Varicella seroprevalence in individuals with a negative or unknown varicella history – results from a large travel clinic in Switzerland between 2008 and 2015
DOI: https://doi.org/10.4414/smw.2016.14342
Michael
Freuler, Susan
De Crom, Christoph
Hatz, Silja
Bühler
Summary
QUESTION UNDER STUDY: In Switzerland, vaccination against varicella is not recommended in the basic immunisation schedule. However, for individuals aged 11–40 years who do not have a reliable varicella history the Swiss Federal Office of Public Health recommends either (i) a vaccination or (ii) a serology test and vaccination of those with a negative result. In the Travel Clinic of the University of Zurich, the second strategy is followed. In this study we retrospectively assessed the overall percentage of individuals with varicella-specific antibodies despite a negative history and we examined the influence of age, number of siblings, order of siblings, age difference to siblings and nationality on varicella seropositivity.
METHODS: Between December 2008 and August 2015, the sera of 1757 individuals with a negative varicella history were tested for varicella antibodies.
RESULTS: A total of 1593 individuals (91%) had a positive result. We found an increasing trend for varicella seropositivity with increasing age. Those aged less than 40 years were significantly more often seronegative (9.5%) than those aged 40 years and above (6.0%, p = 0.049). Seropositivity was associated with nationality. The percentage of seropositives increased with the number of siblings.
CONCLUSIONS: Our results indicate that, despite the significant varicella seropositivity differences between those aged below and above, the age of 40 may not be an ideal threshold for performing a varicella serology in individuals with a negative or unknown varicella history. In the age groups above 40, testing for varicella antibodies may be especially reasonable in individuals with no or a small number of siblings and in those of specific nationalities.
Introduction
Varicella is the primary infection caused by the highly contagious varicella zoster virus (VZV). Most individuals contract varicella during childhood, when varicella in immunocompetent individuals is usually a self-limited disease and only rarely leads to complications [1].
According to previous studies conducted in Switzerland between 1994 and 2005, VZV seroprevalence in adolescents and young adults ranges between 94% and 97% [1–5]. Although seroprevalences show regional differences, studies from other Western European countries report comparable VZV prevalences [6–8]. Seroprevalence has increased in some tropical countries over recent years, but it still remains significantly lower than in Europe, and varicella infections in tropical countries tend to occur at later ages [9, 10]. If varicella infection is contracted in adulthood, the risk of complications such as pneumonia, encephalitis or meningitis is higher than in children [11]; in Switzerland, the frequency of hospitalisations for adults is 16 times higher and mortality is increased 40 times [12].
The prevalence of VZV infection appears to be associated with the number of siblings a person grew up with [2, 13]. Similarly, in a study conducted in Guinea Bissau, household size was a critical risk factor for varicella transmission [10]. However, in Swiss and other European studies, neither socioeconomic status, living in urban versus rural areas nor gender predicted the prevalence of VZV antibodies [2, 8].
Vaccination against varicella is recommended as part of routine childhood vaccination schedules in several countries, such as the United States and Germany [14, 15]. In Switzerland, vaccination against varicella during childhood is not generally recommended and varicella is not a notifiable disease. The Swiss Federal Office of Public Health recommends the following approach in individuals aged 11 to <40 years who have not contracted varicella naturally in the past (i.e. who do not have a reliable varicella history), to either (i) vaccinate or (ii) to perform VZV serology and vaccinate those with a negative result. The threshold of 40 years was chosen on the assumption that almost all individuals will have contracted varicella by the age of 40 and thus testing for varicella seropositivity is unnecessary above this age. The Travel Clinic of the University of Zurich follows the second strategy.
In the current study, we retrospectively assessed VZV serology data obtained from all individuals seeking pre-travel advice at our clinic between December 2008 and August 2015 with a negative or uncertain varicella history who were tested for VZV antibodies.
The objectives were (i) to evaluate the overall percentage of individuals who previously had varicella despite a negative or unclear history, (ii) to assess whether the current Swiss recommendation age of 40 is reasonable by comparing seropositivity percentages above and below this age, and (iii) to examine the influence of age, number of siblings, order of siblings, age difference from siblings and nationality on likelihood of seropositivity. Furthermore, we examined whether any of these factors could identify potential risk groups amongst individuals with a negative varicella history and refine the recommendations on testing for VZV antibodies. In addition (iv), we aimed to compare the costs of the two approaches recommended by the Swiss Federal Office of Public Health.
Methods
Study design
This was a retrospective study of the prevalence of serum varicella IgG in individuals seeking pre-travel advice in the Travel Clinic of the Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Switzerland. In the Travel Clinic we advise individuals with a negative or unknown varicella history (i.e. individuals who do not have a reliable varicella history) and who have had no previous varicella vaccination to be tested for varicella antibodies and we vaccinate those with a negative result. Generally only individuals aged <40 years are tested in accordance with Swiss recommendations. However, some individuals above this age were also tested upon their request. Anonymised VZV serology results from December 2008 to August 2015 were analysed retrospectively. No ethics approval was necessary for this study as it was a retrospective analysis of anonymised data.
Available data
The following anonymous data were analysed: age, gender, year of birth, nationality, number of siblings, year of birth of siblings, VZV serology results.
Varicella-zoster virus serology
All VZV serology tests were conducted in the laboratory of the Institute of Virology at the University of Zurich. The VIDAS bioMérieux VZV IgG, a multiparametric immunoassay system for medium throughput, based on enzyme-linked fluorescent assay (ELFA) technology was used. In September 2012, the laboratory switched to a new system, Virion/Serion VZV IgG kit enzyme-linked immunosorbent assay (ELISA).
Vaccination cost analysis
We compared emerging costs of the two approaches recommended by The Swiss Federal Office of Public Health: (i) to vaccinate individuals between 11 and <40 years of age who had not contracted varicella naturally in the past or (ii) to perform a VZV serology test and vaccinate those with a negative result.
Statistical analysis
Data were analysed with Stata 13.0 (Stata Corp, Texas). Univariate and bivariate analyses were performed. Differences in proportions were assessed using a χ2 test or Fisher’s exact test, where applicable. A test for trend was applied for ordered categorical variables. For continuous variables with a non-normal distribution the median was reported. A logistic regression was performed to adjust for potential confounders. All variables that were considered to be possibly associated with the result of the VZV IgG test result were included in the logistic regression model.
Results
Demographics
Overall, the sera of 1757 individuals with a negative or unknown varicella history were tested for VZV antibodies. Out of these, 860 (49.0%) were male and the median age was 31.6 years (range 11–72 years).
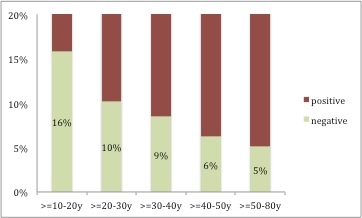
Figure 1
Seronegativity for varicella according to age group in travellers seeking pre-travel advice at the Travel Clinic of the University of Zurich between December 2008 and August 2015 who had an uncertain or negative varicella history (n = 1749).
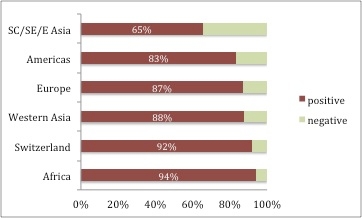
Figure 2
Varicella seropositivity according to nationality in travellers seeking pre-travel advice at the Travel Clinic of the University of Zurich between December 2008 and August 2015 who had an uncertain or negative varicella history (n = 1749).
SC = South Central; SE = South-East; E = East; Europe = European countries except Switzerland; χ2 test p <0.001
In 1560 individuals (88.8%) the nationality was known; 1270 (81.5%) were Swiss, 219 (14.0%) from other European countries, 27 (1.7%) from South Central, Eastern or South-Eastern Asia, 8 (0.5%) from Western Asia, 18 (1.2%) from the Americas and 17 (1.1%) from Africa.
In 1474 individuals (83.9%), the number of siblings was known; 322 (22.0%) had no sibling, 622 (42.4%) had one sibling, 331 (22.6%) had two siblings, 130 (8.9%) had three siblings and 61 (4.1%) at least four. Out of all travellers with siblings, 393 (35.6%) were first-born, 487 (44.1%) second, 147 (13.3%) third and 78 (7.1%) were born in the fourth or a later position.
Univariate analysis
Out of all tested individuals, 1593 (90.7%) had a positive, 156 (8.9%) a negative and 8 (0.4%) an inconclusive result. Individuals with inconclusive results were not included in the further analysis.
We found a decreasing trend for varicella seronegativity with increasing age (p = 0.008, fig. 1). Those aged less than 40 years were significantly more often seronegative (137/1435, 9.5%) than those aged 40 years and above (19/314, 6.0%, p = 0.049). Overall, slightly more males were seropositive than females (92.7% vs 89.6%, p = 0.024).
Individuals from Africa had the highest percentage of seropositivity (16/17, 94.1%) followed by persons of Swiss nationality (1166/1270, 92.0%). Seropositivity of individuals from other European countries, the Americas or Western Asia ranged between 83% and 88%, and persons from South Central, South-Eastern or Eastern Asia had the lowest seropositivity (65.4%, fig. 2). Seroprevalence varied between European countries; for example, VZV seroprevalence was 91% (107/118) in travellers from Germany, 91% in those from Spain (10/11), 71% in individuals from France (5/7), and 100% in travellers from the UK (5/5) or Austria (8/8).
The percentage of seropositivity increased considerably with number of siblings. Whereas 85.4% of persons without siblings had a positive varicella titre, 95.1% of those with at least four siblings were seropositive (p <0.001, table 1).
We found a trend for increasing seropositivity with declining age difference between siblings. This trend was observed in those with older siblings as well as in those with younger siblings (table 2). However, the differences were not statistically significant.
We did not find that the position within family birth order was associated with varicella seropositivity (data not shown).
Multivariate analysis
After gender, number of siblings, order within siblings and nationality were taken into account, age was significantly associated with a positive varicella titre. With every 10-year increase the odds of a positive varicella titre rose by 1.37 (95% confidence interval [CI] 1.09–1.76, p = 0.007, table 3). The number of siblings was also significantly associated with varicella seropositivity after adjusting for gender, age, order within siblings and nationality (odds ratio [OR] 1.34, 95% CI 0.98–1.82, p = 0.065).
Individuals from other European countries (except Switzerland) and persons from South Central / South-Eastern / Eastern Asia had a significantly lower prevalence of seropositivity compared with Swiss citizens, even after taking gender, age, order within siblings and number of siblings into account (Europe: OR 0.59, 95% CI 0.36–0.95, p = 0.03 and South Central / South-Eastern / Eastern Asia: OR 0.16, 95% CI 0.06–0.41, p <0.001).
Vaccination costs
In Switzerland, two varicella doses cost around 170 Swiss francs including all charges while the expenses for varicella serology are around 65 Swiss francs. For the individuals tested in this study, costs for varicella serology plus vaccination of those with a negative result added up to some 141 000 Swiss francs. If all persons had received two vaccine doses without previous testing, costs would have added up to approximately 298 500 Swiss francs.
|
Table 1: Results of varicella serology according to number of siblings in travellers seeking pre-travel advice at the Travel Clinic of the University of Zurich between December 2008 and August 2015 who had an uncertain or negative varicella history (n = 1757). |
|
Number of siblings
|
Varicella serology
|
|
Negative n (%)
|
Positive n (%)
|
|
47 (14.6) |
275 (85.4) |
| 1 |
56 (9.0) |
566 (91.0) |
| 2 |
19 (5.7) |
312 (94.3) |
| 3 |
9 (6.9) |
121 (93.1) |
| ≥4 |
3 (4.9) |
58 (95.1) |
| Test for trend p <0.001 |
|
Table 2:Does age difference between siblings influence varicella seropositivity in travellers seeking pre-travel advice at the Travel Clinic of the University of Zurich between December 2008 and August 2015 who had an uncertain or negative varicella history (n = 1757)? |
|
Age difference
|
Varicella serology
|
|
Negative n (%)
|
Positive n (%)
|
|
(a) Smallest age difference from older siblings
|
| <2 years |
12 (5.3) |
213 (94.7) |
| 2 to <4 years |
22 (8.7) |
232 (91.3) |
| 4 to <6 years |
11 (8.4) |
120 (91.6) |
| ≥6 years |
11 (10.1) |
98 (89.1) |
| Test for trend: p = 0.128 |
| |
|
(b) Smallest age difference from younger siblings
|
| <2 years |
9 (6.9) |
122 (93.1) |
| 2 to <4 years |
10 (4.6) |
210 (95.5) |
| 4 to <6 years |
9 (7.8) |
106 (92.2) |
| 6 to <8 years |
4 (12.9) |
27 (87.1) |
| ≥8 years |
4 (10.3) |
35 (89.7) |
| Test for trend: p = 0.163 |
Discussion
In the current study, more than 90% of individuals, aged 11 to 72 years, with an unknown or negative varicella history were seropositive for VZV antibodies. Seropositivity increased with age and number of siblings, whereas the age difference between siblings and the order of birth did not significantly influence a person’s varicella serostatus. In all individuals from Switzerland and Africa seroprevalence was higher than 90%. In those persons originating from other countries in Europe or Western Asia seroprevalence ranged between 85 and 90%, while seroprevalence was only 83% in individuals from the Americas and 65% in individuals from South Central, South-East and East Asia. However, the numbers of travellers originating from countries other than Switzerland were small. Thus seroprevalence data in these groups should be interpreted with caution.
Age, number of siblings and nationality (origin) have previously been shown to be associated with VZV serostatus in children and adolescents [1, 2]. Heininger et al. demonstrated a positive correlation between presence of VZV antibodies and the number of siblings living in the same household (90.1% occurrence of antibodies in people with no siblings and more than 96% seroprevalence in people with one or more siblings). Similar results were observed in a study conducted by Jerant et al. in young US-military soldiers [13]. Seroprevalence to varicella is generally lower in tropical countries [17, 18] and thus the finding of 65% seronegatives in those from large parts of Asia is not surprising.
Between 1999 and 2000, the reliability of varicella history was investigated among children and adolescents aged 1 to 18 years who were hospitalised at the University Children's Hospital of Basel, Switzerland [5]. Among those with a positive varicella history, serum samples tested positive in 98%. In those who reported no or a possible previous varicella history, 15.7% (31/197) tested positive. When looking at age groups, a different picture was observed: 3% of those aged 1 to 4 years, 23% of those aged 5 to 8 years and 74% of older children (aged 9–18 years) tested positive for varicella despite a negative history. In our study, we report varicella titres in persons aged 11 years and older. In those aged 10 to 19 years, we found a varicella positivity of 85% with an increasing trend in higher age groups. Thus, our findings are in line with the results from the study conducted between 1999 and 2000.
We showed that the strategy of applying two vaccine doses to all individuals with an unknown or negative varicella history would have been twice as costly as the approach of performing a serology test and vaccinating only those with a negative result.
Limitations
Underlying conditions, including immunocompromising conditions, were not recorded and could not be assessed in our study sample. Antibody memory towards varicella has been shown to be impaired in immunocompromised persons [19]. Furthermore, the sample sizes for individuals aged 40 years and above, as well as for individuals from regions outside Switzerland, were low, thus our sample may not be representative for these groups. In addition, we have information only on the nationality of individuals, but not on the country where they grew up in.
We tested for VZV antibodies in every traveller with a negative or unknown varicella history who wished and agreed to be tested. It is possible that results would have been different in those who did not agree to be tested. Unfortunately, because of data limitations we could not assess how many individuals reported a positive varicella history or how many travellers with a negative or unknown varicella history did not agree to be tested. All travellers receive a written report on their serology results 10 to 14 days after testing. Those with a negative VZV serology receive the recommendation to be vaccinated twice. However, owing to the anonymous nature of the data we could not check whether these individuals actually followed our recommendation. In a worst-case scenario, none of those with a VZV-negative test result received the vaccinations and we may have missed vaccinating 156 travellers with no protection against varicella.
Conclusion
From our results it is questionable whether the age of 40 is a reasonable threshold for performing VZV serology in individuals with an unknown or negative varicella history in Switzerland, as 6.0% of tested individuals above the age of 40 were still VZV negative. In age groups above 40, testing for VZV antibodies may be reasonable, especially in those with no or a small number of siblings as well as in those originating from the Americas or from South Central, South-East and East Asia.
Furthermore, our study shows how important pre-travel advice is in updating vaccination and immunity status.
Acknowledgement:We thank Dr. med. Andrea Zbinden Cipolat at the Institute of Virology of the University of Zurich for providing us with details about the VZV-IgG assays.
References
1 Aebi C, Fischer K, Gorgievski M, Matter L, Mühlemann K. Age-specific seroprevalence to varicella-zoster virus: study in Swiss children and analysis of European data. Vaccine. 2001;19(23-24):3097–103.
2 Heininger U, Braun-Fahrländer C, Desgrandchamps D, Glaus J, Grize L, Wutzler P, et al. Seroprevalence of varicella-zoster virus immunoglobulin G antibodies in Swiss adolescents and risk factor analysis for seronegativity. Pediatr Infect Dis J. 2001;20(8):775–8.
3 Baer G, Bonhoeffer J, Schaad UB, Heininger U. Seroprevalence and immunization history of selected vaccine preventable diseases in medical students. Vaccine. 2005;23(16):2016–20.
4 Loutan L, Maitre B, Zuber P. [Are medical students sufficiently vaccinated? Results of a serological survey and of vaccine coverage.] Sozial- und Präventivmedizin. 1994;39(2):86–92. French.
5 Heininger U, Baer G, Bonhoeffer J, Schaad UB. Reliability of varicella history in children and adolescents. Swiss Med Wkly. 2005;135(17–18):252–5.
6 Thiry N, Beutels P, Shkedy Z, Vranckx R, Vandermeulen C, Wielen M Van Der, et al. The seroepidemiology of primary varicella-zoster virus infection in Flanders (Belgium). Eur J Pediatr. 2002;161(11):588–93.
7 Vyse AJ, Gay NJ, Hesketh LM, Morgan-Capner P, Miller E. Seroprevalence of antibody to varicella zoster virus in England and Wales in children and young adults. Epidemiol Infect. 2004;132(6):1129–34.
8 Salleras L, Domínguez A, Vidal J, Plans P, Salleras M, Taberner JL. Seroepidemiology of varicella-zoster virus infection in Catalonia (Spain). Rationale for universal vaccination programmes. Vaccine. 2000;19(2-3):183–8.
9 Fatha N, Ang LW, Goh KT. Changing seroprevalence of varicella zoster virus infection in a tropical city state, Singapore. Int J Infect Dis. 2014;22:73–7.
10 Nichols RA, Averbeck KT, Poulsen AG, al Bassam MM, Cabral F, Aaby P, et al. Household size is critical to varicella-zoster virus transmission in the tropics despite lower viral infectivity. Epidemics. 2011;3(1):12–8.
11 Choo PW, Donahue JG, Manson JE, Platt R. The epidemiology of varicella and its complications. J Infect Dis. 1995;172(3):706–12.
12 Bundesamt für Gesundheit (BAG). Varizellenimpfung [Internet]. 2004 [cited 2015 Sep 28]. Available from: http://www.bag.admin.ch/themen/medizin/00682/00684/02535/index.html?lang=de
13 Jerant AF, DeGaetano JS, Epperly TD, Hannapel AC, Miller DR, Lloyd AJ. Varicella susceptibility and vaccination strategies in young adults. J Am Board Fam Pract. 1998;11(4):296–306.
14 Centers for Disease Control and Prevention. 2015 Recommended Immunizations for Children from Birth Through 6 Years Old [Internet]. 2015. [cited 2015 Dec 18]. Available from: http://www.cdc.gov/vaccines/parents/downloads/parent-ver-sch-0-6yrs.pdf
15 Ständige Impfkommission (STIKO). Impfkalender [Internet]. 2015 [cited 2015 Dec 16]. Available from: http://www.rki.de/DE/Content/Infekt/Impfen/Materialien/Downloads-Impfkalender/Impfkalender_Deutsch.pdf?__blob=publicationFile
16 Ooi PL, Goh KT, Doraisingham S, Ling AE. Prevalence of varicella-zoster virus infection in Singapore. Southeast Asian J Trop Med Public Health. 1992;23(1):22–5.
17 Srichomkwun P, Apisarnthanarak A, Thongphubeth K, Yuekyen C, Mundy LM. Evidence of vaccine protection among thai medical students and implications for occupational health. Infect Control Hosp Epidemiol. 2009;30(6):585–8.
18 L’Huillier AG, Ferry T, Courvoisier DS, Aebi C, Cheseaux J-J, Kind C, et al. Impaired antibody memory to varicella zoster virus in HIV-infected children: low antibody levels and avidity*. HIV Med. 2012;13(1):54–61.

