = deep vein thromboembolism; PE = pulmonary embolism; VTE = venous thromboembolism
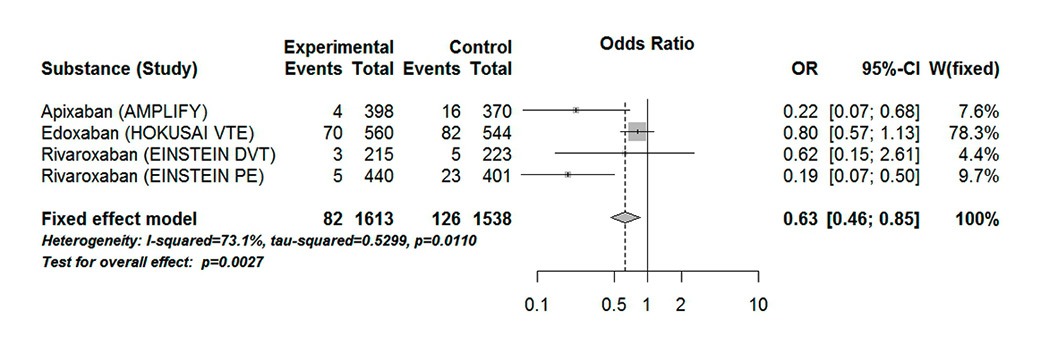
Figure 8
Forest plot of composite safety endpoint major bleeding and clinically significant non-major bleeding in patients with VTE aged 75 years or over.
CI = confidence interval; experimental = direct oral anticoagulants; control = vitamin K antagonists
In accordance with previous meta-analyses of the same age group, pooled analysis of DOACs by indication (nonvalvular atrial fibrillation or VTE) revealed that, in terms of efficacy, DOACs were superior to VKAs in prevention of stroke or systemic embolism as well as VTE or VTE-related death [31, 50]. Although pooled analysis of safety outcomes did not reveal statistically significant differences from VKAs, these results were associated with a moderate to high heterogeneity, suggesting that they should be interpreted with caution. Several explanations for this heterogeneity are possible.
First, included studies used different definitions for primary safety endpoints. Indeed, whereas some studies focused primarily on major clinical bleeding, others included clinically relevant non-major bleeding. In their review, Sharma et al. examined major and clinically relevant non-major bleeding separately [31]. Although we agree that this differentiation might bring additional precision in the safety profile of DOACs compared with VKAs, we consider that the impact of clinically relevant non-major bleeding in the elderly in terms of morbidity might be important enough to be considered a primary safety endpoint, hence our choice of a composite safety endpoint of major bleeding and clinically relevant non-major bleeding. As suggested by previous reports, the fear of bleeding risk as well as a prior history of bleeding (independent of severity of bleeding) impact on the prescriber’s choice of beginning or amending anticoagulation therapy [36].
Second, baseline differences in risk of bleeding in this age group cannot be excluded. Limited information was available regarding the profile of the participants profile in this age group in terms of renal function, concomitant antiplatelet therapy or history of gastrointestinal bleeding, which are known risk factors for bleeding under VKA administration [51]. It remains, therefore, unclear if those risk factors have a similar impact on bleeding risk with DOACs in this age group.
Finally, differences in pharmacological profiles can possibly count for some part of the heterogeneity found in our analysis.
Although pooling of DOACs helped to provide a general overview of their position compared with VKAs, some trends for differences between DOACs became noticeable and were eventually strengthened in our indirect comparisons analysis.
Previous reports from the ARISTOLE trial have highlighted the favourable efficacy-safety profile of apixaban in elderly patients, as well as in patients with impaired renal function [52, 53]. Our meta-analysis included two phase III trials, ARISTOTLE and AMPLIFY, comparing apixaban to VKAs. In the prevention of stroke or systemic embolism, apixaban was superior to VKAs, while non-inferiority to VKAs was found in patients with VTE. Indirect comparisons between apixaban and other DOACs did not reveal statistically significant differences in terms of efficacy. Interestingly, while apixaban was associated with a decreased risk of bleeding compared with VKAs, indirect comparisons revealed that bleeding risk in patients with nonvalvular atrial fibrillation under apixaban was significantly less than with rivaroxaban and both doses of dabigatran, although the difference was more pronounced with high-dose dabigatran. Two factors could explain these results: differences in trial design (ARISTOTLE was a randomised controlled trial, RE-LY was a PROBE trial) and differences in trial populations. In particular, in patients aged 75 years or more, the mean CHADS2 score was 3.7 in the ROCKET trial, but only 2.7 in the ARISTOTLE trial, suggesting the inclusion of lower-risk patients. Similarly, nearly 40% of the elderly participants were under aspirin therapy in the ROCKET trial compared with 31% in the ARISTOTLE trial, whereas in the RE-LY trial, the number of patients taking aspirin was higher than in the ARISTOTLE trial (D110: 40%, D150: 38.7%). As such, the exact superiority of apixaban over other DOACs has still to be confirmed, in particular because comparison of apixaban with rivaroxaban and edoxaban in patients with VTE did not confirm superiority of apixaban over rivaroxaban. This suggests that differences in study populations might have explained its superiority over other DOACs in patients with nonvalvular atrial fibrillation. Notably, a recent study directly comparing dabigatran, rivaroxaban and apixaban showed that apixaban was associated with lower bleeding risk compared with the other DOACs in patients with nonvalvular atrial fibrillation [54]. Although this was not a randomised controlled study, the conclusions confirm our results. In order to further explore the safety and efficacy of apixaban under “real-world” conditions, the J-ELD AD multicentre observational study in Japanese elderly patients was launched in 2016 and should provide additional insights regarding the use of apixaban in the elderly [55].
The safety and efficacy profile of dabigatran in stroke and systemic embolism prevention in elderly patients seems to be dependent on the dose used. Indeed, although dabigatran 150 mg was associated with a reduction in stroke or systemic embolism compared with VKAs, this might come at the expense of safety, suggested by a trend for increased risk of bleeding compared with VKAs. In contrast, dabigatran 110 mg showed non-inferiority to VKAs in terms of efficacy as well as safety. Real-world data have provided some valuable insights into the role of dabigatran in elderly patients. An audit conducted in New Zealand showed that age was a risk factor associated with bleeding events under dabigatran. This risk was compounded by renal impairment and low body weight [56]. A recent retrospective cohort study among 219 027 patients in the USA showed that by the age of 76, the risk of gastrointestinal bleeding with dabigatran or rivaroxaban exceeded that with warfarin [57]. The effect of a dose reduction of dabigatran on bleeding events could not be tested as dabigatran 110 mg has not been approved by the FDA. The importance of a dose reduction in the elderly is supported by a recent Danish cohort study, which confirmed that patients under low-dose dabigatran did not show a statistically significant difference in major bleeding events compared with VKA patients [58]. A post hoc analysis of the RE-LY trial revealed that the bleeding rates with dabigatran 110 mg were not different from those in VKA in patients aged ≥80 years, whereas for patients under dabigatran 150 mg, an increased bleeding rate was seen [59]. Recently, a population-based study using administrative databases in Quebec and including a total of 42 478 elderly patients with nonvalvular atrial fibrillation showed that neither dabigatran dose differed from VKAs in terms of safety. However, low-dose dabigatran was associated with reduced risk for intracranial bleeding but increased risk for gastrointestinal bleeding [60].
Apart from the difference between apixaban and dabigatran already discussed, indirect comparisons suggest that the two might differ in terms of safety profile. Interestingly, higher CHADS2 score in the ENGAGE AF TIMI 48 (mean 3.2) compared with the RE-LY group (dabigatran 110 mg: 2.1, dabigatran 150 mg: 2.2) suggest the inclusion of higher risk patients in the ENGAGE AF-TIMI 48 trial than in the RE-LY trial. However, the proportion of participants under aspirin therapy in the ENGAGE AF-TIMI 48 trial (29%) was less than in the RE-LY trial (D110: 40%, D150: 38.7%). Whether indirect comparisons reflect differences in risk of bleeding in the included population or a true difference in pharmacological profile has still to be confirmed.
We found that high-dose edoxaban was associated with a reduction in stroke and systemic embolism, as well as bleeding events, compared with VKAs in patients with nonvalvular atrial fibrillation. Low dose edoxaban showed non-inferiority compared with VKA in terms of efficacy but with fewer bleeding events than VKAs. Forty-one percent of patients aged 75 years or older received low-dose edoxaban, most commonly (89%) owing to the presence of moderate renal dysfunction (creatinine clearance <50 ml/min). The effect of low-dose edoxaban might be explained by a decrease in median edoxaban plasma concentration (from 30% to 40%) and a decrease in median anti-factor Xa activity of 20% to 40% [61]. Unfortunately, we were not able to confirm the effect of dose reduction in patients with VTE, as no subgroup analysis for both dosages of edoxaban was available from the HOKUSAI VTE study. Interestingly, indirect comparisons of edoxaban with rivaroxaban and dabigatran revealed superiority in terms of bleeding risk reduction. Similar results were reported in two previous meta-analyses that provided indirect comparisons between edoxaban and other DOACs in patients with nonvalvular atrial fibrillation as well as in a subgroup of nonvalvular atrial fibrillation patients with CHADS2 Score ≥2 [62, 63]. Here again, a difference in baseline risk between the populations cannot be excluded and head-to-head comparisons are mandatory before drawing conclusions.
Rivaroxaban was non-inferior to VKAs in patients with nonvalvular atrial fibrillation and VTE. However, in the ROCKET trial, patients under rivaroxaban treatment had higher bleeding rates. When compared with other DOACs, rivaroxaban showed a less favourable safety profile. These results have, of course, to be interpreted with extreme caution, particularly because of the already discussed heterogeneity of the included populations. Real-world data are needed to confirm these suspicions.
Real-world data have addressed some limitations associated with our included studies. The percentage of patients aged 75 years or more ranged from 12.76 to 43.21%, depending on the study. By inspection of the exclusion criteria applied in these studies, it is legitimate to question whether the elderly group in the included trials is sufficiently representative of all potential elderly patients who could benefit from DOACs. Patients with severe comorbidities with reduced life expectancy (ranging from 1 year to 2 years depending on the trial) were systematically excluded from the studies. Most elderly people, though, are suffering from multiple comorbidities such as chronic heart or renal failure with known high mortality [64–66]. In addition to this, the exclusion of persons with known psychosocial reasons that made participation in the trial impracticable and of patients hospitalised for psychiatric issues represents another uncertainty about the external validity of these results. Demented persons, for whom the informed consent process is challenging, and disabled elderly people living isolated at home or in nursing homes were likely to be excluded.
Most of the current recommendations include low bodyweight and impaired renal clearance as criteria in addition to age for dose adaptation in the elderly (tables 3 and 4). Nevertheless, the lack of proper clinical evidence on the use of DOACs in the frail elderly raises concerns on whether these recommendations apply for this particular group. Frailty is a clinical syndrome with varying community prevalence from 4.0 to 59.1% [67]. It is defined by unintentional weight loss (10 lbs in past year), self-reported exhaustion, weakness, slow walking speed and low physical activity [68]. Frail elderly are at higher risk for falls, disability, hospitalisation and mortality [69–71]. The right choice for anticoagulation in this patient group is therefore of utmost importance. The evidence for DOACs in the frail elderly remains very sparse. Prescribers are asked to carry a thorough risk-benefit analysis before starting DOAC therapy in this particular group. Recent reviews have offered recommendations for the use of DOACs in the frail elderly based on real clinical settings and previous VKA experience [72–74]. Nevertheless, further studies, from pharmacological to clinical, should be conducted in this particular group to provide clinicians with the necessary evidence to choose between a VKA and a DOAC.
Our study has a number of limitations. First, we conducted a meta-analysis on aggregated published data from randomised controlled trials, instead of individual patient data, which can be a potential source of bias. Second, we are aware that there is also some heterogeneity as we included studies with various populations, interventions and follow up. Third, we had no access to the baseline characteristics (body mass index, weight, concurrent medication) for our population of interest. This would have helped to further understand the efficacy and safety profile of the DOAC in the elderly. Fourth, we could not perform meta-regression owing to an insufficient number of trials. Fifth, we are aware that the population included in the randomised controlled trials is not always totally representative of everyday practice. Sixth, for the data analysis, we used the median follow-up to calculate the number of events from the rates given, assuming that the risk stayed constant over time. Seventh, we cannot exclude a reporting bias regarding the safety outcome in the open-label studies. Eighth, while we tried to avoid inter-trial heterogeneity when conducting indirect comparisons by selecting a subgroup of patients (75 years or more), we cannot exclude the possibility that some of the differences in trial design and baseline characteristics of participants might have an impact on our results.
To conclude, DOACs are associated with at least non-inferiority in stroke and systemic embolism prevention in patients with atrial fibrillation and in recurrent VTE or VTE-related death in patients with VTE. Although pooled analysis revealed no difference in bleeding events between DOACs and VKAs, these results were associated with moderate to high heterogeneity. Indirect comparisons between DOACs confirmed some tendencies reported in previous studies and reviews. Direct comparative studies of the DOACs should be undertaken in order to detect possible pharmacological differences among DOACs, which could help to adapt the prescription to a particular patient profile. Also, caution should be applied in interpreting these results, as the participants might not be representative of the elderly population in general. In particular, proper evidence for the frail elderly is missing, preventing conclusions on the efficacy and safety of DOACs in this specific patient group. Further studies should be undertaken in this age group to offer a confident basis of evidence for prescribers.
| Table 3:Dose recommendation for stroke prevention in patients ≥80 years old with nonvalvular atrial fibrillation. | ||||||||
| Creatinine clearance | ||||||||
| ≤15 ml/min | 16–30 ml/min | 31–50 ml/min | >50 ml/min | |||||
| Weight | ≤50 kg | Contraindicated | ■ NR | ● 30 mg q.d. | ■ 15 mg q.d. | ● 30 mg q.d. | ■ 20 mg q.d. | ● 30 mg q.d. |
| ▲ 2.5 mg b.i.d. (1) | ◆ NR 75 mg b.i.d. (2) | ▲ 2.5 mg b.i.d. (1) | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (2) | ▲ 2.5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (2) | |||
| 51–59 kg | ■ NR | ● 30 mg q.d. | ■ 15 mg q.d. | ● 30 mg q.d. | ■ 20 mg q.d. | ● 30 mg q.d. | ||
| ▲ 2.5 mg b.i.d. (1) | ◆ NR 75 mg b.i.d. (2) | ▲ 2.5 mg b.i.d. (1) | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (2) | ▲ 2.5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (2) | |||
| ≥60 kg | ■ NR | ● 30 mg q.d. | ■ 15 mg q.d. | ● 30 mg q.d. | ■ 20 mg q.d. | ● 60 mg q.d. | ||
| ▲ 2.5 mg b.i.d. (1) | ◆ NR 75 mg b.i.d. (2) | ▲ 2.5 mg b.i.d. (1) | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (2) | ▲ 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (2) | |||
| Rivaroxaban ■, Apixaban ▲, Edoxaban ●, Dabigatran ◆ NR = Not recommended (1) USA: no adaptation recommended (2) USA only | ||||||||
| Table 4:Dose recommendation for venous thromboembolism treatment in patients ≥80 years old. | ||||||||
| Creatinine clearance | ||||||||
| ≤15 ml/min | 16–30 ml/min | 31–50 ml/min | >50 ml/min | |||||
| Weight | ≤50 kg | Contraindicated | ■ NR | ● 30 mg q.d. | ∎ 15 mg b.i.d. for 3 weeks then 20 mg q.d. | ● 30 mg q.d. | ∎ 15 mg b.i.d. for 3 weeks then 20 mg q.d. | ● 30 mg q.d. |
| ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ NR | ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (1) | ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (1) | |||
| 51–59 kg | ■ NR | ● 30 mg q.d. | ∎ 15 mg b.i.d. for 3 weeks then 20 mg q.d. | ● 30 mg q.d. | ∎ 15 mg b.i.d. for 3 weeks then 20 mg q.d. | ● 30 mg q.d. | ||
| ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ NR | ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (1) | ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (1) | |||
| ≥60 kg | ■ NR | ● 30 mg q.d. | ∎ 15 mg b.i.d. for 3 weeks then 20 mg q.d. | ● 30 mg q.d. | ∎ 15 mg b.i.d. for 3 weeks then 20 mg q.d. | ● 60 mg q.d. | ||
| ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ NR | ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (1) | ▲ 10 mg b.i.d. for 1 week then 5 mg b.i.d. | ◆ 110 mg b.i.d. ◆ 150 mg b.i.d. (1) | |||
| Rivaroxaban ■, Apixaban ▲, Edoxaban ●, Dabigatran ◆ NR = Not recommended (1) USA only Sources: FDA Drug Database, European Medicines Agency | ||||||||
| EINSTEIN DVT EINSTEIN PE | 1. Suspected (recurrent) pulmonary embolism (PE) with one of the following findings: A (new) intraluminal filling defect in segmental or more proximal branches on spiral computed tomography (sCT) A (new) intraluminal filling defect or an extension of an existing defect or a new sudden cut-off of vessels more than 2.5 mm in diameter on the pulmonary angiogram, A (new) perfusion defect of at least 75% of a segment with a local normal ventilation result (high-probability) on ventilation/perfusion lung scintigraphy Integrated inconclusive sCT, pulmonary angiography or lung scintigraphy with demonstration of deep venous thrombosis (DVT) in the lower extremities by compression ultrasound or venography. 2. Suspected (recurrent) DVT with one of the following findings: If there were no previous DVT investigations Abnormal compression ultrasound (CUS) An intraluminal filling defect on venography If there was a DVT investigation at screening Abnormal CUS where compression had been normal or, if noncompressible during screening, a substantial increase (4 mm or more) in diameter of the thrombus during full compression An extension of an intraluminal filling defect, or a new intraluminal filling defect or an extension of non-visualisation of veins in the presence of a sudden cut-off on venography. 3. Fatal PE PE based on objective diagnostic testing, autopsy, or Death which cannot be attributed to a documented cause and for which PE/DVT, cannot be ruled out (unexplained death) In the absence of objective testing, a suspected episode of DVT or PE will be considered as confirmed if it led to a change in anticoagulant treatment at therapeutic dosages for more than 48 hours. |
| HOKUSAI VTE | Diagnosis of symptomatic recurrent PE requires meeting one or more of the following criteria: A (new) intraluminal filling defect in (sub)-segmental or more proximal branches on sCT scan A (new) intraluminal filling defect or an extension of an existing defect or a new sudden cut-off of vessels more than 2.5 mm in diameter on the pulmonary angiogram A (new) perfusion defect of at least 75% of a segment with a local normal ventilation result (high-probability) on ventilation/perfusion lung scintigraphy (VPLS) A nondiagnostic lung scan accompanied by documentation of new DVT by ultrasonography or venography In the absence of previous DVT investigations at baseline, diagnosis of symptomatic recurrent DVT requires one of the following: A noncompressible venous segment on ultrasonography An intraluminal filling defect on venography An intraluminal filling defect on spiral/contrast CT of the leg When DVT investigations are performed at baseline, diagnosis of symptomatic recurrent DVT requires one of the following: Abnormal compression CUS where compression had been normal or, if noncompressible during screening, a substantial increase (3–4 mm) in diameter of the thrombus during full compression An extension of an intraluminal filling defect, or a new intraluminal filling defect, or an extension of non-visualisation of veins in the presence of a sudden cut-off on venography An extension of an intraluminal filling defect, or a new intraluminal filling defect on spiral/contrast CT of the leg Diagnosis of fatal PE is based on one or more of the following: Objective diagnostic testing Autopsy Death which cannot be attributed to a documented cause and for which PE/DVT cannot be ruled out. |
| AMPLIFY | 1. Suspected (new or recurrent) PE with one of the following findings: A new intraluminal filling defect in segmental or more proximal branches on spiral CT scan A new intraluminal filling defect, or an extension of an existing defect, or a new sudden cut-off of vessels more than 2.5 mm in diameter on the pulmonary angiogram A new perfusion defect of at least 75% of a segment with a local normal ventilation result (high-probability) on VPLS) Inconclusive sCT, pulmonary angiography or VPLS evidence of new or recurrent PE, with demonstration of a new or extended DVT in the lower extremities by compression ultrasound or venography 2. Suspected (new or recurrent) DVT with one of the following findings: For a NEW DVT, criteria include abnormal compression ultrasound (CUS), including grey-scale or color-coded Doppler, or an intraluminal filling defect on venography For a RECURRENT DVT, criteria include Abnormal CUS where compression had been normal or, if noncompressible previously, a substantial increase (4 mm or more) in diameter of the thrombus during full compression, or An extension of an intraluminal filling defect, or a new intraluminal filling defect or an extension of non-visualisation of veins in the presence of a sudden cut-off on venography |
| ENGAGE AF-TIMI 48 | A stroke is defined as an abrupt onset, over minutes to hours, of a focal neurological deficit in the distribution of a single brain artery that is not due to an identifiable non-vascular cause (i.e., brain tumour or trauma), and that either lasts at least 24 hours or results in death within 24 hours of onset. A systemic embolic event (SEE) is defined as an arterial embolism resulting in clinical ischaemia, excluding the central nervous system, coronary and pulmonary arterial circulation. |
| ARISTOTLE | Diagnosis of stroke will require the abrupt onset of focal neurological symptoms lasting at least 24 hours. It is strongly recommended (but not required) that an imaging procedure such as a CT scan or magnetic resonance imaging (MRI) be performed. All strokes will be classified as definite ischaemic, definite haemorrhagic or type uncertain. A vascular imaging procedure such as a carotid ultrasound is recommended whenever possible (but not required) for subclassification of ischaemic strokes into cardioembolic, lacunar or large artery. The level of disability and stroke severity will be assessed at presentation and at the next two regularly scheduled follow-up visits using the modified Rankin score. Systemic embolism will be judged to occur where there is a clinical history consistent with an acute loss of blood flow to a peripheral artery (or arteries), which is supported by evidence of embolism from surgical specimens, autopsy, angiography, or other objective testing. |
| ROCKET-AF | Stroke is defined as a new, sudden, focal neurological deficit resulting from a presumed cerebrovascular cause that is not reversible within 24 hours and not due to a readily identifiable cause such as a tumour or seizure. If an event matching this definition lasts less than 24 hours it will be considered a transient ischaemic attack (TIA). The duration of symptoms for a TIA will be recorded as will the results of any imaging procedures. Transient ischaemic attack events with documented cerebral infarction in the appropriate location to explain the clinical syndrome will be recorded. All suspected strokes (including TIA) will be reviewed and adjudicated by the CEC. Whenever possible, the use of CT scanning or MRI should be employed to assist in the classification of strokes. Further, the CEC will consider all clinically relevant information and imaging studies to classify all strokes as: Primary haemorrhagic – stroke with focal collections of intracerebral blood. Events of subarachnoid, subdural, and epidural haemorrhage will be recorded, but these events will not be considered part of the primary efficacy endpoint. Primary ischaemic infarction – stroke without focal collections of intracranial blood. The occurrence of haemorrhagic conversion of a primary ischaemic infarction will be recorded including whether it was symptomatic or asymptomatic. Stroke subtype will be assessed as cardioembolic, non-cardioembolic (e.g., atherothrombotic, lacunar, other known cause) and uncertain. Uncertain – no imaging or autopsy data available Subjects who die within 30 days of the onset of the stroke will be regarded as having had a fatal stroke. Subjects who have a stroke and then die more than 30 days after the onset of the stroke will be regarded as having non-stroke death. Non-CNS systemic embolism Non-CNS systemic embolism is defined as abrupt vascular insufficiency associated with clinical or radiological evidence of arterial occlusion in the absence of other likely mechanisms, (e.g., trauma, atherosclerosis, instrumentation). In the presence of atherosclerotic peripheral vascular disease, diagnosis of embolism to the lower extremities should be made with caution and requires angiographic demonstration of abrupt arterial occlusion. |
| RE-LY | Stroke was defined as the sudden onset of a focal neurological deficit in a location consistent with the territory of a major cerebral artery and categorised as ischaemic, haemorrhagic, or unspecified. Haemorrhagic transformation of ischaemic stroke was not considered to be haemorrhagic stroke. Intracranial haemorrhage consisted of haemorrhagic stroke and subdural or subarachnoid haemorrhage. Systemic embolism was defined as an acute vascular occlusion of an extremity or organ, documented by means of imaging, surgery, or autopsy. |
| Information extracted from each study protocol. For RE-LY trial, endpoint definitions were extracted from the published review. | |
| EINSTEIN DVT EINSTEIN PE ROCKET-AF | Overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, unscheduled contact (visit or telephone call) with a physician, (temporary) cessation of study treatment, or associated with any other discomfort such as pain, or impairment of activities of daily life. |
| HOKUSAI VTE | ‒ Any bleeding compromising haemodynamics‒ Any bleeding leading to hospitalisation‒ Subcutaneous haematoma larger than 25 cm2, or 100 cm2 if there was a traumatic cause‒ Intramuscular haematoma documented by ultrasonography‒ Epistaxis that lasted for more than 5 minutes, was repetitive (i.e., two or more episodes of bleeding more extensive than spots on a handkerchief within 24 hours), or led to an intervention (e.g., packing or electrocoagulation)‒ Gingival bleeding occurring spontaneously (i.e., unrelated to eating or tooth brushing) or lasting for more than 5 minutes‒ Haematuria that was macroscopic and was spontaneous or lasted for more than 24 hours after instrumentation (e.g., catheter placement or surgery) of the urogenital tract‒ Macroscopic gastrointestinal haemorrhage, including at least one episode of melaena or haematemesis, if clinically apparent with positive results on a faecal occult blood test‒ Rectal blood loss, if more than a few spots on toilet paper‒ Haemoptysis, if more than a few speckles in the sputum and not occurring within the context of pulmonary embolism‒ Any other bleeding type considered to have clinical consequences for a patient such as medical intervention, the need for unscheduled contact (visit or telephone call) with a physician, or temporary cessation of a study drug, or associated with pain or impairment of activities of daily life |
| Information extracted from each study protocol. | |
1. exp heart atrium fibrillation/ or heart atrium flutter/
2. ((atrial or auricular) adj5 (fibrillation$ or flutter$)).tw.
3. AF.tw.
4. 1 or 2 or 3
5. exp blood clotting factor 10a inhibitor/
6. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw.
7. (activated adj5 (factor X or factor 10) adj5 inhib$).tw.
8. xabans.tw.
9. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E).tw.
10. 5 or 6 or 7 or 8 or 9
11. exp thrombin inhibitor/
12. (direct$ adj5 thrombin adj5 inhib$).tw.
13. DTI$1.tw.
14. (argatroban or MD805 or MD-805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw.
15. 11 or 12 or 14 or 14
16. exp coumarin anticoagulant/
17. antivitamin K/
18. (warfarin$ or adoisine or aldocumar or athrombin$ k or carfin or coumadin$ or coumafene or coumaphene or jantoven or kumatox or lawarin or marevan or panwarfarin or panwarfin or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).tw.
19. (vitamin K antagonist$ or VKA or VKAs).tw.
20. (coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione).tw.
21. 16 or 17 or 18 or 19 or 20
22. (PE or SSPE).ti,ab.
23. vte.ti,ab.
24. (pulmonary adj4 embolis$).ti,ab.
25. (pulmonary adj4 clot$).ti,ab.
26. (lung adj4 embolis$).ti,ab.
27. (lung adj4 clot$).ti,ab.
28. exp lung embolism/
29. thromboembolism/
30. venous thromboembolism/
31. or/22-30
32. subsegment$.ti,ab.
33. 31 and 32
34. (((4 or 33) and 10) or 15) and 21
35. randomized-controlled-trial/
36. randomization/
37. controlled-study/
38. multicenter-study/
39. phase-3-clinical-trial/
40. phase-4-clinical-trial/
41. double-blind-procedure/
42. single-blind-procedure/
43. (random* or cross?over* ormulticenter* or factorial* or placebo*or volunteer*).mp.
44. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.
45. (latin adj square).mp.
46. animals.mp. not (humans and animals).sh. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
47. 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46
48. 34 and 47
1. atrial fibrillation/ or atrial flutter/
2. ((atrial or auricular) adj5 (fibrillation$ or flutter$)).tw.
3. AF.tw.
4. 1 or 2 or 3
5. exp Pulmonary Embolism/
6. exp thromboembolism/
7. (PE or SSPE).ti,ab.
8. vte.ti,ab.
9. (pulmonary adj4 embolis$).ti,ab.
10. (pulmonary adj4 clot$).ti,ab.
11. (lung adj4 embolis$).ti,ab.
12. (lung adj4 clot$).ti,ab.
13. or/5-12
14. subsegmen$.ti,ab.
15. 13 and 14
16. factor Xa/ai
17. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw.
18. (activated adj5 (factor X or factor 10) adj5 inhib$).tw.
19. xabans.tw.
20. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E).tw.
21. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E).nm.
22. 16 or 17 or 18 or 19 or 20 or 21
23. thrombin/ai
24. (direct$ adj5 thrombin adj5 inhib$).tw.
25. DTI$1.tw.
26. (argatroban or MD805 or MD-805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).tw.
27. (argatroban or MD805 or MD-805 or dabigatran or ximelagatran or melagatran or efegatran or flovagatran or inogatran or napsagatran or bivalirudin or lepirudin or hirudin$ or desirudin or desulfatohirudin or hirugen or hirulog or AZD0837 or bothrojaracin or odiparcil).nm.
28. 23 or 24 or 25 or 26 or 27
29. Warfarin/
30. (warfarin$ or adoisine or aldocumar or athrombin$ k or carfin or coumadin$ or coumafene or coumaphene or jantoven or kumatox or lawarin or marevan or panwarfarin or panwarfin or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).tw.
31. (warfarin$ or adoisine or aldocumar or athrombin$ k or carfin or coumadin$ or coumafene or coumaphene or jantoven or kumatox or lawarin or marevan or panwarfarin or panwarfin or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).nm.
32. (vitamin K antagonist or VKA or VKAs).tw.
33. 4-hydroxycoumarins/ or acenocoumarol/ or coumarins/ or dicumarol/ or ethyl biscoumacetate/ or phenindione/ or phenprocoumon/
34. (coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione).tw.
35. (coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione).nm.
36. 29 or 30 or 31 or 32 or 33 or 34 or 35
37. (((4 or 15) and 22) or 28) and 36
38. limit 37 to human
39. randomized controlled trial.pt.
40. controlled clinical trial.pt.
41. randomized.ab.
42. placebo.ab.
43. drug therapy.fs.
44. randomly.ab.
45. trial.ab.
46. groups.ab.
47. 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46
48. exp animals/ not humans.sh.
49. 47 not 48
50. 37 and 49
ID Search
#1 MeSH descriptor Thrombosis, this term only
#2 MeSH descriptor Thromboembolism, this term only
#3 MeSH descriptor Venous Thromboembolism this term only
#4 MeSH descriptor Venous Thrombosis this term only
#5 (thromboprophyla* or thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):ti,ab,kw
#6 MeSH descriptor Pulmonary Embolism explode all trees
#7 PE or DVT or VTE:ti,ab,kw
#8 (vein* or ven*) near thromb*:ti,ab,kw
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 MeSH descriptor Atrial Fibrillation, this term only
#11 MeSH descriptor Atrial Flutter, this term only
#12 (atrial or atrium or auricular) near/5 (fibrillation* or arrhythmia* or flutter*):ti,ab,kw
#13 (AF):ti,ab,kw
#14 [7-#13]
#15 (new near/3 anticoagulant*):ti,ab
#16 dabigatran:ti,ab,kw
#17 apixaban:ti,ab,kw
#18 rivaroxaban:ti,ab,kw
#19 edoxaban:ti,ab,kw
#20 (thrombin next inhibit*):ti,ab,kw
#21 ((factor next xa next inhibit*) or (factor next 10a next inhibit*)):ti,ab,kw
#22 MeSH descriptor Anticoagulants this term only
#23 MeSH descriptor: [Factor Xa] explode all trees
#24 #22 or #23
#25 [12-#21, #24]
#26 {or #9, #14}
#27 #25 and #26
Forest plots of indirect comparisons between direct oral anticoagulants for composite endpoints recurrent venous thromboembolism or venous thromboembolism-related death (a–d) and clinically relevant non-major bleeding (e–h).
api = apixaban; CI = confidence interval; edo = edoxaban OR = odds ratio; riva = rivaroxaban; VKA = vitamin K antagonist
1 Marinigh R, Lip GY, Fiotti N, Giansante C, Lane DA. Age as a risk factor for stroke in atrial fibrillation patients: implications for thromboprophylaxis. J Am Coll Cardiol. 2010;56(11):827–37.
2 Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. ESC Committee for Practice Guidelines-CPG; Document Reviewers. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation – developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14(10):1385–413.
3 White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23, Suppl 1):I4–8.
4 Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–9.
5 Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–510
6 Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. AMPLIFY Investigators. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. doi:http://dx.doi.org/10.1056/NEJMoa1302507.
7 Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92.
8 Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Hokusai-VTE Investigators. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–15. doi:http://dx.doi.org/10.1056/NEJMoa1306638.
9 Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. EINSTEIN–PE Investigators. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–97. doi:http://dx.doi.org/10.1056/NEJMoa1113572.
10 Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104.
11 Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi:http://dx.doi.org/10.1056/NEJMoa1009638.
12 Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. Erratum in: N Engl J Med. 2010;363(19):1877.
13 Ng KH, Hart RG, Eikelboom JW. Anticoagulation in patients aged ≥75 years with atrial fibrillation: Role of novel oral anticoagulants. Cardiol Ther. 2013;2(2):135–49.
14 Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8.
15 Kim MH, Lin J, Hussein M, Kreilick C, Battleman D. Cost of atrial fibrillation in United States managed care organizations. Adv Ther. 2009;26(9):847–57.
16 Stein PD, Hull RD, Kayali F, Ghali WA, Alshab AK, Olson RE. Venous thromboembolism according to age: the impact of an aging population. Arch Intern Med. 2004;164(20):2260–5.
17 Pautas E, Gouin-Thibault I, Debray M, Gaussem P, Siguret V. Haemorrhagic complications of vitamin k antagonists in the elderly: risk factors and management. Drugs Aging. 2006;23(1):13–25.
18 Pengo V, Legnani C, Noventa F, Palareti G; ISCOAT Study Group (Italian Study on Complications of Oral Anticoagulant Therapy). Oral anticoagulant therapy in patients with nonrheumatic atrial fibrillation and risk of bleeding. A Multicenter Inception Cohort Study. Thromb Haemost. 2001;85(3):418–22.
19 Hajjar ER, Hanlon JT, Artz MB, Lindblad CI, Pieper CF, Sloane RJ, et al. Adverse drug reaction risk factors in older outpatients. Am J Geriatr Pharmacother. 2003;1(2):82–9.
20 Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, et al. Euro Heart Survey Investigators. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27(24):3018–26.
21 Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37(4):1075–80.
22 Birman-Deych E, Radford MJ, Nilasena DS, Gage BF. Use and effectiveness of warfarin in Medicare beneficiaries with atrial fibrillation. Stroke. 2006;37(4):1070–4.
23 Waldo AL, Becker RC, Tapson VF, Colgan KJ; NABOR Steering Committee. Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol. 2005;46(9):1729–36.
24 Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131(12):927–34.
25 Laliberté F, Cloutier M, Nelson WW, Coleman CI, Pilon D, Olson WH, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30(7):1317–25.
26 Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Prefer Adherence. 2010;4:51–60.
27 Vogel T, Geny B, Kaltenbach G, Lang PO. L’anticoagulation dans la fibrillation atriale du sujet âgé : point de vue du gériatre avec un focus sur les anticoagulants oraux directs. [Anticoagulation in atrial fibrillation in the elderly: the geriatrician point of view with a focus on the direct oral anticoagulants]. Rev Med Interne. 2015;36(1):22–30. French.
28 Stöllberger C, Finsterer J. Concerns about the use of new oral anticoagulants for stroke prevention in elderly patients with atrial fibrillation. Drugs Aging. 2013;30(12):949–58.
29 Ho P, Brooy BL, Hayes L, Lim WK. Direct oral anticoagulants in frail older adults: a geriatric perspective. Semin Thromb Hemost. 2015;41(4):389–94.
30 Sardar P, Chatterjee S, Chaudhari S, Lip GYH. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857–64.
31 Sharma M, Cornelius VR, Patel JP, Davies JG, Molokhia M. Efficacy and harms of direct oral anticoagulants in the elderly for stroke prevention in atrial fibrillation and secondary prevention of venous thromboembolism: Systematic review and meta-analysis. Circulation. 2015;132(3):194–204.
32 Castellucci LA, Cameron C, Gal GL, Rodger MA, Coyle D, Wells PS, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: Systematic review and network meta-analysis. BMJ (Online). 2013;347:f5133.
33 Cohen AT, Hamilton M, Mitchell SA, Phatak H, Liu X, Bird A, et al. Comparison of the novel oral anticoagulants apixaban, dabigatran, edoxaban, and rivaroxaban in the initial and long-term treatment and prevention of venous thromboembolism: Systematic review and network meta-analysis. PLoS One. 2015;10(12):e0144856.
34 Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D’Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348(9025):423–8.
35 Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med. 1993;95(3):315–28.
36 Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–83.
37 Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–4.
38 The Cochrane Collaboration: Data collection form.
39 Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3(4):312–24.
40 Rücker G, Schwarzer G. Reduce dimension or reduce weights? Comparing two approaches to multi-arm studies in network meta-analysis. Stat Med. 2014;33(25):4353–69.
41 Rothstein H. Publication bias in meta-analysis : Prevention, assessment and adjustments. Chichester, Wiley, 2005.
42 Higgins JPT. Cochrane handbook for systematic reviews of interventions. Chichester, Wiley-Blackwell, 2009.
43 Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
44 Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35(28):1864–72.
45 Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363–72.
46 Beyer-Westendorf J, Büller H. External and internal validity of open label or double-blind trials in oral anticoagulation: better, worse or just different? J Thromb Haemost. 2011;9(11):2153–8.
47 Kohro T, Yamazaki T. Cardiovascular clinical trials in Japan and controversies regarding prospective randomized open-label blinded end-point design. Hypertens Res. 2009;32(2):109–14.
48 Lega JC, Mismetti P, Cucherat M, Fassier T, Bertoletti L, Chapelle C, Laporte S.: Impact of double-blind vs open study design on the observed treatment effects of new oral anticoagulants in atrial fibrillation: A meta-analysis (provisional abstract). J Thromb Haemost. 2013;11(7):1240–50.
49 O’Neil WM, Welner SA, Lip GYH. Do open label blinded outcome studies of novel anticoagulants versus warfarin have equivalent validity to those carried out under double-blind conditions? Thromb Haemost. 2013;109(3):497–503. doi:http://dx.doi.org/10.1160/TH12-10-0715.
50 Sardar P, Chatterjee S, Chaudhari S, Lip GY.: New oral anticoagulants in elderly adults: Evidence from a meta-analysis of randomized trials (provisional abstract). Database of Abstracts of Reviews of Effects.
51 Linkins L-A. Bleeding risks associated with vitamin K antagonists. Blood Rev. 2013;27(3):111–8.
52 Halvorsen S, Wallentin L, Yang H, De Caterina R, Erol C, Garcia DA, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation. Can J Cardiol. 2013;1:S255.
53 Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: Insightsinsights from the aristotleARISTOTLE trial. Eur Heart J. 2012;33(22):2821–30. doi:http://dx.doi.org/10.1093/eurheartj/ehs274.
54 Noseworthy P, Yao X, Sangaralingham LR, Abraham N, McBane R, Gersh B, Shah Net al. Direct comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;67:692.
55 Akao M, Yamashita T, Okumura K; J-ELD AF Investigators. Study design of J-ELD AF: A multicenter prospective cohort study to investigate the efficacy and safety of apixaban in Japanese elderly patients. J Cardiol. 2016;S0914-5087(15)00404-9. [Epub ahead of print]
56 Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med. 2012;366(9):864–6.
57 Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah NDet al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857.
58 Larsen TB, Gorst-Rasmussen A, Rasmussen LH, Skjøth F, Rosenzweig M, Lip GY. Bleeding events among new starters and switchers to dabigatran compared with warfarin in atrial fibrillation. Am J Med. 2014;127(7):650–656.
59 Coppens, M., Eikelboom, J., Ezekowitz, M., Clemens, A., Healy, J., Wallentin, L., Noack, H., Yusuf, S., and Connolly, S. (2016). Abstract 15537: Dabigatran Versus Warfarin in Very Elderly Patients with Atrial Fibrillation: Results from the RE-LY Trial. Circulation 126, A15537 LP-A15537.
60 Avgil-Tsadok M, Jackevicius CA, Essebag V, Eisenberg MJ, Rahme E, Behlouli H, et al. Dabigatran use in elderly patients with atrial fibrillation. Thromb Haemost. 2016;115(1):152–60.
61 Kato ET, Giugliano RP, Ruff CT, Koretsune Y, Yamashita T, Kiss RG, et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the engage AF-TIMI 48 trial. J Am Heart Assoc. 2016;5(5):e003432.
62 Lip GY, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, et al. Relative efficacy and safety of non-Vitamin K oral anticoagulants for non-valvular atrial fibrillation: Network meta-analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Int J Cardiol. 2016;204:88–94.
63 Skjøth F, Larsen TB, Rasmussen LH, Lip GY. Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Thromb Haemost. 2014;111(5):981–8.
64 Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC, Jr. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27(3):699–703.
65 Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, et al. Incidence and prevalence of heart failure in elderly persons, 1994-2003. Arch Intern Med. 2008;168(4):418–24.
66 Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, et al.; workshop participants. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009;20(6):1199–209.
67 Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–92.
68 Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al.; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56.
69 Speechley M, Tinetti M. Falls and injuries in frail and vigorous community elderly persons. J Am Geriatr Soc. 1991;39(1):46–52.
70 Winograd CH. Targeting strategies: an overview of criteria and outcomes. J Am Geriatr Soc. 1991;39(9 Pt 2):25S–35S.
71 Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205–6.
72 Turagam MK, Velagapudi P, Flaker GC. Stroke prevention in the elderly atrial fibrillation patient with comorbid conditions: focus on non-vitamin K antagonist oral anticoagulants. Clin Interv Aging. 2015;10:1431–44.
73 Granziera S, Cohen AT, Nante G, Manzato E, Sergi G. Thromboembolic prevention in frail elderly patients with atrial fibrillation: a practical algorithm. J Am Med Dir Assoc. 2015;16(5):358–64.
74 Fernández CS, Formiga F, Camafort M, Rodrigo JM, Díez-Manglano J, Reino AP, et al.; Grupo de trabajo de Riesgo vascular de la SEMI. Erratum: Antithrombotic treatment in elderly patients with atrial fibrillation: a practical approach. BMC Cardiovasc Disord. 2015;15(1):157.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.