
Figure 1
Proportion of methicillin-resistant S. aureus (MRSA) among S. aureusisolates by region.
Red = German-speaking; green = French-speaking; yellow = Italian-speaking
DOI: https://doi.org/10.4414/smw.2016.14339
Methicillin-resistant Staphylococcus aureus (MRSA) combines virulence and resistance with remarkable adaptive qualities to the human host and is associated with either carriage or a broad spectrum of infectious conditions causing significant clinical and health-economic adverse outcomes [1]. Initially, MRSA infections were largely a healthcare-associated problem, but over time they have emerged increasingly in the community as well. Currently, global MRSA epidemiology remains heterogeneous, with several Asian countries [2] reporting some of the highest MRSA rates worldwide [3].
Regarding Europe, recent data from the European antimicrobial resistance interactive database (EARS-Net), which covers a wide network of national surveillance systems in Europe, show that, after a general rise in prevalence, MRSA proportions among S. aureus isolates seem to be decreasing in Europe since the beginning of this decade, as observed also in North America [3]. It is, however, a heterogeneous picture as Sweden, Estonia, Austria and Romania still show rising trends in MRSA prevalence (European Centre for Disease Prevention and Control, ECDC 2013). There is a paucity of comparable epidemiological data from Switzerland, which offers a unique perspective for epidemiological analysis of temporal MRSA trends because of its central position in Europe and its three main linguistic regions (German, French and Italian), which have strong cultural and economic ties to neighbouring countries. The last national survey on MRSA epidemiology in Switzerland was conducted in 1997 [4] and showed low rates of MRSA in most hospitals.
The objective of this study was to evaluate the epidemiology and temporal trends of MRSA and methicillin-susceptible S. aureus (MSSA) infections in Switzerland from 2004 to 2014. This analysis of Swiss antimicrobial resistance rates was intended to complement the European picture and demonstrate that important differences may be observed even in different regions within the same country.
This observational laboratory-based study was conducted with use of the database from the Swiss antimicrobial resistance surveillance network (ANRESIS, http://www.anresis.ch ) available since 2004. This database provides antibiotic resistance data for all routinely collected microbiological samples from 20 clinical microbiology laboratories, distributed all over Switzerland and representing at least 70% of annual hospitalisation days and 30% of all Swiss general practitioners [5]. For this study we included data from the 12 laboratories that continuously contributed data from 2004 to 2014. While the number of ambulatory samples increased over time in this subset, for in-patient samples (which were used for calculation of incidence rates) we restricted the analysis to the 36 hospitals linked to these laboratories, which sent continuous data during the whole study period. These hospitals represent at least 40% of annual hospitalisation days in Switzerland. This surveillance data includes data from primary and tertiary care hospitals, outpatient clinics and ambulatory practices.
Antimicrobial susceptibility was tested at local laboratories according to Clinical and Laboratory Standards Institute (CLSI, http://www.clsi.org ) or European Committee on Antimicrobial Susceptibility Testing (EUCAST, http://www.eucast.org ) guidelines. Most of the participating laboratories switched from CLSI to EUCAST breakpoints between 2011 and 2013. All participating laboratories are participating in at least one external quality programme out of the National External Quality Assessment Service (NEQAS, http://www.ukneqasmicro.org.uk/ ) or the Swiss quality control programme from the Institute for Medical Microbiology, University of Zürich (http://www.imm.uzh.ch/services/qc.html). Results from MRSA screening swabs and from duplicates, defined as isolates with identical antimicrobial resistance profiles isolated from the same patient within 1 year, were excluded from the analyses. For calculation of incidence rates for bloodstream infections, we used data from inpatients only, and the duplicate algorithm was restricted to blood cultures. Methicillin resistance was defined as nonsusceptibility to at least one out of oxacillin, flucloxacillin, methicillin or cefoxitin. Isolates not tested against any of these antibiotics were excluded from analysis (n = 795).
The epidemiological data allowed stratification of isolates by sex, age group (<16, 16–65, >65 years), linguistic region (German, French or Italian), in- versus outpatients, and site of isolation (invasive [isolation from usually sterile body sites] versus noninvasive). Bloodstream infections were analysed separately, too.
We used the term non-multidrug resistant MRSA (NmMRSA) as a potential marker for community-associated strains of MRSA (CA-MRSA), because of similarities in epidemiology [6] and phenotypic antimicrobial susceptibility [7]. NmMRSA was defined as MRSA susceptible to at least three of the following agents – ciprofloxacin, clindamycin, tetracycline or trimethoprim-sulfamethoxazole (TMP/SMX) – while remaining resistant to all beta-lactam antibiotics.
We calculated the annual incidence of MRSA, NmMRSA and MSSA bacteraemia and invasive isolates per 100 000 hospital admissions. Assuming that patients with bacteraemia and other invasive isolates are usually hospitalised, we restricted our dataset to inpatients only for this analysis. Univariate logistic regression was performed to determine the association between age, sex, in-/outpatient, region, invasive/noninvasive sample origin, as explanatory variables, and MRSA versus MSSA, and NmMRSA versus mMRSA (multidrug-resistant MRSA), as dependent variables. The explanatory variables were selected on the basis of data availability. We considered at least 10 events for each explanatory variable analysed. Using the explanatory variables from the univariate analysis with a value of p <0.2, we built two multivariate regression models (NmMRSA vs mMRSA; and MRSA vs MSSA). However, there are some potential limitations with this method of selecting explanatory variables because it is known that more complex models tend to give overoptimistic predictions, especially when extensive variable selection has been performed [8].
We conducted several time series analyses of MRSA trends stratified by age group, in- versus outpatient population and linguistic region. The same analysis was performed with the proportion of resistance to selected drugs (such as ciprofloxacin, fusidic acid, clindamycin and erythromycin) among MRSA strains. To perform the time series analyses, we used an autoregressive integrated moving average (ARIMA) model, which is considered a suitable method to monitor and predict the trend of bacterial resistance prevalence when using surveillance data [9]. With the Box-Jenkins method, checks were made to determine whether the time series was stationary by use of the augmented Dickey-Fuller test for unit root [10]. The models were identified by determining the ARIMA model orders (p, q, d) through consideration of the autocorrelation and partial autocorrelation profiles. Finally, we evaluated the adequacy of the models using a Durbin Watson test for the statistical significance of the parameters [11]. In general, the analyses sought to identify the most parsimonious model with the fewest parameters. The time series analyses were performed in Eviews version 18, all other analyses were carried out in STATA 13.
Since the database contains routinely collected anonymised non-genetic surveillance data, ethical consent was not required according to the Swiss law for research on human beings (Art. 33 al. 2 LRH).
The 12 laboratories included in the analysis reported 130 565 samples positive for S. aureus, including 115 917 MSSA and 14 648 MRSA (with 3179 [22%] NmMRSA) isolates, over the period from January 2004 to December 2014. Demographic and clinical characteristics of patients in the different groups are shown in table 1.
| Table 1:Characteristics of patients with MRSA, MSSA, or NmMRSA infections (2004–2014). | |||
| MSSA in % of S. aureus (n = 115 917) | MRSA in % of S. aureus (n = 14 648) | NmMRSA in % of MRSA (n = 3179) | |
| Linguistic region | |||
| German | 69 054 (95.3%) | 3389 (4.7%) | 1349 (39.8%) |
| French | 36 764 (79.9%) | 9279 (20.1%) | 1099 (11.8%) |
| Italian | 10 008 (83.5%) | 1980 (16.5%) | 731 (36.9%) |
| Sex | |||
| Male | 62 019 (88%) | 8649 (12%) | 1680 (19.4%) |
| Female | 53 773 (90%) | 5982 (10%) | 1496 (25%) |
| In- versus outpatient | |||
| Inpatient | 55 830 (84.1%) | 10 604 (15.9%) | 1858 (17.5%) |
| Outpatient | 59 781 (93.9%) | 3897 (6.1%) | 1265 (32.5%) |
| ICU versus other departments | |||
| ICU | 7588 (90.7%) | 776 (9.3%) | 139 (17.9%) |
| Non-ICU | 48 241 (83.1%) | 9828 (16.9%) | 1719 (17.5%) |
| Age group | |||
| <16 years | 22 372 (95.9%) | 971 (4.1%) | 397 (40.9%) |
| 16–65 years | 60 583 (92.4%) | 4984 (7.6%) | 1498 (30%) |
| >65 years | 32 962 (79.1%) | 8693 (20.9%) | 1284 (14.7%) |
| Invasive versus noninvasive | |||
| Invasive | 19 384 (91.6%) | 1775 (8.4%) | 530 (29.9%) |
| Noninvasive | 96 533 (88.3%) | 12 873 (11.7%) | 2649 (20.6%) |
| ICU = intensive care unit; MRSA = methicillin-resistant S. aureus; MSSA = methicillin-susceptible S. aureus; NmMRSA = non-multidrug resistant MRSA In the first column, for each variable (linguistic regions, sex…), the distribution of MSSA in every category is shown (per row). There are some missing data for each variable. Within each row the numbers in the first two columns add up to 100%. The percentages in the third column are a proportion of NmMRSA of all MRSA in each subcategory. | |||
The overall proportion of MRSA among S. aureus isolates decreased from 14% in 2004 to 8% in 2014, with a decreasing trend (–0.08% per quarter, p <0.01). Conversely, the relative proportion of NmMRSA among all MRSA increased from 8 % in 2004 to 43% in 2014 (+0.92% per quarter, p = 0.04).

Figure 1
Proportion of methicillin-resistant S. aureus (MRSA) among S. aureusisolates by region.
Red = German-speaking; green = French-speaking; yellow = Italian-speaking
Differences were found across the linguistic regions. In the French- and Italian-speaking regions, MRSA proportions were decreasing over time (–0.28% and –0.15% per quarter, respectively, p <0.01; fig. 1), while in the German-speaking region MRSA trends were on a slightly upward trajectory (+0.03% per quarter, p <0.01; fig. 1). Nevertheless, MRSA rates remained significantly higher in the French- and Italian-speaking regions throughout the whole time period. Conversely, NmMRSA was increasing in all regions (Italian region, +0.42% per quarter, p <0.01; French region, +0.8% per quarter, p <0.01; German-speaking region, +0.92% per quarter, p <0.01).
MRSA rates were significantly lower in outpatients, but increased from 2008 to 2014, (0.03% per quarter, p <0.01), while MRSA prevalence decreased in inpatients during the same time period (–0.18% per quarter, p <0.01).
For NmMRSA strains, however, an increasing trend was noticed for both in- and outpatients (+1.07% and +0.61% per quarter, respectively, p <0.01).
MRSA was increasing in the younger population (+0.05% per quarter, p <0.01), but decreasing in the adult and elderly groups (–0.30% and – 0.02% per quarter, respectively, p <0.01). However, the proportion of NmMRSA strains in all three age groups increased over time (data not shown).
Males were more likely to be infected by MRSA. This finding was stable over the 11 years of surveillance (table 1).
Over the study period, resistance to ciprofloxacin, clindamycin, gentamicin and erythromycin in MRSA strains decreased (fig. 2). In contrast, resistance to tetracycline, TMP/SMX, fusidic acid and rifampicin remained constant and low over the study period (data not shown). Antibiotic resistance to clindamycin and ciprofloxacin was highest in the French- and Italian-speaking regions. In comparison, in the German speaking-region, resistance to gentamicin and erythromycin was more prevalent (see appendix).

Figure 2
Time series analysis of antibiotic resistance among methicillin-resistant S. aureus (MRSA) isolates. Trends per quarter are given in parentheses.
The proportion of MRSA amongS. aureus decreased for invasive isolates (–0.10% per quarter, p <0.01) and noninvasive isolates (–0.09% per quarter, p <0.01), whereas the proportion for NmMRSA among MRSA increased for invasive (+1.04% per quarter, p <0.01) and noninvasive isolates (+0.83% per quarter, p = 0.02).
Considering invasive isolates only, we found decreasing MRSA proportions in blood cultures from 14% in 2004 to 5% in 2014 for S. aureus and for other invasive infections from 16% to 13%. After extrapolation to the whole Swiss population, the incidence of mMRSA bacteraemia decreased from 21 to 7 per 100 000 admissions, whereas the incidence of other invasive mMRSA infections decreased only from 11 to 10 per 100 000 admissions (fig. 3a). Although we observed a true increase in NmMRSA in invasive infections (from 4 to 14 per 100 000 admissions), incidence of NmMRSA in bloodstream infections remained stable over time (from 4 to 3 per 100 000 admissions, fig. 3b). While the incidence of MSSA bacteraemia decreased until 2013, this was not the case for other invasive MSSA infections.
The results of the univariate regression analyses are reported in table 2A. Location in the French and Italian linguistic regions, inpatient status and elderly age were significant risk factors for MRSA, but not for NmMRSA. Invasive isolates, younger age and outpatient status were identified as risk factors for NmMRSA but not for MRSA in general. Outpatient status was protective against MRSA. Similar results were found in the multivariate regression models (table 2B) using the dependent variables of MRSA versus MSSA and NmMRSA versus mMRSA. The first model confirmed the same risk factors for MRSA (French and Italian linguistic regions and elderly) and the same protective factors (younger age group, invasive isolates and outpatient group); conversely, in the second model young age, outpatient status, invasive isolates and the Italian-speaking region were risk factors for NmMRSA isolation; whereas location in the French-speaking region and being elderly were protective against NmMRSA.
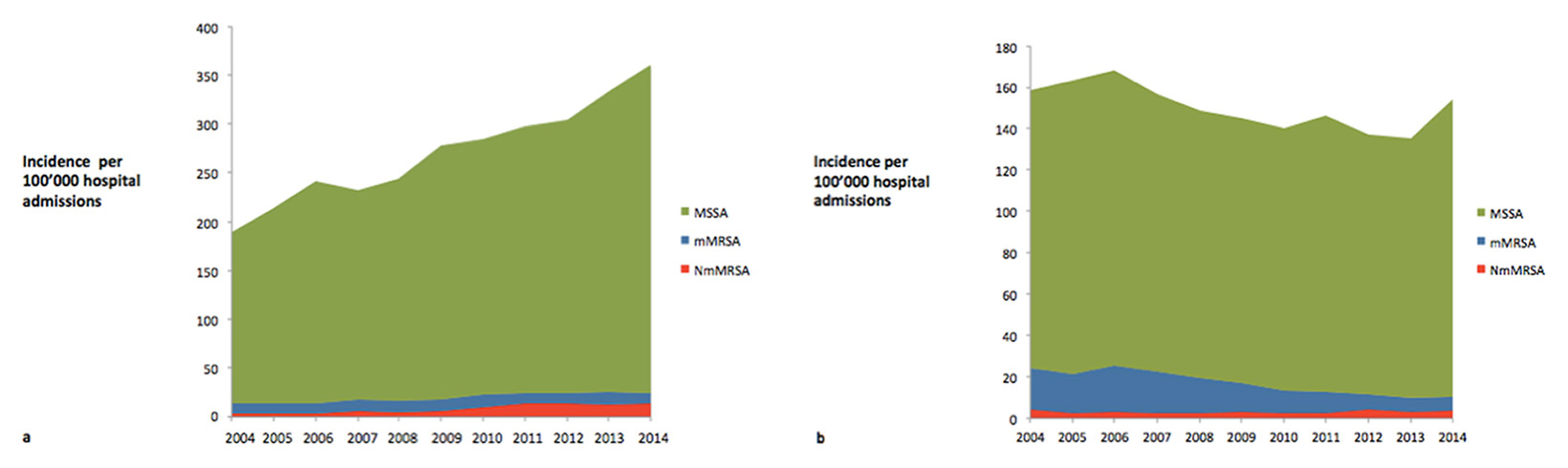
Figure 3
Incidence of methicillin-susceptible S. aureus (MSSA), non-multidrug resistant methicillin-resistant S. aureus (NmMRSA) and multidrug resistant methicillin-resistant S. aureus (mMRSA) per 100 000 hospital admissions (a) in invasive isolates (excluding bacteraemic isolates) and (b) in bacteraemia.
| Table 2A:Risk factors for MRSA (vs MSSA) and NmMRSA (vs mMRSA) by univariable logistic regression analysis. | ||||
| MRSA vs MSSA | NmMRSA vs mMRSA | |||
| Stratification | Odds ratio | 95% CI | Odds ratio | 95% CI |
| Linguistic region | ||||
| German | 1* | 1* | ||
| French | 5.14 | 4.90–5.30 | 0.20 | 0.10–0.20 |
| Italian | 4.03 | 3.70–4.20 | 0.80 | 0.70–0.90 |
| In- versus outpatient | ||||
| Inpatient | 1* | 1* | ||
| Outpatient | 0.34 | 0.33–0.35 | 2.26 | 2.08–2.45 |
| ICU versus other departments | ||||
| Non-ICU | 1* | 1* | ||
| ICU | 0.50 | 0.46–0.54 | 1.02 | 0.85–1.24 |
| Age group | ||||
| <16 years | 0.52 | 0.49–0.56 | 1.60 | 1.39–1.85 |
| 16–65 years | 1* | 1* | ||
| >65 years | 3.20 | 3.08–3.33 | 0.40 | 0.37–0.44 |
| Invasive versus noninvasive | ||||
| Noninvasive | 1* | 1* | ||
| Invasive | 0.68 | 0.65–0.72 | 1.64 | 1.47–1.83 |
| CI = confidence interval; ICU = intensive care unit; mMRSA = multidrug-resistant MRSA; MRSA = methicillin-resistant S. aureus; MSSA = methicillin-susceptible S. aureus; NmMRSA = non-multidrug resistant MRSA * Reference category, adjusted for all other variables listed in the table | ||||
| Table 2B:Results from multivariate logistic regression comparing the risk factors for MRSA versus MSSA and NmMRSA versus mMRSA. | ||||
| MRSA vs MSSA | NmMRSA vs mMRSA | |||
| Stratification | Odds ratio | 95% CI | Odds ratio | 95% CI |
| Linguistic regions | ||||
| German | 1* | 1* | ||
| French | 4.41 | 4.23–4.61 | 0.28 | 0.26–0.32 |
| Italian | 3.19 | 3.00–3.40 | 1.30 | 1.14–1.46 |
| In- versus outpatient | ||||
| Inpatient | 1* | 1* | ||
| Outpatient | 0.38 | 0.37–0.40 | 1.57 | 1.43–1.72 |
| ICU versus other departments | ||||
| ICU | Omitted because of collinearity† | 1.02 | 0.85–1.24 | |
| Age group | ||||
| <16 years | 0.71 | 0.66–0.76 | 1.60 | 1.39–1.85 |
| 16–65 years | 1* | 1* | ||
| >65 years | 2.49 | 2.40–2.59 | 0.40 | 0.37–0.44 |
| Invasive versus noninvasive | ||||
| Noninvasive | 1* | 1* | ||
| Invasive | 0.61 | 0.57–0.64 | 1.25 | 1.40–2.40 |
| CI = confidence interval; ICU = intensive care unit; mMRSA = multidrug-resistant MRSA; MRSA = methicillin-resistant S. aureus; MSSA = methicillin-susceptible S. aureus; NmMRSA = non-multidrug resistant MRSA * Reference category, adjusted for all other variables listed in the table † Collinearity with variable "inpatient" | ||||
This large-scale national resistance surveillance study described the epidemiology of MRSA in Switzerland over 11 years, revealing an overall downward trend across the various patient groups. In particular, the French- and Italian-speaking regions moved from endemic MRSA prevalence to moderate MRSA rates. In contrast to the general MRSA trend, we found a general increase in NmMRSA (as marker of CA-MRSA) during these 11 years, in particular for younger age groups, outpatients and invasive isolates.
Considering the regional distribution of MRSA rates and trends, we noted relevant regional differences with both a north-south and an east-west gradient. The geographical, cultural and economic ties of the Italian- and French-speaking regions of Switzerland to Italy and France (where the prevalences of MRSA as reported by ECDC were 36% and 17%, respectively, ECDC 2013) may in part explain the higher MRSA prevalence in these regions compared with the German-speaking region that borders with countries with lower MRSA rates (Germany, 12.7%; Austria, 9.2% in 2013, ECDC data).
Several lines of argumentation have been tried to explain the different geographic distribution of antibiotic-resistant microorganism between countries and continents. Important determinants are country-specific factors related to antibiotic resistance, like the organisation of the healthcare system with reimbursement structures and incentives, diagnostic practices, laboratory recognition, antibiotic use, and physician and patient attitudes and expectations [12, 13]. In fact, differences in the approaches to control MRSA dissemination amongst countries neighbouring Switzerland help to explain their differences in MRSA endemicity. For example Italy, which delayed the implementation of strict control measures, now is endemic for MRSA. On the other hand, France, which implemented control measures faster, was able to stabilise or even decrease MRSA prevalence in confined geographic areas. Even more striking was the case of central European countries like Germany that managed to maintain MRSA at a low level using multiple approaches such as restricting antibiotic usage, and enforcement of screening and contact precautions [3].
Switzerland had adopted more local than national approaches to control MRSA dissemination. Following the first national MRSA surveillance study in 1997 [4], limited surveillance, elaboration of guidelines and interventions to control MRSA dissemination were conducted at a national level [14]. This has led to marked heterogeneity between regions.
Our finding of a general upward trend of NmMRSA in all of Switzerland is in line with the rapid global dissemination of CA-MRSA with different clonal outbreaks in Europe, the USA and the rest of the world, especially among younger age groups and outpatients [15]. The first imported case of CA-MRSA in Switzerland was reported in 2002 [16], followed by other reports, such as the outbreak of a Panton–Valentine leukocidin (PVL)-positive ST5-IV strain in a neonatal intensive care unit in Geneva [17].
Antibiotic resistance rates in MRSA strains against non-beta-lactam antibiotics such as ciprofloxacin, clindamycin, gentamicin and erythromycin decreased, while resistance rates to other compounds (tetracycline, TMP/SMX, fusidic acid and rifampicin) remained stable. These findings could be explained by a decrease in multiresistant hospital-acquired (HA-MRSA) isolates in regions with previously endemic MRSA incidence. This decrease is potentially linked to an interplay between a loss of biological fitness in endemic HA-MRSA clones [18] and improved infection control measures with increased hand hygiene compliance and other infection control measures [19–21].
A further explanation could be the replacement of HA-MRSA clones with CA-MRSA strains [22], which are characterised by lower rates of resistance and greater fitness by virtue of their smaller SCCmec cassettes. In the absence of molecular characterisation in this study, we used antibiotic resistance phenotypes as correlates, which have been used to distinguish between HA-MRSA and CA-MRSA strains in the past [23]. Thus, dissemination of CA-MRSA strains is likely to be the main reason for the observed increase in the sensitivity of MRSA to some antibiotics, such as gentamicin, as described by De Angelis et al., with the replacement of older strains containing the SCCmec I cassette (a marker of HA-MRSA strains) by newer strains with the SCCmec IV cassette (a marker for CA-MRSA) [24].
The decrease in resistance of MRSA strains to ciprofloxacin can also be partially attributed to the reduction of ciprofloxacin use in Switzerland [25]. In fact there is ample evidence [19, 26] that there is a strong correlation between the use of fluoroquinolones and predisposition to colonisation or infection with MRSA. Indeed, Charbonneau et al. [27] demonstrated how a restriction of fluroroquinolones use led to a decreased rate of MRSA.
This study has a number of limitations. Firstly, not all hospitals and medical practitioners were included in the analysis, and the regions were not equally represented (in particular the German-speaking region was underrepresented). Secondly, we did not have molecular epidemiology analyses at our disposal requiring us to approximate the phenotypic definition of NmMRSA to represent CA-MRSA. However, this approach has been taken repeatedly in the past, as several investigators have used NmMRSA as a surrogate of CA-MRSA [6, 7]. Thirdly, similarly to most national surveillance databases, we lacked detailed clinical data to enable distinction between MRSA carriage and infection. Furthermore the choice of antimicrobials in the antibiograms in respiratory and wound samples could vary from one centre to another. Considering these limiting factors, we recommend that our findings be interpreted with caution.
In conclusion, our study represents the most recent and comprehensive national S. aureus surveillance study in Switzerland and is intended to fill a gap in the European MRSA map. It confirms the low MRSA prevalence in Switzerland compared to the rest of Europe. At the same time our study illustrates the regional differences and trends which may be affected by surrounding countries and thus cultural influences. Compared to many other national S. aureus databases, ANRESIS contains data on both invasive and noninvasive infections and MSSA, MRSA and NmMRSA. This comprehensive dataset mirrors the successful efforts to reduce MRSA incidences observed in many countries worldwide with a simultaneous increase in NmMRSA likely reflecting the concomitant emergence of CA-MRSA.
| Table S1:Time series analysis. | ||||||||
| All MRSA | NmMRSA | |||||||
| R2 | SEa | t-statistic (p-value) | R2 | SEa | t-statistic (p-value) | |||
| Switzerland | 0.52 | C:12.98 | 0.48 | 26.90 (<0.01) | 0.88 | C:2.33 | 1.13 | 2.06 (0.04) |
| T:–0.08% | 0.01 | –4.81 (<0.01) | T:0.92% | 0.04 | 22.75 (<0.01) | |||
| AR(3) | 0.18 | 2.53 (<0.01) | AR(1) | 0.07 | 10.71 (<0.01) | |||
| MA(3) | 0.65 | –13.66 (<0.01) | MA(1) | 0.09 | –10.63 (<0.01) | |||
| Linguistic region | ||||||||
| German | 0.39 | C:3.87 | 0.34 | 11.35 (<0.01) | 0.68 | C:18.61 | 6.5 | 2.86 (<0.01) |
| T:0.03% | 0.01 | 2.68 (<0.01) | T:0.92% | 0.24 | 3.72 (<0.01) | |||
| AR(1) | 0.14 | 2.83 (<0.01) | AR(1) | 0.13 | 3.89 (<0.01) | |||
| Italian | 0.46 | C:19.76 | 0.78 | 25.21 (<0.01) | 0.37 | C:29.78 | 1.17 | 25.40 (<0.01) |
| T:–0.15% | 0.03 | –4.93 (<0.01) | T:0.42% | 0.08 | 4.66 (<0.01) | |||
| AR(1) | 0.10 | –7.39 (<0.01) | AR(3) | 0.63 | –11.91 (<0.01) | |||
| MA(1) | 0.05 | 17.29 (<0.01) | MA(1) | 0.68 | 15.88 (<0.01) | |||
| French | 0.66 | C:26.19 | 1.06 | 24.49 (<0.01) | 0.86 | 4.61 | 1.44 | –3.19 (<0.01 |
| T:–0.28% | 0.04 | –6.81 (<0.01) | T:0.8% | 0.05 | 15.15 (<0.01) | |||
| AR(0) | – | – | AR(3) | 0.17 | 2.27 (0.02) | |||
| MA(4) | 0.14 | 2.62 (<0.01) | MA(3) | 0.04 | –19.49 (<0.01) | |||
| In- versus outpatient | ||||||||
| Inpatient | 0.59 | C:19.69 | 0.83 | 23.55 (<0.01) | 0.62b | C:0.47 | 0.16 | 2.90 (<0.01) |
| T:–0.18% | 0.03 | –5.5 (<0.01) | T:1.07% | 0.01 | 2.81 (<0.01) | |||
| MA(1) | 0.14 | 2.79 (<0.01) | MA(1) | 0.20 | –6.29 (<0.01) | |||
| Outpatient | 0.28 | C:5.44 | 0.15 | 35.3 (<0.01) | 0.70 | C:18.42 | 1.87 | 9.82 (<0.01) |
| T:0.03% | 0.01 | 4.78 (<0.01) | T:0.61% | 0.07 | 8.76 (<0.01) | |||
| AR(0) | – | – | AR(2) | 0.04 | 19.61 (<0.01) | |||
| MA(2) | 0.14 | –2.75 (<0.01) | MA(2) | 0.08 | –8.50 (<0.01) | |||
| Age-group | ||||||||
| <16 years | 0.59 | C:3.21 | 0.31 | 10.27 (<0.01) | 0.61 | C:12.63 | 5.16 | 2.44 (<0.01) |
| T:0.05 | 0.01 | 4.78 (<0.01) | T:1.14% | 0.20 | 5.56 (<0.01) | |||
| AR(2) | 0.11 | 4.59 (<0.01) | AR(0) | – | – | |||
| MA(2) | 0.02 | –42.83 (<0.01) | MA(1) | 0.14 | 2.60 (<0.01) | |||
| 16–65 years | 0.21 | C:8.01 | 0.19 | 40.45 (<0.01) | C:6.01 | 2.53 | 2.37 (0.02) | |
| T:–0.02% | 0.01 | –2.98 (<0.01) | 0.84 | T:1.17% | 0.09 | 11.76 (<0.01) | ||
| MA(2) | 0.14 | –3.1 (<0.01) | MA(4) | 0.15 | 2.00 (0.05) | |||
| >65 years | 0.77 | C:27.42 | 0.33 | 82.85 (<0.01) | 0.76b | C:3.45 | 1.01 | 3.41 (<0.01) |
| T:–0.3% | 0.01 | –21.78 (<0.01) | T:0.53% | 0.05 | 9.72 (<0.01) | |||
| AR(0) | – | – | AR(1) | 0.11 | 6.3 (<0.01) | |||
| MA(3) | 0.06 | –13.66 (<0.01) | MA(1) | 0.15 | 1.43 (0.15) | |||
| Invasive versus noninvasive | ||||||||
| Noninvasive | 0.45 | C:13.94 | 0.55 | 25.09 (<0.01) | 0.85 | C:4.17 | 1.81 | 2.29 (0.02) |
| T:–0.09% | 0.02 | –4.48 (<0.01) | T:0.83% | 0.07 | 11.50 (<0.01) | |||
| MA(1) | 0.15 | 2.22 (<0.01) | MA(1) | 0.14 | 2.23 (0.03) | |||
| Invasive | 0.50 | C:10.77 | 0.45 | 23.77 (<0.01) | 0.70c | C:2.56 | 0.11 | 21.44 (<0.01) |
| T:–0.10% | 0.14 | –7.1 (<0.01) | T:1.04% | 0.15 | 6.96 (<0.01) | |||
| AR(2) | 0.06 | –14.20 (<0.01) | AR(0) | – | – | |||
| MA(2) | 0.05 | 18.78 (<0.01) | MA(2) | 0.14 | 2.70 (<0.01) | |||
| AR = autoregressive term; C = constant; MA = moving average term; T = trend per quarter; a standard error; b first difference; c logarithm | ||||||||
| Table S2: Percentage of antibiotic resistance among MRSA strains. | |||||||
| Linguistic region | Ciprofloxacin | Clindamycin | Erythromycin | Fusidic acid | Gentamicin | Rifampicin | TMP/SMX |
| German | |||||||
| 2004 | 67 | 46 | 61 | 9 | 14 | 4 | 7 |
| 2005 | 64 | 38 | 61 | 16 | 25 | 7 | 8 |
| 2006 | 62 | 32 | 53 | 13 | 21 | 4 | 3 |
| 2007 | 55 | 27 | 47 | 18 | 29 | 4 | 4 |
| 2008 | 60 | 42 | 54 | 7 | 14 | 4 | 6 |
| 2009 | 51 | 30 | 41 | 10 | 19 | 3 | 7 |
| 2010 | 52 | 29 | 46 | 10 | 12 | 4 | 5 |
| 2011 | 48 | 24 | 46 | 9 | 20 | 1 | 7 |
| 2012 | 35 | 22 | 43 | 9 | 21 | 1 | 7 |
| 2013 | 44 | 24 | 50 | 7 | 20 | 4 | 12 |
| 2014 | 49 | 25 | 51 | 7 | 19 | 4 | 7 |
| French | |||||||
| 2004 | 97 | 89 | 91 | 5 | 56 | 3 | 1 |
| 2005 | 95 | 85 | 88 | 7 | 75 | 3 | 3 |
| 2006 | 95 | 86 | 90 | 4 | 41 | 4 | 1 |
| 2007 | 95 | 87 | 90 | 5 | 42 | 3 | 2 |
| 2008 | 93 | 84 | 86 | 4 | 30 | 4 | 2 |
| 2009 | 85 | 77 | 80 | 6 | 44 | 3 | 2 |
| 2010 | 83 | 72 | 77 | 7 | 43 | 3 | 2 |
| 2011 | 86 | 76 | 81 | 5 | 37 | 3 | 3 |
| 2012 | 78 | 65 | 70 | 8 | 55 | 2 | 2 |
| 2013 | 76 | 61 | 66 | 7 | 47 | 5 | 2 |
| 2014 | 70 | 51 | 56 | 6 | 26 | 5 | 4 |
| Italian | |||||||
| 2004 | 97 | 61 | 73 | 19 | 12 | 3 | 0 |
| 2005 | 93 | 59 | 68 | 16 | 27 | 1 | |
| 2006 | 98 | 67 | 70 | 5 | 10 | 2 | 1 |
| 2007 | 93 | 60 | 62 | 3 | 6 | 2 | |
| 2008 | 97 | 54 | 58 | 1 | 2 | 3 | |
| 2009 | 92 | 60 | 63 | 4 | 6 | 2 | 4 |
| 2010 | 93 | 40 | 46 | 6 | 2 | 1 | |
| 2011 | 88 | 46 | 48 | 7 | 1 | 4 | 2 |
| 2012 | 84 | 48 | 49 | 20 | 2 | 6 | 3 |
| 2013 | 88 | 52 | 54 | 0 | 0 | 1 | 5 |
| 2014 | 83 | 44 | 51 | 12 | 1 | 7 | |
| TMP/SMX = trimethoprim-sulfamethoxazole | |||||||
Acknowledgement: Institute for Laboratory Medicine, Cantonal Hospital Aarau; Central Laboratory, Microbiology Section, Cantonal Hospital Baden; Clinical Microbiology, University Hospital, Basel; Viollier AG, Basel; Laboratory Medicine EOLAB, Department of Microbiology, Bellinzona; Institute for Infectious Diseases, University Bern; Microbiology Laboratory, Unilabs, Coppet; Central Laboratory, Cantonal Hospital Graubünden; Microbiology Laboratory, Hospital Thurgau; Microbiology Laboratory Hôpital Fribourgeois, Fribourg; Bacteriology Laboratory, Geneva University Hospital, Geneva; ADMED Microbiology, La Chaux-de-Fonds; Institute for Microbiology, Université de Lausanne; Centre for Laboratory Medicine, Cantonal Hospital Luzern; Centre for Laboratory Medicine, Cantonal Hospital Schaffhausen; Centre for Laboratory Medicine Dr. Risch, Schaan; Central Institute, Hôpitaux Valaisans (ICHV), Sion; Centre for Laboratory Medicine St. Gallen; Institute for Medical Microbiology, University Hospital Zürich; Laboratory for Infectious Diseases, University Children’s Hospital Zürich.
Studies by Drs Olearo and Harbarth on surveillance and prevention of S. aureus infection leading to this publication have received support from the Innovative Medicines Initiative Joint Undertaking under the Combatting Bacterial Resistance in Europe (COMBACTE-Net) grant agreement no. 115523, resources of which are composed of financial contribution from the EU’s 7th Framework Programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contribution.
1 Karchmer TB, Gianetta ET, Muto CA, Strain BA, Farr BM. A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Arch Intern Med. 2000;160:3294–8.
2 Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20:605–23.
3 Boyce JM, Cookson B, Christiansen K, Hori S, Vuopio-Varkila J, Kocagöz S, Oztop AY, et al. Meticillin-resistant Staphylococcus aureus. Lancet Infect Dis. 2005,5:653–63.
4 Blanc DS, Pittet D, Ruef C, Widmer AF, Mühlemann K, Petignat C, Harbarth S, et al. Epidemiology of methicillin-resistant Staphylococcus aureus: results of a nation-wide survey in Switzerland. Swiss Med Wkly. 2002;132:223–9.
5 Kronenberg A, Zanetti B, Piffaretti J, Mühlemann K. Antibiotikaresistenzdaten der Schweiz: jetzt online. Schweiz Med Forum. 2008,8:415–8.
6 Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–84.
7 Munckhof WJ, Nimmo GR, Carney J, Schooneveldt JM, Huygens F, Inman-Bamber J, Tong E, et al. Methicillin-susceptible, non-multiresistant methicillin-resistant and multiresistant methicillin-resistant Staphylococcus aureus infections: a clinical, epidemiological and microbiological comparative study. Eur J Clin Microbiol Infect Dis. 2008;27:355–64.
8 Altman DG, Vergouwe Y, Royston P, Moons KG. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009,338:b605.
9 Vernaz N, Huttner B, Muscionico D, Salomon JL, Bonnabry P, Lopez-Lozano JM, et al. Modelling the impact of antibiotic use on antibiotic-resistant Escherichia coli using population-based data from a large hospital and its surrounding community. J Antimicrob Chemother. 2011,66:928–35.
10 Dickey DA, Wayne A. Distribution of the estimators for autoregressive time series with a unit root. J Am Stat Assoc. 1979;74:427–31.
11 Durbin J, Watson GS. Testing for serial correlation in least squares regression. I. Biometrika. 1950;37:409–28.
12 Harbarth S, Albrich W, Goldmann DA, Huebner J. Control of multiply resistant cocci: do international comparisons help? Lancet Infect Dis. 2001;1:251–61.
13 Filippini M, Masiero G, Moschetti K. Socioeconomic determinants of regional differences in outpatient antibiotic consumption: evidence from Switzerland. Health Policy. 2006;78:77–92.
14 Harbarth S, Sprumont D, Francioli P. Recensement, surveillance et contrôle des infections dues au staphylocoque doré résistant à la méticilline («MRSA»): la déclaration doit-elle être rendue obligatoire? Swiss-NOSO 2007;13.
15 Otter JA, Kearns AM, French GL, Ellington MJ. Panton-Valentine leukocidin-encoding bacteriophage and gene sequence variation in community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2010;16:68–73.
16 Liassine N, Auckenthaler R, Descombes MC, Bes M, Vandenesch F, Etienne J. Community-acquired methicillin-resistant Staphylococcus aureus isolated in Switzerland contains the Panton-Valentine leukocidin or exfoliative toxin genes. J Clin Microbiol. 2004,42:825–8.
17 Sax H, Posfay-Barbe K, Harbarth S, Francois P, Touveneau S, Pessoa-Silva CL, Schrenzel J, et al. Control of a cluster of community-associated, methicillin-resistant Staphylococcus aureus in neonatology. J Hosp Infect. 2006;63:93–100.
18 Köck R, Becker K, Cookson B, van Gemert-Pijnen JE, Harbarth S, Kluytmans J, Mielke M, et al. Methicillin-resistant Staphylococcus aureus (MRSA): burden of disease and control challenges in Europe. Euro Surveill. 2010;15:19688.
19 Harbarth S, Martin Y, Rohner P, Henry N, Auckenthaler R, Pittet D. Effect of delayed infection control measures on a hospital outbreak of methicillin-resistant Staphylococcus aureus. J Hosp Infect. 2000;46:43–9.
20 Pittet D, Hugonnet S, Harbarth S, Mourouga P, Sauvan V, Touveneau S, Perneger TV. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356:1307–12.
21 Landelle C, , Marimuthu K, Harbarth S. Infection control measures to decrease the burden of antimicrobial resistance in the critical care setting. Curr Opin Crit Care. 2014;20:499–506.
22 Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8:747–63.
23 LaPlante KL, Rybak MJ, Amjad M, Kaatz GW. Antimicrobial susceptibility and staphylococcal chromosomal cassette mec type in community- and hospital-associated methicillin-resistant Staphylococcus aureus. Pharmacotherapy. 2007;27:3–10.
24 De Angelis G, Francois P, Lee A, Schrenzel J, Renzi G, Girard M, Pittet D, Harbarth S. Molecular and epidemiological evaluation of strain replacement in patients previously harboring gentamicin-resistant MRSA. J Clin Microbiol. 2011;49:3880–4.
25 C. Plüss-Suard AK, Kronenberg RA, Zanetti G and the Swiss Centre for Antibiotic Resistance. Antibiotic use in 61 acute care hospitals in Switzerland: trends over the years 2004–2012 and comparison with Europe. Joint Annual Meeting, Aarau, 2014.
26 Graffunder EM, Venezia RA. Risk factors associated with nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection including previous use of antimicrobials. J Antimicrob Chemother. 2002;49:999–1005.
27 Charbonneau P, Parienti JJ, Thibon P, Ramakers M, Daubin C, du Cheyron D, Lebouvier G, et al. Fluoroquinolone use and methicillin-resistant Staphylococcus aureus isolation rates in hospitalized patients: a quasi experimental study. Clin Infect Dis. 2006;42:778–84.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article were reported.
* Co-first authors. ** Steering committee: R. Auckenthaler, Synlab Suisse, Switzerland; A. Cherkaoui, Bacteriology Laboratory, Geneva University Hospitals, Switzerland; O. Dubuis, Viollier AG, Basel, Switzerland; A. Egli, Clinical Microbiology, University Hospital Basel, Switzerland; V. Gaia, Department of Microbiology, EOLAB, Bellinzona, Switzerland; D. Koch, Federal Office of Public Health, Bern, Switzerland; A. Kronenberg, Institute for Infectious Diseases, University of Bern, Switzerland; S. Leib, Institute for Infectious Diseases, University of Bern, Switzerland; S. Luyet, Swiss Conference of the Cantonal Ministers of Public Health, Switzerland; P. Nordmann, Molecular and Medical Microbiology, Department of Medicine, University Fribourg, Switzerland; V. Perreten, Institute of Veterinary Bacteriology, University of Bern, Switzerland; J.-C. Piffaretti, Interlifescience, Massagno, Switzerland; G. Prod’hom, Institute of Microbiology, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; J. Schrenzel, Bacteriology Laboratory, Geneva University Hospitals, Geneva, Switzerland; A. F. Widmer, Division of Infectious Diseases & Hospital Epidemiology, University of Basel, Switzerland; G. Zanetti, Service of Hospital Preventive Medicine, Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland; R. Zbinden, Institute of Medical Microbiology, University of Zürich, Switzerland.