Heterogeneity in testing practices for infections during pregnancy: national survey across Switzerland
DOI: https://doi.org/10.4414/smw.2016.14325
Karoline
Aebi-Popp, Christian
Kahlert, Andri
Rauch, Beatrice
Mosimann, David
Baud, Nicola
Low, Daniel
Surbek
Summary
QUESTION: Detection and treatment of infections during pregnancy are important for both maternal and child health. The objective of this study was to describe testing practices and adherence to current national guidelines in Switzerland.
METHODS: We invited all registered practicing obstetricians and gynaecologists in Switzerland to complete an anonymous web-based questionnaire about strategies for testing for 14 infections during pregnancy. We conducted a descriptive analysis according to demographic characteristics.
RESULTS: Of 1138 invited clinicians, 537 (47.2%) responded and 520 (45.6%) were eligible as they are currently caring for pregnant women. Nearly all eligible respondents tested all pregnant women for group B streptococcus (98.0%), hepatitis B virus (HBV) (96.5%) and human immunodeficiency virus (HIV) (94.7%), in accordance with national guidelines. Although testing for toxoplasmosis is not recommended, 24.1% of respondents tested all women and 32.9% tested at the request of the patient. Hospital doctors were more likely not to test for toxoplasmosis than doctors working in private practice (odds ratio [OR] 2.52, 95% confidence interval [CI] 1.04–6.13, p = 0.04). Only 80.4% of respondents tested all women for syphilis. There were regional differences in testing for some infections. The proportion of clinicians testing all women for HIV, HBV and syphilis was lower in Eastern Switzerland and the Zurich region (69.4% and 61.2%, respectively) than in other regions (range 77.1–88.1%, p <0.001). Most respondents (74.5%) said they would appreciate national guidelines about testing for infections during pregnancy.
CONCLUSIONS: Testing practices for infections in pregnant women vary widely in Switzerland. More extensive national guidelines could improve consistency of testing practices.
Introduction
Untreated infections in pregnancy can cause substantial morbidity in pregnant women and the fetus or newborn. Transmission of infection can result from transplacental transmission, amniotic fluid infection or during labour. Adverse pregnancy outcomes include miscarriage, preterm labour, premature rupture of membranes, preterm birth, stillbirth and perinatal infectious complications [1–4]. Consequences for the fetus and newborn range from asymptomatic infection to sepsis, fetal malformations and fetal death [5, 6]. Several infections are acquired through sexual intercourse (human immunodeficiency virus [HIV], syphilis, chlamydia, gonorrhoea, hepatitis B virus [HBV] and herpes simplex virus [HSV]) so there are implications for sexual history taking and partner treatment. HBV, varicella and rubella infections are preventable with vaccinations, if administered before pregnancy. Other infections like cytomegalovirus (CMV), parvovirus and toxoplasmosis can be tested for if abnormalities occur during pregnancy, but there is no established treatment during pregnancy for these infections.
Policies and practices for testing pregnant women for infections during pregnancy differ between countries, depending on the incidence and prevalence of infection, on historical precedents, on interpretation of the research evidence and on the type of health care system [7–9]. To date, there is no European consensus on routine testing for infections during pregnancy. In Switzerland, there is no single guideline about testing for infections in pregnancy and no single body responsible for guidelines. The Federal Office of Public Health (FOPH) and the Swiss Society of Gynaecology and Obstetrics (SSGO) have issued recommendations for specific infections: to screen all women for hepatitis B, HIV, group B streptococcus, varicella and rubella [10–15]; further to screen pregnant women for syphilis [16], and not to screen for toxoplasmosis [17]. Testing practices in antenatal care and adherence to national recommendations have not been investigated at a national level in Switzerland. This survey was designed to describe current practices followed by gynaecologists and obstetricians in Switzerland, to identify regional differences and to determine factors associated with testing for specific infections during antenatal care.
Methods
A multidisciplinary team of gynaecologists/obstetricians and specialists in infectious diseases, paediatrics and public health at University Hospital Bern, Cantonal Hospital St. Gallen and the Institute of Social and Preventive Medicine in Bern in Switzerland developed the survey “Testing for infections during pregnancy in Switzerland”.
We used a web-based questionnaire created with an online application (SurveyMonkey®). The questionnaire was available in three languages (German, French, Italian) and the questions were pretested by 50 gynaecologists at the Bern University Hospital to assess readability and content validity. A total of 12 questions were included to characterise the responding doctors and their infection screening practices. The information collected about the doctors included the number of pregnant patients attended per year, place of work (private practice, hospital), year of medical board certification (specialisation) in obstetrics and gynaecology, region of work in Switzerland (according to the Swiss Federal Statistics Office [18]: Eastern Switzerland, Zürich region, Central Switzerland, North-western Switzerland, Midland region [Bern, Solothurn, Neuchâtel, Jura and Fribourg], Lake Geneva region [Geneva, Vaud and Valais] and Ticino) and gender of the physician. In addition, we asked whether doctors took a sexual history from pregnant women at increased risk of sexually transmitted infection (STI), defined as having more than one partner and unprotected vaginal intercourse with different partners during pregnancy. Clinicians were then instructed to indicate the strategy they applied, based on their clinical practice, for testing for each of the following infections (in alphabetical order): bacterial vaginosis (BV), Chlamydia trachomatis (chlamydia), cytomegalovirus (CMV), gonorrhoea, hepatitis B virus (HBV), human Immunodeficiency virus (HIV), herpes simplex virus (HSV), hepatitis C virus (HCV), parvovirus B19, rubella, group B streptococcus (GBS), syphilis, toxoplasmosis and varicella zoster virus (VZV). There were five mutually exclusive categories of testing strategy: universal screening, testing women at high risk (multiple sex partners, a history of injecting drug use or any other risk judged relevant to the infection), testing if the pregnant woman had clinical symptoms or if there were fetal signs (e.g. on ultrasound), testing at the request of the women, and not testing at all.
The initial survey invitation was distributed to all clinicians who are active members of the SSGO, which covers 98% of all practicing obstetricians and gynaecologists in Switzerland. The invitation e-mail contained a short description of the survey as well as the web link for the online survey. Two reminders were sent out over a total time period of 5 months between February and June 2015. This study was exempt from approval of the ethics committee, as it did not include patients’ data but collected anonymous information about the views and practices of healthcare professionals only.
Statistical analyses were conducted using STATA 12 (Stata Corporation, College Station, TX). We described the frequency of testing strategies for each infection as percentages, excluding missing values. We considered region of work as the main exposure variable and analysed overall differences between regions with chi-square tests. Three outcomes were analysed with logistic regression models: testing according to recommendation (i.e. testing all women for HIV, HBV and syphilis), not testing for toxoplasmosis, and inquiring about a sexual history. For each outcome, we examined univariable associations with region of work, workplace (hospital or private practice), years since specialisation and gender. Results are expressed as odds ratios (OR) and 95% confidence intervals (95% CI). For testing according to recommendation a multivariable model of the association between region of work and testing practice, controlling for workplace, years since specialisation and gender. We present the probability of testing in each region (with 95% CI), after controlling for potential confounding. We present results about screening practices in two groups: infections that are mentioned in guidelines and infections that are not mentioned.
Results
Characteristics of participants
A total of 1138 clinicians were sent an email invitation and 537 (47.2%) responded. Seventeen of 537 (3.2%) the responding doctors were not seeing pregnant patients and thus did not answer further questions. The characteristics of the 520 eligible participating doctors are shown in table 1. The distributions of eligible participants according to sex and region of work did not differ from those of all members of the SSGO (see appendix, supplementary table S3).
Sexual history taking
Only 94/515 (18.3%) of the respondents reported that they took a sexual history from all their patients during antenatal care and 31.7% never did so (table 1). Overall, 137 (26.6 %) asked only single women about their sexual risks and 98 (19.0%) asked only at the beginning of pregnancy. Male doctors were more likely to ask about sexual history and sexual risk (26.4%) than female doctors (16.1%; OR 1.87, 95% CI 1.19–2.96, p = 0.007) during antenatal care. The evaluation of sexual history was not associated with region of work, years since specialization or place of work (supplementary table S1).
Infections mentioned in guidelines
We found that nearly all respondents reported that they tested all women for GBS (98.0%, 502/512), HBV (96.5%, 497/515) and HIV (94.7%, 479/506) during antenatal care, in accordance with national recommendations (fig. 1). Amongst these infections, the time at which testing was done varied most for HBV with 55.5% (287/517) reporting testing during the first trimester, 7.5% (39/517) in the second and 31.3% (162 /517) routinely in the third trimester.
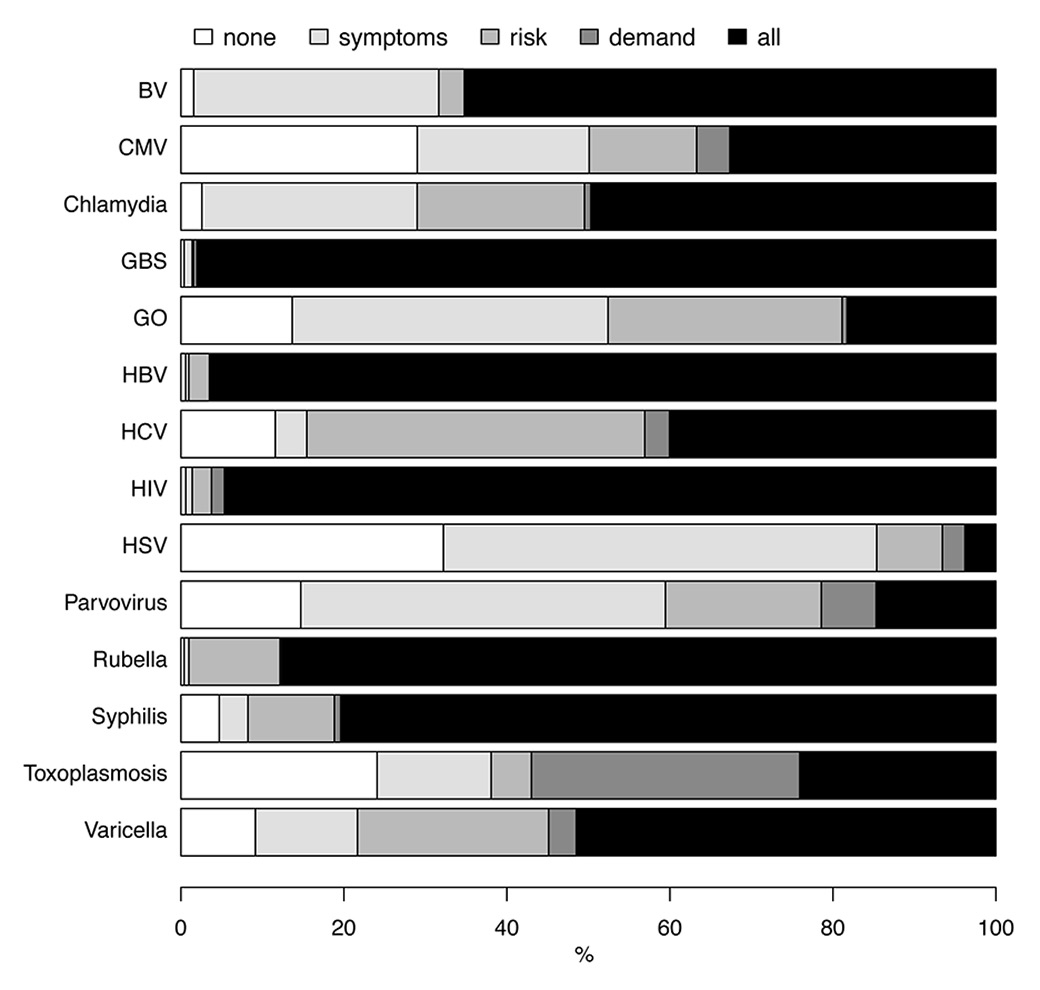
Figure 1
Testing practice for each infection, percentage of respondents reporting each practice.
None = no testing for this infection; symptoms = test only women with symptoms; risk = test only women with specific risk factors; demand = test women who request it; all = test all women for this infection
BV = bacterial vaginosis; CMV = cytomegalovirus; GBS = group B streptococcus; GO = gonorrhoea; HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; HSV, herpes simplex virus
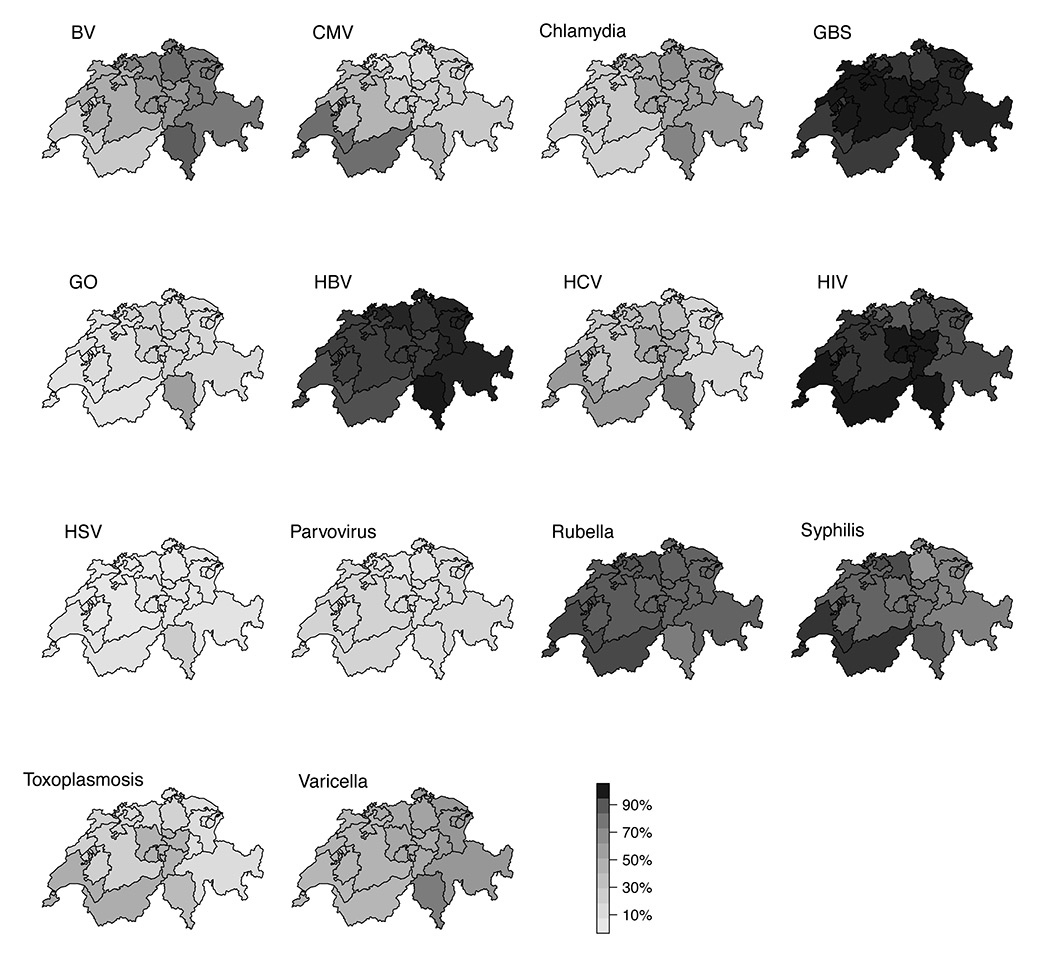
Figure 2
Percentage of participants reporting testing all pregnant women for the indicated disease, by region.
BV = bacterial vaginosis; CMV = cytomegalovirus; GBS = group B streptococcus; GO = gonorrhoea; HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus; HSV = herpes simplex virus.
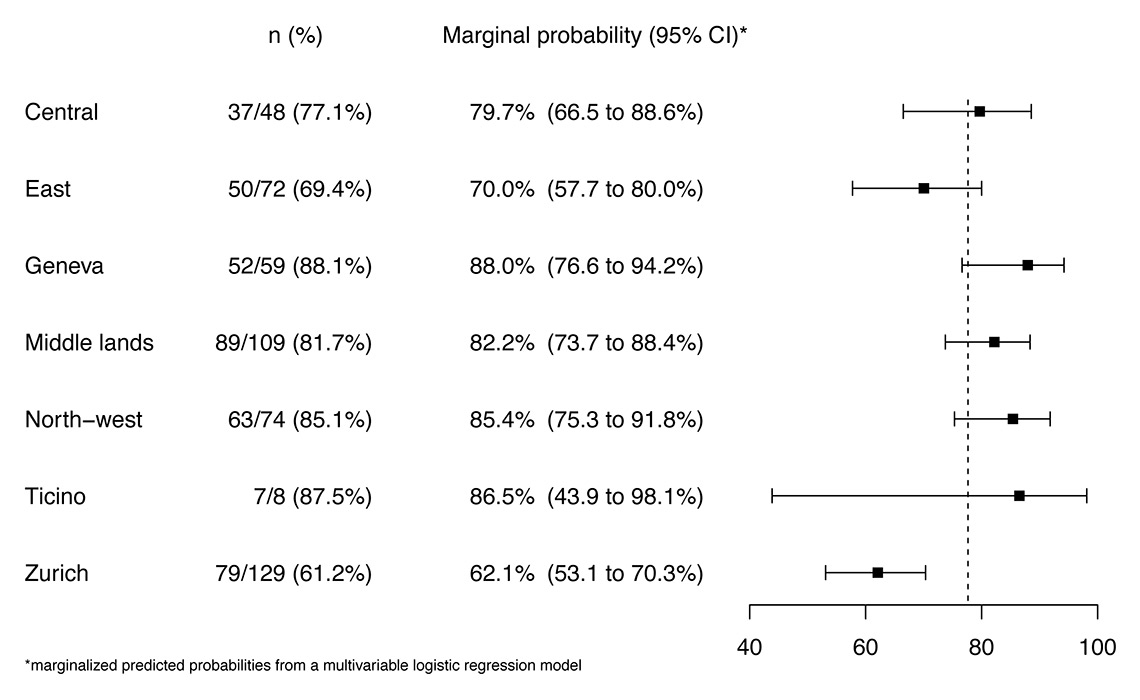
Figure 3
Probability of reporting testing of all women for human immunodeficiency virus (HIV), hepatitis B virus (HBV) and syphilis, by region adjusted for place of work, year of specialist certification, and gender. The dashed line indicates the overall mean, boxes are the point estimate, bars are 95% confidence intervals (CIs).
Syphilis testing for all pregnant women was reported by 80.4% (410/510) of respondents and 10.6% (54/510) said that they tested women at high risk of infection; only 4.7% (24/510) reported that they never test for syphilis during pregnancy. There were geographic differences in the proportion of clinicians that tested all women for syphilis (table 2, fig. 2); more than 90% of physicians in most regions (96.6% in the Geneva region and 92.0% in North-western Switzerland) but only 65.4% in the Zurich region and 73.3% in Eastern Switzerland (p <0.001). In these regions 10.4% and 7.9%, respectively, reported that they tested only women at high risk of syphilis. For women with positive syphilis serology test results, 45.1% (227/503) doctors reported that they send their patients to infectious disease specialists for antibiotic treatment.
When we focused on doctors who reported that they tested all pregnant women for HIV, HBV and syphilis, there were no differences according to place of work (hospital or private practice), year of specialisation or sex of the participant compared with doctors who did not test all women. There was a difference between regions, with lower rates of reported testing in Eastern Switzerland and Zurich region (69.4% and 61.2%, respectively) compared with the other regions (range 77.1–88.1%, p <0.001). This difference remained after adjusting for doctor’s gender, place of work and year of specialist certification (fig. 3, supplementary table S2).
The majority (447/510, 87.6%) of respondents reported testing women for rubella and 51.5% (259/503) for varicella immunity, with no geographical variation.
For toxoplasmosis, 24.1% (117/486) of all respondents reported testing all women, 32.9% (160/486) tested at the request of the pregnant woman and 24.1% (117/486) did not test any women. Doctors in the Geneva region reported testing 47.4% (27/57) of all women and those in Eastern Switzerland only 10.5% (8/75, p across region <0.001) (table 2). Hospital doctors were more likely than doctors working in private practice not to test for toxoplasmosis (OR 2.52, 95% CI 1.04–6.13, p = 0.04), but there was no association with gender or year of specialisation.
Infections not mentioned in guidelines
For genital tract infections, 65.0% (333/512) respondents reported testing all women for BV, 49.7% (252/507) for chlamydia and 18.3% (91/498) for gonorrhoea. There were geographical differences in testing for all three infections. In general, doctors in German-speaking regions and the Ticino reported testing all women for these infections more often than doctors in the French-speaking region. Doctors practicing in hospitals were more likely than those in private practice to report testing all women for chlamydia (52/67, 77.6% and 145/352 41.2%, respectively) and gonorrhoea (27/65, 41.5% and 44/346, 12.7%, respectively; p <0.001 for both comparisons).
Overall, 32.7% (161/493) of respondents reported testing all pregnant women and 13.2% (65/493) reported testing women at high risk of CMV (fig. 1). Testing practices for CMV were geographically heterogeneous (table 2, fig. 2): 80.7% of respondents in the Geneva region, compared with 50% or fewer of respondents in all other regions reported testing all women for CMV (p <0.001).
For hepatitis C, 40.0% (197/492) of respondents reported testing all women and another 41.5% (204/492) reported testing women at high risk (e.g. women with a history of injection drug use). The doctors most likely to report testing all pregnant women for hepatitis C during pregnancy were found in the Geneva region (34/56, 60.7%) and Central Switzerland (25/46, 54.3%), compared with 18.6% (13/70) in Eastern Switzerland (p across all regions <0.001).
Only 18.0% (81/520) respondents reported that they had, according to their opinion, enough information to decide about testing strategies and 388 (74.5%) of all responding clinicians mentioned that they would appreciate clearer guidelines about testing for infections during pregnancy in the future.
|
Table 1: Characteristics of participating doctors. |
| Number of participants |
520 |
| Gender (male) |
n = 517, 171 (33%) |
| Number of pregnancies managed (>100) |
n = 519, 218 (42%) |
| Workplace |
n = 519, |
| Private practice |
363 (70%) |
| Cantonal hospital |
67 (13%) |
| Regional hospital |
54 (10%) |
|
| University hospital |
24 (5%) |
| Others |
11 (2%) |
| Year of specialist training |
n = 517 |
| 1986–1995 |
163 (32%) |
| 1996–2005 |
166 (32%) |
| 2006–2015 |
118 (23%) |
| In progress |
39 (8%) |
| No specialist training |
31 (6%) |
| Region |
n = 519, |
| Middle lands |
111 (21%) |
| North-west |
78 (15%) |
| East |
76 (15%) |
| Geneva |
59% (11%) |
| Ticino |
8 (2%) |
| Central |
51 (10%) |
| Zurich |
136 (26%) |
| Sexual history inquired |
n = 515, |
| Yes |
94 (18%) |
| No |
163 (32%) |
| Only single women |
137 (27%) |
| Only start of pregnancy |
98 (19%) |
| Other |
23 (4%) |
| Availability of information |
n = 511, |
| Guidelines would be helpful |
381 (75%) |
| Not enough information but can search for it |
38 (7%) |
| I have enough information |
92 (18%) |
|
Table 2: Number and percentage of participants testing all pregnant women by region. |
| |
Middle lands
(n = 111)
|
North-west
(n = 78)
|
East
(n = 76)
|
Geneva
(n = 59)
|
Ticino
(n = 8)
|
Central
(n = 51)
|
Zurich
(n = 136)
|
|
| All pregnant women tested for: |
Number (percentage) of participants |
p value |
| Bacterial vaginosis |
53 (48%) |
57 (73%) |
59 (78%) |
15 (27%) |
7 (88%) |
32 (64%) |
110 (82%) |
<0.001 |
| Group B streptococcus |
110 (100%) |
75 (97%) |
74 (99%) |
55 (96%) |
8 (100%) |
51 (100%) |
129 (96%) |
0.36 |
| Chlamydia |
31 (28%) |
43 (56%) |
45 (59%) |
12 (22%) |
5 (71%) |
27 (55%) |
88 (66%) |
<0.001 |
| Cytomegalovirus |
45 (42%) |
11 (15%) |
20 (28%) |
46 (81%) |
4 (50%) |
13 (29%) |
22 (17%) |
<0.001 |
| Gonorrhoea |
13 (12%) |
20 (26%) |
13 (17%) |
3 (6%) |
4 (57%) |
11 (24%) |
27 (21%) |
0.003 |
| Hepatitis B virus |
105 (95%) |
77 (99%) |
74 (99%) |
54 (92%) |
8 (100%) |
49 (96%) |
129 (97%) |
0.30 |
| Hepatitis C virus |
38 (36%) |
33 (43%) |
13 (19%) |
34 (61%) |
6 (75%) |
25 (54%) |
48 (37%) |
<0.001 |
| Human immunodeficiency virus |
106 (97%) |
68 (89%) |
66 (92%) |
59 (100%) |
8 (100%) |
49 (100%) |
122 (92%) |
0.024 |
| Herpes simplex virus |
2 (2%) |
2 (3%) |
3 (4%) |
3 (6%) |
2 (25%) |
3 (7%) |
3 (2%) |
0.039 |
| Parvovirus |
20 (20%) |
7 (10%) |
13 (18%) |
10 (19%) |
1 (14%) |
7 (16%) |
12 (9%) |
0.28 |
| Rubella |
95 (88%) |
69 (91%) |
65 (86%) |
55 (93%) |
6 (75%) |
42 (86%) |
114 (86%) |
0.61 |
| Syphilis |
94 (85%) |
69 (92%) |
55 (73%) |
57 (97%) |
7 (88%) |
40 (82%) |
87 (65%) |
<0.001 |
| Toxoplasmosis |
22 (22%) |
12 (16%) |
8 (11%) |
27 (47%) |
3 (38%) |
19 (44%) |
26 (20%) |
<0.001 |
| Varicella |
47 (44%) |
39 (51%) |
46 (61%) |
23 (42%) |
6 (75%) |
25 (51%) |
72 (55%) |
0.12 |
Discussion
This is the first national study in Switzerland to have assessed obstetricians’ and gynaecologists’ testing practices for 14 infections during pregnancy. More than 90% of respondents reported that they tested all women for HIV, HBV and GBS and 88% for rubella, in accordance with national recommendations. Overall, 80% of respondents reported testing all women for syphilis. We found that 24% of respondents reported testing all women for toxoplasmosis, 7 years after the publication of recommendations not to screen. Our study demonstrated geographical variations in testing strategies for several infections. Most respondents said that they would like to have national guidelines on testing for infections in pregnancy.
The strength of this study was the large sample of practicing obstetricians and gynaecologists in Switzerland. The participation rate was 47% and respondents were representative of all SSGO members according to sex and region of work. Limitations of the survey include the potential for participation bias. If respondents are more likely to be those who test for infections, we will have overestimated the coverage of antenatal screening for infections. Reported levels of testing do not necessarily correspond to testing of individual women in practice. These levels of testing might also be overestimated if respondents felt that they were expected to say that they tested. The anonymous nature of the survey should have reduced such a bias.
Adherence to national recommendations for universal screening in pregnancy
We found very high adherence to specific recommendations published on the SSGO website stating that all pregnant women should be tested for GBS [11], HBV [10] and HIV [11]. Studies in Switzerland have shown successful prevention of mother to child transmission of GBS by intrapartum antibiotics in women with GBS colonisation [19] and very low levels of early onset sepsis with GBS in newborns (0.12/1000 live births) [20]. Testing for GBS is almost universal practice in Switzerland according to our survey results. This might be explained by a clear Swiss recommendation for GBS screening in pregnant women and is an example of effective implementation of a national screening strategy that was introduced by the SSGO.
International recommendations for universal screening: global campaign to eliminate congenital syphilis
Our results show that antenatal syphilis screening could be improved in Switzerland. There are large geographical variations, which might suggest that doctors in some regions screen all women but others only test women at high risk of syphilis. The World Health Organization (WHO) aims to eliminate mother to child transmission of syphilis and recommends screening of all pregnant women to allow timely diagnosis and treatment of infected women and their partners [21]. The number of infectious syphilis cases in pregnant women in Switzerland increased between 2006 and 2009 [16]. Furthermore, the United States Centers for Disease Control and Prevention reported an increase in cases of congenital syphilis in 2014 [22]. This finding underlines the importance of screening all women for syphilis during pregnancy.
Screening for rubella and varicella and opportunities for vaccination
We found 88% of Swiss obstetricians and gynaecologists test all pregnant women for rubella antibodies, as recommended by the SFOPH. Serological testing might reflect the fact that many women do not have their immunisation records. According to a serological study of women who gave birth, rates of seronegative status for rubella were as low as 3.2% in Swiss/German/Austrian women [23] compared with 7.8% of patients from other European countries. Even with low seronegative rates, the postpartum period gives an important opportunity to vaccinate seronegative women for rubella and varicella in order to provide protection in subsequent pregnancies.
Withdrawen recommendations for screening: toxoplasmosis
In 2009, Swiss recommendations about screening for toxoplasmosis changed and stated that routine screening for toxoplasmosis in pregnancy should not be done [17]. Instead, hygienic measures (e.g. avoiding consumption of raw meat) are recommended for all pregnant women. The main reasons for this decision were the low prevalence of toxoplasmosis in Switzerland, the limited options for therapy following seroconversion during pregnancy, and the low specificity of toxoplasmosis serology testing resulting in many false positive tests. False positive test results lead to unnecessary anxiety and might even result in termination of pregnancy of a unaffected fetus. Our survey results indicate that nearly a quarter of clinicians still test all pregnant women for toxoplasmosis, and one third are still testing at the request of the patient. Neighbouring countries, especially France, still recommend screening for toxoplasmosis during pregnancy [24]. Accordingly, the highest proportion of doctors testing for toxoplasmosis was found in the French-speaking part of Switzerland (48%). These findings show the difficulty of changing practice after a shift in screening recommendations.
Controversies about screening for infections in pregnancy: bacterial vaginosis and chlamydia
Heterogeneity in testing practices for infections in pregnancy can reflect ongoing debates about the effectiveness of screening. Testing rates for BV in asymptomatic pregnant women varied geographically, from a quarter in the Geneva region to more than 80% in the Zurich region. Although the correlation between BV and severe complications in pregnancy and postpartum (preterm birth, late miscarriage, postpartum endometritis) is well established, the benefits of treatment of BV in pregnancy have been debated because of inconsistent study results [25]. Nevertheless, if BV is treated early in pregnancy (before 16 weeks), the risk for preterm birth might be reduced [26, 27]. Furthermore, the prevalence of BV in Switzerland has been shown to be as high as 32% [28]. Similarly, observational studies show associations between chlamydia infection in pregnancy [2, 4] but the lack of randomised controlled trials [29] perpetuates debate about whether routine screening of asymptomatic pregnant women reduces these outcomes [30]. Screening of all pregnant women for chlamydia is recommended in countries such as the USA [31], Estonia, Germany and Latvia, but other countries such as the UK actively recommend not screening, based on an evidence review [30].
History taking for sexual risk and sexually transmitted infections
Adverse outcomes of STIs such as chlamydia, gonorrhoea, syphilis, HBV, HIV and HSV in pregnancy are well documented [1–4]. For example, the risk of HIV transmission to the newborn is highest in women with incident HIV infection during pregnancy [32]. Numbers of reported cases of syphilis, chlamydia and gonorrhoea are increasing in Switzerland [33]. Acquisition of these infections is associated with the number of current and past sexual partners, with non-use of condoms and with the behaviours of sexual partners [34]. Sexual history taking is a sensitive topic, especially in pregnant women as they often attend consultations together with their male partner, so assessing risk in regard to STI is challenging [35]. This survey shows a need to improve sexual history taking during pregnancy, especially since levels of sexual risk assessment were low, irrespective of the time since specialist certification.
New evidence from research: perinatal treatment for HCV and HBV
Hepatitis C screening is carried out by 40% of our survey respondents. Until now, there have been no measures to reduce transmission risk of HCV (about 6%) to the newborn during pregnancy and delivery [36]. New directly acting antivirals, which are able to clear HCV infection in more than 90% within 10–12 weeks of therapy, should stimulate a re-evaluation of testing and treatment strategies before and during pregnancy [37]. Treatment of the mother in the pregnancy interval or before conception could avoid any HCV exposure of newborns of subsequent pregnancies [38]. A recent UK study suggests that general HCV screening during pregnancy could be cost effective [39].
Timely HBV screening during pregnancy has important implications for prevention of mother to child transmission. Nearly all doctors in our survey routinely test for HBV (96.5%), which is recommended by the Swiss national health authorities and the SSGO. Treatment of HBV-infected pregnant women with high viral load reduces mother to child transmission in both actively and passively vaccinated newborns [40]. Therefore, women positive for HBV surface antigen should be assessed for viral load and offered treatment during the third trimester of gestation. This is not yet included in the Swiss national guidelines, which were last published in 2007 and should be updated.
Conclusion
Our survey reached nearly half of all registered obstetricians and gynaecologists in Switzerland and found that three quarters would appreciate clear guidelines about testing for infections during pregnancy. National guidelines for HIV, HBV and GBS testing show that wide dissemination and clear recommendations allow high and consistent implementation.
We conclude that measures should be taken to provide a consistent format for recommendations for all relevant infections in pregnancy. Such recommendations should be evidence-based, straightforward and transparent, as well as practice- and patient-oriented, in order to achieve high adherence by doctors. As an additional benefit, a more uniform national strategy with high adherence will allow regular evaluation of the current national epidemiological situation of infections in pregnant women. The results of this survey can be used by healthcare authorities to make decisions about the evaluation and implementation of antenatal infection screening programmes in Switzerland. More extensive national guidelines could improve consistency of testing practices.
Appendix: Heterogeneity in testing practices for infections during pregnancy: national survey across Switzerland
|
Table S1: Univariable logistic regression models for testing for HIV, HBV and syphilis, not testing for toxoplasmosis and taking a sexual history, according to region, clinic characteristic, year of specialist training and gender.. |
|
Testing for HIV, HBV and syphilis
|
Not testing for toxoplasmosis
|
Sexual history taking
|
| Odds ratio (95% CI) and p value |
|
Region
|
| Zurich |
1 (reference) |
|
1 (reference) |
|
1 (reference) |
|
| Middle lands |
2.82 (1.54–5.13) |
<0.001 |
0.82 (0.44–1.53) |
0.54 |
0.79 (0.39–1.57) |
0.49 |
| North-west |
3.62 (1.74–7.54) |
<0.001 |
1.21 (0.64–2.31) |
0.56 |
0.77 (0.35–1.66) |
0.50 |
| East |
1.44 (0.78–2.66) |
0.25 |
1.50 (0.80–2.80) |
0.20 |
1.19 (0.58–2.43) |
0.64 |
| Geneva |
4.70 (1.98–11.17) |
<0.001 |
0.42 (0.17–1.02) |
0.06 |
1.38 (0.64–2.95) |
0.41 |
| Central |
2.13 (1.00–4.55) |
0.05 |
0.69 (0.29–1.63) |
0.39 |
1.32 (0.59–2.93) |
0.50 |
| Ticino |
4.43 (0.53–37.10) |
0.17 |
1.00 (0.19–5.20) |
1.00 |
4.67 (1.09–19.98) |
0.038 |
|
Clinic characteristic
|
| Private practice |
1 (reference) |
|
1 (reference) |
|
1 (reference) |
|
| Cantonal hospital |
1.10 (0.59–2.06) |
0.77 |
1.27 (0.68–2.37) |
0.46 |
0.47 (0.21–1.08) |
0.08 |
| Regional hospital |
1.12 (0.56–2.23) |
0.75 |
1.93 (1.03–3.61) |
0.040 |
0.70 (0.31–1.54) |
0.37 |
| University Hospital |
1.72 (0.57–5.16) |
0.34 |
2.52 (1.04–6.13) |
0.041 |
1.11 (0.40–3.09) |
0.84 |
| Others |
2.75 (0.34–22.28) |
0.34 |
0.40 (0.05–3.25) |
0.39 |
0.89 (0.19–4.20) |
0.88 |
|
Year of specialist training
|
| 1986–1995 |
1 (reference) |
|
1 (reference) |
|
1 (reference) |
|
| 1996–2005 |
0.93 (0.55–1.55) |
0.77 |
0.60 (0.35–1.03) |
0.06 |
0.73 (0.42–1.27) |
0.27 |
| 2006–2015 |
1.31 (0.73–2.38) |
0.37 |
0.84 (0.48–1.48) |
0.55 |
0.62 (0.33–1.17) |
0.14 |
| Still In training |
0.87 (0.39–1.96) |
0.74 |
0.98 (0.42–2.26) |
0.96 |
0.41 (0.14–1.24) |
0.11 |
| No specialist training |
0.59 (0.25–1.38) |
0.22 |
1.76 (0.76–4.06) |
0.19 |
1.80 (0.77–4.20) |
0.17 |
|
Gender
|
| Female |
1 (reference) |
|
1 (reference) |
|
1 (reference) |
|
| Male |
0.73 (0.48–1.12) |
0.15 |
1.40 (0.90–2.16) |
0.13 |
1.87 (1.19–2.96) |
0.007 |
| CI = confidence interval; HBV = hepatitis B virus; HIV = human immunodeficiency virus |
|
Table S2: Multivariable logistic regression for testing for HIV, HBV and syphilis according to region, clinic characteristics, year of specialist training and gender. |
| |
Odds ratio (95% CI)
|
p-value
|
|
Region
|
| Zurich |
1 (reference) |
|
| Middle lands |
2.82 (1.52–5.24) |
0.001 |
| North-west |
3.57 (1.69–7.57) |
<0.001 |
| East |
1.43 (0.74–2.76) |
0.29 |
| Geneva |
4.46 (1.84–10.78) |
<0.001 |
| Ticino |
3.92 (0.46–33.16) |
0.21 |
| Central |
2.39 (1.10–5.20) |
0.028 |
|
Workplace
|
| Private practice |
1 (reference) |
|
| Cantonal hospital |
1.05 (0.49–2.24) |
0.90 |
| Regional hospital |
1.38 (0.64–2.97) |
0.41 |
| University Hospital |
2.31 (0.54–9.85) |
0.26 |
| Others |
2.65 (0.25–28.19) |
0.42 |
|
Year of specialist training
|
|
|
| 1986–1995 |
1 (reference) |
|
| 1996–2005 |
0.95 (0.55–1.65) |
0.86 |
| 2006–2015 |
1.25 (0.65–2.41) |
0.50 |
| Still in training |
0.64 (0.22–1.86) |
0.41 |
| No specialist training |
0.62 (0.25–1.56) |
0.31 |
|
Gender
|
|
|
| Female |
1 (reference) |
|
| Male |
0.64 (0.39–1.04) |
0.07 |
| CI = confidence interval; HBV = hepatitis B virus; HIV = human immunodeficiency virus |
|
Table S3: Characteristics of the members of the Swiss Society of Gynaecology and Obstetrics. |
|
1. Place of work
|
|
Region
|
Cantons
|
|
| Lake Geneva region |
Geneva, Vaud, Valais |
18.7% |
| Midland region |
Berne, Fribourg, Jura, Neuchâtel, Solothurn |
18% |
| North-western Switzerland |
Aargau, Basel-Landschaft, Basel-Stadt |
12.6% |
| Zurich |
Zurich |
24.8% |
| Eastern Switzerland |
Appenzell Ausserrhoden, Appenzell Innerrhoden, Glarus, Graubunden, St-Gallen, Schaffhausen, Thurgau |
11.1% |
| Central Switzerland |
Luzern, Nidwalden, Obwalden, Schwyz, Uri, Zug |
8.4% |
| Ticino |
Ticino |
3% |
| Other |
Foreign (German/Austrian/French) |
3.4% |
|
2. Percentage male and female
|
| – 63% women |
| – 37% men |
|
3. Mean age
|
| Mean age 47 years |
|
NB:For date of birth, information given by members is incomplete. |
Acknowledgements: We thank the Swiss Society of Obstetrics and Gynaecology SSOG / gynécologie suisse for providing their support. Furthermore, we thank all participating obstetricians/gynaecologists for their commitment in this study. We also would like to thank Lukas Buetikofer for his great support in regards to the statistical analysis and Charles Béguelin, MD, and Elia Francesco Lo Priore, MD, for translation of the questionnaire in French and Italian.
References
1 Cunnington M, Kortsalioudaki C, Heath P. Genitourinary pathogens and preterm birth. Curr Opin Infect Dis. 2013;26:219–30. doi:10.1097/QCO.0b013e328360dc31.
2 Liu B, Roberts CL, Clarke M, Jorm L, Hunt J, Ward J. Chlamydia and gonorrhoea infections and the risk of adverse obstetric outcomes: a retrospective cohort study. Sex Transm Infect. 2013;89:672–8. doi:10.1136/sextrans-2013-051118.
3 Newman L, Kamb M, Hawkes S, Gomez G, Say L, Seuc A, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med. 2013;10:e1001396. doi:10.1371/journal.pmed.1001396.
4 Baud D, Goy G, Jaton K, Osterheld M-C, Blumer S, Borel N, et al. Role of Chlamydia trachomatis in miscarriage. Emerg Infect Dis. 2011;17:1630–5. doi:10.3201/eid1709.100865.
5 Lamont RF, Sobel JD, Carrington D, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, et al. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG Int J Obstet Gynaecol. 2011;118:1155–62. doi:10.1111/j.1471-0528.2011.02983.x.
6 Salemi JL, Whiteman VE, August EM, Chandler K, Mbah AK, Salihu HM. Maternal hepatitis B and hepatitis C infection and neonatal neurological outcomes. J Viral Hepat. 2014;21:e144-153. doi:10.1111/jvh.12250.
7 Moore DL, MacDonald NE, Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Preventing ophthalmia neonatorum. Can J Infect Dis Med Microbiol J Can Mal Infect Microbiol Médicale AMMI Can. 2015;26:122–5.
8 Hill MG, Menon S, Smith S, Zhang H, Tong X, Browne PC. Screening for Chlamydia and Gonorrhea Cervicitis and Implications for Pregnancy Outcome. Are We Testing and Treating at the Right Time? J Reprod Med. 2015;60:301–8.
9 Ville Y, Leruez-Ville M. Managing infections in pregnancy. Curr Opin Infect Dis. 2014;27:251–7. doi:10.1097/QCO.0000000000000066.
10 Bundesamt für Gesundheit – Hepatitis B http://www.bag.admin.ch/themen/medizin/00682/00684/01077/index.html?lang=de (accessed May 22, 2016).
11 Expertenbriefe – SGGG 2015. http://www.sggg.ch/fachthemen/expertenbriefe/ (accessed November 17, 2015).
12 Mueller M, Henle A, Droz S, Kind AB, Rohner S, Baumann M, et al. Intrapartum detection of Group B streptococci colonization by rapid PCR-test on labor ward. Eur J Obstet Gynecol Reprod Biol. 2014;176:137–41. doi:10.1016/j.ejogrb.2014.02.039.
13 Rausch A-V, Gross A, Droz S, Bodmer T, Surbek DV. Group B Streptococcus colonization in pregnancy: prevalence and prevention strategies of neonatal sepsis. J Perinat Med. 2009;37:124–9. doi:10.1515/JPM.2009.020.
14 Renner RM, Renner A, Schmid S, Hoesli I, Nars P, Holzgreve W, et al. Efficacy of a strategy to prevent neonatal early-onset group B streptococcal (GBS) sepsis. J Perinat Med. 2006;34:32–8. doi:10.1515/JPM.2006.005.
15 Empfohlene Impfungen für Frauen vor, während und nach der Schwangerschaft: Eidgenössische Kommission für Impffragen (EKIF), Sektion Impfprogramme und Bekämpfungsmassnahmen. Bern: Bundesamt für Gesundheit (BAG). Internet: http://www.bag.admin.ch/themen/medizin/00682/00685/03212/
16 Meyer Sauteur PM, Trück J, Bosshard PP, Tomaske M, Morán Cadenas F, Lautenschlager S, et al. Congenital syphilis in Switzerland: gone, forgotten, on the return. Swiss Med Wkly. 2012;141:w13325. doi:10.4414/smw.2011.13325.
17 Boubaker K, Raeber PA, Vaudaux B, Bucher HC, Garweg JG, Hoesli I, et al. Toxoplasmosis during pregnancy and infancy. A new approach for Switzerland. Swiss Med Wkly. 2008;138:1–8.
18 Swiss cantons and major regions http://www.bfs.admin.ch/bfs/portal/en/index/regionen/thematische_karten/maps/raumgliederung/institutionelle_gliederungen.NewWindow.parsys.0002.1.Preview.html (accessed May 22, 2016).
19 Fröhlicher S, Reichen-Fahrni G, Müller M, Surbek D, Droz S, Spellerberg B, et al. Serotype distribution and antimicrobial susceptibility of group B streptococci in pregnant women: results from a Swiss tertiary centre. Swiss Med Wkly. 2014;144:w13935. doi:10.4414/smw.2014.13935.
20 Giannoni E, Berger C, Stocker M, Agyeman P, Posfay-Barbe KM, Heininger U, et al. Incidence and Outcome of Group B Streptococcal Sepsis in Infants in Switzerland. Pediatr Infect Dis J. 2016;35:222–4. doi:10.1097/INF.0000000000000974.
21 World Health Organization. WHO | Investment case for eliminating mother-to-child transmission of syphilis. WHO http://www.who.int/reproductivehealth/publications/rtis/9789241504348/en/ (accessed November 17, 2015).
22 Increase in Incidence of Congenital Syphilis – United States, 2012–2014 http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6444a3.htm (accessed November 17, 2015).
23 Frischknecht F, Sell W, Trummer I, Brühwiler H. Serological testing for infectious diseases in pregnant women: are the guidelines followed? Swiss Med Wkly. 2011;140:w13138. doi:10.4414/smw.2010.13138.
24 Team EC for DP and C (ECDC)-HCU-E editorial. Congenital toxoplasmosis in France in 2007: first results from a national surveillance system 2010. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19600 (accessed February 18, 2016).
25 Redelinghuys MJ, Ehlers MM, Dreyer AW, Kock MM. Normal flora and bacterial vaginosis in pregnancy: an overview. Crit Rev Microbiol. 2015:1–12. doi:10.3109/1040841X.2014.954522.
26 Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2013;1:CD000262. doi:10.1002/14651858.CD000262.pub4.
27 Sangkomkamhang US, Lumbiganon P, Prasertcharoensuk W, Laopaiboon M. Antenatal lower genital tract infection screening and treatment programs for preventing preterm delivery. In: The Cochrane Collaboration, editor. Cochrane Database Syst. Rev., Chichester, UK: John Wiley & Sons, Ltd; 2015.
28 Surbek DV, Hoesli IM, Holzgreve W. Morphology assessed by transvaginal ultrasonography differs in patients in preterm labor with vs. without bacterial vaginosis. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2000;15:242–5. doi:10.1046/j.1469-0705.2000.00102.x.
29 Low N, Redmond S, Uusküla A, van Bergen J, Ward H, Andersen B, et al. Low N, Redmond S, Uusküla A, van Bergen J, Ward H, Andersen B, et al. Screening for genital chlamydia infection. Cochrane Database Syst Rev. 2016: CD010866 (In press). In: The Cochrane Collaboration, editor. Cochrane Database Syst. Rev., Chichester, UK: John Wiley & Sons, Ltd; 2013.
30 Current UK NSC recommendations n.d. http://legacy.screening.nhs.uk/screening-recommendations.php (accessed February 22, 2016).
31 Workowski KA, Berman S, Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep Cent Dis Control. 2010;59:1–110.
32 Kinuthia J, Drake AL, Matemo D, Richardson BA, Zeh C, Osborn L, et al. HIV acquisition during pregnancy and postpartum is associated with genital infections and partnership characteristics. AIDS Lond Engl. 2015;29:2025–33. doi:10.1097/QAD.0000000000000793.
33 Bundesamt für Gesundheit – HIV/STI-Statistiken, Analysen und Trends http://www.bag.admin.ch/hiv_aids/05464/12908/12909/12913/index.html?lang=de (accessed December 23, 2015).
34 Fenton KA, Lowndes CM. Recent trends in the epidemiology of sexually transmitted infections in the European Union. Sex Transm Infect. 2004;80:255–63. doi:10.1136/sti.2004.009415.
35 Meystre-Agustoni G, Jeannin A, de Heller K, Pécoud A, Bodenmann P, Dubois-Arber F. Talking about sexuality with the physician: are patients receiving what they wish? Swiss Med Wkly. 2011. doi:10.4414/smw.2011.13178.
36 Cottrell EB, Chou R, Wasson N, Rahman B, Guise J-M. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158:109–13. doi:10.7326/0003-4819-158-2-201301150-00575.
37 Aebi-Popp K, Duppenhtaler A, Rauch A, De Gottardi A, Kahlert C. Vertical transmission of hepatitis C: towards universal antenatal screening in the era of new direct acting antivirals (DAAs)? Short review and analysis of the situation in Switzerland. J Virus Eradication. 2016:52–4.
38 Kanninen TT, Dieterich D, Asciutti S. HCV vertical transmission in pregnancy: New horizons in the era of DAAs. Hepatol Baltim Md 2015. doi:10.1002/hep.28032.
39 Selvapatt N, Ward T, Bailey H, Bennett H, Thorne C, See L-M, et al. Is antenatal screening for hepatitis C virus cost-effective? A decade’s experience at a London centre. J Hepatol. 2015. doi:10.1016/j.jhep.2015.05.015.
40 Brown RS, McMahon BJ, Lok ASF, Wong JB, Ahmed AT, Mouchli MA, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: A systematic review and meta-analysis. Hepatol Baltim Md. 2016;63:319–33. doi:10.1002/hep.28302.


