Learn, simplify and implement: developmental re-engineering strategies for cartilage repair
DOI: https://doi.org/10.4414/smw.2016.14346
Paola
Occhetta, Chiara
Stüdle, Andrea
Barbero, Ivan
Martin
Summary
The limited self-healing capacity of cartilage in adult individuals, and its tendency to deteriorate once structurally damaged, makes the search for therapeutic strategies following cartilage-related traumas relevant and urgent. To date, autologous cell-based therapies represent the most advanced treatments, but their clinical success is still hampered by the long-term tendency to form fibrous as opposed to hyaline cartilage tissue. Would the efficiency and robustness of therapies be enhanced if cartilage regeneration approaches were based on the attempt to recapitulate processes occurring during cartilage development (“developmental engineering”)? And from this perspective, shouldn’t cartilage repair strategies be inspired by development, but adapted to be effective in a context (an injured joint in an adult individual) that is different from the embryo (“developmental re-engineering”)? Here, starting from mesenchymal stem/stromal cells (MSCs) as an adult cell source possibly resembling features of the embryonic mesenchyme, we propose a developmental re-engineering roadmap based on the following three steps: (i) learn from embryonic cartilage development which are the key pathways involved in MSC differentiation towards stable cartilage, (ii) simplify the complex developmental events by approximation to essential molecular pathways, possibly by using in vitro high-throughput models and, finally, (iii) implement the outcomes at the site of the injury by establishing an appropriate interface between the delivered signals and the recipient environment (e.g., by controlling inflammation and angiogenesis). The proposed re-design of developmental machinery by establishing artificial developmental events may offer a chance for regeneration to those tissues, like cartilage, with limited capacity to recover from injuries.
Cartilage regeneration: state of the art
Cartilage is a tissue with poor intrinsic regeneration capacity. Therefore, trauma affecting articular cartilage, if not properly treated, predisposes to osteoarthritis, a pathological condition that provokes joint pain and loss of motility. For this degenerative joint disease, which causes a reduction of the life quality for millions of people world-wide, no effective disease-modifying therapies are available [1].
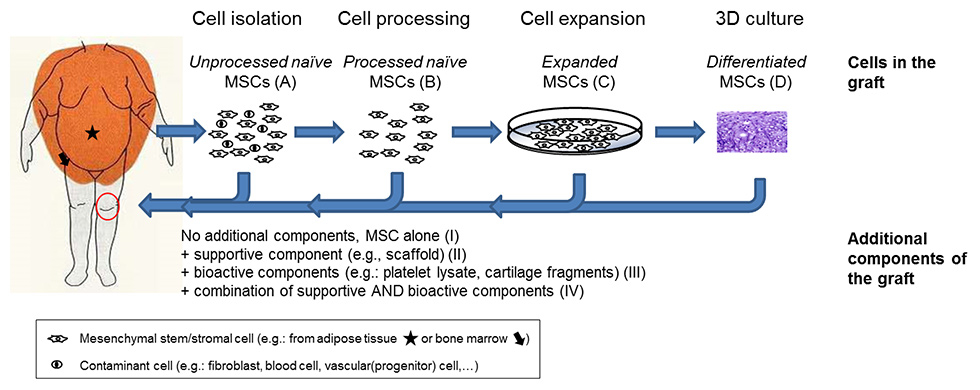
Figure 1
Classification of mesenchymal stromal/stem cell (MSC)-based strategies based on the type and differentiation stage of cells (capital letters in parenthesis) and additional components (Roman numbers in parenthesis) present in the graft. Below are listed examples of MSC-based graft material used in selected clinical trials registered at http://clinicaltrials.gov (keywords used for the research: stem cells AND cartilage repair).
A–II: bone marrow MSC aspirate in a scaffold (identifier NCT00885729).
B–III: MSCs isolated from adipose tissue specimens (liposuction) combined with platelet-rich plasma (identifier NCT01739504).
B–IV: concentrated bone marrow-derived cells on a collagen scaffold, covered with a platelet gel (identifier NCT02005861); MSCs separated from redundant joint tissues (bone marrow or synovium) combined with cartilage fragments in a fibrin gel (identifier NCT01301664).
C–I: expanded adipose tissue-derived MSCs alone (identifier NCT01399749).
C–III: expanded bone marrow MSCs combined with platelet lysate (identifier NCT02118519).
C–IV: expanded bone marrow MSCs combined with chondrocytes in a fibrin gel (identifier NCT02037204).
D–II: chondrogenic pellets derived by bone marrow MSCs embedded in a scaffold (identifier NCT00891501).
Therefore there is a need to repair cartilage defects in order to prevent or delay the onset of osteoarthritis. Among the various techniques to heal cartilage traumas [2], cell-based therapy is the most advanced [3, 4]. Autologous chondrocytes expanded ex vivo are the favourite source of cells for such therapies. However, clinical outcomes of chondrocyte-based cartilage repair approaches are not predictable [5]. This can be explained by the inability of articular chondrocytes to form cartilage after expansion [6], a step required to increment the initially limited number of cells available from a normal-sized cartilage biopsy [5]. Therefore, the following sources of chondrogenic cells have been proposed: nasal chondrocytes [7]; chondro-progenitors isolated from the superficial zone of articular cartilage [8, 9] or fetal cartilage tissues [10, 11]; mesenchymal stem/stromal cells (MSCs) from various adult [12] or fetal tissues [13, 14]; pluripotent stem cells (embryonic stem cells) [15], or inducible pluripotent stem cells [16]. Table 1 summarises the advantages and disadvantages of using each of these candidate cell sources for cartilage repair.
Ideally, for efficient cartilage repair the therapeutic cells may have to recapitulate in the adult joint processes occurring during cartilage development. Mesenchymal condensation is a critical transitional stage leading to cartilage formation in the embryo. During this stage, undifferentiated mesenchymal cells migrate from the lateral plate mesenchyme and aggregate, forming a cartilaginous anlage. Within this, two distinct populations of chondrocytes arise: one will differentiate into growth plate chondrocytes (i.e., cells that further mature into hypertrophic chondrocytes, ultimately die and are replaced by bone cells); the other, instead, will differentiate in stable chondrocytes, thus contributing to articular cartilage [17]. For durable cartilage repair, the therapeutic/targeted cells must be capable of efficiently differentiating into articular chondrocytes and not activating the endochondral programme.
Although it is not yet clear to what extent adult MSCs resemble the cells in the condensing embryonic mesenchyme, these cells represent the obvious candidate to recapitulate processes leading to articular cartilage formation. Moreover, MSCs are abundantly available in the human body and have an intrinsic tissue-repair capacity under inflammatory/stress conditions, as well as immunomodulatory effects [18-20]. Indeed, positive structural/functional outcomes of MSC-based cartilage repair have been reported in several clinical case reports and trials of the application of MSCs for cartilage repair [20–22].
MSC-based cartilage repair approaches can be classified on the basis of the type and differentiation stage of cells present in the graft, as follows: cell preparation containing (a) unprocessed
naïve MSCs together with contaminant cells present in the native tissues (e.g., bone marrow, adipose tissue, synovium), (b) processed naïve MSCs (to enrich for MSCs and/or to remove cell contaminants), (c) expanded MSCs, (d) differentiated MSCs after chondrogenic culture (thus within tissue-engineered cartilage tissue). These cell preparations have been clinically used without (I) or with additional supportive (II) or bioactive (III) components or with combinations of supportive and bioactive components (IV) (fig. 1). It is important to consider that additional bioactive component(s) (matrices and/or growth factors) in association with MSCs on the one hand might allow enhancement of the reparative/regenerative properties of the grafted (and of the resident) MSCs, but on the other hand render the clinical outcome difficult to interpret. In this article the different cell-free approaches investigated for cartilage repair will not be discussed, in order to allow a more focused analysis (for an overview of this topic see the references [23, 24]).
Despite the increased utilisation of MSCs for articular cartilage repair, before these cells can be a widely accepted cell source for the treatment of diseased joints, it will be necessary to identify isolation/culture conditions (and bioactive components) enabling them to trigger orderly and durable cartilage tissue repair. In this review article we discuss the main limitations of the MSC-based approaches and we outline possible innovative strategies to use them to induce cartilage regeneration.
|
Table 1:Cell sources for cartilage repair. |
|
Cell source
|
Advantage
|
Disadvantage
|
| Differentiated chondrocytes |
Adult articular chondrocytes |
Competent to produce cartilage matrix |
Limited amount from cartilage biopsyAge-related difference in the differentiation capacityPossible phenotypic alteration in post-traumatic/pre-osteoarthritis joints |
| Adult nasal chondrocytes |
High proliferation and chondrogenic capacity |
Limited amount from cartilage biopsy |
| Neonatal, juvenile articular chondrocytes |
High proliferation and chondrogenic capacityImmune-privileged cells |
Limited tissue availabilityUse of the cells associated with ethical concerns |
| Chondrocyte progenitors |
Chondrocyte progenitors from adult jointEpiphyseal chondroprogenitorsFetal cartilage-derived progenitor cells |
High proliferation capacityCells maintain a commitment to their differentiation programme |
No conclusive protocols available for the isolation/culture of these cellsLimited studies on the stability of the acquired cartilage phenotype |
| Mesenchymal stromal/stem cells |
From adult tissues:– bone marrow,– adipose tissues– synovium– … |
Large availabilityHigh proliferation capacityImmunomodulatory effectsTrophic effects |
Large donor-donor variability in the differentiation capacityInstability of the acquired cartilage phenotype |
| From fetal tissues:– placenta– umbilical cord– umbilical cord blood |
Large availabilityHigh proliferation capacityCells with immuno-privileged statusImmunomodulatory effectsNo ethical concerns |
No conclusive protocols available for the isolation and culture of these cellsControversial evidence on the capacity of these cells to form hyaline-like cartilage |
| Pluripotent stem cells |
Embryonic stem cellsInducible pluripotent stem cells |
Potentially unlimited source of chondrocytesPossibility for personalised medicine |
No conclusive protocols availableProblems associated with tumour formationUse of the cells associated with ethical concerns |
Developmental (re-)engineering: a new paradigm for tissue engineering
Traditional MSC-based tissue engineering (TE) strategies suffer from critical drawbacks, which limit robust and routinely accepted clinical translation [25]. MSCs are indeed intrinsically affected by intra- and inter-donor variability, and currently there is no consensus on common markers to predict their chondrogenic potential or therapeutic effect [26, 27]. Moreover, cartilaginous grafts engineered from MSCs typically undergo hypertrophic differentiation when transplanted ectopically in vivo[28]. This finally leads to limited structural and functional similarities between the implanted graft and native cartilage, causing, in most cases, the failure of interface integration [29]. A currently investigated explanation for these issues is the possibility that adult MSCs are intrinsically committed towards terminal, hypertrophic chondrocyte differentiation and cannot stably differentiate into articular chondrocytes. A strategy for “re-programming” the fate of MSCs by exposing them to specific signals may be used to reverse this tendency and guide these cells towards differentiation into stable cartilage. From this perspective, defining an effective, and possibly temporally staged, combination of stimuli represents an important challenge for strengthening MSC-based TE protocols for cartilage repair.
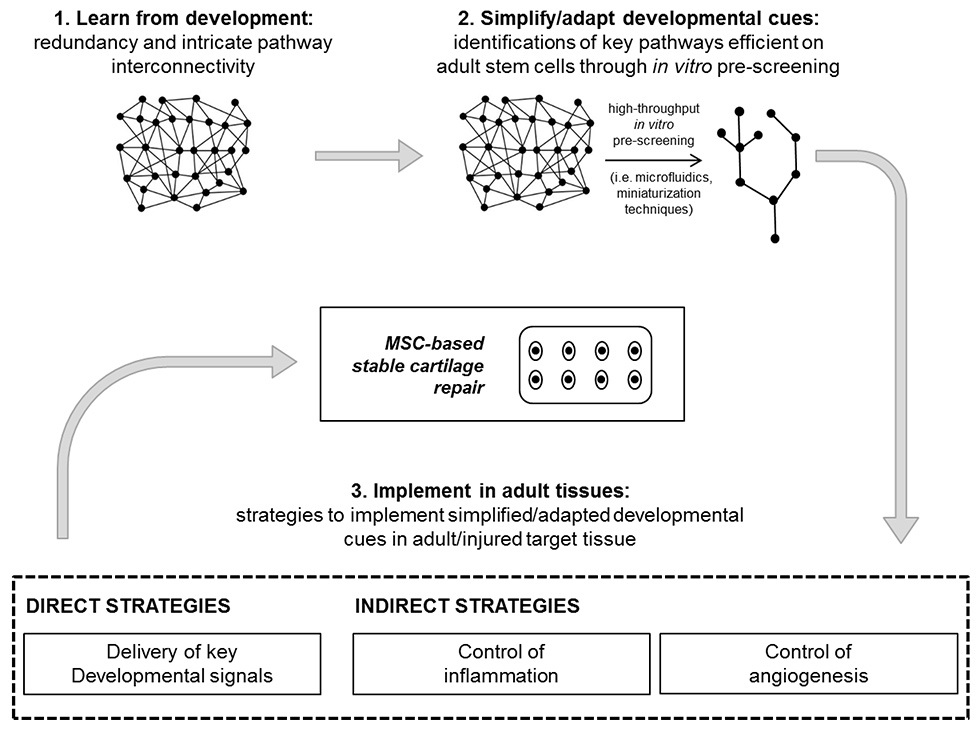
Figure 2
Developmental re-engineering concept: learn, simplify/adapt, implement. After the understanding of pathways involved in the embryonic development of the targeted tissue to repair (1), a simplification/adaptation step is required to extrapolate from the in-vivo complexity the key signals that are effective in adult stem cells models. This step (2) could be accelerated by using recently introduced high-throughput in-vitro screening tools. The most effective developmentally inspired protocol defined in vitro for the acquisition of functional cartilage is then implemented in the adult/injured target tissue. For this aim, different strategies, either direct or indirect, can be identified (3).
Here, we introduce the concept of “developmental re-engineering” [30] as a strategy for stable cartilage repair. This term describes a combination of the controlled recapitulation of embryonic morphogenetic events as an approach for engineering adult tissues (“developmental engineering”), with the recognition that such events need to be implemented in the different adult environment, where in-vivo repair processes take place and thus require a possible “re-engineering” of processes. We propose that the paradigm of developmental re-engineering is summarised in three main steps: learn, simplify/adapt and implement (fig. 2). The first requirement for engineering a functional tissue is to understand the processes and pathways involved in its embryonic development [31] (“learn”) (fig. 2 step 1). However, before successfully applying concepts learnt from developmental biology to TE strategies, a further simplification and adaptation step is required (“simplify”) (fig. 2 step 2). Indeed, developmental morphoregulatory systems feature an inherent redundancy and intricate pathway interconnectivity, finally contributing to their robustness. This eventually makes them probably too complex to be faithfully reproduced in vitro, especially with the perspective of a clinical translation [30]. Key pathways necessary and sufficient for guiding early progenitors commitment in vitro have thus to be extrapolated from the intricate redundancy found in vivo,and eventually adapted according to the level of commitment of MSCs [32]. To accomplish this aim, reliable and high-throughput in-vitro models, mainly based on microfluidics [33–36] or miniaturisation techniques [37–39], have been recently introduced, with the potential to speed up the screening process. Finally, key pathways selected from developmental events need to be correctly interfaced at the site of the injury, thanks to either direct or indirect strategies (“implement”) (fig. 2 step 3).
Developmental re-engineering strategies for cartilage repair
In this section we provide a more detailed description of our vision for exploiting the paradigm of developmental re-engineering towards MSC-based approaches for cartilage regeneration and repair.
Step 1: Learn – development of articular cartilage
During limb development, two different chondrogenic phenotypes are formed, (i) the stable articular cartilage, which acts as a crucial joint component throughout life and (ii) the transient cartilage of the cartilage anlage and the growth plate responsible for long bone growth that eventually is replaced by bone tissue [17]. In order to design a successful cartilage repair strategy it is essential to understand the genesis and maintenance of articular cartilage. Its development is linked to joint formation, which manifests first with the creation of the so-called interzone interrupting the cartilage anlage of the skeletal elements at prospective joint sites [40–42]. The interzone is demarcated by down-regulation of chondrogenic genes such as type II collagen and Sox9, as well as the prominent expression of growth differentiation factor-5 (GDF-5, a member of the bone morphogenetic protein [BMP] superfamily), wing-less type proteins (Wnt) such as Wnt9a, Wnt4, chordin and noggin (specific antagonists of BMPs). The precise molecular mechanisms governing the induction of interzone formation are not yet completely known. Clearly, Wnt signalling, in particular Wnt9a secreted from interzone cells, plays an important role since its ectopic application causes heterotopic joint-like structures [43, 44]. However, it has been demonstrated that Wnt is not a prerequisite for joint induction; rather, it is involved in the regulation of joint integrity by inhibiting chondrogenesis and by regulating Ihh expression [45]. Indian hedgehog (Ihh), in turn, is not only involved in controlling the phenotype of growth-plate chondrocytes, but also seems to be implicated in joint formation [46]. Tight regulation of BMP signalling is also crucial for joint formation [47]. Recently, it has been shown that proliferating cells in the distal part of the interzone contribute to both embryonic articular and growth plate cartilage formation, and that inhibition of BMP signalling by noggin next to the presumptive joint site allows preferential differentiation towards the embryonic articular cartilage phenotype [48]. However, the trigger for these cells to acquire a chondrogenic phenotype has not been elucidated yet. Formerly, it was demonstrated that articular cartilage initially shares a history with growth-plate chondrocytes (both are derived from cells expressing Sox9, collagen type II and doublecortin [49, 50]) until they eventually split their fates. Indeed, cells descended from GDF-5-positive cells do not contribute to growth plate development [51], but only to joint elements, including articular cartilage. On the other hand, cells of matrillin lineage give rise to growth plate chondrocytes [52].
Starting from the complexity of the described developmental processes (extensively reviewed elsewhere [17, 40-42]) and the intricate interplay between the occurrence of stable and transient cartilage, the developmental events leading to stable cartilage need to be extrapolated and validated for application on a model based on adult cells.
Step 2: Simplify and adapt – key pathways for adult MSC differentiation into stable cartilage
Recently, it has been demonstrated that the in-vitro activation/inhibition of pathways hypothesised to be active in development sequentially guides embryonic stem cell differentiation through mesoderm intermediates to a final chondrocyte population [53]. Interestingly, the differentiation of mouse chondrogenic mesodermal cells derived from embryonic stem cells (ESCs) could be directed in vitro either towards hypertrophy, under the influence of BMP4, or towards an articular cartilage phenotype by exposing them to GDF-5 and simultaneously inhibiting hedgehog and BMP pathways [54]. These results confirm the abovementioned postulated importance of a spatial restriction of BMP signalling for the in-vivo development of stable articular cartilage [48]. Similar outcomes have been achieved through human induced pluripotent stem cells (hiPSCs). In recent studies, hiPSC-derived chondrogenic progenitors, obtained in vitro through an intermediate state of mesoderm induction, were guided towards the acquisition of a stable cartilaginous phenotype (characterised by absence of BMP receptor type 2b expression) [55] by administration of transforming growth factor-beta (TGFβ) and leukaemia inhibitory factor (LIF) signals [56].
The direct application of developmental cues to embryonic or induced pluripotent cell sources thus confirms the potential of developmentally inspired approaches in generating cartilage templates. However, the translation of key developmental pathways to more clinically relevant adult stem cell sources, which are in different stages of commitment, requires further adaptation steps. Regarding the differentiation of MSCs to stable chondrocytes, several studies have focused on key signalling factors that are known to regulate hypertrophy during growth plate development (i.e., members of TGFβ, Wnt and fibroblast growth factor [FGF] protein families and the parathyroid hormone-related protein [PTHrP] / Ihh regulatory loop) [57].
Among the different signalling pathways, BMP is involved in many phases of limb development [58, 59]. BMP is indeed a key chondrogenic factor [60], but it is also involved in triggering endochondral ossification [48, 54]. Recently, it has, for example, been demonstrated how exogenous over-expression of Sox9 potentiates BMP2-induced chondrogenic differentiation while inhibiting BMP2-mediated hypertrophic maturation [61]. Temporally dynamic regulation of BMP pathways can thus be exploited to modulate MSC differentiation towards stable cartilage.
Temporal modulation of Wnt signalling is another currently investigated approach. Initial exposure to Wnt3a (either alone or in combination with FGF2) was indeed shown to enhance undifferentiated proliferation of MSCs, while priming cells towards more efficient chondrogenic differentiation [33, 62]. Endogenous Wnt signals, however, were also discovered to be the main driver of late hypertrophic maturation, suggesting the late inhibition of Wnt as a possible strategy for preventing calcification of cartilaginous templates [63]. Gremlin, a BMP inhibitor, and two inhibitors of Wnt signalling (frizzled-related protein [FRP] and dickkopf-related protein 1 [DKK1]) were also recently identified as distinctive markers of adult human articular cartilage. This further suggests that the inhibition of these pathways has to be considered for achieving stable chondrogenic differentiation of MSCs [64].
FGF family members have been shown to play a dynamic and time-dependent role during MSC chondrogenesis. Early exposure to FGF2 is well known to maximise the expansion potential of MSCs [65], but it has been associated with the early appearance of hypertrophy-related features as well [66]. Interestingly, Correa and colleagues recently demonstrated that this tendency can be modulated through late exposure of MSCs to the combination of endogenous FGF9 and FGF18 signals. FGF9 and FGF18, signalling mainly through FGF receptor 3, have been shown to induce both an anabolic effect on extracellular matrix production and a delay in the maturation of MSC-derived chondrocytes towards hypertrophy in vitro [66].
Finally, the PTHrP/Ihh regulatory loop is recognised as one of the main pathways involved in mediating chondrocyte hypertrophy [67]. Mueller and coworkers found that PTHrP treatment reduced alkaline phosphatase expression in MSC 3D pellet culture in a dose-dependent manner; however, when cultured under hypertrophy-enhancing conditions, PTHrP could not diminish the induced enhancement of hypertrophy in MSC pellets [68]. The intermittent supplementation of 3D pellet culture with PTHrP was also demonstrated to stimulate MSCs chondrogenesis (through an upregulation of collagen type II gene expression), while reducing endochondral ossification (through a reduction of Ihh and alkaline phosphatase activity) [69]. In contrast, Weiss and colleagues observed a concomitant down-regulation of chondrogenic and hypertrophic factors upon PTHrP treatment in MSC chondrogenic cultures that also resulted in unstable in-vivo cartilage formation [70].
These studies are examples of how complex developmental events may be approximated to discrete molecular pathways and highlight the importance of the temporal stage for delivery of instructive factors modulating MSC commitment. However, the stability of generated cartilaginous templates once implanted in vivo is the main limitation of all the above-mentioned approaches, suggesting the necessity for further refinements. The next required step will be to investigate interconnections among different pathways in MSC models in order to identify the most effective spatiotemporal sequence of instructive signals. Moreover, as mechanical factors are also involved in the development of articular cartilage, mechanotransduction can be considered as an alternative strategy to activate key articular chondrogenic pathways. Preliminary studies indeed showed that dynamic compressive loading suppressed a number of hypertrophic markers (collagen type X, matrix metalloproteinase-13 and ALP gene expression) in hMSC-derived constructs exposed to hypertrophic conditioning [71]. However, a thorough understanding of how individual mechanical factors influence hMSC is needed to utilise them predictably for mechanically-induced stable chondrogenesis [72].
To accomplish these aims, high-throughput in-vitro models [33, 34, 36] have been recently introduced as powerful tools for testing the effect of different combinations/concentrations of soluble factors, immobilised cues or mechanical stimuli [73] on MSC differentiation fate, in a fast and reliable fashion. They will thus be promising candidates for speeding up this preliminary screening step.
Step 3: Implement – developmental re-engineering strategies for cartilage repair
Once the network of signalling pathways necessary and sufficient for the generation of articular cartilage has been identified, strategies for transferring them to the site of the injury need to be implemented. In our vision, this can be achieved either by a direct approach, namely localised delivery of selected developmental signals, or indirectly through the exploitation of environmental features characterising the target adult/injured tissue.
Direct strategies
The direct delivery of key agonists and/or antagonists, defined in the previous section, at the site of the injury is a promising strategy to guide resident progenitor cells and/or implanted MSCs towards the generation of stable cartilage in the context of a traumatic joint environment. The envisioned implanted graft should thus ensure the timed delivery of factors for activating/inhibiting selected pathways on targeted cells. In this regard, possible strategies for the controlled and localised delivery of soluble signals are addressed in the next section.
Concerning the targeted cells for the repair process, a few studies have recently led to the identification of putative joint progenitor cells. They were defined within the joint site as slow-cycling proliferative cells showing numerous stem cell markers, as well as specific traits such as TGFβ receptor II [74] or proteoglycan 4 expression [75] in combination with in vitroassays of colony forming units and differentiation capacity. These cells localise to various joint tissues such as the superficial zone of articular cartilage, the infrapatellar fat pat, the synovium and the groove of Ranvier, and were shown to persist also in the (young) adult organism (reviewed in [76]). The relationship between these hypothetical progenitor cells and their role in articular cartilage regeneration and repair, however, remains largely unknown. If they could be activated to migrate to the site of injury and induced to differentiate, they would represent a promising cell source in addition to, or as a substitute for, implanted MSCs in cartilage TE approaches (reviewed in [25, 77]). In this regard, microfracture has traditionally been exploited as the main strategy for recruiting progenitor cells from the subchondral bone to the site of the injury [78, 79]. Moreover, a number of studies have recently addressed the use of acellular natural or synthetic scaffolds decorated with chemotactic factors as a promising approach to improve the homing of endogenous cells for cartilage regeneration. Generally, these scaffolds demonstrated in rabbit cartilage defect models a superior repair potential compared with control scaffolds. Zhang and colleagues used stromal derived factor-1 (SDF-1) in collagen type 1-based scaffolds for the repair of partial thickness defects. In this study, in contrast to other reports mentioned below, stem/progenitor cells from tissues other than bone marrow were recruited, since the subchondral bone plate was intact [80]. Huang and colleagues utilised a “MSC-affinity peptide” to functionalise a demineralised bone matrix filled with chitosan-based hydrogel in order to enhance full-thickness osteochondral defect repair by a microfracture procedure [81]. Luo et al. demonstrated the synergistic effect of mechanogrowth factor (MFG), an isoform of insulin-growth factor-1 with a chemokine-like function, with a TGFβ3-decorated spongy silk fibroin-based scaffold in osteochondral defect repair in terms of increased MSC recruitment and suppression of fibrocartilage formation [82].
Alternatively, the use of devitalised cartilaginous templates generated by genetically modified MSCs for the generation of the extracellular matrix template [83, 84] may presumably be applied for cartilage TE in order to attract resident stem/progenitor cells. As an example, MSCs overexpressing a potent, matrix-interacting antiangiogenic protein and undergoing early in-vitro chondrogenic differentiation could serve as a template for later decellularisation.
Indirect strategies
Alternative strategies consist of providing cues at the site of the injury to influence the recipient’s environment, which in turn would induce differentiation by indirect activation/inhibition of key molecular pathways. The main cues of a traumatic joint environment have been extensively described in recent reviews [85, 86], and they are beyond the scope of this article. Briefly, key elements playing a role within a traumatic joint environment include (i) the trigger of inflammatory and immune responses and (ii) the increase of vascularisation. These events are not present during embryonic development of cartilage and they have to be considered while building up an efficient MSC-based TE therapeutic approach for cartilage repair, eventually favouring final cartilage regeneration after the implantation of a tissue-engineered graft.
As a first example, inflammatory cues could be exploited and/or modulated in order to improve cartilage repair and the integration of a restored chondral surface to the subchondral bone. It is well known that inflammation is the first phase of tissue repair and that inflammatory cells are crucially involved in the initiation of chondrogenic differentiation and repair processes [87]. Inflammatory cells (i.e., macrophages) have indeed been demonstrated to be key players in healing processes by orchestrating the early regenerative response to injuries [88]. Recent studies have shown that monocytes polarised towards tissue repair – namely anti-inflammatory macrophages (M2) [89] – consistently had a synergistic effect on the cartilage-forming capacity of MSCs in in-vitro co-culture models [90]. Inflammatory cells infiltrating the damaged cartilage area could thus be exploited as a strategy to improve osteochondral repair at injured joint sites by directly enhancing the chondrogenic capacity of implanted/recruited MSCs. This can be accomplished by the development of scaffolds capable of promoting the recruitment/polarisation of tissue-repair macrophages [91] and concomitantly stimulating MSC chondrogenesis through the controlled release of instructive factors.
It is well known that continuous inflammation at the defect site leads to aberrant angiogenesis [92]. This may affect the fate of both cartilage generated through an implanted graft and the repaired tissue itself. Indeed, chondrocytes are exposed to a hypoxic environment from development throughout adulthood and a beneficial effect of low-oxygen conditions on chondrogenic phenotype has been observed in cultured human articular chondrocytes [93, 94]. In contrast, vascular invasion through vascular endothelial growth factor (VEGF) signalling is essential for progression from cartilaginous towards bone tissue during endochondral ossification [95]. Based on this, several studies have addressed the influence of blocking angiogenesis and of low oxygen tension on the stability of engineered cartilage. When muscle-derived stem cells were genetically modified to overexpress a soluble VEGF inhibitor and BMP-4, cartilage formation by these cells, in comparison with cells modified for BMP-4 only, was improved in a rat articular cartilage defect model in both healthy [96] and osteoarthritic conditions [97]. Hyaluronic acid- / fibrin-based scaffold functionalised with bevacizumab (an anti-VEGF drug currently in use) and seeded with nasal chondrocytes reliably developed into cartilaginous tissue stable upon subcutaneous implantation, whereas nonfunctionalised scaffolds were mostly resorbed as a result of vessel-mediated ingrowth of matrix-digesting monocytes [98]. Moreover, in a comparison of the effect of hypoxia and normoxia on MSCs cultured under standard 3D chondrogenic conditions in the presence of TGFβ, suppression of hypertrophic markers and a phenotype resembling articular cartilage was observed [99]. Such hypoxic conditions were necessary for the whole in-vitro culture period and the hypoxia-primed cartilage templates showed a reduced extent of calcification upon ectopic implantation [100]. Collectively, these findings indicate that the inhibition of angiogenesis and the maintenance of a hypoxic environment might be key requisites for functional performance of engineered cartilage repair tissue.
Biomaterials for the controlled delivery of bioactive signals for developmental re-engineering
Once the effective stimuli for stable cartilage repair have been identified (see previous section), a strategy for their efficient delivery to the injury site has to be defined. To this end, we envision immobilising them within grafts and controlling their spatiotemporal release (fig. 3). This requires the exploitation of innovative technologies in the field of biomaterials.

Figure 3
Developmental re-engineering strategy for cartilage repair. We envision the implantation of a graft fitting the site of the injury and able to integrate and release in a spatiotemporally controlled manner either one or several bioactive factors among: (a) developmentally inspired signals to guide either implanted or (b) resident progenitor cells towards a chondrogenic phenotype, (c) factors for the recruitment and polarisation of tissue-repair macrophages or (d) anti-angiogenic factors.
MSC = mesenchymal stromal/stem cell
There are currently numerous techniques available for simple localised delivery of growth factors, in particular by hydrogels based either on biomolecules such as extracellular matrix proteins or polysaccharides, or on synthetic polymers such as poly(ethylene)glycol. Growth factors can be physically incorporated into and immobilised in the gel network by covalent (reviewed in [101]) or affinity binding (reviewed in [102, 103]). Specific tethering of the growth factors allows their controlled release. In the case of covalent attachment, the release can be tuned by additionally inserting cell-responsive linker sequences which are, for example, recognition-sites for metalloproteases. With affinity binding, the affinity of the specific interaction partners governs release kinetics. Furthermore, affinity binding offers the possibility of manipulating the activity of the growth factor. There are hydrogel systems using naturally derived growth factor binding segments from heparin, fibrin and akin that either do not interfere or enhance the activity of the growth factor. In contrast, bound factors are inactive in hydrogels in which binding happens to the active site of the morphogen as, for example, when peptides derived from receptors are included in the gel backbone. Thus, these hydrogel systems can act to sequester and eventually to release growth factors, possibly generating gradients [102].
For the dynamic adjustability of biomaterials targeting temporal control over growth factor release, light-mediated chemistries have been recently introduced as an emerging tool (reviewed in [104]). Methods such as light-activated enzymatic patterning of synthetic hydrogels, in which the light-reversible protection group blocks the recognition site for the enzyme in the gel network, allow the functionalisation of the hydrogel with signalling factors with high spatial resolution while maintaining their bioactivity [105]. However, these strategies allow the material to be tuneable only in vitro prior to implantation. Cartilage is naturally exposed to mechanical loading; therefore, systems in which growth factor release is governed mechanically might lead to the possibility to adjust growth factor delivery after implantation. Moghadam et al. showed that cyclic loading of a hydrogel consisting of thermosensitive nanoparticles and a physically entrapped drug triggers, within a few minutes, a temperature-mediated shrinkage of the nanoparticles that results in higher permeability of the hydrogel and thereby facilitates release of the drug [106]. Moreover, the field of drug release from photosensitive beads has been growing [107] and may also be adapted for TE applications. Lee and colleagues demonstrated that photo-caged adhesion ligand RGD (the tripeptide Arg-Gly-Asp) in subcutaneously implanted hydrogels can be activated and host cell colonisation can be controlled spatiotemporally upon exposure to transdermal light [108].
Moreover, there is increasing evidence that physical properties of the scaffold can steer chondrogenic differentiation. It was shown that glycosaminoglycan-based scaffolds of lower crosslinking density and lower stiffness support cartilaginous matrix accumulation by human MSCs in a chondrogenic medium [109] or induce increased SOX9 expression in rat MSCs in absence of any other differentiation supplements [110]. Therefore, in addition to biochemical factors, the physical parameters of scaffolds also should be taken into account.
Conclusions
In this review, we discuss the possibility to apply the paradigm of “developmental re-engineering” to cartilage repair and regeneration, by following three main steps: learn, simplify/adapt and implement.
According to this vision, the regeneration of functional and stable cartilaginous tissues should start with an understanding of the pathways involved in cartilage embryonic development. A simplification/adaptation step is then proposed to extrapolate from the in-vivo complexity key signals that are specifically efficient on clinically relevant adult stem cells models. Developmentally inspired protocols obtained as outcomes of this second step have finally to be implemented in the native tissue, while considering and possibly exploiting the peculiar features of an adult environment. This would lead to the design of artificial developmental pathways in the attempt to either recapitulate directly developmental events or to alter the adult environment to become compatible with developmental processes. The latter case is exemplified by an anti-angiogenic strategy. During development and throughout life articular cartilage is an avascular tissue, but upon injury vascular ingrowth can occur. Therefore, re-establishing the physiological low oxygen tension by blocking angiogenesis may restore conditions that steer resident or injected mesenchymal progenitor cells towards chondrogenic differentiation [111]. Ultimately, the proposed design of artificial developmental pathways may offer a chance for increasing the robustness of regeneration strategies, especially for those tissues, like cartilage, where after development the self-healing capacity remains limited.
References
1 Tonge D, Pearson M, Jones S. The hallmarks of osteoarthritis and the potential to develop personalised disease-modifying pharmacological therapeutics. Osteoarthritis Cartilage. 2014;22(5):609–21.
2 Minas T. A primer in cartilage repair. J Bone Joint Surg Br, British Volume, 2012. 94(11 Suppl A):141–6.
3 Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, et al. Tissue engineering for articular cartilage repair – the state of the art. Eur Cell Mater. 2013;25(248):e67.
4 Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. Biomed Res Int. 2014. Epub 2014 Aug 18. doi: 10.1155/2014/272481.
5 Niemeyer P, Pestka JM, Kreuz PC, Salzmann GM, Köstler W, Südkamp NP, et al. Standardized cartilage biopsies from the intercondylar notch for autologous chondrocyte implantation (ACI). Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1122–7.
6 Barbero A, Grogan S, Schäfer D, Heberer M, Mainil-Varlet P, Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12(6):476–84.
7 Pelttari K, Pippenger B, Mumme M, Feliciano S, Scotti C, Mainil-Varlet P, et al. Adult human neural crest–derived cells for articular cartilage repair. Sci Transl Med. 2014;6(251):251ra119-251ra119.
8 Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, et al. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(6):889–97.
9 Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, et al. Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PloS one. 2010;5(10):e13246.
10 Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal Cartilage-derived Cells have Stem Cell Properties and are a Highly Potent Cell Source for Cartilage Regeneration. Cell Transplant. 2016;25(3):449–61.
11 Darwiche S, Scaletta C, Raffoul W, Pioletti DP, Applegate LA. Epiphyseal chondroprogenitors provide a stable cell source for cartilage cell therapy. Cell Med. 2012;4(1):23–32.
12 Khan WS, Johnson DS, Hardingham TE. The potential of stem cells in the treatment of knee cartilage defects. Knee. 2010;17(6):369–74.
13 Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155–62.
14 Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3(4):248–69.
15 Cheng A, Hardingham TE, Kimber SJ. Generating cartilage repair from pluripotent stem cells. Tissue Eng Part B Rev. 2013;20(4):257–66.
16 Diekman BO, Christoforou N, Willard VP, Sun H, Sanchez-Adams J, Leong KW, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Natl Acad Sci. 2012;109(47):19172–7.
17 Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6.
18 Mariani E, Facchini A. Clinical applications and biosafety of human adult mesenchymal stem cells. Curr Pharm Des. 2012;18(13):1821–45.
19 Jorgensen C, Noël D. Mesenchymal stem cells in osteoarticular diseases. Regen Med. 2011;6(6s):44–51.
20 Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3(4):25.
21 Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res Ther. 2014;16(5):432.
22 Baugé C, Boumédiene K. Use of adult stem cells for cartilage tissue engineering: current status and future developments. Stem Cells Int. 2015;2015:438026.
23 Kon E, Roffi A, Filardo G, Tesei G, Marcacci M. Scaffold-based cartilage treatments: with or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy. 2015;31(4):767–75.
24 Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev. 2014;20(5):468–76.
25 Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic differentiation of mesenchymal stem cells: challenges and unfulfilled expectations. Tissue Eng Part B Rev. 2014;20(6):596–608.
26 Lv F-J, Tuan RS, Cheung K, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19.
27 Jones E, Schäfer R. Where is the common ground between bone marrow mesenchymal stem/stromal cells from different donors and species? Stem Cell Res Ther. 2015;6(1):1–8.
28 Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, et al. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–66.
29 Gaut C, Sugaya K. Critical review on the physical and mechanical factors involved in tissue engineering of cartilage. Regen Med. 2015;10(5):665–79.
30 Tonnarelli B, Centola M, Barbero A, Zeller R, Martin I. Re-engineering development to instruct tissue regeneration. Curr Top Dev Biol. 2014;108(4):319–38.
31 Lenas P, Moos M Jr, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B Rev. 2009;15(4):381–94.
32 Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42.
33 Occhetta P, Centola M, Tonnarelli B, Redaelli A, Martin I, Rasponi M. High-throughput microfluidic platform for 3D cultures of mesenchymal stem cells, towards engineering developmental processes. Sci Rep. 2015;5:10288.
34 Kim J-Y, Fluri DA, Marchan R, Boonen K, Mohanty S, Singh P, et al. 3D spherical microtissues and microfluidic technology for multi-tissue experiments and analysis. J Biotechnol. 2015;205:24–35.
35 Occhetta P, Glass N, Otte E, Rasponi M, Cooper-White JJ. Stoichiometric control of live cell mixing to enable fluidically-encoded co-culture models in perfused microbioreactor arrays. Integr Biol (Camb). 2016;8(2):194–204.
36 Occhetta P, Malloggi C, Gazaneo A, Redaelli A, Candiani G, Rasponi M. High-throughput microfluidic platform for adherent single cells non-viral gene delivery. RSC Adv. 2015;5(7):5087–95.
37 Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8(11):949–55.
38 Klumpers DD, Mooney DJ, Smit TH. From skeletal development to tissue engineering: lessons from the micromass assay. Tissue Eng Part B Rev. 2015;21(5):427–37.
39 Futrega K, Palmer JS, Kinney M, Lott WB, Ungrin MD, Zandstra PW, et al. The microwell-mesh: A novel device and protocol for the high throughput manufacturing of cartilage microtissues. Biomaterials. 2015;62:1–12.
40 Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014;39:5–10.
41 Decker RS, Koyama E, Pacifici M. Articular Cartilage: Structural and Developmental Intricacies and Questions. Curr Osteoporos Rep. 2015;13(6):407–14.
42 Iwamoto M, Ohta Y, Larmour C, Enomoto-Iwamoto M. Toward regeneration of articular cartilage. Birth Defects Res C Embryo Today. 2013;99(3):192–202.
43 Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104(3):341–51.
44 Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev. 2004;18(19):2404–17.
45 Später D, Hill TP, O’Sullivan RJ, Gruber M, Conner DA, Hartmann C. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis. Development. 2006;133(15):3039–49.
46 Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, et al. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development. 2007;134(11):2159–69.
47 Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280(5368):1455–7.
48 Ray A, Singh PNP, Sohaskey ML, Harland RM, Bandyopadhyay A. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development. 2015;142(6):1169–79.
49 Soeda T, Deng JM, de Crombrugghe B, Behringer RR, Nakamura T, Akiyama H. Sox9-expressing precursors are the cellular origin of the cruciate ligament of the knee joint and the limb tendons. Genesis. 2010;48(11):635–44.
50 Zhang Q, Cigan AD, Marrero L, Lopreore C, Liu S, Ge D, et al. Expression of doublecortin reveals articular chondrocyte lineage in mouse embryonic limbs. Genesis. 2011;49(2):75–82.
51 Koyama E, ShibukawaY, Nagayama M, Sugito H, Young B, Yuasa T, et al. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol. 2008;316(1):62–73.
52 Hyde G, DoverS, Aszodi A, Wallis GA, Boot-Handford RP. Lineage tracing using matrilin-1 gene expression reveals that articular chondrocytes exist as the joint interzone forms. Dev Biol. 2007;304(2):825–33.
53 Oldershaw RA, Baxter MA, LoweET, Bates N, Grady LM, Soncin F, et al. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28(11):1187–94.
54 Craft AM, Ahmed N, Rockel JS, Baht GS, Alman BA, Kandel RA, et al. Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development. 2013;140(12):2597–610.
55 Wu L, Bluguermann C, Kyupelyan L, Latour B, Gonzalez S, Shah S, et al. Human developmental chondrogenesis as a basis for engineering chondrocytes from pluripotent stem cells. Stem Cell Rep. 2013;1(6):575–89.
56 Craft AM, Rockel JS, Nartiss Y, Kandel RA, Alman BA, Keller GM. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(6):638–45.
57 Cleary MA, Osch GJ, Brama PA, Hellingman CA, Narcisi R. FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen Med. 2015;9(4):332–42.
58 Pignatti E, Zeller R, Zuniga A. To BMP or not to BMP during vertebrate limb bud development. Semin Cell Dev Biol. 2014;32:119–27.
59 Bénazet J-D, Pignatti E, Nugent A, Unal E, Laurent F, Zeller R. Smad4 is required to induce digit ray primordia and to initiate the aggregation and differentiation of chondrogenic progenitors in mouse limb buds. Development. 2012;139(22):4250–60.
60 Karamboulas K, Dranse HJ, Underhill TM. Regulation of BMP-dependent chondrogenesis in early limb mesenchyme by TGFβ signals. J Cell Sci. 2010;123(12):2068–76.
61 Liao J, Hu N, Zhou N, Lin L, Zhao C, Yi S. et al., Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PloS one. 2014;9(2):e89025.
62 Centola M, Tonnarelli B, Schären S, Glaser N, Barbero A, Martin I. Priming 3D cultures of human mesenchymal stromal cells toward cartilage formation via developmental pathways. Stem Cells Dev. 2013;22(21):2849–58.
63 Narcisi R, Cleary MA, Brama PA, Hoogduijn MJ, Tüysüz N, ten Berge D, et al. Long-term expansion, enhanced chondrogenic potential, and suppression of endochondral ossification of adult human MSCs via WNT signaling modulation. Stem Cell Rep. 2015;4(3):459–72.
64 Leijten J, Emons J, Sticht C, Van Gool S, Decker E, Uitterlinden A, et al. Gremlin 1, Frizzled‐related protein, and Dkk‐1 are key regulators of human articular cartilage homeostasis. Arthritis Rheum. 2012;64(10):3302–12.
65 Martin I, Muraglia A, Campanile G, Cancedda R, Quarto R. Fibroblast Growth Factor-2 Supports ex Vivo Expansion and Maintenance of Osteogenic Precursors from Human Bone Marrow 1. Endocrinology. 1997;138(10):4456–62.
66 Correa D, Somoza R, LinP, Greenberg S, Rom E, Duesler L, et al. Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation. Osteoarthritis Cartilage. 2015;23(3):443–53.
67 Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–6.
68 Mueller MB, Tuan RS. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008;58(5):1377–88.
69 Fischer J, Aulmann A, Dexheimer V, Grossner T, Richter W. Intermittent PTHrP (1–34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev. 2014;23(20):2513–23.
70 Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223(1):84–93.
71 Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng Part A. 2011;18(7-8):715–24.
72 O’Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther. 2013;4(4):1.
73 Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16(3):599–610.
74 Li T, Longobardi L, Myers TJ, Temple JD, Chandler RL, Ozkan H, et al, Joint TGF-β type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev. 2012;22(9):1342–59.
75 Kozhemyakina E, ZhangM, Ionescu A, Ayturk UM, Ono N, Kobayashi A, et al. Identification of a Prg4-Expressing Articular Cartilage Progenitor Cell Population in Mice. Arthritis Rheumatol. 2015;67(5):1261–73.
76 Iwamoto M, Ohta Y, Larmour C, Enomoto-Iwamoto M. Toward regeneration of articular cartilage. Birth Defects Res C Embryo Today. 2013;99(3):192–202.
77 Stoddart MJ, Bara J, Alini M. Cells and secretome – towards endogenous cell re-activation for cartilage repair. Adv Drug Deliv Rev. 2015;84:135–45.
78 Kristjánsson B, Honsawek S. Current perspectives in mesenchymal stem cell therapies for osteoarthritis. Stem Cells Int. 2014. Epub 2014 Dec 8. doi: 10.1155/2014/194318. .
79 Park MS, Kim YH, Jung Y, Kim SH, Park JC, Yoon DS, et al. In situ recruitment of human bone marrow-derived mesenchymal stem cells using chemokines for articular cartilage regeneration. Cell Transplant. 2015;24(6):1067–83.
80 Zhang W, Chen J, Tao J, Jiang Y, Hu C, Huang L, et al. The use of type 1 collagen scaffold containing stromal cell-derived factor-1 to create a matrix environment conducive to partial-thickness cartilage defects repair. Biomaterials. 2013;34(3):713–23.
81 Huang H, Zhang X, Hu X, Shao Z, Zhu J, Dai L, et al. A functional biphasic biomaterial homing mesenchymal stem cells for in vivo cartilage regeneration. Biomaterials. 2014;35(36):9608–19.
82 Luo Z, Jiang L, Xu Y, Li H, Xu W, Wu S, et al. Mechano growth factor (MGF) and transforming growth factor (TGF)-β3 functionalized silk scaffolds enhance articular hyaline cartilage regeneration in rabbit model. Biomaterials. 2015;52:463–75.
83 Bourgine PE, Scotti C, Pigeot S, Tchang LA, Todorov A, Martin I. Osteoinductivity of engineered cartilaginous templates devitalized by inducible apoptosis. Proc Natl Acad Sci. 2014;111(49):17426–31.
84 Gawlitta D, Benders KE, Visser J, van der Sar AS, Kempen DH, Theyse LF, et al. Decellularized cartilage-derived matrix as substrate for endochondral bone regeneration. Tissue Eng Part A. 2014;21(3-4):694–703.
85 Caldwell K, Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthritis Cartilage. 2015;23(3):351–62.
86 Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4(4):269–85.
87 Caron MM, Welting TJ, van Rhijn LW, Emans. Targeting Inflammatory Processes for Optimization of Cartilage Homeostasis and Repair Techniques. In: Emans P., Peterson L, editors. Developing Insights in Cartilage Repair. 1st ed. London: Springer-Verlag; 2014. p. 43–63.
88 Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013;110(23):9415–20.
89 Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55.
90 Sesia SB, Duhr R, Medeiros da Cunha C, Todorov A, Schaeren S, Padovan E, et al. Anti-Inflammatory/Tissue Repair Macrophages Enhance the Cartilage-Forming Capacity of Human Bone Marrow‐Derived Mesenchymal Stromal Cells. J Cell Physiol. 2015;230(6):1258–69.
91 Hoemann CD, Chen G, Marchand C, Tran-Khanh N, Thibault M, Chevrier A, et al. Scaffold-guided subchondral bone repair implication of neutrophils and alternatively activated arginase-1+ macrophages. Am J Sports Med. 2010;38(9):1845–56.
92 Suri S, Gill SE, de Camin SM, McWilliams DF, Wilson D, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheum Dis. 2007;66(11):1423–8.
93 Lafont JE, Talma S, Hopfgarten C, Murphy CL. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and-independent pathways. J Biol Chem. 2008;283(8):4778–86.
94 Lafont JE, Talma S, Murphy CL. Hypoxia-inducible factor 2α is essential for hypoxic induction of the human articular chondrocyte phenotype. Arthritis Rheum. 2007;56(10):3297–306.
95 Gerber H-P, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–8.
96 Kubo S, Cooper GM, Matsumoto T, Phillippi JA, Corsi KA, Usas A, et al. Blocking vascular endothelial growth factor with soluble Flt-1 improves the chondrogenic potential of mouse skeletal muscle–derived stem cells. Arthritis Rheum. 2009;60(1):155–65.
97 Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, et al. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble flt-1. Arthritis Rheum. 2009;60(5):1390–405.
98 Centola M, Abbruzzese F, Scotti C, Barbero A, Vadala G, Denaro V, et al. Scaffold-based delivery of a clinically relevant anti-angiogenic drug promotes the formation of in vivo stable cartilage. Tissue Eng Part A. 2013;19(17-18):1960–71.
99 Lee H-H, Chang C-C, Shieh M-J, Wang J-P, Chen Y-T, Young T-H, et al. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Scientific Rep. 2013. 3.
100 Leijten J, Georgi N, Teixeira LM, van Blitterswijk CA, Post JN, Karperien M. Metabolic programming of mesenchymal stromal cells by oxygen tension directs chondrogenic cell fate. Proc Natl Acad Sci U S A. 2014;111(38):13954–9.
101 Reed S, Wu B. Sustained growth factor delivery in tissue engineering applications. Ann Biomed Eng. 2014;42(7):1528–36.
102 Belair DG, Le NN, Murphy WL. Design of growth factor sequestering biomaterials. Chem Commun (Camb). 2014;50(99):15651–68.
103 Vulic K, Shoichet MS. Affinity-based drug delivery systems for tissue repair and regeneration. Biomacromolecules. 2014;15(11):3867–80.
104 Grim JC, Marozas IA, Anseth KS. Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels. J Control Release. 2015;219:95–106.
105 Mosiewicz KA, Kolb L, van der Vlies AJ, Martino MM, Lienemann PS, Hubbell JA, et al. In situ cell manipulation through enzymatic hydrogel photopatterning. Nat Mater. 2013;12(11):1072–8.
106 Moghadam MN, Kolesov V, Vogel A, Klok H-A, Pioletti DP. Controlled release from a mechanically-stimulated thermosensitive self-heating composite hydrogel. Biomaterials. 2014;35(1):450–5.
107 Timko BP, Arruebo M, Shankarappa SA, McAlvin JB, Okonkwo OS, Mizrahi B, et al. Near-infrared–actuated devices for remotely controlled drug delivery. Proc Natl Acad Sci U S A. 2014;111(4):1349–54.
108 Lee TT, García JR, Paez JI, Singh A, Phelps EA, Weis S, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mat. 2015;14(3):352–60.
109 Bian L, Hou C, Tous E, Rai R, Mauck RL, Burdick JA. The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials. 2013;34(2):413–21.
110 Murphy CM, Matsiko A, Haugh MG, Gleeson JP, O’Brien FJ. Mesenchymal stem cell fate is regulated by the composition and mechanical properties of collagen–glycosaminoglycan scaffolds. J Mech Behav Biomed Mater. 2012;11:53–62.
111 Marsano A, Medeiros da Cunha CM, Ghanaati S, Gueven S, Centola M, Tsaryk R, et al. Spontaneous in vivo chondrogenesis of bone barrow-derived mesenchymal progenitor cells by blocking vascular endothelial growth factor signaling. Stem Celle Transl Med. 2016 Jul 26. pii: sctm.2015-0321. [Epub ahead of print]


