Postponed pregnancies and risks of very advanced maternal age
DOI: https://doi.org/10.4414/smw.2016.14330
Christian
Haslinger, Bernhard
Stoiber, Federica
Capanna, Marie-Kristin
Schäffer, Roland
Zimmermann, Leonhard
Schäffer
Summary
QUESTIONS UNDER STUDY: To evaluate pregnancy outcome in pregnant women aged ≥45 years, termed very advanced maternal age (VAMA).
METHODS: We retrospectively compared the outcome of pregnancies in VAMA patients with controls aged 30 years at time of delivery. Subgroups of singleton and multiple pregnancies were also analysed. Incidences of maternal and fetal adverse outcomes were measured. Statistical significance was set at p <0.05. Odds ratios (ORs) were adjusted where necessary.
RESULTS: One hundred and twenty-seven VAMA pregnancies and 2066 control pregnancies of women aged 30 years were analysed. VAMA pregnancies had a higher rate of maternal complications such as gestational hypertension (3.9% vs 0.6%; OR 6.5), preeclampsia (14.2% vs 3.0%; OR 5.4, adjusted OR 4.4) and gestational diabetes (12.6% vs 3.6%; OR 3.8). Likewise, increased need for blood transfusion (3.2% vs 0.7%; OR 4.8, adjusted OR 4.4) and prolonged hospitalisation >7 days (37.8% vs 15.1%; OR 3.42) was found. Infant complications such as prematurity (44.9% vs 16.2%; OR 4.2) and low birthweight <5th percentile (11.0% vs 5.6%; OR 2.1) were also increased.
CONCLUSION: Pregnant women of very advanced maternal age (≥45 years) have significantly increased maternal and fetal risks. Women postponing pregnancy or planning a pregnancy in very advanced age should be informed about these risks, in particular before artificial reproductive technologies are applied or “social freezing”.
Introduction
In recent decades, a trend of shifted family planning towards higher maternal age for socioeconomic and lifestyle reasons could be observed in western countries [1]. At the same time there were reasonable advances in artificial reproductive technologies (ART) and these became more and more easily accessible. Lately, some big companies even began to offer egg freezing to their female employees (social freezing) in order to keep them longer at work without a baby break. These trends might lead to an increase in pregnancies of women of perimenopausal age. Social freezing may be the optimal strategy for companies to employ young, cheap and powerful females and keep them attached to the company in their most productive phase. However, the propagated advantages of total gender equality owing to biological independence may be at the expense of these mothers and their children. Most importantly, the risk of stillbirth clearly increases with maternal age [2–4]. In a Californian cohort, stillbirth was twice as common in women 35 years of age or older as in those younger than 35 years [2]. Women of very advanced maternal age (VAMA) had a 2.4-fold risk increase for stillbirth compared with women aged 20–24 years in a Canadian study [3] and a 2.2-fold risk increase as compared to women aged 25–30 years in the US [4]. While there are some data about increasing risks for adverse perinatal outcome with increasing maternal age [5, 6], others show “generally good” outcomes as well [7, 8]. Very advanced maternal age is defined as maternal age of 45 years or older at the time of delivery [7, 9]. Women of VAMA have a higher likelihood for multiple pregnancies due to multiple ovarian follicular development after extensive follicle-stimulating hormone (FSH) release as well as due to the use of ART [10], which in itself leads to increased risks during pregnancy. However, little is known concerning risks in singleton compared with multiple pregnancies in VAMA women, with separate controls. The objective of this study was, therefore, not only to evaluate perinatal risks in VAMA pregnancies as compared with 30-year-old controls in a single tertiary centre, but also to stratify between the outcome of singleton and multiple pregnancies.
Methods
A retrospective cohort study was conducted using data from our electronic database Perinat 5.0. This database is a local comprehensive electronic health record containing all diagnoses and parameterised detailed clinical data about the course of pregnancy, delivery, maternal, and infant outcome.
The study had ethical approval according to the Institutional Review Board decision for the use of anonymised patient data for medical research (13 April 2000 and 1 March 2012).
All deliveries in the University Hospital of Zürich between January 1996 and December 2012 were analysed. A total of 36 328 deliveries fulfilled the inclusion criteria (≥24 weeks of gestation or ≥500 g fetal weight).
Information on the following parameters was collected: maternal age at delivery, parity, gestational age at delivery, delivery mode, number of fetuses, occurrence of pregestational diabetes mellitus, gestational diabetes mellitus, chronic hypertension, gestational hypertension, preeclampsia, HELLP syndrome (haemolysis, elevated liver enzymes, low platelet count), transfusion of blood products, infant birthweight, obesity and duration of hospitalisation after delivery.
The main study group consisted of all women with VAMA (aged 45 years or older at time of delivery). This group of women was compared with all women aged 30 years at time of delivery during the same study period. Thirty-year-old women were chosen as controls because the mean maternal age at delivery in Switzerland during the observed period was 30.4 years [11].
Statistical methods
Statistical analysis was conducted using Sigmaplot 12.0 (Systat Software Inc., California, USA). The level of statistical significance was set at p <0.05. Baseline characteristics (age, parity, prior caesarean section, obesity (defined as body mass index ≥30 kg/m2), chronic hypertension, pregestational diabetes mellitus) were compared using the chi-square test. Incidence of multiple fetus pregnancies, hypertensive disorders (gestational hypertension, preeclampsia, eclampsia and HELLP syndrome), metabolic disorders (gestational diabetes mellitus), transfusion of packed red cells, prolonged hospital stay (>7 days) after delivery, placenta praevia, caesarean section, and infant outcome (preterm birth <37/34/32 weeks of gestation, infant birthweight <5th percentile) in both groups was compared using the chi-square test. Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) for the occurrence of the above mentioned diagnoses in VAMA women as compared with 30-year-old controls were calculated. A logistic regression analysis was conducted with calculation of adjusted odds ratios for the incidence of preeclampsia (adjusted for chronic hypertension) and transfusion of blood products (adjusted for prior caesarean section), as baseline analysis revealed a statistically significant difference regarding chronic hypertension and prior caesarean section between groups. In a subgroup analysis, singleton and multiple fetus pregnancies in VAMA and 30-year-old women were compared separately for the most important diagnoses.
Results
From 36 328 included deliveries, 127 VAMA women (0.35%) and 2066 30-year-old women (5.69%) were identified. Mean maternal age was 47.0 (standard deviation 2.5) and 30.0 (SD 0.4) years, respectively. In our study population, a statistically significant difference for prior caesarean section (9.5% vs 3.4%) and chronic hypertension (7.9% vs 0.7%) between VAMA and 30-year-old women (p <0.01 for each) was observed. No difference was observed for parity, obesity and pre-existing diabetes mellitus (table 1).
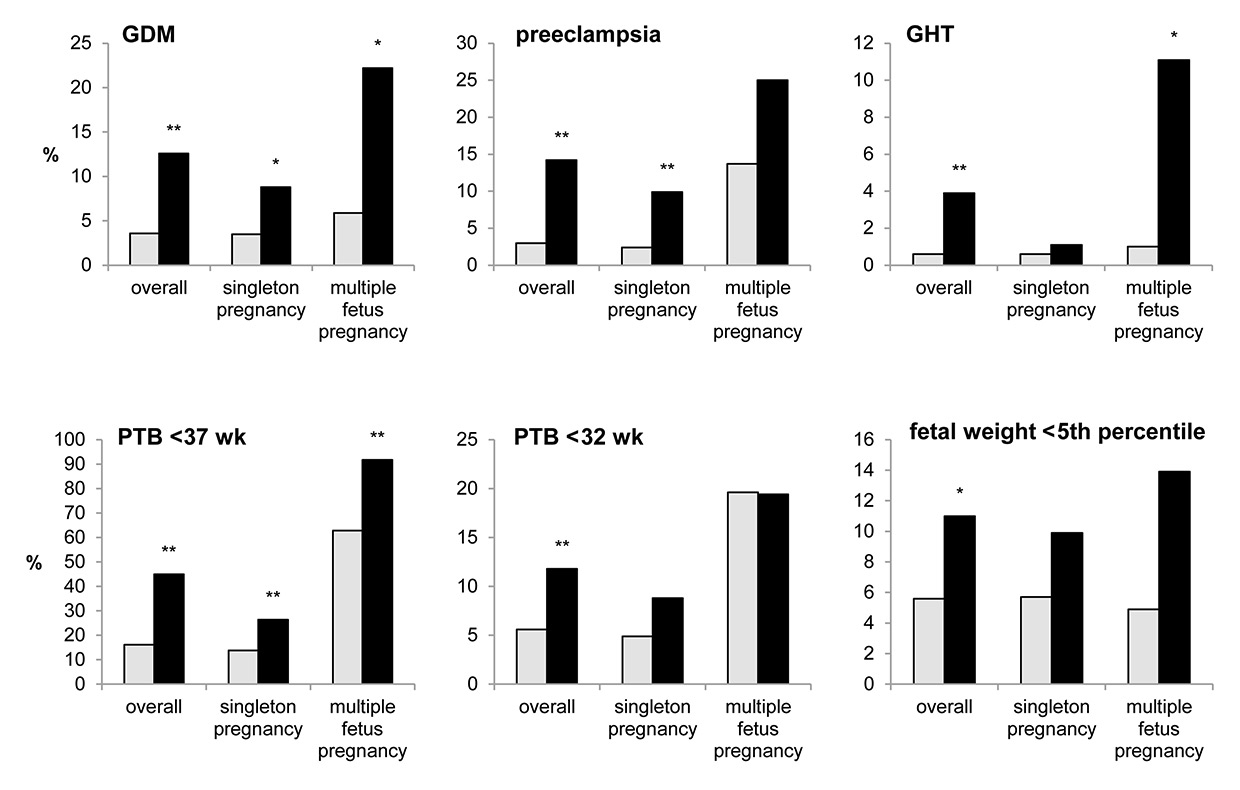
Figure 1
Incidence (%) of adverse maternal and infant outcome in very advanced maternal age (VAMA) patients (black) and 30-year-old patients (grey) within the overall group and within subgroups (singleton and multiple-fetus pregnancies).
GDM = gestational diabetes mellitus; GHT =: gestational hypertension; PTB = preterm birth; wk = weeks of gestation
** p<0.01, * p<0.05 (chi-square test)
In pregnancies of VAMA mothers, maternal morbidity was significantly increased. Compared with 30-year-old women, the presence of gestational hypertension (3.9% vs 0.6%, OR 6.5 95% CI 2.3–18.5, p <0.001), preeclampsia (14.2% vs 3.0%, OR 5.4 95% CI 3.1–9.5, p <0.001) and gestational diabetes mellitus (12.6% vs 3.6%, OR 3.8 95% CI 2.2-6.8, p <0.001) was significantly higher. Likewise, the rate of caesarean section (76.4% vs 37.5%, OR 5.4 95% CI 3.5–8.2, p <0.001), transfusion of packed red cells (3.2% vs 0.7%, OR 4.8 95% CI 1.6-14.7, p = 0.01) and prolonged hospitalisation after delivery >7 days (37.8% vs 15.1%, OR 3.4 95% CI 2.3–5.0, p <0.001) was increased. With respect to infants, VAMA pregnancies had a significantly higher incidence of multiple pregnancy (28.4% vs 4.9%, OR 7.6 95% CI 4.9–11.8, p <0.001), preterm birth (PTB) <37 weeks of gestation (44.9% vs 16.2%, OR 4.2 95% CI 2.9–6.1, p <0.001), PTB <34 weeks of gestation (17.3% vs 8.6%, OR 2.2 95% CI 1.4–3.6, p <0.01), PTB <32 weeks of gestation (11.8% vs 5.6%, OR 2.3 95% CI 1.3–4.0, p <0.01) and infant birthweight <5th percentile (11.0% vs 5.6%, OR 2.1 95% CI 1.3–2.7, p = 0.02) as compared with 30-year-old women (table 2, fig. 1). After adjustment of preeclampsia and maternal transfusion for relevant factors, morbidity remained significant. Preeclampsia was adjusted for chronic hypertension (adjusted OR 4.4 95% CI 2.4–8.0) and transfusion of packed red cells was adjusted for prior caesarean section (adjusted OR 4.4 95% CI 1.4–13.8).
In a subgroup analysis, singleton and multiple pregnancies in VAMA and 30-year-old women were compared separately. In VAMA women, 91 singleton pregnancies and 36 multiple pregnancies were observed and compared with 1964 singleton and 102 multiple pregnancies in 30-year-old women. For singleton pregnancies, the development of preeclampsia (9.9% vs 2.4 %, OR 4.5, adjusted OR 8.6 95% CI 2.9–25.8, p <0.001), gestational diabetes mellitus (8.8% vs 3.5%, OR 2.7 95% CI 1.2–5.7, p = 0.02) and preterm birth <37 weeks of gestation (26.4% vs 13.8%, OR 2.3 95% CI 1.4–3.7, p = 0.001) was found to be significantly increased in VAMA pregnancies compared with those of 30-year-old women (table 3, fig. 1).
In multiple pregnancies, VAMA women had a statistically significant higher incidence of gestational diabetes mellitus (22.2% vs 5.9%, OR 4.6 95% CI 1.5–14.3, p = 0.01), gestational hypertension (11.1% vs 1.0%, OR 12.6 95% CI 1.4–117.1, p = 0.02), prolonged hospitalisation >7 days after delivery (83.3% vs 40.2%, OR 7.4 95% CI 2.8–19.5, p <0.001) and preterm birth <37 weeks of gestation (91.7% vs 62.8%, OR 6.5 95% CI 1.9–22.8, p <0.01) (table 3, fig. 1).
|
Table 1:Study population. |
| |
30-year-old women
(n = 2066)
|
VAMA
(n = 127)
|
p-value
|
OR (95% CI)
|
| Age (mean (SD); in years) |
30.0
(0.4) |
47.0
(2.5) |
|
|
| Chronic hypertension |
0.7%
(15) |
7.9%
(10) |
<0.001 |
11.69 (5.14–26.58) |
| Pregestational diabetes mellitus |
0.6%
(12) |
1.6%
(2) |
0.43 |
2.74 (0.61–12.37) |
| Obesity (BMI ≥30 kg/m2) |
6.0%
(123) |
6.3%
(8) |
0.97 |
1.06 (0.51–2.22) |
| Primiparity |
50.4%
(1041) |
43.3%
(55) |
0.15 |
|
| Prior caesarean section |
3.4%
(70) |
9.5%
(12) |
0.001 |
2.98 (1.57–5.65) |
| BMI = body mass index; CI = confidence Interval; OR = odds ratio; SD = standard deviation; VAMA = very advanced maternal age (≥45 years)
Data are expressed as % (n) or as indicated; p-values are from the chi-square test; ORs in bold type are statistically significant |
|
Table 2:Outcome of VAMA women in comparison with 30-year-old women. |
| |
30-year-old women
(n = 2066)
|
VAMA
(n = 127)
|
OR
(95% CI)
|
p-value
|
Adjusted OR
(95% CI)
|
| Maternal diagnoses |
Gestational diabetes mellitus* |
3.6% (75) |
12.6% (16) |
3.83 (2.16–6.78) |
<0.001 |
|
| Gestational hypertension* |
0.6% (13) |
3.9% (5) |
6.47 (2.27–18.45) |
<0.001 |
|
| Preeclampsia* |
3.0% (61) |
14.2% (18) |
5.43 (3.10–9.50) |
<0.001 |
4.41 (2.46–7.97)†
|
| Eclampsia + HELLP |
1.1% (22) |
1.6% (2) |
1.46 (0.34–6.29) |
0.94 |
|
| Peripartum
diagnoses |
Twin pregnancy* |
4.7% (98) |
26.0% (33) |
7.05 (4.52–11.01) |
<0.001 |
|
| Triplet pregnancy* |
0.2% (4) |
2.4% (3) |
12.47 (2.76–56.34) |
<0.001 |
|
| Multiple pregnancy* |
4.9% (102) |
28.4% (36) |
7.62 (4.93–11.76) |
<0.001 |
|
| Placenta praevia |
0.7% (15) |
1.6% (2) |
2.19 (0.50–9.67) |
0.59 |
|
| Caesarean section* |
37.5% (775) |
76.4% (97) |
5.39 (3.54–8.19) |
<0.001 |
|
| Transfusion of packed red cells* |
0.7% (14) |
3.2% (4) |
4.77 (1.55–14.70) |
0.01 |
4.41 (1.41–13.78)‡
|
| Prolonged hospitalisation (>7 days)* |
15.1% (312) |
37.8% (48) |
3.42(2.34–4.99) |
<0.001 |
|
| Neonatal diagnoses |
PTB <37 weeks* |
16.2% (334) |
44.9% (57) |
4.22 (2.92–6.11) |
<0.001 |
|
| PTB <34 weeks* |
8.6% (178) |
17.3% (22) |
2.22 (1.37–3.61) |
<0.01 |
|
| PTB <32 weeks* |
5.6% (116) |
11.8% (15) |
2.25 (1.27–3.98) |
<0.01 |
|
| Birthweight <5th percentile* |
5.6% (116) |
11.0% (14) |
2.08 (1.16–3.74) |
0.02 |
|
| CI = Confidence Interval; HELLP = Haemolysis, Elevated Liver enzymes, Low Platelet count; OR = Odds ratio; PTB = preterm birth; VAMA = very advanced maternal age (≥45 years)
Data are expressed as % (n); p-values are from the chi-square test; ORs and adjusted ORs in bold type are statistically significant
* p <0.05
† adjusted for chronic hypertension
‡ adjusted for prior caesarean section |
|
Table 3:Subgroup analysis: singleton and multiple pregnancies. |
| |
|
30-year-old patients
(n = 2066)
|
VAMA
(n = 127)
|
OR (95% CI)
|
p-value
|
Adjusted OR
(95% CI)
|
| Maternal diagnoses |
Gestational diabetes mellitus |
Single* |
3.5% (69) |
8.8% (8) |
2.65 (1.23–5.69) |
0.02 |
|
| Multiple* |
5.9% (6) |
22.2% (8) |
4.57 (1.46–14.28) |
0.01 |
|
| Gestational hypertension |
Single |
0.6% (12) |
1.1% (1) |
1.81 (0.23–14.05) |
0.92 |
|
| Multiple* |
1.0% (1) |
11.1% (4) |
12.63 (1.36–117.07) |
0.02 |
|
| Preeclampsia |
Single* |
2.4% (47) |
9.9% (9) |
4.48 (2.12–9.45) |
<0.001 |
8.58 (2.85–25.84)†
|
| Multiple |
13.7% (14) |
25% (8) |
2.1 (0.82–5.37) |
0.19 |
1.76 (0.64–4.83)†
|
| Eclampsia + HELLP |
Single |
0.9% (18) |
2.2% (2) |
2.43 (0.56–10.63) |
0.50 |
|
| Multiple |
3.9% (4) |
0% (0) |
|
0.53 |
|
| Peripartum diagnoses |
Transfusion of packed red cells |
Single |
0.6% (11) |
2.2% (2) |
3.99 (0.87–18.27) |
0.21 |
3.36 (0.71–15.89)‡
|
| Multiple |
2.9% (3) |
5.6% (2) |
1.94 (0.31–12.12) |
0.84 |
1.98 (0.32–12.37)‡
|
| Prolonged hospitalisation (>7d) |
Single |
13.8% (271) |
19.8% (18) |
1.54 (0.91–2.62) |
0.15 |
|
| Multiple* |
40.2% (41) |
83.3% (30) |
7.44 (2.84–19.46) |
<0.001 |
|
| Neonatal diagnoses |
PTB <37 weeks |
Single* |
13.8% (270) |
26.4% (24) |
2.25 (1.39–3.65) |
0.001 |
|
| Multiple* |
62.8% (64) |
91.7% (33) |
6.53 (1.88–22.76) |
0.002 |
|
| PTB <34 weeks |
Single |
7.4% (146) |
12.1% (11) |
1.71 (0.89–3.29) |
0.15 |
|
| Multiple |
31.4% (32) |
30.6% (11) |
0.96 (0.42–2.19) |
0.91 |
|
| PTB <32 weeks |
Single |
4.9% (96) |
8.8% (8) |
0.53 (0.25–1.13) |
0.16 |
|
| Multiple |
19.6% (20) |
19.4% (7) |
0.99 (0.38–2.58) |
0.82 |
|
| Birth weight <5th percentile |
Single |
5.7% (111) |
9.9% (9) |
1.83 (0.90–3.74) |
0.15 |
|
| Multiple |
4.9% (5) |
13.9% (5) |
3.13 (0.85–11.53) |
0.16 |
|
| CI = confidence interval; HELLP = Haemolysis, Elevated Liver enzymes, Low Platelet count; OR = odds ratio; PTB = preterm birth; VAMA = very advanced maternal age (≥45 years)
Data are expressed as % (n); p-values are from the chi-square test; ORs and adjusted ORs in bold type are statistically significant
* p <0.05
† adjusted for chronic hypertension
‡ adjusted for prior caesarean section |
Discussion
Our data show statistically significant higher risks for pregnancies in women of very advanced maternal age (≥45 years) for maternal and infant morbidity. Thirty-year-old women were selected as control group because the mean age of women at the time of delivery was 30.4 years in Switzerland during the observed period (1996–2012) [11]. As expected, there were significantly more multiple pregnancies in VAMA women than in 30-year-old women. Even though we believe that this fact should not be seen as a confounder but rather as a consequence of VAMA, as these women have a higher likelihood for multiple pregnancies, we conducted a subgroup analysis of singleton and multiple pregnancies separately to exclude this putative confounder. Our data reveal that not only in multiple but also in singleton pregnancies increased risks for maternal and infant morbidity are clearly present. Some parameters missed the level of statistical significance, possibly owing to smaller numbers in the subgroup analysis. Therefore, multiple pregnancy is not the only cause for the increased occurrence of adverse outcome in VAMA women.
Our data confirm the results of previous studies that describe higher rates of pregnancy and perinatal complications for VAMA women [5, 9, 12, 13]. However, although an increased rate of multiple pregnancies was observed, none of these studies analysed singleton and multiple pregnancies separately in detail. Interestingly, there are two studies that report a generally good pregnancy outcome for VAMA women [7, 8]. In both of these studies, the rate of naturally conceiving VAMA women was relatively high (96% [7] and 84% [8]). However, both studies lacked an adequate control group and thus conclusions are difficult to draw. Unfortunately, in our study we did not survey the rate of women with previous use of ART. It might be interesting to compare the outcome of VAMA women who conceived naturally with VAMA women who became pregnant through ART in a further study. The organism of naturally conceiving VAMA women might be biologically younger and thus more stable during the strain of pregnancy. As our data did not include information about stillbirth, this important outcome variable could not be analysed.
A strength of our study is the relatively large number of women included in a single centre study. Our electronic database allows solid documentation of the requested information, which is filled in prospectively. Documentation is completed for every woman before discharge and supervised by the attending consultant. Hence, the database allows a reliable retrospective analysis. At the same time, as in any single centre study, a possible selection bias cannot be excluded as the experience of only one centre is included in the analysis. A possible drawback is the diminished number of women with adverse outcome in the subgroup analysis, above all in multiple pregnancies.
A clinically useful aspect of our study is the subgroup analysis, which allows a personalised risk assessment in pregnant VAMA women. Of our VAMA women, 28.4% had multiple pregnancies, and thus it is important to know the risks for singleton and multiple pregnancies on a separate basis.
Furthermore, implications for the public healthcare system should be considered, because with rising rates of VAMA women these implications will gain increasing public and political attention. We can show that VAMA is associated with increased direct costs (prolonged hospital stay). Increased indirect costs (maternal complications, infant complications due to prematurity) for the general public can be assumed.
There is a tendency towards increasing maternal age in western countries. Due to favourable advances in artificial reproductive medicine and changes of socioeconomic and lifestyle issues, very advanced maternal age is also likely to increase. We believe that women who plan a pregnancy at the age of 45 years or older should be informed about the increased risks for metabolic, hypertensive, peripartum and neonatal adverse outcome, especially before the use of ART and even more when they choose to postpone a wished pregnancy to a period of their life that is associated with higher risks for themselves and their offspring. This topic is even more relevant in times of company-paid egg-freezing proposals.
Authors contribution: C Haslinger: Protocol development, Data collection, Data analysis, Manuscript writing, B Stoiber: Data collection, Manuscript editing, F Capanna: Data analysis, Manuscript editing, MK Schäffer: Data collection, Manuscript editing, R Zimmermann: Manuscript editing, Supervision, L Schäffer: Protocol development, Manuscript writing
References
1 Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews T. Births: final data for 2011. Natl Vital Stat Rep. 2013;62:1–69, 72.
2 Miller DA. Is advanced maternal age an independent risk factor for uteroplacental insufficiency? Am J Obstet Gynecol. 2005;192:1974–1980; discussion 1980–1982. doi:10.1016/j.ajog.2005.02.050
3 Balayla J, Azoulay L, Assayag J, Benjamin A, Abenhaim HA. Effect of maternal age on the risk of stillbirth: a population-based cohort study on 37 million births in the United States. Am J Perinatol. 2011;28:643–50. doi:10.1055/s-0031-1276739
4 Canterino JC, Ananth CV, Smulian J, Harrigan JT, Vintzileos AM. Maternal age and risk of fetal death in singleton gestations: USA, 1995–2000. J Matern Fetal Neonatal Med. 2004;15:193–7. doi:10.1080/14767050410001668301
5 Yogev Y, Melamed N, Bardin R, Tenenbaum-Gavish K, Ben-Shitrit G, Ben-Haroush A. Pregnancy outcome at extremely advanced maternal age. Am J Obstet Gynecol. 2010;203:558.e1–7. doi:10.1016/j.ajog.2010.07.039
6 Simchen MJ, Yinon Y, Moran O, Schiff E, Sivan E. Pregnancy outcome after age 50. Obstet Gynecol. 2006;108:1084–8. doi:10.1097/01.AOG.0000240139.46018.bd
7 Dildy GA, Jackson GM, Fowers GK, Oshiro BT, Varner MW, Clark SL. Very advanced maternal age: pregnancy after age 45. Am J Obstet Gynecol. 1996;175:668–74.
8 Callaway LK, Lust K, McIntyre HD. Pregnancy outcomes in women of very advanced maternal age. Aust N Z J Obstet Gynaecol. 2005;45:12–6. doi:10.1111/j.1479-828X.2005.00333.x
9 Glasser S, Segev-Zahav A, Fortinsky P, Gedal-Beer D, Schiff E, Lerner-Geva L. Primiparity at very advanced maternal age (≥45 years). Fertil Steril. 2011;95:2548–51. doi:10.1016/j.fertnstert.2011.02.031
10 Beemsterboer SN, Homburg R, Gorter NA, Schats R, Hompes PG, Lambalk CB. The paradox of declining fertility but increasing twinning rates with advancing maternal age. Hum Reprod. 2006;21:1531–2. doi:10.1093/humrep/del009
11 Bundesamt für Statistik Schweiz. Lebendgeburten nach Alter der Mutter 1970–2013. 2014. http://www.bfs.admin.ch/bfs/portal/de/index/themen/01/06/blank/data/01.html
12 Carolan MC, Davey M-A, Biro M, Kealy M. Very advanced maternal age and morbidity in Victoria, Australia: a population based study. BMC Pregnancy Childbirth. 2013;13:80. doi:10.1186/1471-2393-13-80
13 Jacobsson B, Ladfors L, Milsom I. Advanced maternal age and adverse perinatal outcome. Obstet Gynecol. 2004;104:727–33. doi:10.1097/01.AOG.0000140682.63746.be
