Host response to fungal infections – how immunology and host genetics could help to identify and treat patients at risk
DOI: https://doi.org/10.4414/smw.2016.14350
Nina
Khanna, Claudia
Stuehler, Anna
Lünemann, Agnieszka
Wójtowicz, Pierre-Yves
Bochud, Salomé
Leibundgut-Landmann
Summary
In spite of the ever-increasing incidence and poor outcome of invasive fungal infections in immune compromised patients, there is currently no reliable method to accurately predict the risk, to monitor the outcome and to treat these infections. Protective immunity against Candida and Aspergillus depends on a highly coordinated interaction between the innate and adaptive immune systems. Genetic and immunological defects in components of these networks result in increased risk of invasive fungal infections among patients undergoing chemotherapy or transplant recipients.
We review the most important genetic and immunological factors that influence human susceptibility to Candida and Aspergillus infections and discuss the potential role of basic research to promote precision medicine for infectious diseases. We discuss how immunogenetic studies can help to provide tools for improved identification of high-risk patients and the development of tailored antifungal therapies.
Epidemiology of invasive fungal diseases in immune compromised patients
Invasive fungal infections caused by Candida species or Aspergillus fumigatus and other filamentous moulds are devastating in immune compromised patients. Patients at risk include transplant recipients, patients receiving chemotherapy, patients infected with human immunodeficiency virus-1 (HIV1) or patients with underlying autoimmune diseases. Allogeneic haematopoietic stem cell transplant (HSCT) recipients and patients treated for acute leukaemia are predominately affected [1–5].
In these populations, Candida infections are associated with a mortality of around 20 to 40%, whereas invasive mould infections carry even higher mortality reaching up to 80% [6–11]. Prior to the routine use of antifungal prophylaxis, Candida species (spp.) accounted for the majority of fungal infections among these patients. However, over the last two decades, the incidence of Aspergillus infections has surpassed that of Candida infections. This mainly results from the use of effective antifungal prophylaxis targeting Candida spp. [12, 13].
In recent years, posaconazole and voriconazole prophylaxis have led to a great reduction of invasive mould and yeast infections in randomised controlled trials [14, 15] and are therefore recommended in high-risk populations (e.g., during neutropenia or graft-versus-host diseases [GVHD]). However, these prophylaxes are not uniformly adopted in Switzerland and other countries because of concerns about high costs, drug interactions, toxicity and, most importantly, limited clinical efficacy with the emergence of resistant fungal strains and breakthrough infections [14, 15]. Indeed, new and highly treatment-resistant fungal species, including yeasts resistant to azole therapies like Candida krusei and Candida glabrata, as well as highly resistant moulds such as Aspergillus fumigatuswith mutations in the cyp51A gene, Aspergillus terreus, Fusariumspp., Zygomycetesspp. andScedosporiumspp. have emerged as serious pathogens in transplant recipients [13, 16–20]. Hence, reliable tools to identify patients at risk and tailored treatment strategies to improve patient outcome are urgently needed.
The introduction of precision medicine, which takes into account individual genetic and environmental factors in the choice of the most promising therapy for each patient, has led to impressive achievements, especially in the field of oncology [21]. Large-scale efforts, such as the United States Precision Medicine Initiative or the United Kingdom 100 000 Genomes Project, aim at enhancing the impact of this concept in oncology and extending its application to other clinical areas including infectious diseases [22, 23]. A comprehensive understanding of the genetic and functional basis of immune protection in fungal infection will promote precision medicine for infectious diseases through classification of patients according to a specific risk score and personalised therapy to restore the impaired host immunity in high-risk patients [24].
Host immune response to fungal infection
The innate immune system plays a pivotal role in protection from acute fungal infections [25–27]. Innate immune cells including neutrophils, monocytes/macrophages and dendritic cells rapidly detect the presence of fungi and induce an antimicrobial response. Fungal recognition is mediated by a variety of surface-bound and soluble pattern recognition receptors (PRRs) recognizing fungal cell wall components and nucleic acids including Toll-like receptors (TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR9), C-type lectins (Dectin-1, Dectin-2, Mincle, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin [DC-SIGN], mannose binding lectin 2 [MBL2]), and long pentraxin 3 (PTX3) [28–33]. The importance of these PRRs for fungal control has been demonstrated in a variety of animal studies [34–39]
Neutropenia is a critical risk factor for the development of invasive fungal infections. Neutrophils possess a wide range of effector mechanisms that contribute to intra- and extracellular elimination of fungi, including production of reactive oxygen species, release of antimicrobial peptides, or the formation of neutrophil extracellular traps (NETs) to restrict fungal spread [27, 40–43]. However, recent findings show that neutrophil function is not only restricted to elimination of microorganisms. These cells can show extensive heterogeneity and plasticity, can greatly extend their life span and have important functions in immunoregulatory networks through contact-dependent mechanisms or by de novo production of cytokines [44, 45]. Neutrophils can further prevent morphotypic switching (e.g., transition from yeast to filamentous growth), a key virulence trait of C. albicans [46–48]. Taken together, although a large arsenal of different antifungal activities of neutrophils have been described, it is still not well understood which ones are most relevant in vivo in infected tissues.
Innate antifungal defence also relies on mononuclear phagocytes. Tissue-infiltrating monocytes have been described in the context of Candida and Aspergillus infections [49, 50] and tissue-resident macrophages, such as CX3CR1+ macrophages in the kidney, were protective against systemic candidiasis [51]. Monocytes and macrophages display a remarkable ability to internalise fungi, to secrete several proinflammatory cytokines and chemokines and to exert significant fungicidal activity [27]. Their contribution to antifungal defence may be of particular relevance in neutropenic settings [52].
Natural killer (NK) cell recruitment was previously reported to be essential for antifungal defence in neutropenic mice [53, 54] and NK cell proliferation was associated with inhibition of fungal growth [55]. Moreover, adoptive NK cell transfer led to enhanced fungal clearance in neutropenic animals [56]. NK cells might also be important in the context of invasive fungal infections in patients after HSCT, as we observed that patients with invasive aspergillosis had reduced NK cell counts, and lower NK cell counts were associated with a poor outcome [57]. NK cells may exert direct antifungal activity by secretion of interferon-γ (IFN-γ) and perforin [58, 59], or contribute to fungal clearance by regulation of other innate and adaptive immune cells via cytokine production [60] such as granulocyte macrophage colony stimulating factor (GM-CSF), which promotes the mobilisation and antimicrobial activity of granulocytes and macrophages [54, 59, 61–63]. In humans, NK cells may directly respond to fungal stimuli via the NK receptor NKp30 and a still unknown fungal ligand [64] or become activated through accessory cells such as monocytes/macrophages or dendritic cells [65–67]. It will be interesting to determine whether particular NK cell subsets are important for fungal control, as we have previously shown for the control of viral infections [68].
Additionally to innate immunity, adaptive immune responses seem to play a crucial role in fungal control. The protective role of T cell responses in Candida and Aspergillusinfections has been studied intensively in different experimental systems and some human studies [69–72]. Fungus-specific T helper (Th) 1 responses characterised by production of IFN-γ, GM-CSF and tumour necrosis factor (TNF-α) are protective, and impaired Th1 cell numbers and cytokine responses correlate with higher fungal burden [73–75]. A protective role of Th17 cells in fungal immunity has also been observed, in particular in mucocutaneous Candida infections [76]. Moreover, it has been recently demonstrated in a mouse model of oropharyngeal candidiasis that other sources of interleukin (IL)-17, including innate lymphoid cells, also contribute to fungal control [77]. Whereas the contribution of CD4+ T cells in anti-fungal adaptive immunity is well characterised, recent studies have also unrevealed protective CD8+ T-cell immunity against Aspergillusand Candida infections [78]. We have recently shown that after HSCT patients have a defective A. fumigatus-specific T cell response for up to a year after transplantation, correlating with the period when patients are at highest risk for infection [57]. Moreover, A. fumigatus-specific T cell responses to different cell-wall and cytosolic antigens were higher in patients recovering from invasive aspergillosis than in patients with progressive disease [79]. Consistent with the observations in transplant patients, an inverse correlation between CD4+ T cell numbers and the incidence of invasive fungal infections has been observed in HIV-infected patients [73, 80].
In summary, antifungal immunity relies on many different immune pathways and functional defects in many of those have been associated with the occurrence or severity of invasive fungal infections in animal as well as human studies.
Association of genetic polymorphisms with increased risk for invasive candidiasis and aspergillosis
It is striking that despite similar clinical risk factors such as chemotherapy, graft source or GVHD and/or similar immunosuppressive conditions, some patients rapidly develop invasive fungal infections, while others seem to be protected and never do so. Such differences may result, at least in part, from the individual genetic makeup that would increase or decrease susceptibility to infection. Based on this hypothesis, many investigators analysed whether single nucleotide polymorphisms (SNPs) in genes involved in immune responses against fungal pathogens influenced susceptibility to infections (table 1 and fig. 1) [81].
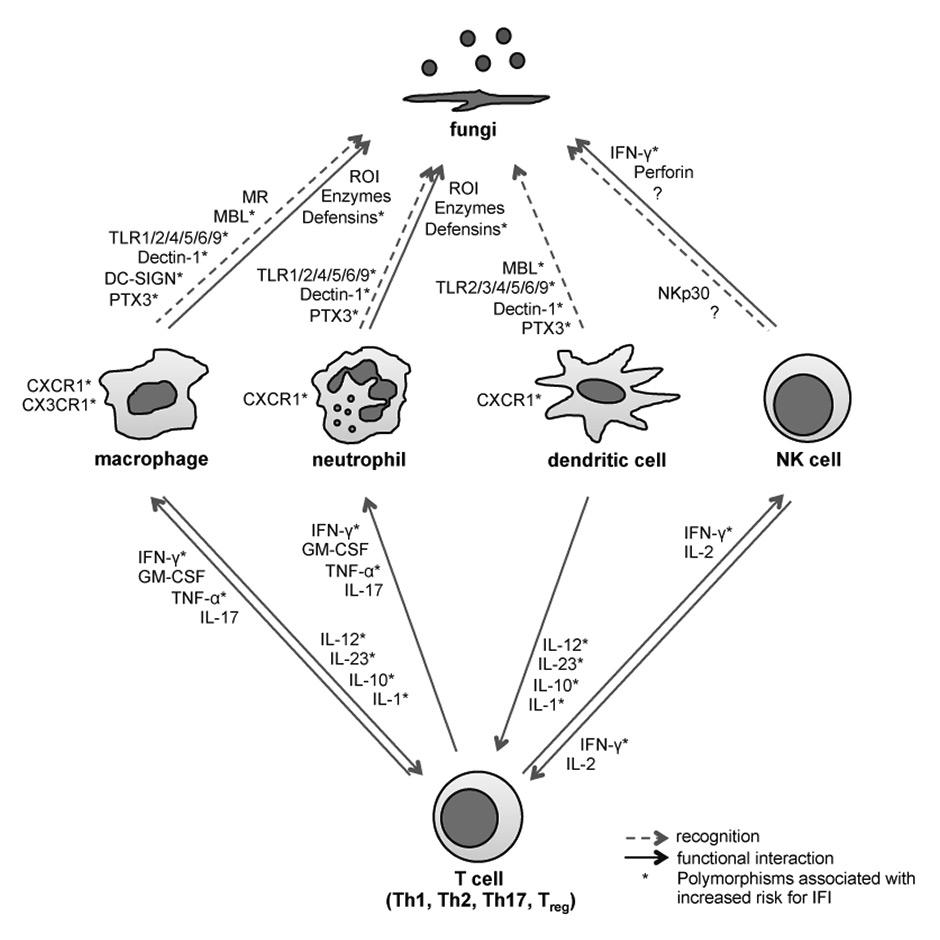
Figure 1
Single nucleotide polymorphisms pathways of innate and adaptive antifungal immunity associated with increased risk for invasive fungal infections.
NK cell = natural killer cell; MR = mannose receptor; MBL = mannose-binding lectin; TLR = Toll-like receptor; PTX3 = pentraxin 3; ROI = reactive oxygen intermediates; IFN = interferon; GM-CSF = granulocyte macrophage colony stimulating factor; TNF = tumour necrosis factor; IL = interleukin ; IFI = invasive fungal infection
Among the most studied candidate genes are those encoding PRRs, as well as those encoding cytokines, chemokines and their receptors and/or antagonists. So far, more than 35 association studies of such polymorphisms with invasive fungal infections have been published [81]. However, many studies were limited by several factors, including small sample size, lack of replication, and/or lack of functional evidence supporting the association. Many studies also had a problematic design, such as cases and controls not exposed to the same risk, inhomogeneous ethnic background, failure to account for relevant non-genetic risk factors or time at risk and/or lack of correction for multiple testing to obtain definite evidence. However, some studies reported relatively robust associations, which were either replicated by several investigators and/or are supported by strong functional evidence.
One of the first studies that included a replication group identified an association between two missense polymorphisms within TLR4 (D299G and T399I) and susceptibility to invasive aspergillosis after HSCT [82]. The relevant polymorphisms were issued from the donor, i.e., affected the engrafted immune cells, but not the recipient, and could be combined with other risk factors such as cytomegalovirus serostatus for pretransplant risk stratification. Both polymorphisms were also associated with disseminated candidiasis in a small cohort of non-neutropenic patients [83]. However, the association of these TLR4 polymorphisms with invasive aspergillosis was not universally confirmed in other HSCT studies [84–86], possibly as a result of differences in the patients’ characteristics, conditioning and/or antifungal prophylactic regimen across different studies or over time. The limited reproducibility could also be due to the very low frequencies of both TLR4 polymorphisms, thereby requiring very large patient numbers for replication.
A further study supported by strong functional evidence was the association of a stop-codon polymorphism in Dectin-1 (Y238X) in both recipient and donor with an increased risk for invasive aspergillosis after HSCT [87]. In vitro studies showed that Dectin-1 silencing in respiratory epithelial cells resulted in impaired Aspergillus-driven proinflammatory responses. The Dectin-1 polymorphism was associated with diminished IFN-γ and IL-10 secretion in peripheral blood mononuclear cells (PBMCs) upon stimulation with this fungus in vitro [87]. In vivo mouse studies further revealed that both donor (haematopoietic cells) and recipient (nonhaematopoietic cells) Dectin-1 was needed to complement a protective role against invasive aspergillosis after HSCT [87]. In addition to its relevance for mould infections, the polymorphism in Dectin-1 was further associated with inherited forms of chronic mucocutaneous candidiasis [88] and oral and gastrointestinal Candida colonisation, but not with invasive candidiasis after HSCT [89].
|
Table 1:Genetic risk factors for invasive fungal infections. |
|
Gene
|
SNPs ID
|
Genetic association1
|
Replication
|
Functional
evidence
|
| IA |
IC |
|
Pattern recognition receptors
|
| TLR1 |
rs574361 |
↑ |
↑ |
Controversial |
+/– |
|
rs4833095, rs5743618 |
↑ |
↓ |
Controversial |
+/– |
| TLR6 |
rs5743810 |
↑ |
NA |
Controversial |
– |
| TLR4 |
rs4986790, rs4986791 |
↑ |
↑ |
Controversial |
+/– |
| TLR2 |
rs5743708 |
NA |
↑ |
Controversial |
+ |
| TLR3 |
rs3775296 |
↑ |
NS |
No |
+ |
| TLR5 |
rs5744168 |
↑ |
NS |
No |
– |
| TLR9 |
rs5743836 |
NA |
NA |
No |
– |
| CLEC7A |
rs16910526 |
↑ |
NA |
Controversial |
+ |
|
rs7309123, rs3901533 |
↑ |
NS |
No |
+ |
| CD209 |
rs4804800, rs11465384, rs7248637, rs7252229 |
↑ |
NS |
No |
– |
| MBL |
Low genotype |
↑ |
↑ |
No |
+/– |
| PTX3 |
rs2305619/rs3816527 |
↑ |
NS |
Yes |
+ |
|
Cytokines and related genes
|
| IL1A |
rs1800587 |
↑ |
NS |
No |
+ |
| IL1B |
rs1143627, rs16944 |
↑ |
NA |
Controversial |
+ |
| IL1RN |
82bp VNTR |
↑ |
NS |
No |
+ |
|
rs419598 |
↑ |
NS |
No |
+ |
| IL4 |
rs2243250, rs2070874, rs2243248 |
NS |
↑ |
No |
– |
| IL8 |
rs2227307 |
↑ |
NS |
No |
– |
| IL10 |
rs1800896, rs1800871, rs1800872 |
↑ |
↑ |
Controversial |
+/– |
| IL12B |
rs41292470 |
NA |
↑ |
No |
+ |
|
rs3212227 |
↓ |
NS |
No |
– |
| IL23R |
rs11209026 |
↓ |
NS |
No |
+/– |
| TNFA |
rs1800629 |
NA |
↑ |
No |
– |
| TNFR1 |
rs4149570 |
↑ |
NS |
No |
+ |
| TNFR2 |
-322 VNTR |
↑ |
NS |
No |
– |
| IFNG |
rs2069705 |
↓ |
NS |
Yes |
+ |
| |
rs2430561 |
NA |
NS |
No |
– |
| CCL8 |
1-kg-17-29697448 |
NS |
↑ |
No |
+ |
| CXCL10 |
rs3921, rs1554013, rs4257674 |
↑ |
NS |
No |
+ |
| CXCR1 |
rs2234671 |
NS |
↑ |
No |
+ |
| CX3CR1 |
rs3732378 |
NS |
↑ |
Yes |
+ |
|
Other
|
| VEGFA |
rs3024994 |
↑ |
NS |
No |
– |
| |
rs2146323, rs6900017 |
↑ |
NS |
No |
– |
| DEFB1 |
rs1800972 |
↑ |
↑ |
No |
– |
| MASP2 |
rs72550870 |
↑ |
NS |
No |
– |
| RAGE |
rs1800624 |
↑ |
NS |
No |
+ |
| S100B |
rs9722 |
↑ |
NS |
No |
+ |
| PLG |
rs4252125 |
↑ |
NS |
No |
+/– |
| STAT1 |
rs16833172 |
NS |
↑ |
No |
+ |
| SP110 |
rs3769845 |
NS |
↑ |
No |
+ |
| PSMB8 |
rs3198005 |
NS |
↑ |
No |
+ |
| CD58 |
rs17035850, rs12025416 |
NS |
↑ |
Controversial |
+ |
| LCE4A/C1orf68 |
rs4845320 |
NS |
↑ |
Controversial |
– |
| TAGAP |
rs3127214 |
NS |
↑ |
Controversial |
+ |
| C1orf68 = chromosome 1 open reading frame 68; CCL8 = chemokine C-C motif ligand 8; CD209 = cluster of differentiation 209 (known as DC-SIGN); CLEC7A = C-type lectin domain 7 (known as Dectin-1); CXCL10 = CXC-chemokine ligand-10; CXCR1 = Chemokine C-X-C Motif Receptor 1; CX3CR1 = Chemokine C-X3-C Motif Receptor 1; DEFB1 = β-defensin 1; IA = invasive aspergillosis; IC = invasive candidiasis; IL = interleukin; IL1RN = interleukin-1 receptor antagonist; IL23R = interleukin 23 receptor; IFNG = interferon gamma; LCE4A = late cornified envelope (LCE) protein 4 A; MASP2 = mannan-binding lectin serine peptidase 2; MBL = mannose banding lectin; PLG = plasminogen; PSMB8 = proteasome (prosome, macropain) subunit beta type 8; PTX3 = pentraxin 3; RAGE = advanced glycosylation end product-specific receptor; SNP = single nucleotide polymorphism; S100B = S100 calcium binding protein B; SP110 = speckled 110 KDa; STAT1 = signal transducer and activator of transcription 1; TAGAP = T cell activation RhoGTPase-activating protein; TLR = toll-like receptor; TNFA = tumor necrosis factor alpha; TNFR = tumour necrosis factor receptor; VAGFA = vascular endothelial growth factor A
1 Effect of minor allele on susceptibility to either invasive aspergillosis (IA) or invasive candidiasis (IC); the arrow symbol “↑” refers to variants that were associated with increased susceptibility; the arrow symbol “↓ “ refers to variants that showed protective effect; “NA” refers to variants that were studied but were not associated; “NS” refers to variants that were not studied. |
Polymorphisms and/or haplotypic combinations of three IL-1 cluster genes including IL-1β (-31T/C and -511C/T), natural IL-1 receptor antagonist (IL1RN; 2018T/C, VNTR2) as well as IL-1α (-889C/T) were associated with invasive aspergillosis in solid organ transplant recipients [90] and acute leukaemia patients [91]. IL-1β is a key proinflammatory cytokine, involved in promoting both innate and adaptive responses during infection with A. fumigatus, and its action can be controlled by its natural antagonist IL1RN. PBMCs from individuals carrying one or two copies of the -511C/T polymorphism in the promoter of IL-1β or the 2018T/C polymorphism in IL1RN showed decreased production of IL-1β and TNF-α upon stimulation with Aspergillus[90].
Moreover, polymorphisms in the chemokine receptors CXCR1 (CXCR1-T276) and CX3CR1 (CX3CR1-M280) were associated with an increased risk for disseminated candidiasis [92, 93]. These factors regulate neutrophil- and macrophage-mediated innate defence against fungal infections as discussed above.
The most promising results in the field of fungal immunogenetics have been provided by recent studies uncovering two frequent polymorphisms (281A/G and 734A/C) in the long PTX3 gene as susceptibility markers of invasive aspergillosis in two different populations: HSCT [94] and solid organ transplant [95] recipients. The associations with invasive aspergillosis resulted from the presence of the risk allele in the donor for HSCT and in the recipient for solid organ transplants, which is consistent with the source of circulating immune cells in both situations. These data were strongly supported by functional work [94] and confirm the protective role of PTX3 observed in animal studies [34, 37, 38, 96]. The h2/h2 haplotype (composed of 281G and 734A alleles) was associated with lower expression of PTX3 by neutrophil precursors in vitro upon exposure to Aspergillus, most likely owing to changes in messenger RNA (mRNA) folding [94]. Lower PTX3 levels were consistently observed in bronchoalveolar lavage and lung biopsies from patients with invasive aspergillosis carrying the h2/h2 haplotype, compared with controls. PTX3 is released by circulating immune cells such as neutrophils upon infection with Aspergillus to promote fungal recognition and killing. Neutrophils from patients carrying the D48A PTX3 polymorphism had reduced ability to phagocytose and kill Aspergillus conidia as compared with other neutrophils [94].
In summary, diverse genetic studies linking the presence of polymorphisms with susceptibility to invasive fungal infections have considerably contributed to our understanding of antifungal immune mechanisms and might help to promote precision medicine for invasive fungal infections.
How immunology and host genetics could help to identify and improve the treatment of patients at risk for invasive fungal infections
Basic research over recent years has provided important insights into the mechanisms of protection against invasive Candida and Aspergillus infections. These studies demonstrated the contribution of various immune cells including neutrophils, macrophages, dendritic cells, NK cells and T cells to antifungal immunity, and partly revealed the molecular mechanisms of protection. Genetic studies demonstrated how genetic polymorphisms, especially in signalling pathways of innate immune cells, can predispose to the development of invasive fungal infections.

Figure 2
Possible risk assessment and personalised therapy scheme for patients at risk for invasive fungal infections.
*Clinical risk factors include graft-versus-host disease, steroid treatment, T cell-depleted graft and acute myeloid leukemia.
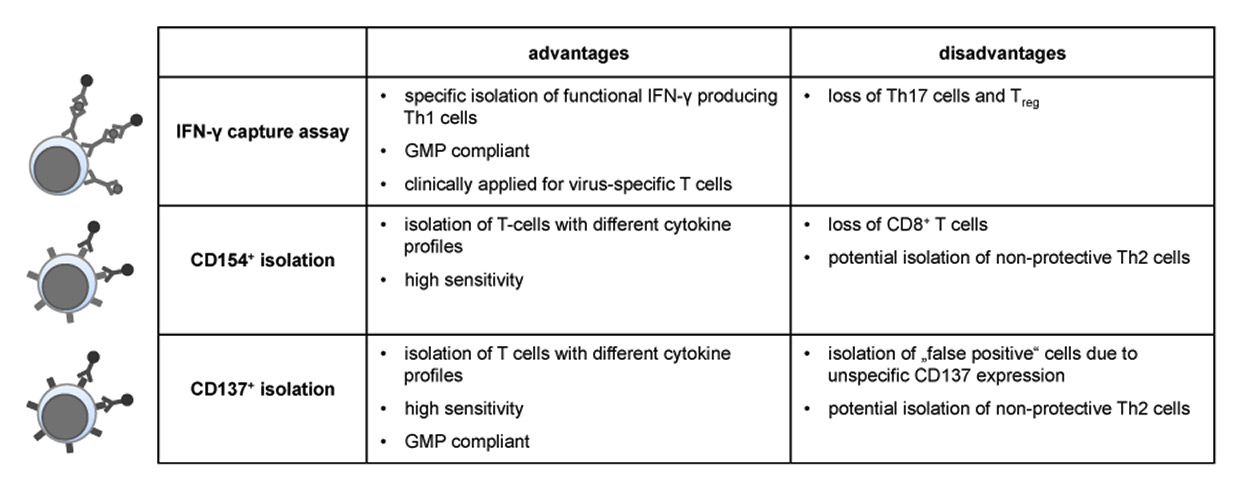
Figure 3
Advantages and disadvantages of different enrichment strategies for antigen-specific T cells.
GMP = Good Manufacturing Practice; IFN = interferon
Techniques such as high throughput SNP genotyping (e.g., large arrays of selected SNPs) or genome-wide association studies (GWAS) have become more easily accessible in the clinical setting. Therefore, the chance to assign each patient an individual risk score based on the genetic background of the donor and/or the recipient is coming within reach. This knowledge might lead to personalised prophylaxis and treatment schemes comparable to the approach used in modern oncology and would counter overtreatment with antifungal therapy (fig. 2).
Early immunotherapeutic approaches such as granulocyte infusion [97] or administration of GM-CSF or IFN-γ tried to restore the deficient number and function of innate immune cells in patients with invasive fungal infections. Although these therapies were promising in patients with chronic granulomatous disease, they were used only reluctantly in transplant recipients becaue of their limited clinical efficacy and the potential to induce GVHD or graft loss [98, 99].
Another approach improving antifungal immunity might be administration of the soluble PRR PTX3 alone or in combination with antifungal drugs, especially in patients with the PTX3 h2/h2 haplotype. The protective effect of PTX3 alone or together with amphotericin B was shown in a murine model of invasive aspergillosis [34]. PTX3-mediated protection was associated with accelerated recovery of lung phagocytic cells and Th1 lymphocytes, and a concomitant decrease of inflammatory pathology. PTX3 administration also potentiated the therapeutic efficacy of suboptimal doses of amphotericin B. These encouraging results could be reproduced in rats treated with PTX3 alone or in combination with posaconazole or voriconazole [37, 38, 96].
Similar to the protective effect of PTX3, administration of MBL might be another therapeutic option. In mice with invasive aspergillosis, MBL administration significantly increased survival and production of the proinflammatory cytokines TNF-α and IL-1α [36], and human studies showed significantly lower serum MBL levels in patients with invasive aspergillosis than in control patients [100].
|
Table 2: Immunogenic A. fumigatus proteins in mice, healthy individuals and haematopoietic stem cell transplant recipients with invasive aspergillosis. |
|
Antigen
|
Localisation
|
Study design
|
Responding T cells
|
Cytokine profile
|
Additional findings
|
Ref
|
| Crf1 (Asp f9/16) |
GPI-anchored cell wall protein |
Rechallenge of vaccinated mice (CTX-treated or BMT) with A. fumigatus
|
CD4 |
IFN-γ, IL-10, (IL-17) |
Protective; cross-protective against C. albicans
|
[111, 120] |
| Characterisation of PBMC, T cell lines or T cell clones from healthy donors |
CD4, (CD8) |
IFN-γ,
(TNF‑α,
GM-CSF,
IL-10, IL-17, IL-4) |
(Cross-reactive to C. albicans
in vitro) |
[79, 111, 115, 120–125] |
| Characterisation of PBMC or T cell clones from HSCT recipients with IA |
(CD4) |
(IFN-γ,
IL-10, IL-17) |
Patients with better IFN-γ response show favourable outcome; specific T cells appear at regression of IA lesion |
[79, 107, 123] |
| Gel1 |
GPI-anchored cell wall protein |
Rechallenge of vaccinated mice (CTX-treated or BMT) with A. fumigatus
|
CD4 |
IFN-γ, IL-10, (IL-17) |
Protective; increased survival |
[120] |
| Characterisation of PBMC or T cell clones from healthy donors |
CD4, (CD8) |
(IFN-γ,
TNF-α, IL-10, IL-17) |
|
[79, 120, 121, 123] |
| Characterisation of PBMC from HSCT recipients with IA |
n.d. |
(IFN-γ,
IL-10) |
Patients with better IFN-γ response show favourable outcome |
[79, 123] |
| Pmp20 (Asp f3) |
Peroxisomal protein |
Rechallenge of vaccinated WT, cortisone acetate-immunosuppressed or PMN-depleted mice with A. fumigatus or adoptive transfer of CD4 T cells from vaccinated mice to naive mice |
CD4 |
|
Protective in WT mice, partly protective in immunosuppressed and PMN-depleted mice |
[126, 127] |
| Characterisation of PBMC from healthy donors |
CD4, CD8 |
IFN-γ, IL-4, IL-17,
(TNF-α,
IL-10) |
|
[79, 121, 122] |
| Characterisation of PBMC from HSCT recipients with IA |
n.d. |
(IFN-γ) |
Higher response in patients with favourable outcome |
[79] |
| Pep1 |
Secreted protein |
Rechallenge of vaccinated mice (CTX-treated or BMT) with A. fumigatus
|
CD4 |
IFN-γ, IL-10, (IL-17) |
Protective |
[120] |
| Characterisation of PBMC or T cell clones from healthy donors |
CD4, (CD8) |
IL-17 (IFN-γ, IL-10) |
|
[120, 123] |
| Characterisation of PBMC from HSCT recipients with IA |
n.d. |
(IFN-γ),
IL-10 |
Patients with IFN-γ response show favourable outcome |
[123] |
| Cat1 |
Secreted protein |
Rechallenge of vaccinated mice with A. fumigatus
|
CD4, Th2 |
IL-4 |
Non-protective |
[120] |
| Characterisation of PBMC or T cell clones from healthy donors |
CD4 |
IFN-γ, (IL-4, IL-10, IL-17) |
|
[115, 120, 128] |
| Characterisation of T cell clones from HSCT recipients with IA |
CD4 |
IFN-γ
(IL-17) |
Specific T cells appear at regression of IA lesion |
[107] |
| Sod |
Secreted protein |
Rechallenge of vaccinated mice with A. fumigatus
|
CD4, Th2 |
IL-4 |
Nonprotective |
[120] |
| Characterisation of PBMC or T cell clones from healthy donors |
CD4 (CD8) |
IL-17, IL-10, (IFN-γ,
TNF-α, IL-4) |
|
[120, 121, 123] |
| Characterisation of PBMC from HSCT recipients with IA |
n.d. |
(IFN-γ),
IL-10 |
Patients with IFN-γ response show favourable outcome |
[123] |
| Crf1 = extracellular cell wall glucanase Crf1; Gel1 = 1 =3-β-glucanosyl-transferase Gel1; Pmp20 = cytosolic peroxisomal peroxiredoxin Pmp20 (Asp f3); Pep1 = aspartic protease Pep1; Cat1 = mycelial Catalase 1; Sod = superoxide dismutase; GPI = glycosylphosphatidylinositol; CTX = cyclophosphamide; BMT = bone marrow transplanted; HSCT = hematopoietic stem cell transplantation; IA = invasive Aspergillosis; IFN = interferon; IL = interleukin; n.d. = not determined; PBMC = peripheral blood mononuclear cells; PMN = polymorphonuclear cells; TNF = tumor necrosis factor; WT = wild type
Data in brackets apply only to a part of the studies. |
The identification of NK cells and T cells as important players in antifungal immunity also encourages cellular therapies such as adoptive transfer of NK cells or antigen-specific T cells. Many studies have assessed the potency of NK cell transfer in antitumour therapy [101, 102], but there is still limited knowledge of this approach in the context of infectious diseases. However, the observation that HSCT and solid organ transplant recipients with invasive fungal infections have lower NK cell counts compared with control patients, endorses adoptive NK cell transfer.
Adoptive transfer of donor-derived pathogen-specific T cells is to date the most promising and feasible immunotherapeutic strategy in transplant recipients restoring the lacking T cell function [103–105]. So far, only one study targeting fungal infections has been performed, in haploidentical HSCT recipients with invasive aspergillosis. In this study, infusion of donor-derived Aspergillus-specific Th1 clones generated by stimulation with inactivated A. fumigatus conidia controlled Aspergillus galactomannan and helped to clear invasive aspergillosis in 9 of 10 patients [106]. However, reproducibility is difficult due to variation of stimuli and the elaborate production under good manufacturing practice (GMP). Recent research has therefore focused on identification of suitable recombinant antigens for fungus-specific cells and on a simple, reproducible and reliable isolation or culture method to generate fungus-specific T cells.
The identification of fungal antigens is challenging and until now, only few immunogenic proteins and T cell epitopes specific for A. fumigatus have been characterised in healthy individuals and mice (table 2). Only recently, we and others have shown that T cell responses to some of these proteins correlate with a beneficial outcome in patients with invasive aspergillosis [107, 108]. Furthermore, it would be favourable if the transferred T cells would target various clinically relevant moulds [3, 109, 110]. We have previously shown that CD4+ cells specific for the A. fumigatus Crf1/p41 epitope confer cross-reactivity to C. albicansin a mouse model, thereby targeting the two most important fungal pathogens in HSCT recipients [111]. In a further study, we showed that T cell lines specific for A. fumigatus Crf1, Gel1 and Pmp20 proteins not only efficiently recognised naturally processed A. fumigatus, but additionally cross-reacted with different clinically relevant Aspergillus and Mucoralesspecies, suggesting that adoptively transferred T cells could very likely protect the recipients against a variety of fungal infections [79].
Various isolation and expansion methods for fungus-specific T cells have been assessedin vitro (fig. 3, reviewed in [112]). The cytokine capture assay is GMP-compliant and has been shown to be efficacious and safe for the isolation of virus-specific T cells [113]. This method has, however, limited sensitivity when fungus-specific peptide pools are used (unpublished data N.K.). Therefore other selection methods based on activation-dependent expression of CD154 or CD137 have been investigated [79, 107, 114, 115]. Although all isolation strategies were able to enrich antigen-specific T cells from PBMC, the relatively low specificity and cell number after isolation probably hinders direct infusion and additional in vitro expansion would be required [116–118].
A thorough understanding of antifungal immune pathways could further lead to novel treatment approaches such as the development of bioengineered T cells with antifungal activity. This was exemplified by Cooper and colleagues who showed that T cells expressing a chimeric antigen receptor based on the PRR Dectin-1 were able to inhibit hyphal growth of Aspergillusboth in vitro and in vivo [119] and thereby provided an interesting alternative to conventional T cell therapy.
Future challenges in invasive fungal infections
The identification of patients who are at increased risk for development of these infections, the lack of biomarkers to define the net state of immunosuppression and the problem of treating invasive fungal infections are remaining difficulties. It is debatable which patients would benefit the most from antifungal prophylaxis and who should be treated with combination therapies or even with immunotherapy. Ideally, these clinical decisions should be individualised based on clinical factors, genetic polymorphisms and immune function, which could be integrated into complex diagnostic algorithms or risk-stratification scores for personalised therapy. Well-designed intervention studies are needed to explore whether this concept can be translated into clinical practice, but previous experience in the field of oncology and the great progress of precision medicine raise confidence that the outcome of invasive fungal infections will be improved in the future.
References
1 Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv113.
2 Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
3 Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–100.
4 Marr KA. Fungal infections in hematopoietic stem cell transplant recipients. Med Mycol. 2008;46:293–302.
5 Pagano L, Caira M, Nosari A, Van Lint MT, Candoni A, Offidani M, et al. Fungal infections in recipients of hematopoietic stem cell transplants: results of the SEIFEM B-2004 study-Sorveglianza Epidemiologica Infezioni Fungine Nelle Emopatie Maligne. Clin Infect Dis. 2007;45:1161–70.
6 Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, et al. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore). 2000;79:250–60.
7 Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, et al. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46–52.
8 Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–75.
9 Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Ame S, Fohrer C, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008;47:1176–84.
10 Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–40.
11 Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infec.t 2012;65:453–64.
12 Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–51.
13 Trifilio SM, Bennett CL, Yarnold PR, McKoy JM, Parada J, Mehta J, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007;39:425–9.
14 Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–47.
15 Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Baden LR, et al. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–8.
16 Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42:4419–31.
17 Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997–2006. Emerg Infect Dis. 2009;15:1395–401.
18 Kontoyiannis DP, Lionakis MS, Lewis RE, Chamilos G, Healy M, Perego C, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–60.
19 Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Piccardi M, et al. Mucormycosis in hematologic patients. Haematologica. 2004;89:207–14.
20 Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, et al. Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother. 2015;70:1522–6.
21 de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–9.
22 Bahcall O. Precision medicine. Nature. 2015;526:335.
23 Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015,372:793–5.
24 Oliveira-Coelho A, Rodrigues F, Campos A, Jr., Lacerda JF, Carvalho A, Cunha C. Paving the way for predictive diagnostics and personalized treatment of invasive aspergillosis. Front Microbiol. 2015;6:411.
25 Lionakis MS. New insights into innate immune control of systemic candidiasis. Med Mycol. 2014;52:555–64.
26 Romani L. Immunity to fungal infections. Nat Rev Immunol. 2004;4:1–23.
27 Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21.
28 Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–64.
29 Plato A, Hardison SE, Brown GD. Pattern recognition receptors in antifungal immunity. Semin Immunopathol. 2015;37:97–106.
30 Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat Immunol. 2012;13:817–22.
31 Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–88.
32 Netea MG, Gow NA, Joosten LA, Verschueren I, van der Meer JW, Kullberg BJ. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med Mycol. 2010;48:897-903.
33 Marakalala MJ, Vautier S, Potrykus J, Walker LA, Shepardson KM, Hopke A, et al. Differential adaptation of Candida albicans in vivo modulates immune recognition by dectin-1. PLoS Pathog. 2013;9:e1003315.
34 Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, et al. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother. 2004;48:4414–21.
35 Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, et al. Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus. Infect Immun. 2012;80:410–17.
36 Kaur S, Gupta VK, Thiel S, Sarma PU, Madan T. Protective role of mannan-binding lectin in a murine model of invasive pulmonary aspergillosis. Clin Exp Immunol. 2007;148:382–9.
37 Lo Giudice P, Campo S, Verdoliva A, Rivieccio V, Borsini F, De Santis R, et al. Efficacy of PTX3 in a rat model of invasive aspergillosis. Antimicrob Agents Chemother. 2010;54:4513–5.
38 Marra E, Sousa VL, Gaziano R, Pacello ML, Arseni B, Aurisicchio L, et al. Efficacy of PTX3 and posaconazole combination in a rat model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2014;58:6284–6.
39 Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–46.
40 Feldmesser M. Role of neutrophils in invasive aspergillosis. Infect Immun. 2006;74:6514–6.
41 Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–25.
42 Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–22.
43 Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5:e1000639.
44 Taylor PR, Roy S, Leal SM, Jr., Sun Y, Howell SJ, Cobb BA, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2013;15:143–51.
45 Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124:710–9.
46 Wozniok I, Hornbach A, Schmitt C, Frosch M, Einsele H, Hube B, et al. Induction of ERK-kinase signalling triggers morphotype-specific killing of Candida albicans filaments by human neutrophils. Cell Microbiol. 2008;10:807–20.
47 Ermert D, Niemiec MJ, Rohm M, Glenthoj A, Borregaard N, Urban CF. Candida albicans escapes from mouse neutrophils. J Leukoc Biol. 2013;94:223–36.
48 Lionakis MS, Netea MG. Candida and host determinants of susceptibility to invasive candidiasis. PLoS Pathog. 2013;9:e1003079.
49 Ngo LY, Kasahara S, Kumasaka DK, Knoblaugh SE, Jhingran A, Hohl TM. Inflammatory monocytes mediate early and organ-specific innate defense during systemic candidiasis. J Infect Dis. 2014;209:109–19.
50 Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS Pathog. 2014;10:e1003940.
51 Break TJ, Jaeger M, Solis NV, Filler SG, Rodriguez CA, Lim JK, et al. CX3CR1 is dispensable for control of mucosal Candida albicans infections in mice and humans. Infect Immun. 2015;83:958-965.
52 Park SJ, Burdick MD, Brix WK, Stoler MH, Askew DS, Strieter RM, et al. Neutropenia enhances lung dendritic cell recruitment in response to Aspergillus via a cytokine-to-chemokine amplification loop. J Immunol. 2010;185:6190–7.
53 Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112:1862–70.
54 Bar E, Whitney PG, Moor K, Reis e Sousa C, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014;40:117–27.
55 Benedetto N, Sabatini P, Sellitto C, Romano Carratelli C. Interleukin-2 and increased natural killer activity in mice experimentally infected with Aspergillus niger. Microbiologica. 1988;11:339–45.
56 Park SJ, Hughes MA, Burdick M, Strieter RM, Mehrad B. Early NK cell-derived IFN-{gamma} is essential to host defense in neutropenic invasive aspergillosis. J Immunol. 2009;182:4306–12.
57 Stuehler C, Kuenzli E, Jaeger VK, Baettig V, Ferracin F, Rajacic Z, et al. Immune Reconstitution After Allogeneic Hematopoietic Stem Cell Transplantation and Association With Occurrence and Outcome of Invasive Aspergillosis. J Infect Dis. 2015;212:959–67.
58 Schmidt S, Tramsen L, Hanisch M, Latge JP, Huenecke S, Koehl U, et al. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis. 2011;203:430–5.
59 Bouzani M, Ok M, McCormick A, Ebel F, Kurzai O, Morton CO, et al. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-gamma release. J Immunol. 2011;187:1369–76.
60 Lünemann A, Lunemann JD, Munz C. Regulatory NK. Cell Functions in Inflammation and Autoimmunity. Mol Med. 2009.
61 Bhatnagar N, Hong HS, Krishnaswamy JK, Haghikia A, Behrens GM, Schmidt RE, et al. Cytokine-activated NK cells inhibit PMN apoptosis and preserve their functional capacity. Blood. 2010;116:1308–16.
62 Costantini C, Micheletti A, Calzetti F, Perbellini O, Pizzolo G, Cassatella MA. Neutrophil activation and survival are modulated by interaction with NK cells. Int Immunol. 2010;22:827–38.
63 Voigt J, Hunniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, et al. Human natural killer cells acting as phagocytes against Candida albicans and mounting an inflammatory response that modulates neutrophil antifungal activity. J Infect Dis. 2014;209:616–26.
64 Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, et al. The NK receptor NKp30 mediates direct fungal recognition and killing and is diminished in NK cells from HIV-infected patients. Cell Host Microbe. 2013;14:387–97.
65 Michel T, Hentges F, Zimmer J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front Immunol. 2012;3:403.
66 Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–91.
67 Whitney PG, Bar E, Osorio F, Rogers NC, Schraml BU, Deddouche S, et al. Syk signaling in dendritic cells orchestrates innate resistance to systemic fungal infection. PLoS Pathog. 2014,10:e1004276.
68 Lünemann A, Vanoaica LD, Azzi T, Nadal D, Munz C. A distinct subpopulation of human NK cells restricts B cell transformation by EBV. Journal of Immunology. 2013;191:4989–95.
69 Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–81.
70 LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–8.
71 Rivera A, Ro G, Van Epps HL, Simpson T, Leiner I, Sant'Angelo DB, et al. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006,25:665–75.
72 Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-gamma or IL-10 and are regulated by IL-1beta. Nature. 2012;484:514–18.
73 Marukutira T, Huprikar S, Azie N, Quan SP, Meier-Kriesche HU, Horn DL. Clinical characteristics and outcomes in 303 HIV-infected patients with invasive fungal infections: data from the Prospective Antifungal Therapy Alliance registry, a multicenter, observational study. HIV AIDS (Auckl). 2014;6:39–47.
74 Nagai H, Guo J, Choi H, Kurup V. Interferon-gamma and tumor necrosis factor-alpha protect mice from invasive aspergillosis. J Infect Dis. 1995;172:1554–60.
75 Roilides E, Dimitriadou-Georgiadou A, Sein T, Kadiltsoglou I, Walsh TJ. Tumor necrosis factor alpha enhances antifungal activities of polymorphonuclear and mononuclear phagocytes against Aspergillus fumigatus. Infect Immun. 1998;66:5999–6003.
76 Sparber F, LeibundGut-Landmann S. Interleukin 17-Mediated Host Defense against Candida albicans. Pathogens. 2015;4:606–19.
77 Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521-525.
78 Carvalho A, De Luca A, Bozza S, Cunha C, D'Angelo C, Moretti S, et al. TLR3 essentially promotes protective class I-restricted memory CD8(+) T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood. 2012;119:967–77.
79 Stuehler C, Nowakowska J, Bernardini C, Topp MS, Battegay M, Passweg J, et al. Multispecific Aspergillus T cells selected by CD137 or CD154 induce protective immune responses against the most relevant mold infections. J Infect Dis. 2015;211:1251–61.
80 Stuehler C, Bernardini C, Elzi L, Stoeckle M, Zimmerli S, Furrer H, et al. Immune recovery in HIV-infected patients after candida esophagitis is impaired despite long-term antiretroviral therapy. AIDS. 2016;30(12):1923–33.
81 Wojtowicz A, Bochud PY. Host genetics of invasive Aspergillus and Candida infections. Semin Immunopathol. 2015;37:173–86.
82 Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008;359:1766–77.
83 Van der Graaf CA, Netea MG, Morre SA, Den Heijer M, Verweij PE, Van der Meer JW, et al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur Cytokine Netw. 2006;17:29–34.
84 Richardson ED, Malloy PF, Grace J. Othello syndrome secondary to right cerebrovascular infarction. J Geriatr Psychiatry Neurol. 1991;4:160–5.
85 Kesh S, Mensah NY, Peterlongo P, Jaffe D, Hsu K, van den Brink MB, et al. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann N Y Acad Sci. 2005;1062:95–103.
86 Koldehoff M, Beelen DW, Elmaagacli AH. Increased susceptibility for aspergillosis and post-transplant immune deficiency in patients with gene variants of TLR4 after stem cell transplantation. Transpl Infect Dis. 2013;15:533–9.
87 Cunha C, Di Ianni M, Bozza S, Giovannini G, Zagarella S, Zelante T, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116:5394–402.
88 Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7.
89 Plantinga TS, van der Velden WJ, Ferwerda B, van Spriel AB, Adema G, Feuth T, et al. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32.
90 Wojtowicz A, Gresnigt MS, Lecompte T, Bibert S, Manuel O, Joosten LA, et al. IL1B and DEFB1 Polymorphisms Increase Susceptibility to Invasive Mold Infection After Solid-Organ Transplantation. J Infect Dis. 2015;211:1646–57.
91 Sainz J, Perez E, Gomez-Lopera S, Jurado M. IL1 gene cluster polymorphisms and its haplotypes may predict the risk to develop invasive pulmonary aspergillosis and modulate C-reactive protein level. J Clin Immunol. 2008;28:473–85.
92 Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, et al. CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest. 2013,123:5035–51.
93 Swamydas M, Gao JL, Break TJ, Johnson MD, Jaeger M, Rodriguez CA, et al. CXCR1-mediated neutrophil degranulation and fungal killing promote Candida clearance and host survival. Sci Transl Med. 2016;8:322ra310.
94 Cunha C, Aversa F, Lacerda JF, Busca A, Kurzai O, Grube M, et al. Genetic PTX3 deficiency and aspergillosis in stem-cell transplantation. N Engl J Med. 2014;370:421–32.
95 Wojtowicz A, Lecompte TD, Bibert S, Manuel O, Rueger S, Berger C, et al. PTX3 Polymorphisms and Invasive Mold Infections After Solid Organ Transplant. Clin Infect Dis. 2015;61:619–22.
96 Lo Giudice P, Campo S, De Santis R, Salvatori G. Effect of PTX3 and voriconazole combination in a rat model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2012;56:6400–2.
97 Estcourt LJ, Stanworth S, Doree C, Blanco P, Hopewell S, Trivella M, et al. Granulocyte transfusions for preventing infections in people with neutropenia or neutrophil dysfunction. Cochrane Database Syst Rev. 2015;CD005341.
98 Armstrong-James D, Harrison TS. Immunotherapy for fungal infections. Curr Opin Microbiol. 2012;15:434–9.
99 Wan L, Zhang Y, Lai Y, Jiang M, Song Y, Zhou J, et al. Effect of Granulocyte-Macrophage Colony-Stimulating Factor on Prevention and Treatment of Invasive Fungal Disease in Recipients of Allogeneic Stem-Cell Transplantation: A Prospective Multicenter Randomized Phase IV Trial. J Clin Oncol. 2015,33:3999–4006.
100 Lambourne J, Agranoff D, Herbrecht R, Troke PF, Buchbinder A, Willis F, et al. Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2009;49:1486–491.
101 Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Semin Immunol. 2014;26:161–72.
102 Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk acute leukaemia. Semin Immunol. 2014;26:173–9.
103 Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011,52:49–57.
104 Papadopoulou A, Gerdemann U, Katari UL, Tzannou I, Liu H, Martinez C, et al. Activity of Broad-Spectrum T Cells as Treatment for AdV, EBV, CMV, BKV, and HHV6 Infections after HSCT. Sci Transl Med. 2014;6:242ra283.
105 Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360:436–42.
106 Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106:4397–406.
107 Jolink H, Hagedoorn RS, Lagendijk EL, Drijfhout JW, van Dissel JT, Falkenburg JH, et al. Induction of A. fumigatus-specific CD4-positive T-cells in patients recovering from invasive aspergillosis. Haematologica. 2014;99(7):1255–63.
108 Potenza L, Vallerini D, Barozzi P, Riva G, Forghieri F, Beauvais A, et al. Characterization of specific immune responses to different Aspergillus antigens during the course of invasive Aspergillosis in hematologic patients. PLoS One. 2013;8:e74326.
109 Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53.
110 Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859–67.
111 Stuehler C, Khanna N, Bozza S, Zelante T, Moretti S, Kruhm M, et al. Cross-protective TH1 immunity against Aspergillus fumigatus and Candida albicans. Blood. 2011;117:5881–91.
112 Deo SS, Gottlieb DJ. Adoptive T-cell therapy for fungal infections in haematology patients. Clin Transl Immunology. 2015;4:e40.
113 Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76.
114 Khanna N, Stuehler C, Conrad B, Lurati S, Krappmann S, Einsele H, et al. Generation of a multipathogen-specific T-cell product for adoptive immunotherapy based on activation-dependent expression of CD154. Blood. 2011;118:1121–31.
115 Jolink H, Meijssen IC, Hagedoorn RS, Arentshorst M, Drijfhout JW, Mulder A, et al. Characterization of the T-cell-mediated immune response against the Aspergillus fumigatus proteins Crf1 and catalase 1 in healthy individuals. J Infect Dis. 2013;208:847–56.
116 Tramsen L, Koehl U, Tonn T, Latge JP, Schuster FR, Borkhardt A, et al. Clinical-scale generation of human anti-Aspergillus T cells for adoptive immunotherapy. Bone Marrow Transplant. 2009;43:13–9.
117 Tramsen L, Schmidt S, Boenig H, Latge JP, Lass-Florl C, Roeger F, et al. Clinical-scale generation of multi-specific anti-fungal T cells targeting Candida, Aspergillus and mucormycetes. Cytotherapy. 2013;15:344–51.
118 Beck O, Topp MS, Koehl U, Roilides E, Simitsopoulou M, Hanisch M, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107:2562–9.
119 Kumaresan PR, Manuri PR, Albert ND, Maiti S, Singh H, Mi T, et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc Natl Acad Sci U S A. 2014;111:10660–5.
120 Bozza S, Clavaud C, Giovannini G, Fontaine T, Beauvais A, Sarfati J, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol. 2009;183:2407–14.
121 Bacher P, Kniemeyer O, Teutschbein J, Thon M, Vodisch M, Wartenberg D, et al. Identification of Immunogenic Antigens from Aspergillus fumigatus by Direct Multiparameter Characterization of Specific Conventional and Regulatory CD4+ T Cells. J Immunol. 2014;193(7):3332–43.
122 Chaudhary N, Staab JF, Marr KA. Healthy human T-Cell Responses to Aspergillus fumigatus antigens. PLoS One. 2010;5:e9036.
123 Potenza L, Vallerini D, Barozzi P, Riva G, Forghieri F, Beauvais A, et al. Characterization of specific immune responses to different Aspergillus antigens during the course of invasive Aspergillosis in hematologic patients. PLoS One. 2013;8:e74326.
124 Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of cytotoxic T cell responses directed to human leucocyte antigen Class I restricted epitopes from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;140:81–91.
125 Ramadan G, Davies B, Kurup VP, Keever-Taylor CA. Generation of Th1 T cell responses directed to a HLA Class II restricted epitope from the Aspergillus f16 allergen. Clin Exp Immunol. 2005;139:257–67.
126 Diaz-Arevalo D, Bagramyan K, Hong TB, Ito JI, Kalkum M. CD4+ T cells mediate the protective effect of the recombinant Asp f3-based anti-aspergillosis vaccine. Infect Immun. 2011;79:2257–66.
127 Diaz-Arevalo D, Ito JI, Kalkum M. Protective Effector Cells of the Recombinant Asp f3 Anti-Aspergillosis Vaccine. Front Microbiol. 2012;3:299.
128 Hebart H, Bollinger C, Fisch P, Sarfati J, Meisner C, Baur M, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–8.


