Prolonged impairment of deglutition in supratentorial ischaemic stroke: the predictive value of Parramatta Hospitals’ Assessment of Dysphagia
DOI: https://doi.org/10.4414/smw.2016.14355
Georg
Kägi, Natascha
Leisi, Marian
Galovic, Marlise
Müller-Baumberger, Werner
Krammer, Bruno
Weder
Summary
BACKGROUND: Up to 50% of ischaemic stroke patients show initial dysphagia, which may persist for months. Guidelines recommend switching nasogastric (NG) to percutaneous endoscopic gastrostomy (PEG) tube feeding at the second week after the stroke if impaired deglutition is expected for another 4 weeks. Precise prognostic criteria are lacking. We hypothesised that the Parramatta Hospitals’ Assessment of Dysphagia (PAHD) performed 8 to 10 days after the stroke predicts impaired deglutition for another 4 weeks.
METHODS: After a first dysphagia assessment (buccolingual motor function, liquid and semisolid swallow tests, “two-out-of-six” scale) within 48 hours of onset, patients with a first hemispheric stroke and risk of aspiration, defined as a two-out-of-six scale score of ≥2 (dysphonia, dysarthria, abnormal gag reflex, abnormal volitional cough, cough / voice change after swallowing) were included and were assessed by a blinded rater using the PHAD. The same dysphagia assessments were repeated 8 to 10 days after the stroke (second assessment) and patients remained in the study if the two-out-of-six scale score remained ≥2. At a final evaluation by telephone after 4 weeks, impaired deglutition was assessed with the Bogenhausen dysphagia score (BODS-2). Exclusion criteria were infratentorial or recurrent stroke and pre-existing dysphagia. The primary objective was to define a threshold score and value of the PHAD at second assessment that predicted impaired deglutition as assessed with the BODS-2 (score ≥4) at the final evaluation. The secondary objective was to explore the value of the PHAD assessed within 48 hours to predict impaired deglutition (BODS-2 ≥4) at final evaluation. To evaluate the predictive value of the PHAD score assessed 8 to 10 days after stroke onset for impaired deglutition for another 4 weeks, we determined the area under the receiver operating curve (ROC AUC).
RESULTS: Over a 1-year period, 29 out of 252 assessed patients remained at risk of aspiration after the second assessment. In these patients, ROC analysis of PHAD recorded 8 to 10 days after the stroke showed excellent accuracy with an AUC of 0.971 (cut-off 71.5) predicting a BODS-2 score of ≥4 at final evaluation. The accuracy of ROC analysis of the PHAD score assessed within 48 hours of stroke onset to predict prolonged impairment of deglutition was poor (AUC 0.685).
CONCLUSIONS: In a selected population at risk of aspiration, the PHAD with a threshold of 70 assessed in the second week after stroke onset may be a valuable tool to predict prolonged impairment of deglutition for another 4 weeks and to guide the decision about switching from NG to PEG tube feeding after supratentorial ischaemic stroke.
Clinical trial registration number: EKSG10/157
Introduction
Dysphagia is a common complication of ischaemic stroke with an incidence of up to 55% [1]. About half of the patients with acute dysphagia will recover safe swallowing within the first week [2, 3]. Nevertheless, a significant proportion of up to 17% may have prolonged dysphagia for 4 weeks or longer [2]. Furthermore, dysphagia is recognised as an important cause of morbidity and mortality [1, 2, 4, 5], which is mainly driven by pulmonary complications secondary to aspiration [6, 7]. Known risk factors associated with aspiration are stroke severity, increasing age, dysphagia and various stroke locations [1, 8–10, 11] To reduce these complications, early screening for dysphagia is important [12, 13]. If safe swallowing is not possible, early (<72 h after stroke onset) enteral feeding via a nasogastric (NG) tube is recommended because malnutrition is an independent prognostic factor of poor outcome in the first week after stroke [14–16]. Current guidelines recommend switching from NG to PEG (percutaneous endoscopic gastrostomy) tube feeding not before the second week after stroke onset and only if the need for further enteral feeding is expected for 4 weeks or longer [17]. In the acute stroke phase, the Parramatta Hospitals’ Assessment of Dysphagia (PHAD) has proved to be a reliable predictor for dysphagia of at least 2 weeks [18, 19]. However, prognostic criteria predicting prolonged impairment of deglutition for at least 4 weeks are not available (i.e., timing and validity of prognosis and the associated time window of significantly disturbed swallowing). Consequently, and based on current guidelines, the primary aim of this study in patients with hemispheric ischaemic stroke was to explore the value of the PHAD recorded between 8 and 10 days after stroke onset to predict impaired deglutition for another 4 weeks. Our hypothesis was that the PHAD at this time-point predicts impaired deglutition requiring tube feeding at week 4.
Patients and methods
Patients
We performed a prospective, longitudinal, single centre and assessment-blinded, prognostic accuracy, cohort study on patients with hemispheric ischaemic stroke and risk of aspiration, with consecutive enrolment over a period of 12 months. Inclusion criteria were (i) acute and computed tomography / magnetic resonance imaging (CT/MRI) proven hemispheric ischaemic stroke with hospital admission within 48 hours of symptom onset, (ii) relevant risk of aspiration defined as a “two-out-of-six” scale score ≥2. Exclusion criteria were (i) infratentorial stroke, (ii) recurrent stroke, (iii) stroke mimics, (iv) coma, (v) pre-existing dysphagia, (vi) two-out-of-six scale score <2, (vii) death before final assessment.
Methods
Stroke patients considered at risk for dysphagia by the treating physician (i.e., presence of dysphonia, dysphagia, cough or voice change after swallowing) were referred to the speech-language pathologists (SLPs) for standard comprehensive swallowing assessment including the two-out-of-six scale within 48 hours of stroke onset. In a few ventilated patients, first assessment was outside the scheduled interval of the first 48 hours. Patients with relevant risk of aspiration (two-out-of-six scale score ≥2) were included and were assessed by a blinded SLP using the PHAD on the same day; a second standard comprehensive swallowing assessment was carried out 8 to 10 days after stroke onset. Patients with persistent risk of aspiration (two-out-of-six scale score ≥2) were assessed again by a blinded SLP using the PHAD on the same day and remained in the study. A final evaluation was performed 4 weeks later as a telephone interview focussing on impairment of deglutition, assessed with the Bogenhausen dysphagia score part 2 (BODS-2), and return to prestroke diet (fig. 1).
Demographic variables, stroke severity (National Institutes of Health stroke scale [NIHSS]) and stroke aetiology (TOAST criteria) were assessed by the treating physician after standard clinical work up. The CT/MRI images were visually checked for lesions within the insula and/or frontal operculum (yes/no) (table 1).
The primary endpoint was the threshold value and performance of the PHAD score obtained at second assessment to predict severe to seriously impaired deglutition as assessed with the BODS-2 (score ≥4) at final evaluation. The secondary endpoints were (i) performance of the PHAD obtained at first assessment to predict severe to seriously impaired deglutition assessed with the BODS-2 at final evaluation, (ii) return to prestroke diet, (iii) evaluation of PHAD items with regard to functional outcome measured with the BODS-2 score at the final assessment.
Between all assessments, routine therapeutic procedures were performed and treatment decisions relied exclusively on the standard dysphagia assessment without knowledge of the PHAD results.
Swallowing assessments
The comprehensive dysphagia assessment
This represents our in-house standard since 2005 and includes: standardised examination of: oral musculature strength, agility and symmetry; protective reflexes; sensation; systematic testing of swallowing of fluids (50 ml water swallow test) and semisolids; the two-out-of-six scale.
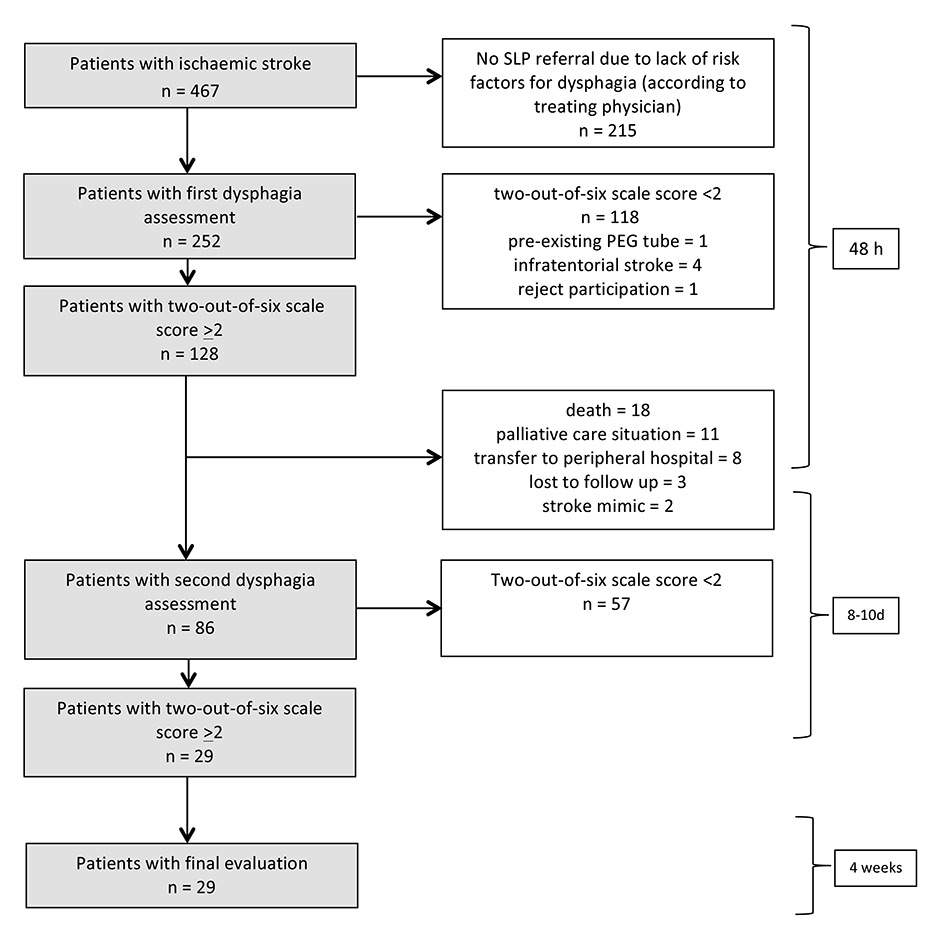
Figure 1
Patient enrolment and exclusion.
First and second dysphagia assessments consisted of: comprehensive dysphagia assessment including the two-out-of-six scale. If the two-out-of-six scale score was ≥2 the Parramatta Hospitals’ Assessment of Dysphagia was added by a speech-language pathologists (SLP) who was unaware of the other dysphagia and clinical assessments. First assessment was performed within 48 h after stroke onset and the second 8–10 d after stroke onset. The final evaluation was performed by an SLP blinded to clinical follow up and consisted of a telephonic interview with assessment of the BODS-2 scale (Bogenhausen-dysphagia-score, part 2) 4 weeks after the second assessment (5–7 weeks after stroke onset).
Two-out-of-six scale
The presence of two or more features in the two-out-of-six scale (dysphonia, dysarthria, abnormal gag reflex, abnormal volitional cough, cough after swallowing, and voice change after swallowing) is an indicator of an elevated risk of aspiration (sensitivity 92%, specificity 67%) as referenced in a video-fluoroscopic swallowing study [20].
Parramatta Hospitals’ Assessment of Dysphagia
The PHAD allows a quantitative rating of swallowing and is validated for neurogenic dysphagia [21]. It consists of 14 items with two main components: (i) documentation of the prerequisite needed to swallow and (ii) a clinical swallow assessment. The maximum score is 100 points when swallowing function is normal or a minimum score of 20 points with a severe disorder. A score of less than 60 indicates serious to severe dysphagia, 61 to 90 mild to serious dysphagia and >90 no dysphagia (see supplemental material in appendix 1).
Bogenhausen dysphagia score
BODS-2 assesses functional oral intake. A score of 4 to 8 represents serious to severe impairment of deglutition ranging from (i) restriction of oral food intake to only one consistency (at a threshold of 4) to (ii) partial/absent oral food intake, justifying enteral tube feeding for full substitution [22] (appendix 2).
Statistics
Data were analysed using the SPSS statistical package for Windows v. 20. Baseline characteristics are presented as mean and standard deviation (SD) if not stated otherwise. To check for group differences, Mann Whitney U-test (age, NIHSS, modified Rankin scale, two-out-of-six, BODS-2 and PHAD scales) or, where applicable, Fisher’s exact test (gender, thrombolysis, lesion location, cardiovascular risk factors, stroke aetiology [TOAST]) were applied. To evaluate our primary endpoint (i.e. the predictive value of the PAHD score, assessed after the first week, regarding serious to severe impaired deglutition [BODS ≥4] at final evaluation) we determined the area under the receiver operating curve (ROC). Furthermore we calculated the Youden index to determine the most appropriate cut-off score of the PHAD. Fisher’s exact test was then applied to test the power (OR, sensitivity/specificity) of the PHAD using this cut-off to predict serious to severe impaired deglutition defined as a BODS-2 score of ≥4 at final evaluation. For our secondary endpoint, the predictive value of the PHAD at first assessment regarding serious to severe impairment of deglutition at final evaluation, we repeated the statistical methods described for the primary endpoint. To evaluate the contribution of each of the 14 subitems of the PHAD we calculated a linear logistic regression with the BODS-2 score at final evaluation as the dependent variable.
|
Table 1: Descriptive statistics of patients with extended risk of aspiration with or without prolonged impairment of deglutition. |
|
|
Extended risk of aspiration*
|
Without prolonged impairment of deglutition **
|
With prolonged impairment of deglutition***
|
p-value
|
| Number |
29 |
22 |
7 |
|
| Age (years) |
72.9 (10.5) |
72.7(11.2) |
73.6 (8.4) |
0.940†
|
| Gender (% males) |
18(62) |
14 (64) |
4 (57) |
1.000††
|
| NIHSS |
12.6 (5.9) |
12(5.0) |
14.6 (8.2) |
0.328†
|
| Premorbid mRS (range) |
0.5 (0–3) |
0.4 (0–3) |
0.7 (0–2) |
0.438†
|
| Thrombolysis (n, %) |
19 (66) |
15 (68) |
4 (57) |
0.665††
|
| Lesion location (frontal operculum or insula) |
24 (83) |
17 (77) |
7 (100) |
0.296††
|
| Stroke aetiology (TOAST): |
|
|
|
|
| Macroangiopathy (n, %) |
9 (31) |
7 (32) |
2 (29) |
1.000††
|
| Cardioembolic (n, %) |
13 (45) |
9 (41) |
4 (57) |
0.667††
|
| Microangiopathy (n, %) |
0 (0) |
0 (0) |
0 (0) |
na††
|
| Other (n, %) |
1 (3) |
1 (5) |
0 (0) |
1.000††
|
| Unclear (n, %) |
6 (21) |
5 (23) |
1 (14) |
1.000††
|
| Cardiovascular risk factors: |
|
|
|
|
| Hypertension (n, %) |
25 (86) |
19 (86) |
6 (86) |
1.000††
|
| Diabetes (n, %) |
10 (34) |
8 (36) |
2 (29) |
1.000††
|
| Dyslipidaemia (n, %) |
25 (86) |
20 (91) |
5 (71) |
0.238††
|
| Obesity (n, %) |
8 (28) |
5 (23) |
3 (43) |
0.357††
|
| Smoking (n, %) |
5 (17) |
5 (23) |
0 (0) |
0.296††
|
| Dysphagia assessments: |
|
|
|
|
| Two-out-of-six scale |
|
|
|
|
| 1st assessment
2nd assessment |
3.9 (1.4)
3.3 (1.3) |
3.7 (1.2)
2.8 (1.0) |
4.6 (1.8)
5 (0.8) |
0.122†
<0.001†
|
| PHAD |
|
|
|
|
| 1st assessment |
65 (14.2) |
68 (9.9) |
55.7 (21.5) |
0.149†
|
| 2nd assessment |
74.5 (12) |
79.5 (8.1) |
59 (8.5) |
<0.001†
|
| BODS-2 |
|
|
|
|
| 1st assessment |
5.3 (2.2) |
5.1 (2.1) |
6 (2.4) |
0.381†
|
| 2nd assessment |
3.9 (2.2) |
2.9 (1.2) |
7 (1.4) |
<0.001†
|
| Final evaluation |
2.2 (1.6) |
1.4 (0.6) |
4.6 (1.1) |
<0.001†
|
| BODS-2 = Bogenhausen dysphagia score, part 2; mRS = modified Rankin scale score; na = not applicable; NIHSS = National Institutes of Health stroke scale; PHAD = Parramatta Hospitals’ Assessment of Dysphagia
* Two-out-of-six scale-score: ≥2 at 1st and 2nd assessment
** BODS-2 <4 at final evaluation
*** BODS-2 ≥4 at final evaluation
Level of significance: 0.05 († Mann-Whitney U test; †† Fisher’s exact test) |
Results
A total of 252 out of 467 patients with acute ischaemic stroke were considered at risk for dysphagia by the treating physician and were referred for a comprehensive clinical dysphagia assessment by an SLP. At first assessment, 128/252 patients were at risk of aspiration, defined as a two-out-of-six scale score of ≥2. Screening failures at first assessment comprised two-out-of-six scale score <2 (n = 118), brainstem infarction (n = 4), PEG tube before stroke onset (n = 1), rejected participation (n = 1). Eighty-six patients reached the second assessment after the first week. Reasons for exclusion at the second assessment were: two-out-of-six scale score <2 (n = 57), death before second assessment (n = 18), palliative care situation (n = 11), incomplete documentation due to transfer to a peripheral hospital (n = 8), lost to follow up (n = 3), stroke mimics (n = 2). Twenty-nine patients remained at risk of aspiration and finished the study per protocol. The mean age of this group with extended risk of aspiration was 72.9 (±10.5, SD) years, 18 (62%) were male and the mean NIHSS score at hospital admission was 12.6 (±5.9) (table 1). Sixteen patients (55%) had right hemispheric, 12 (41%) had left hemispheric and one patient had bi-hemispheric lesions. Within this group, seven patients had relevant functional impairment of deglutition (BODS ≥4) at the final evaluation, in contrast to the other 22 patients who were without relevant functional impairment of deglutition (BODS <4) at the final evaluation (fig. 2). There were no between-group differences regarding age, sex, stroke severity or stroke aetiology (table 1).
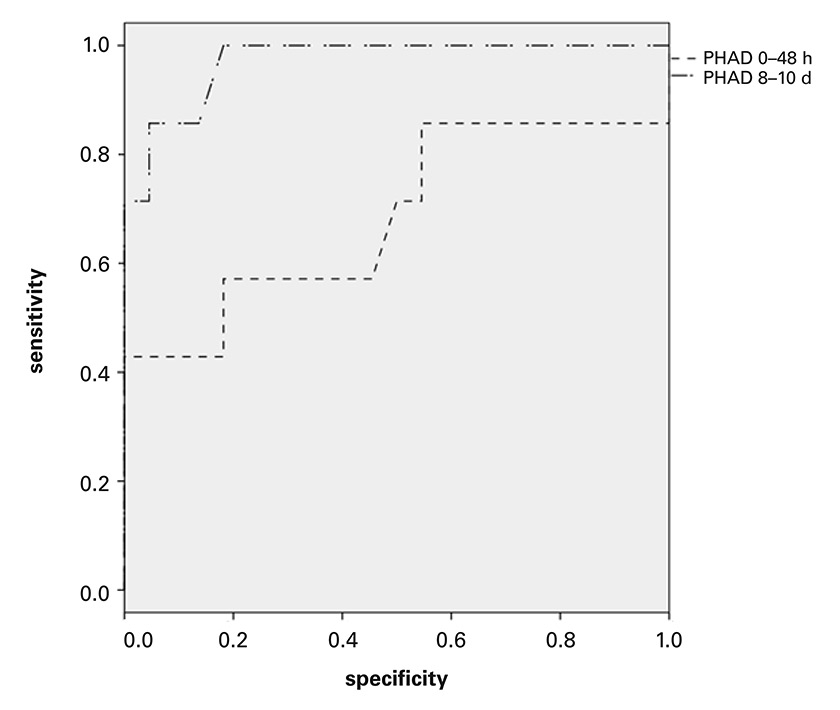
Figure 2
Receiver operator curve (ROC) -analysis of Parramatta Hospitals’ Assessment of Dysphagia (PHAD) for prediction of “prolonged impairment of deglutition”.
At second assessment (mean 8.7 days, SD ± 1.4 post stroke), ROC analysis for PHAD score in relation to serious to severe impairment of oral intake defined as a Bogenhausen-dysphagia-score, part 2 (BODS-2) score of ≥4 at 5–7 weeks after the stroke. Area under the curve (AUC) 0.971, 95% confidence interval 0.915–1.0. ROC analysis for PHAD score assessed in the first week: AUC 0.685, 95% CI 0.412–0.958.
Predicting prolonged impairment of oral food intake with the PHAD
Only 7 out of 86 patients (8.1%) with risk of aspiration in the acute stroke phase had serious to severe impairment of deglutition at the final assessment. At second assessment, ROC analysis showed that the PHAD score was highly accurate in predicting a BODS-2 score of ≥4 at the final evaluation 5 to 7 weeks after stroke onset: area under the curve (AUC) of 0.971 (95% confidence interval [CI] 0.915–1.0); Youden Index of 0.82 at a cut off of 71.5 points (sensitivity 100%, specificity 82%) and a second highest Youden Index of 0.81 (sensitivity 86%, specificity 96%) at a cut off of 66 points. Fisher’s exact test for the groups BODS-2 </≥4 and PHAD ≤/>70 gave a highly significant result (CI 3.3–436.93, OR 38, p <0.001, sensitivity 85%, specificity 86%).
At first assessment, ROC analysis found that the PHAD score was not accurate in predicting a BODS-2 score of ≥4 at the final evaluation, with an AUC of 0.685 (95% CI 0.412–0.958) (fig. 2). Fisher’s exact test for the groups BODS-2 </≥4 and PHAD ≤/>70 at first assessment did not show significance (95% CI 0.05–3.9, OR 0.48, p = 0.67).
Return to prestroke diet was possible in 14/29 patients; 8/29 had mild to moderate and 7/29 serious to severe impairment of deglutition, the latter requiring PEG tube feeding in 6 out of 7 patients decided on the basis of the results of the standard comprehensive assessment (fig. 3).
The linear regression model for the 14 sub-items of the PHAD with the BODS-2 as dependent variable was significant only for the item “pharyngeal phase” (95% CI –1.117 to –0.265, p = 0.004) (table 2).
|
Table 2: PHAD sub-items in patients with extended risk of aspiration. |
|
|
|
Extended risk of aspiration
(n = 29)
|
Extended risk of aspiration without prolonged impairment of deglutition
(n = 22)
|
Extended risk of aspiration with prolonged impairment of deglutition
(n = 7)
|
95% confidence limits from linear regression
|
|
PHAD item
|
Maximal score
|
Mean
|
Median (IQR)
|
Mean
|
Median (IQR)
|
Mean
|
Median (IQR)
|
|
| Alertness |
10 |
9.8 (0.6) |
10 (0) |
9.8 (0.6) |
10 (10) |
9.7 (0.8) |
10 (0) |
–1.382, 0.728 |
| Respiratory function |
10 |
8.2 (2.4) |
10 (3) |
9.1 (1.3) |
10 (2) |
5.4 (2.8) |
4 (4) |
–0.378, 0.329 |
| Language comprehension |
5 |
3.9 (1.3) |
4 (2) |
4.3 (0.9) |
5 (1) |
2.7 (1.5) |
3 (3) |
–0.473, 0.866 |
| Language expression |
5 |
3.3 (1.1) |
4 (1) |
3.6 (0.9) |
3 (1) |
2.4 (1.5) |
2 (3) |
–0.853, 0.497 |
| Motor function of: |
| Lips |
5 |
3.4(0.7) |
3 (1) |
3.6 (0.8) |
3 (1) |
3.1 (0.4) |
3 (0) |
–0.684, 0.789 |
| Tongue |
10 |
6.9 (1.6) |
6 (2) |
7.2 (1.5) |
8 (2) |
6 (1.6) |
6 (4) |
–0.513, 0.264 |
| Palate |
5 |
4.0 (0.8) |
4 (2) |
4.1 (0.8) |
4 (3) |
3.7 (0.9) |
4 (2) |
–0.596, 1.030 |
| Gag reflex |
5 |
3.1 (1.5) |
2 (3) |
3.2 (1.5) |
3 (2) |
2.8 (1.4) |
2 (2) |
–0.410, 0.303 |
| Phonation |
5 |
3.5 (1.3) |
4 (1.5) |
3.9 (1) |
4 (1.25) |
2 (1.2) |
2 (1) |
–0.693, 0.562 |
| Cough |
10 |
7.3 (2.1) |
8 (4) |
8.1 (0.8) |
8 (4) |
4.9 (1.1) |
4 (2) |
–0.580, 0.179 |
| Swallowing: |
| Oral preparation phase |
5 |
3.4 (0.9) |
3 (1) |
3.6 (0.8) |
4 (1) |
2.86 (1.1) |
3 (1) |
–0.898, 1.161 |
| Oral phase |
10 |
7.0 (1.6) |
8 (2) |
7.3 (1.3) |
8 (2) |
6 (2) |
6 (2) |
–0.107, 0.806 |
| Pharyngeal phase |
10 |
7.3 (1.7) |
8 (2) |
8 (1.2) |
8 (0) |
5.1 (1.1) |
6 (2) |
–1.117, –0.265 |
| Tolerance of different food consistencies |
5 |
3.3 (1) |
3 (1) |
3.6 (0.7) |
4 (1) |
2.3 (1) |
2 (1) |
–1.079, 0.673 |
| Total PHAD |
100 |
74.5 (12) |
75 (18.5) |
79.5 (8.1) |
79.5 (14) |
59 (8.5) |
61 (15) |
|
| Total BODS-2 |
8 |
2.2 (1.6) |
2 (2.5) |
1.4 (0.6) |
1 (1) |
4.6 (1.1) |
4 (1) |
|
| BOD-2 = Bogenhausen dysphagia score, part 2 (assessed 5–7 weeks after stroke); IQR = interquartile range; PHAD = Parramatta Hospitals’ Assessment of Dysphagia (assessed 8–10 days after stroke) |
Discussion
In our study we demonstrated that prolonged impairment of deglutition after hemispheric ischaemic stroke can be predicted with the Parramatta Hospitals’ Assessment of Dysphagia (PHAD). Prediction of prolonged impairment of swallowing of at least 4 weeks is very accurate if dysphagia is assessed with the PHAD in the second week after stroke onset but not helpful in this regard if it is assessed within the acute phase of stroke of the first week. These results regarding the timing of evaluation and the observation period are new findings and can add important information for the clinician deciding upon switching to PEG tube feeding.
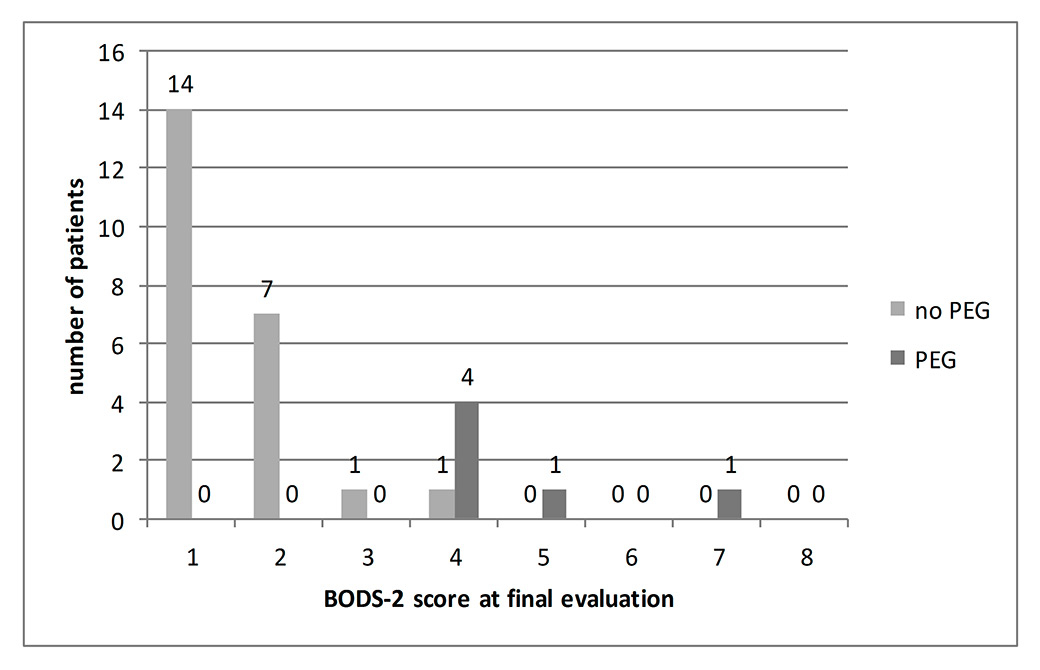
Figure 3
Impairment of deglutition 5 to 7 weeks after stroke.
BODS-2 (Bogenhausen-dysphagia-score, part 2) assessed 5–7 weeks after a stroke (final evaluation) in the group with “extended risk of aspiration” (n = 29). PEG indicates individuals with percutaneous endoscopic gastrostomy. Note partitioning into groups (i) BODS-2 <4 and (ii) BODS-2 ≥4. The latter, representing the group of “prolonged impairment of deglutition” (n = 7), had to be predicted from the PHAD score.
The algorithm of the study was based on “the principle of uncertainty” after the routine comprehensive dysphagia assessment, retaining the patients within the study at relevant risk of aspiration. This uncertainty was mainly due to the high sensitivity but low specificity of the two-out-of-six scale score of ≥2 in this regard. In contrast, the AUC from the ROC curve analysis in the second week indicated the high accuracy of PHAD in predicting prolonged severe to serious impaired deglutition at this time point until the final assessment. It depicted two cut-off points (at scores of 71.5 and 66), with high prognostic accuracy according to the Youden index, for selecting patients with this unfavourable course. From a practical point of view, a cut-off PHAD score of about 70 is in line with previous work evaluating the prognostic value of this score for prolonged impairment of swallowing in patients with acute stroke [18].
At a first glance, the lack of prognostic power at an early time-point seems to contradict the prognostic value of the PHAD reported by Broadley et al. [18]: however, the difference is explained by a different, refined methodology as well as by a different observation period as proposed by other study groups (see below):
1. Different population, i.e. acute ischaemic supratentorial stroke vs any acute stroke except subarachnoid haemorrhage;
2. Exclusion of deceased patients with risk of aspiration vs allocation of these patients to the group “prolonged dysphagia”;
3. Different outcome measures, i.e. gradual assessment according to BODS-2 vs. requirement of non-oral feeding.
In a subsequent study, Broadley et al. suggested a risk score (RAPIDS) that includes components of clinical and imaging findings to predict prolonged dysphagia [19]. The problems of early assessment and a short observation period remained. Our findings suggest that the time-point of assessment is important because dysphagia is a highly dynamic process within the first week, with up to 50% of patients returning to normal swallowing [2]. Further evidence that transient risk of aspiration (less than 7 days) has a pathophysiology different from that of extended risk of aspiration (more than 7 days) comes from our recent work on lesion location in patients at risk of aspiration [11]. Acute risk of aspiration was mainly explained by lesions in the periventricular white matter, the internal capsule and the insular cortex, but poor recovery within 7 days was mainly driven by an additional fronto-opercular lesion [11]. Apart from this specific predictor of extended recovery (combined ischaemic infarction of the frontal operculum and the insular cortex), previous studies defined nonspecific predictors such as stroke severity (NIHSS score), age and lesion volume [8, 10, 23–25]. A recent retrospective study described a predictive model for PEG tube feeding at a very early time point of less than 24 hours [26]. Patients with supra- and infratentorial ischaemic stroke were included, which we feel is critical because recovery from supra- and infratentorial strokes is different. There is no need to predict future PEG tube feeding within the first week because (i) dysphagia improves in an important proportion of patients within the first week and (ii) early PEG tube feeding instead of NG tube feeding can even harm the patient [16].
Like our study, previous neuroimaging studies did not show an overall hemispheric predominance during swallowing, although at an individual level hemispheric predominance cannot be excluded, being out-averaged at the group level [27]. It is noteworthy that all patients with a prolonged impairment of deglutition in our study were struck by a lesion within the insula / frontal operculum complex. This issue has to be reconsidered in order to formulate eventually a compound clinical and morphological lesion score.
The fact that prolonged impairment of deglutition of more than 4 weeks cannot reliably be predicted at an early time-point is expressed in current guidelines, which recommend starting NG tube feeding in the early phase (<72 h). PEG tube feeding is not advocated until the next plateau phase around days 10 to 14 and only if prolonged impairment of deglutition requiring enteral tube feeding is expected for another 4 weeks or more [17]. The recommendation is derived mostly from the large FOOD trial which showed a trend for lower mortality when tube feeding was introduced early (<7 days), but no benefit of early PEG tube feeding compared with NGT feeding [16]. By using the PHAD in the second week after a stroke, our study adds an important and so far missing piece of information needed to decide at the given time-point which patients are at high risk for prolonged dysphagia and will benefit from a PEG tube as proposed by current guidelines [17]. The PHAD is an easily performed and well standardised dysphagia score covering all important aspects of swallowing within its 14 items. With a score from 20 to 100 it is easier to define cut-offs for PEG tube feeding in comparison with our standard dysphagia assessment, which incorporates many different tests. In a stroke network of various bigger and smaller hospitals, this score allows easily applicable and standardised criteria for PEG tube feeding. Of the eight patients with a PHAD score of ≤70, seven had impaired deglutition for another 4 weeks, with six of them receiving PEG tube feeding. On the other hand, only 1 out of 20 patients with a PHAD score of more than 70 points still suffered from seriously impaired deglutition. Therefore, we consider patients with a score of ≤70 in the PHAD 1 week after stroke onset as good candidates for PEG tube insertion.
The linear regression model showed a significant association of the PHAD sub-item “pharyngeal phase” with the BODS-2 score at final evaluation. This finding is in line with the concept that the pharyngeal phase of swallowing may be synaptically linked to airway-protective reflexes [19, 28, 29]. It emphasises that an impaired pharyngeal phase of swallowing is a critical sign pointing to an increased likelihood of an increased and prolonged risk of aspiration.
A few limitations of the study have to be considered. The number of patients with prolonged impairment of deglutition is rather low, but the promising results will ease validation in a larger multicentre cohort. The predication of prolonged impairment of deglutition by the PHAD has been validated exclusively for supratentorial ischaemic strokes. The study was accomplished at the level of dysphagia assessment, requiring an adequately sized group of trained SLPs [30].
To conclude, the PHAD assessed in the second week after stroke onset seems to be a good predictor of prolonged dysphagia for a further 4 weeks or longer in a selected group of patients with hemispheric ischaemic stroke at risk of aspiration for more than 1 week after the stroke. These data can be used as a basis for helping the clinician in everyday practice: to decide about switching from NG tube to PEG tube feeding beyond the first week after ischaemic stroke, and to study therapeutic interventions and the mechanisms of recovery from dysphagia. In the future, the PHAD may help to standardise dysphagia assessment and monitoring for quality control in stroke networks.
Appendix
Appendix 1,
Parramatta Hospitals’ Assessment of Dysphagia, and
Appendix 2,
Bogenhausen dysphagia score, are available in the PDF version of this article.
Georg Kägi, MD
Department of Neurology
Kantonsspital St. Gallen
CH-9007 St.Gallen
georg.kaegi[at]kssg.ch
References
1 Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–63.
2 Smithard DG, O’Neill PA, England RE, Park CL, Wyatt R, Martin DF, et al. The natural history of dysphagia following a stroke. Dysphagia. 1997;12:188–93.
3 Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J. (Clin Res Ed) 1987;295:411–4.
4 Paciaroni M, Mazzotta G, Corea F, Caso V, Venti M, Milia P, et al. Dysphagia following Stroke. Eur Neurol. 2004;51:162–7.
5 Smithard DG, Smeeton NC, Wolfe CD. Long-term outcome after stroke: does dysphagia matter? Age Ageing. 2007;36:90–4.
6 Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–36.
7 Sharma JC, Fletcher S, Vassallo M, Ross I. What influences outcome of stroke – pyrexia or dysphagia? Int J Clin Pract. 2001;55:17–20.
8 Okubo PC, Fábio SR, Domenis DR, Takayanagui OM. Using the National Institute of Health Stroke Scale to predict dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2012;33:501–7.
9 Turhan N, Atalay A, Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: a special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation. 2009;24:321–6.
10 Adams HP, Jr., Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126–31.
11 Galovic M, Leisi N, Muller M, Weber J, Abela E, Kagi G, et al. Lesion location predicts transient and extended risk of aspiration after supratentorial ischemic stroke. Stroke. 2013;44:2760–7.
12 Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36:1972–6.
13 Yeh SJ, Huang KY, Wang TG, Chen YC, Chen CH, Tang SC, et al. Dysphagia screening decreases pneumonia in acute stroke patients admitted to the stroke intensive care unit. J Neurol Sci. 2011;306:38–41.
14 Gariballa SE, Parker SG, Taub N, Castleden CM. Influence of nutritional status on clinical outcome after acute stroke. Am J Clin Nutr. 1998;68:275–81.
15 Finestone HM, Greene-Finestone LS, Wilson ES, Teasell RW. Prolonged length of stay and reduced functional improvement rate in malnourished stroke rehabilitation patients. Arch Phys Med Rehabil. 1996;77:340–5.
16 Dennis MS, Lewis SC, Warlow C. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:764–272.
17 Prosiegel M. Neurogene Dysphagien. In: Diener H.C., Weimar C, eds. Leitlinien für Diagnostik und Therapie in der Neurologie. Stuttgart: Thieme 2012:1078–86.
18 Broadley S, Croser D, Cottrell J, Creevy M, Teo E, Yiu D, et al. Predictors of prolonged dysphagia following acute stroke. J Clin Neurosci. 2003;10:300–5.
19 Broadley S, Cheek A, Salonikis S, Whitham E, Chong V, Cardone D, et al. Predicting prolonged dysphagia in acute stroke: the Royal Adelaide Prognostic Index for Dysphagic Stroke (RAPIDS). Dysphagia. 2005;20:303–10.
20 Daniels SK, McAdam CP, Brailey K, Foundas AL. Clinical Assessment of Swallowing and Prediction of Dysphagia Severity. Am J Speech Lang Pathol. 1997;6:17–24.
21 Warms T, Champion R, Mortensen L. The Parramatta Hospitals’ Assessment of Dysphagia. Sydney: Westmead Hospital and Community Health Services, 1998.
22 Bartolome G, Schroeter-Morasch H, Starrost U. Klinische Eingangsuntersuchung bei Schluckstörungen. In: Bartolome G, Schroeter-Morasch H, eds. Schluckstörungen – Diagnostik und Rehabilitation. München: Urban & Fischer 2010:155–72.
23 Turhan N, Atalay A, Muderrisoglu H. Predictors of functional outcome in first-ever ischemic stroke: a special interest to ischemic subtypes, comorbidity and age. NeuroRehabilitation. 2009;24:321–6.
24 Thijs VN, Lansberg MG, Beaulieu C, Marks MP, Moseley ME, Albers GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke. 2000;31:2597–602.
25 Johnston KC, Wagner DP, Wang XQ, Newman GC, Thijs V, Sen S, et al. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820–5.
26 Dubin PH, Boehme AK, Siegler JE, Shaban A, Juengling J, Albright KC, Martin-Schild S. New model for predicting surgical feeding tube placement in patients with an acute stroke event. Stroke. 2013;44:3232–4.
27 Lowell SY, Reynolds RC, Chen G, Horwitz B, Ludlow CL. Functional connectivity and laterality of the motor and sensory components in the volitional swallowing network. Exp Brain Res. 2012;219:85–96.
28 Broussard DL, Altschuler SM. Central integration of swallow and airway-protective reflexes. Am J Med. 2000;108(Suppl 4a):62S–67S.
29 Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25:323–33.
30 Donovan NJ, Daniels SK, Edmiaston J, Weinhardt J, Summers D, Mitchell PH. Dysphagia screening: state of the art: invitational conference proceeding from the State-of-the-Art Nursing Symposium, International Stroke Conference 2012. Stroke. 2013;44:e24–e31.


