Stroke research at the crossroads – where are we heading?
DOI: https://doi.org/10.4414/smw.2016.14329
Stefan
Roth, Arthur
Liesz
Summary
Stroke causes 5.7 million deaths annually. This ranks stroke as the second most common cause of death and, additionally, it is a major cause of disability. Because of an ageing population, stroke incidence and costs will greatly increase in the future. This makes stroke an ongoing social and economic burden, in contrast to the only very limited therapeutic options. In the last decade vast sums were spent on translational research focused on neuroprotective strategies in the acute phase of ischaemic stroke. A plethora of candidate agents were tested in experimental models and preclinical studies, but none was proven effective in clinical trials. This gave rise to discussions about the possible reasons for this failure, ending up mainly with criticism of methodological aspects of the preclinical and clinical studies, or of the relevance of animal studies in drug development. Indeed, the question could rather be whether neuroprotection is the right target for successful stroke treatment. In this context, a paradigm change can currently be observed: the focus of experimental and translational stroke research is shifting from early neuroprotection to delayed mechanisms such as stroke-associated comorbidities, regeneration and plasticity. In this review we highlight a few recently emerging fields in translational stroke research. One such topic is the crosstalk between immunity and the injured brain as key pathomechanism in stroke. On one hand, innate and adaptive immune cells play an important role in the fate of injured brain tissue after stroke; on the other, peripheral immune alterations are critically involved in post-stroke comorbidities. Another emerging research area is the analysis of mechanisms involved in regeneration and neuronal plasticity after stroke. Here, we discuss the current understanding of basic mechanisms involved after brain injury, clinical imaging approaches and therapeutic strategies to promote regeneration in stroke patients.
Status quo – stroke
Annually, about 16 million first-ever strokes occur worldwide, causing a total of 5.7 million deaths [1]. As a consequence, stroke ranks as the second cause of death in the world population after ischaemic heart disease. Moreover, stroke is a global epidemic: about 85% of all stroke deaths are registered in low- and middle-income countries [2]. In 2002, stroke was the sixth most common reason for reduced disability-adjusted life-years [3]. In the United States, the total direct and indirect cost for stroke was estimated at $ 65.5 billion in 2008 [4]. In all 27 European Union countries the overall costs were estimated € 27 billion in 2008 (European cardiovascular disease statistics 2008). A further increase in stroke incidence and costs can be expected simply as a result of population aging.
Although stroke places such an enormous medical and economic burden on society, thrombolysis with tissue plasminogen activator and mechanical vascular recanalisation are currently the only clinically approved therapies for ischaemic stroke. Moreover, there are well known limitations, including a narrow time window, coagulation abnormalities, intracranial haemorrhage and a list of further contraindications, which make these therapeutic options accessible only to a small percentage of stroke patients [5].
Therefore, prognosis for patients remains poor and the necessity for effective stroke treatment remains an urgent priority. For more than two decades, translational stroke research focused on neuroprotective strategies in the acute phase of ischaemic stroke. More than 1000 neuroprotective compounds have been tested in rodent models with the aim to improve stroke outcome [6]. Early mechanisms of neuronal damage like excitotoxicity, production of reactive oxygen species, cellular energy deficiency and depolarisation were targeted. Indeed, many agents reduced brain damage (in most cases measured as decreased infarct volume) in rodent models of experimental stroke. Out of these candidates approximately 50 neuroprotective agents were tested in more than 100 clinical stroke trials, but none has improved outcome in clinical stroke patients [6].
What are possible reasons for the failure of so many trials? So far, attention in discussions about this failure has been drawn mainly to methodological mistakes. The inappropriate selection of experimental animals in terms of age, sex, comparable physiology and genetic background was discussed, as well as the low replication rate and lack of statistical rigor in preclinical studies [7–9]. Regarding the failure of clinical trials in stroke, other syndromes with strong involvement of the innate immune system, such as sepsis, have been equally resistant to effective drug development. In sepsis, for example, more than 100 randomised phase II and III clinical trials did not result in a single US Food and Drug Administration (FDA) approved drug [10]. Sepsis is a very different disease from stroke, both in preclinical and clinical settings. This poses the question, what are the factors involved in the failure to improve clinical outcome? Is the human immune system really that different from the rodent? Is our focus on suppressing the inflammatory response a dead-end strategy because inflammation is not only harmful but also essential for repair processes and regeneration? These are serious caveats in the varied field of immune-oriented research that will have to be more specifically addressed in future studies. Additionally, after the translation to clinical trials, the time window and dose for administration, as well as patient heterogeneity and inaccurate outcome parameters, were listed as possible sources for failure [11]. This has finally led to the currently widely discussed concept of a “translational roadblock” particularly in stroke research, which becomes obvious through the numerous commentaries, editorials and reviews on this topic [12–14].
However, we have to ask ourselves whether the choice of research tools, protocols and methods are the sole reason for this depressing failure in translational stroke research. Finally, the choice of the therapeutic target itself – acute neuronal death and neuroprotective strategies – also has to be questioned. Consequently, a paradigm shift in translational stroke research can currently be observed: from early neuroprotection to mechanisms involved in subsequent processes such as stroke-associated comorbidities, regeneration and plasticity. In the following text we want to highlight two of these evolving fields.
Brain-immune interactions in stroke
In response to the ischaemic injury, neuroinflammatory responses are relevant pathomechanisms promoting secondary brain injury in the subacute phase after stroke [15]. Brain resident microglia and astrocytes are activated after ischaemic brain injury and release mediators such as free radicals and proinflammatory cytokines that inflict secondary damage on the peri-ischaemic tissue [16]. Activated glial cells play an important role in clearance of cell debris, promoting neuroregenerative processes and controlling the neuroinflammatory reaction, and hence have a beneficial rather than a neurotoxic function after stroke [17–20]. Additionally, the rapid inflammatory response involves infiltration of leukocyte subpopulations (fig. 1; neutrophils, monocytes and lymphocytes). Recruitment seems to occur in a strictly synchronised manner following brain ischaemia; one of the first types of immune cell infiltrating are neutrophils, followed by monocytes and lymphocytes [21, 22].
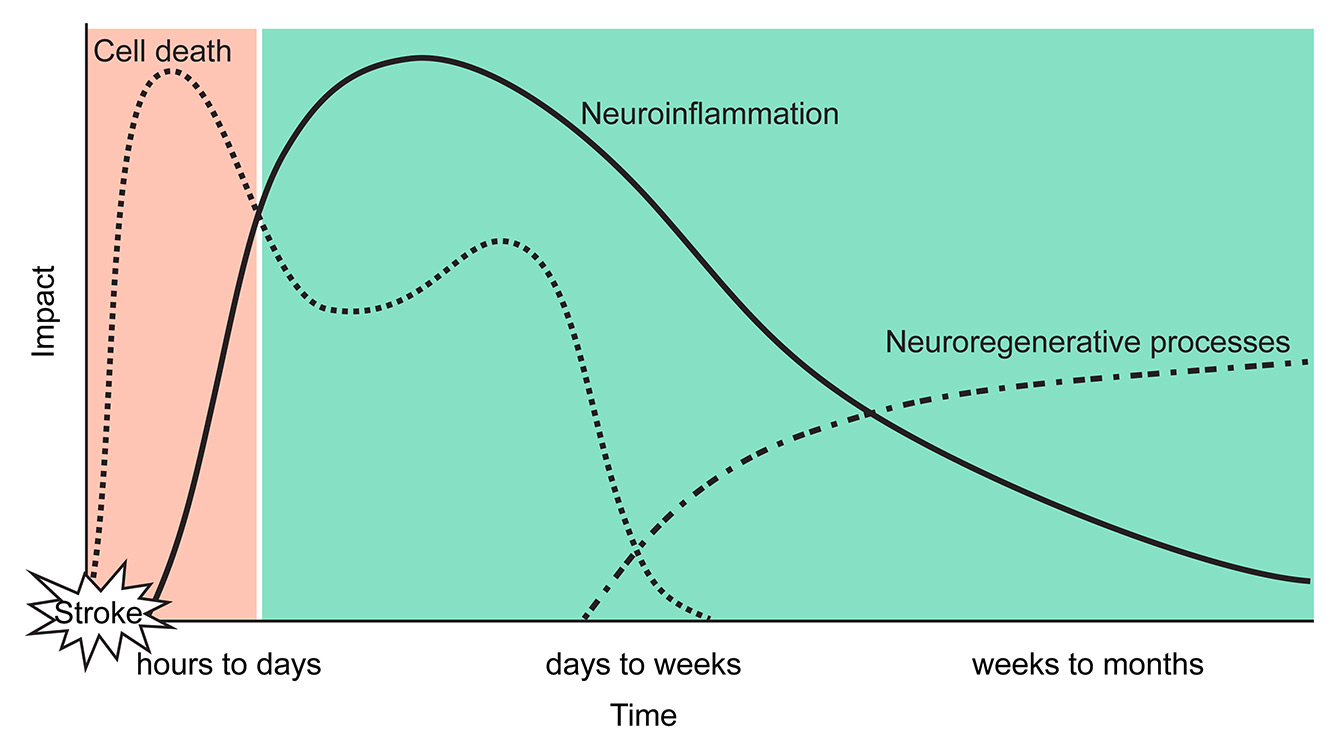
Figure 1
Multiphasic brain interactions after stroke and opportunities for treatment.
Previous neuroprotective strategies targeted pathological mechanisms in a very narrow window of opportunity in the (hyper-) acute phase after stroke (orange). Recently, the focus of translational stroke research has shifted towards understanding pathological processes in the subacute and chronic phase such as neuroinflammation and neuroregeneration (green). These targets have the potential for novel therapeutic approaches which are suitable for a larger population of stroke patients then neuroprotective agents or thrombolysis.
Our current mechanistic insights about the contribution of immunity to stroke pathophysiology were obtained nearly exclusively in rodent stroke models. Despite the only very limited information about neuroinflammatory mechanisms in human stroke, efforts by academia and pharmaceutical companies have prematurely resulted in testing immunomodulatory drugs in human stroke patients (table 1) [23, 24]. These first clinical trials, which aimed to test immunomodulatory drugs (e.g. fingolimod, natalizumab, table 1) in stroke patients, were particularly hampered by the lack of suitable clinical surrogate markers for post-stroke neuroinflammation. However, such novel parameters, for example blood biomarkers or functional imaging of neuroinflammation, will be indispensable for characterising neuroinflammation in stroke subtypes and analysing the efficacy of immunomodulatory drugs in stroke patients. State-of-the-art imaging modalities such as magnetic resonance imaging (MRI) and positron emission tomography (PET) of microglial activation have already provided valuable information in preclinical studies [25]. Molecular MRI seems to be a promising, relatively noninvasive method to image in vivo inflammatory processes in the brain and detect biomarkers (e.g. vascular cell adhesion protein [VCAM] and intercellular adhesion molecule-1 [ICAM-1]) that are not detectable by conventional MRI [26, 27]. Still there are disadvantages, like the low MR sensitivity for cellular neuroinflammation, compared with other molecular imaging modalities such as PET and single-photon-emission-computer-tomography (SPECT). Ultrasmall superparamagnetic iron oxide particles (USPIO) [28], which are used in cancer and cardiac imaging, for example, have high amounts of iron oxide, which can compensate for the low sensitivity. However, repeated usage is limited, as a result of accumulation of the inert particles in liver and kidney. PET imaging of inflammation, using tracers binding the translocator-protein 18kDa (TSPO; formerly known as peripheral benzodiazepine receptor), showed promising results in first clinical approaches in stroke patients [29]. Despite having lower spatial resolution than MRI, the high contrast resolution of PET, offering functional and molecular information with high sensitivity in low molar ranges, makes it a potential imaging modality for future clinical trials targeting neuroinflammatory pathways after stroke.
|
Table 1: Treatment of post-stroke inflammation. |
|
Target
|
Preclinical
|
Model
|
Clinical
|
Treatment effect on:
|
Miscellaneous
|
|
Reference
|
|
Reference
|
Infarct volume
|
Neurol. deficits
|
Inflammation
|
|
| Downregulation of S1P receptors
Fingolimod (FTY720) |
Hasegawa Y. et al. 2010, Stroke [62] |
tMCAo (rat) |
|
+ |
+ |
n.d. |
Decreased Casp-3 expression and number of dying neurons |
| Wei Y. et al., 2011, Ann Neurol [63] |
tMCAo (mouse) |
|
+ |
+ |
Reduction of activated microglia and neutrophil infiltration |
Decreased dying cells in core and peri-infarct area |
| Rolland W. et al., 2013, Exp Neurol [64] |
ICH (rat) |
|
n.d. |
+ |
Reduction of circulating leukocytes and ICAM-1+ T cells in brain |
Ameliorated brain atrophy and memory performance |
| Kraft P. et al. 2013, Stroke [65] |
tMCAo (mouse) |
|
+ |
+ |
No effect on local inflammatory response |
Reduces microvascular thrombosis; no direct neuroprotection or BBB improvement |
| Campos F. et al., 2013, Stroke [66] |
Thrombo-embolic stroke (mouse) |
|
+ |
+ |
n.d. |
Combined alteplase and fingolimod administration; BBB improvement |
| |
|
Fu Y. et al., 2014, PNAS [67] |
+ |
+ |
Reduction of circulating lymphocytes |
Small study: 11 vs 11 patients |
| |
|
Fu Y. et al., 2014, JAMA Neurol [68] |
n.d. |
+ |
Reduction of circulating lymphocytes |
Reduction of PHE and relative PHE after administration |
| |
|
Zhu Z. et al., 2015, Circulation [23] |
+ |
+ |
Reduction of circulating lymphocytes |
Combined alteplase and fingolimod administration |
| Blockage of VLA-4
Natalizumab (α-CD49d) |
Liesz A. et al., 2011, Brain [15] |
pMCAo and 30 min tMCAo (mouse) |
|
+ |
+ |
Reduced number of infiltrating leukocytes |
Anti-CD49d inhibited T cell migration and abrogated their effector mechanisms |
| Langhauser F. et al., 2014, Stroke [69] |
pMCAo and 30 min tMCAo (mouse) |
|
o |
o |
Reduced number of infiltrating leukocytes |
Anti-CD49d did not influence overall stroke outcome irrespective of model or time |
| Llovera G. et al., 2015, Sci Trans Med [70] |
pMCAo and 60 min tMCAo (mouse) |
|
+/o |
o |
Reduced number of infiltrating leukocytes after pMCAo |
First preclinical randomised controlled multicentre trial; reduced infarct volume only in small cortical lesions |
| |
|
Elkins J. et al. “ACTION trial” (ISC 2016) |
o |
+ |
n.d. |
Improvement in functional independence, cognition and patient-reported stroke impact |
| BBB = blood-brain barrier; ICH = intracerebral haemorrhage; PHE = perihaematomal oedema; pMCAo = permanent middle cerebral artery occlusion; tMCAo = transient middle cerebral artery occlusion; VCAM-1 = vascular cell adhesion molecule-1; VLA-4 = very late antigen-4 |
In addition to the neuroinflammatory reaction to the acute brain injury, perturbations of peripheral immune homeostasis have attracted much attention as a relevant complication after stroke. Recent investigations showed that peripheral immune activation has already peaked 4 hours after stroke [30] with highly increased serum concentrations of proinflammatory mediators, both after experimental stroke [31] and in stroke patients [32]. We have recently demonstrated that proinflammatory mediators released from the necrotic brain tissue into the blood circulation after stroke – so-called damage associated molecular patterns (DAMPs) – are critical mediators of a multiphasic peripheral immunomodulation [33]. DAMPs include a plethora of different soluble molecules derived from dying cells and giving rise to new treatment options targeting either DAMPs or their receptors on peripheral immune cells.
Acute immune activation after brain injury is followed by a sudden shift into a subacute immunosuppressive phase caused by exhaustion of innate immune cells and apoptotic lymphocyte death [33]. Subacute immunosuppression results in an increased susceptibility to secondary infections, particularly of the respiratory and urinary tract, which contribute substantially to post-stroke mortality and morbidity [34, 35]. The commonly attributed explanation for this phenomenon is stress-signalling after brain injury, including activation of the hypothalamic–pituitary–adrenal axis and sympathetic innervation of immune organs such as the spleen and bone marrow [36–38]. An alternative explanation proposes exhaustion of innate immune cells upon acute (over)activation and lymphocyte apoptosis due to inadequate costimulatory signalling derived from such exhausted antigen-presenting cells leading to an immunosuppressive phenotype [39]. However, the exact mechanisms of immunodepression following stroke will require further investigation and are a prime example of insufficient reverse translation: while susceptibility to bacterial infections due to functional immunosuppression is a long-standing clinical experience, translation from bedside-to-bench was largely neglected. Future experimental studies will be required for mechanistic understanding and target identification of this clinically important complication after stroke.
|
Table 2: Neuroregenerative approaches after stroke. |
|
Target
|
(Pre)clinical
|
Model
|
Therapeutic effect on:
|
Miscellaneous
|
|
Reference
|
|
Funct. outcome
|
Regeneration
|
|
| Transcranial magnetic or direct current cortical stimulation |
Plautz E. et al., 2003, Neurol Res [71] |
Bipolar coagulation of vasculature in M1 cortex (squirrel monkey) |
+ |
Large-scale plasticity of movement representation in stimulated cortex |
Combination therapy of sub-threshold electrical stimulation and rehabilitative training |
| Hummel F. et al., 2005, Brain [72] |
Six patients (chronic stroke); double-blind crossover study |
+ |
Functional improvement in paretic hand of all patients, which outlasted stimulation period |
Noninvasive cortical stimulation and assessment of functional hand motor skills |
| Khedr EM. et al., 2005, Neurology [73] |
52 stroke patients in a randomised therapeutic trial |
+ |
rTMS led to improvement of disability scores |
10 consecutive days rTMS in addition to best clinical care |
| Takeuchi N. et al., 2005, Stroke [74] |
20 stroke patients with a first-time cerebral infarct |
+ |
Reduction of transcallosal inhibition by reducing the amplitude of motor-evoked potentials in contralesional M1 |
Double blind study of real vs sham rTMS |
| Grefkes C. et al., 2010, NeuroImage [75] |
Eleven patients with unilateral hand weakness after first-ever stroke |
+ |
rTMS over contra-lesional M1 reduced inhibition influence and enables more effective motor processing in lesioned areas |
Usage of DCM to assess rTMS influence on effective connectivity within cortical motor system |
| Zimerman M. et al., 2012, Stroke [61] |
Twelve patients with first-ever subcortical stroke |
+ |
Contra-lesional M1 tDCS improved early online learning period |
Association between an intervention-induced SICI within lesional M1 and enhancement of skill acquisition |
| Motor function therapy & ergorobotics |
Wolf S. et al., 2006, JAMA [76] |
116 stroke patients in a randomised clinical trial |
+ |
CIMT produced improvements in arm motor function that persist ≥1 year |
Measurement of motor function by functional ability and motor activity log |
| Staubli P. et al., 2009, J Neuroeng Rehabil [57] |
Four patients with chronic stroke and left side hemiparesis |
+ |
Three out of four patients showed improvement in motor functions |
Intensive therapy using the robot ARMin II in a functional 3D workspace |
| Lo A. et al., 2010, N Engl J Med [77] |
127 chronic patients in a multicentre, randomised trial |
+ |
Robot-assisted therapy showed motor function improvement after 12 and 36 weeks |
Four-modules robotic system for horizontal, vertical, wrist and grasp movements |
| CIMT = constraint-induced movement therapy; DCM = dynamic causal modelling; M1= primary motor cortex; SICI = short interval intracortical inhibition; tDCS = transcranial direct current stimulation; rTMS = repetitive transcranial magnetic stimulation |
Long-term stroke outcome: regeneration and plasticity
Stroke patients experience continuous functional recovery after the stroke for weeks to years [40]. Due to differences in severity and location of the cerebral lesion, large variability between subjects in terms of functional recovery makes it almost impossible to generalise regenerative processes after the acute brain lesion. Although there are standardised scorings for neurological deficits and recovery after stroke in routine clinical use, such as the NIH Stroke Scale or Rankin Scale, current routine clinical tools are still insufficient to assess functional recovery [41]. Despite impressive progresses and convincing preliminary results obtained by use of imaging modalities to investigate neuronal plasticity, functional imaging of brain plasticity and remapping during the spontaneous recovery after stroke is barely used in clinical practice. Rodent studies [42] as well as clinical observations [43] provide important information about the loss and regain of neuronal connectivity after stroke; nevertheless, systematic network analyses and computational mapping remain demanding and require a high amount of methodological expertise. For integration of functional noninvasive imaging into clinical routine further investigations and the development of robust protocols are needed.
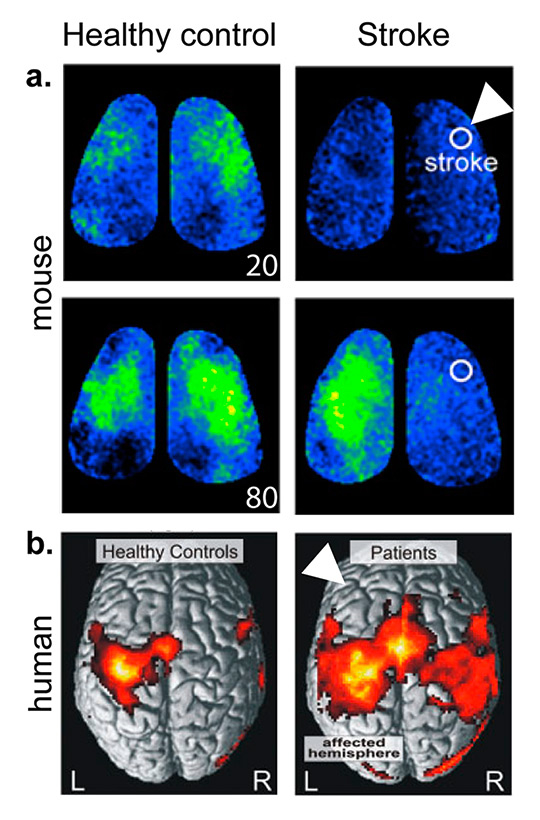
Figure 2
Comparison of neuronal connectivity after stroke in mouse and human.
a. In-vivo imaging of mouse cortex during left forelimb stimulation shows sensory-evoked polarisation in control mice emerging immediately (20 ms) in a confined area representing left forelimb function. In contrast, forelimb stimulation after stroke resulted in a more diffuse signal encompassing mainly the contralateral hemisphere. Additionally, there is a significant shift in timing of signal propagation, with a delayed and prolonged response after stroke (compare signal maps at 20 and 80 ms after forelimb stimulation).
b. Connectivity analysis based on significantly activated voxels (BOLD signal, fMRI) during movement of the right hand shows a distinct single-hemispheric cortical pattern in healthy controls. Hand movement in stroke patients were associated with enhanced and more extended neural activity in both hemispheres. White arrowheads mark the affected hemispheres.( Adapted from Mohajerani et al. 2011 [45] and Grefkes et al. 2008 [43], with permission).
Recently, novel in-vivo imaging modalities visualising cortical neuronal activity in rodent models have advanced our understanding of reorganisation of cortical functional representation and reorganisation after injury [44].After experimental stroke, the loss of functional connectivity, represented by breakdown of cortical connectivity maps, is recovered over weeks by establishing new structural and functional circuits (fig. 2a) [42, 45]. In-vivo imaging of neuronal activity showed that forelimb-evoked responses re-emerge in peri-infarct areas of the cortex after rodent stroke [26, 46]. The processes of re-establishing neuronal circuits, including axonal sprouting, synapse plasticity and neurogenesis, require a distinct micro-milieu of signalling cues to arise during cortical remodelling processes. Therefore, neuronal “re-wiring” becomes a challenge under the conditions of an adult brain, which is generally inhibitory to axonal sprouting [47]. Instead, for post-stroke recovery neurons must engage a neuronal growth programme. Previous reports have shown that growth-inhibitory molecules are reduced after experimental stroke and neurons themselves activate growth-promoting genes in successive waves after ischaemia [47]. Grefkes and colleagues demonstrated, by using functional magnetic resonance imaging (fMRI), the impact of stroke lesions on cerebral network connectivity [43]. They observed, as in the above mentioned rodent studies, that motor deficits of patients with focal ischaemia are associated with pathological intra- and interhemispheric connections between motor areas (fig. 2b). In the future, combining clinical assessment of disabilities and analyses of connectivity by means of imaging modalities such as fMRI might help to determine the patient’s status during the time-course of recovery and to design personalised therapeutic options. Neuronal connectivity analyses will provide insights into how neuromodulatory interventions might target pathological networks that are associated with incomplete recovery. Such novel diagnostic approaches will improve treatment paradigms based on the individual network pathology underlying a particular neurological deficit [48].
Active stimulation of motor function and coordination plays an important role in the recovery and regeneration of cortical circuits. This becomes obvious in experimental stroke studies in which rats housed in an enriched environment, with access to various activities and interaction with other rats, perform significantly better than rats that were housed in a standard environment [49, 50]. Similarly, clinical studies have shown improved cognitive recovery when stroke patients were exposed to music [51], and physical therapy for movement coordination and motor function are well established routine interventions in stroke recovery units [52, 53]. In the last decade, ergo-robotics has become a promising tool in motor-stimulating therapies, although it is not yet used in daily routine. Passively supporting systems like the SwedishHelparm™[54] assist arm movements with counter-weights connected to the arm for fulfilling reach tasks. More advanced systems, like the assisted rehabilitation and measurement (ARM) guide [55], not only support arm movements during therapy, but evaluate the arm impairments to improve further therapy. Recently, state-of-the-art exoskeleton robots provide support, movement guidance and evaluation of movement to individualise rehabilitation therapies, which provided a significantly improved outcome in chronic stroke patients [56, 57]. Additionally, several clinical studies have shown the positive effects of robot-aided neurorehabilitation in comparison with conventional therapy (table 2) [58, 59]. Throughout the last decade, other noninvasive treatment paradigms emerged in the field of chronic stroke regeneration. One paradigm is repetitive transcranial magnetic stimulation (rTMS), relying on the use of an insulated coil placed over the scalp. The coil generates repetitive magnetic pulses, producing changes in brain activity. In several clinical stroke trials, rTMS of the motor cortex led to improved hand function (table 2). A second approach is direct current stimulation (DCS), which uses constant low current delivered via electrodes on the scalp [60]. In a study with patients having a subcortical stroke, contra-lesional M1 area DCS showed an intervention-induced enhancement of skill acquisition [61] (table 2).
Taken together, an integrated view of individual neuronal plasticity after clinical stroke through use of novel imaging modalities and advanced deficit assessment will improve efforts toward personalised and more efficient therapy in stroke recovery.
In summary, after the failure of countless neuroprotective agents in the acute phase, stroke research has to continue transforming from a “neuro-centric” to a multi-disciplinary research field considering the contributions of various brain-resident and invading cell populations to the injured brain, and the complex interplay of the brain and remote organs over a prolonged time course after the stroke. Stroke is more than an acute event, it is a chronic condition and we must not underestimate the potential of therapeutic interventions in the subacute and chronic stages. In the future, promising findings in immune alterations caused by ischaemia and post-stroke brain recovery can provide us with manifold treatment opportunities, which can diminish the burden of stroke.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.
References
1 Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6(2):182–7. Epub 2007/01/24. doi: 10.1016/S1474-4422(07)70031-5. PubMed PMID: 17239805.
2 Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–57. Epub 2006/05/30. doi: 10.1016/S0140-6736(06)68770-9. PubMed PMID: 16731270.
3 Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612–23. Epub 2008/05/13. doi: 10.1016/S0140-6736(08)60694-7. PubMed PMID: 18468545.
4 Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics – 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. Epub 2007/12/19. doi: 10.1161/CIRCULATIONAHA.107.187998. PubMed PMID: 18086926.
5 Jauch EC, Saver JL, Adams HP, Jr., Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. Epub 2013/02/02. doi: 10.1161/STR.0b013e318284056a. PubMed PMID: 23370205.
6 O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–77. Epub 2006/02/03. doi: 10.1002/ana.20741. PubMed PMID: 16453316.
7 Howells DW, Porritt MJ, Rewell SS, O’Collins V, Sena ES, van der Worp HB, et al. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(8):1412–31. Epub 2010/05/21. doi: 10.1038/jcbfm.2010.66. PubMed PMID: 20485296; PubMed Central PMCID: PMC2949237.
8 van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245. Epub 2010/04/03. doi: 10.1371/journal.pmed.1000245. PubMed PMID: 20361020; PubMed Central PMCID: PMC2846855.
9 Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J Gene Med. 2010;12(7):561–3. Epub 2010/07/08. doi: 10.1002/jgm.1473. PubMed PMID: 20607692.
10 Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195–203. Epub 2014/03/04. doi: 10.1016/j.molmed.2014.01.007. PubMed PMID: 24581450.
11 Gladstone DJ, Black SE, Hakim AM, Heart, Stroke Foundation of Ontario Centre of Excellence in Stroke R. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–36. Epub 2002/08/03. PubMed PMID: 12154275.
12 Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61(5):396–402. Epub 2007/04/11. doi: 10.1002/ana.21127. PubMed PMID: 17420989.
13 Grotta J. Neuroprotection is unlikely to be effective in humans using current trial designs. Stroke. 2002;33(1):306–7. Epub 2002/01/10. PubMed PMID: 11779929.
14 Dirnagl U, Endres M. Found in translation: preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke. 2014;45(5):1510-8. Epub 2014/03/22. doi: 10.1161/STROKEAHA.113.004075. PubMed PMID: 24652307.
15 Liesz A, Zhou W, Mracsko E, Karcher S, Bauer H, Schwarting S, et al. Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain. 2011;134(Pt 3):704–20. Epub 2011/03/01. doi: 10.1093/brain/awr008. PubMed PMID: 21354973.
16 Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–568. Epub 1999/10/03. PubMed PMID: 10508238.
17 Hellwig S, Heinrich A, Biber K. The brain’s best friend: microglial neurotoxicity revisited. Front Cell Neurosci. 2013;7:71. Epub 2013/06/05. doi: 10.3389/fncel.2013.00071. PubMed PMID: 23734099; PubMed Central PMCID: PMC3655268.
18 Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. Epub 2011/12/14. doi: 10.1186/1742-2094-8-174. PubMed PMID: 22152337; PubMed Central PMCID: PMC3251548.
19 Perego C, Fumagalli S, De Simoni MG. Three-dimensional confocal analysis of microglia/macrophage markers of polarization in experimental brain injuryJ Vis Exp. 2013(79). Epub 2013/09/24. doi: 10.3791/50605. PubMed PMID: 24056862; PubMed Central PMCID: PMC3857388.
20 Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70. Epub 2012/08/31. doi: 10.1161/STROKEAHA.112.659656. PubMed PMID: 22933588.
21 del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci. 2010;1207:143–8. Epub 2010/10/20. doi: 10.1111/j.1749-6632.2010.05761.x. PubMed PMID: 20955437; PubMed Central PMCID: PMC4552338.
22 Famakin BM. The Immune Response to Acute Focal Cerebral Ischemia and Associated Post-stroke Immunodepression: A Focused Review. Aging Dis. 2014;5(5):307–26. Epub 2014/10/03. doi: 10.14336/AD.2014.0500307. PubMed PMID: 25276490; PubMed Central PMCID: PMC4173797.
23 Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the Immune Modulator Fingolimod With Alteplase in Acute Ischemic Stroke: A Pilot Trial. Circulation. 2015;132(12):1104–12. doi: 10.1161/CIRCULATIONAHA.115.016371. PubMed PMID: 26202811; PubMed Central PMCID: PMC4580515.
24 Schabitz WR, Dirnagl U. Are we ready to translate T-cell transmigration in stroke? Stroke. 2014;45(6):1610–1. Epub 2014/04/20. doi: 10.1161/STROKEAHA.114.005294. PubMed PMID: 24743434.
25 Jacobs AH, Tavitian B, consortium IN. Noninvasive molecular imaging of neuroinflammation. J Cereb Blood Flow Metab. 2012;32(7):1393–415. Epub 2012/05/03. doi: 10.1038/jcbfm.2012.53. PubMed PMID: 22549622; PubMed Central PMCID: PMC3390799.
26 Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, et al. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A. 2001;98(22):12766–71. Epub 2001/10/19. doi: 10.1073/pnas.231235598. PubMed PMID: 11606760; PubMed Central PMCID: PMC60128.
27 Gauberti M, Montagne A, Marcos-Contreras OA, Le Behot A, Maubert E, Vivien D. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke. 2013;44(7):1988–96. Epub 2013/06/08. doi: 10.1161/STROKEAHA.111.000544. PubMed PMID: 23743972.
28 Hoyte LC, Brooks KJ, Nagel S, Akhtar A, Chen R, Mardiguian S, et al. Molecular magnetic resonance imaging of acute vascular cell adhesion molecule-1 expression in a mouse model of cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(6):1178–87. Epub 2010/01/21. doi: 10.1038/jcbfm.2009.287. PubMed PMID: 20087364; PubMed Central PMCID: PMC2949202.
29 Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55(7):1052–4. Epub 2000/11/04. PubMed PMID: 11061271.
30 Chapman KZ, Dale VQ, Denes A, Bennett G, Rothwell NJ, Allan SM, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29(11):1764–8. Epub 2009/08/06. doi: 10.1038/jcbfm.2009.113. PubMed PMID: 19654587.
31 Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, et al. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PloS One. 2014;9(3):e89450. Epub 2014/03/07. doi: 10.1371/journal.pone.0089450. PubMed PMID: 24594628; PubMed Central PMCID: PMC3942385.
32 Schulze J, Zierath D, Tanzi P, Cain K, Shibata D, Dressel A, et al. Severe stroke induces long-lasting alterations of high-mobility group box 1. Stroke. 2013;44(1):246–8. Epub 2012/12/04. doi: 10.1161/STROKEAHA.112.676072. PubMed PMID: 23204053; PubMed Central PMCID: PMC3530419.
33 Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci. 2015;35(2):583–98. Epub 2015/01/16. doi: 10.1523/JNEUROSCI.2439-14.2015. PubMed PMID: 25589753; PubMed Central PMCID: PMC4293412.
34 Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2 Suppl):770–3. Epub 2007/01/31. doi: 10.1161/01.STR.0000251441.89665.bc. PubMed PMID: 17261736.
35 Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198(5):725–36. Epub 2003/08/27. doi: 10.1084/jem.20021098. PubMed PMID: 12939340; PubMed Central PMCID: PMC2194193.
36 Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, et al. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol. 2013;20(1):153–9. Epub 2012/07/28. doi: 10.1111/j.1468-1331.2012.03818.x. PubMed PMID: 22834894.
37 Chamorro A, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nature reviews Neurology. 2012;8(7):401–10. doi: 10.1038/nrneurol.2012.98.
38 Urra X, Obach V, Chamorro A. Stroke induced immunodepression syndrome: from bench to bedside. Curr Mol Med. 2009;9(2):195–202.
39 Singh V, Roth S, Veltkamp R, Liesz A. HMGB1 as a key mediator of immune mechanisms in ischemic stroke. Antioxid Redox Signal. 2015. doi: 10.1089/ars.2015.6397. PubMed PMID: 26493086.
40 Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272–87. Epub 2008/04/03. doi: 10.1002/ana.21393. PubMed PMID: 18383072.
41 Patel AT, Duncan PW, Lai SM, Studenski S. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. 2000;81(10):1357–63. Epub 2000/10/13. doi: 10.1053/apmr.2000.9397. PubMed PMID: 11030501.
42 Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009;29(6):1719–34. Epub 2009/02/13. doi: 10.1523/JNEUROSCI.4249-08.2009. PubMed PMID: 19211879.
43 Grefkes C, Nowak DA, Eickhoff SB, Dafotakis M, Kust J, Karbe H, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236–46. Epub 2007/09/28. doi: 10.1002/ana.21228. PubMed PMID: 17896791.
44 Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193(2):291–311. Epub 2005/05/05. doi: 10.1016/j.expneurol.2005.01.004. PubMed PMID: 15869933.
45 Mohajerani MH, Aminoltejari K, Murphy TH. Targeted mini-strokes produce changes in interhemispheric sensory signal processing that are indicative of disinhibition within minutes. Proc Natl Acad Sci U S A. 2011;108(22):E183–91. Epub 2011/05/18. doi: 10.1073/pnas.1101914108. PubMed PMID: 21576480; PubMed Central PMCID: PMC3107306.
46 Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. Epub 2009/11/06. doi: 10.1038/nrn2735. PubMed PMID: 19888284.
47 Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59(5):735–42. Epub 2006/04/25. doi: 10.1002/ana.20845. PubMed PMID: 16634041.
48 Grefkes C, Fink GR. Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 2014;13(2):206–16. Epub 2014/01/25. doi: 10.1016/S1474-4422(13)70264-3. PubMed PMID: 24457190.
49 Held JM, Gordon J, Gentile AM. Environmental influences on locomotor recovery following cortical lesions in rats. Behav Neurosci. 1985;99(4):678–90. Epub 1985/08/01. PubMed PMID: 3843734.
50 Ohlsson AL, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke. 1995;26(4):644–9. Epub 1995/04/01. PubMed PMID: 7709412.
51 Sarkamo T, Tervaniemi M, Laitinen S, Forsblom A, Soinila S, Mikkonen M, et al. Music listening enhances cognitive recovery and mood after middle cerebral artery stroke. Brain. 2008;131(Pt 3):866–76. Epub 2008/02/22. doi: 10.1093/brain/awn013. PubMed PMID: 18287122.
52 Platz T. [Evidence-based arm rehabilitation – a systematic review of the literature.] Nervenarzt. 2003;74(10):841–9. Epub 2003/10/11. doi: 10.1007/s00115-003-1549-7. PubMed PMID: 14551687. German.
53 Dickstein R, Hocherman S, Pillar T, Shaham R. Stroke rehabilitation. Three exercise therapy approaches. Phys Ther. 1986;66(8):1233–8. Epub 1986/08/01. PubMed PMID: 3737695.
54 Riener R, Nef T, Colombo G. Robot-aided neurorehabilitation of the upper extremities. Med Biol Eng Comput. 2005;43(1):2–10. Epub 2005/03/04. PubMed PMID: 15742713.
55 Reinkensmeyer DJ, Dewald JP, Rymer WZ. Guidance-based quantification of arm impairment following brain injury: a pilot study. IEEE Trans Rehabil Eng. 1999;7(1):1–11. Epub 1999/04/03. PubMed PMID: 10188602.
56 Nilsson A, Vreede KS, Haglund V, Kawamoto H, Sankai Y, Borg J. Gait training early after stroke with a new exoskeleton – the hybrid assistive limb: a study of safety and feasibility. J Neuroeng Rehabil. 2014;11:92. Epub 2014/06/04. doi: 10.1186/1743-0003-11-92. PubMed PMID: 24890413; PubMed Central PMCID: PMC4065313.
57 Staubli P, Nef T, Klamroth-Marganska V, Riener R. Effects of intensive arm training with the rehabilitation robot ARMin II in chronic stroke patients: four single-cases. J Neuroeng Rehabil. 2009;6:46. Epub 2009/12/19. doi: 10.1186/1743-0003-6-46. PubMed PMID: 20017939; PubMed Central PMCID: PMC2807864.
58 Fasoli SE, Krebs HI, Stein J, Frontera WR, Hogan N. Effects of robotic therapy on motor impairment and recovery in chronic stroke. Arch Phys Med Rehabil. 2003;84(4):477–82. Epub 2003/04/12. doi: 10.1053/apmr.2003.50110. PubMed PMID: 12690583.
59 Lum P, Reinkensmeyer D, Mahoney R, Rymer WZ, Burgar C. Robotic devices for movement therapy after stroke: current status and challenges to clinical acceptance. Top Stroke Rehabil. 2002;8(4):40–53. Epub 2003/10/03. doi: 10.1310/9KFM-KF81-P9A4-5WW0. PubMed PMID: 14523729.
60 Feng WW, Bowden MG, Kautz S. Review of transcranial direct current stimulation in poststroke recovery. Topics Stroke Rehabil. 2013;20(1):68–77. Epub 2013/01/24. doi: 10.1310/tsr2001-68. PubMed PMID: 23340073.
61 Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43(8):2185–91. Epub 2012/05/24. doi: 10.1161/STROKEAHA.111.645382. PubMed PMID: 22618381.
62 Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41(2):368–74. Epub 2009/11/27. doi: 10.1161/STROKEAHA.109.568899. PubMed PMID: 19940275; PubMed Central PMCID: PMC2811754.
63 Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, et al. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69(1):119–29. Epub 2011/02/01. doi: 10.1002/ana.22186. PubMed PMID: 21280082; PubMed Central PMCID: PMC3200194.
64 Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, et al. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp Neurol. 2013;241:45–55. Epub 2012/12/25. doi: 10.1016/j.expneurol.2012.12.009. PubMed PMID: 23261767; PubMed Central PMCID: PMC3570752.
65 Kraft P, Gob E, Schuhmann MK, Gobel K, Deppermann C, Thielmann I, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 2013;44(11):3202–10. Epub 2013/09/14. doi: 10.1161/STROKEAHA.113.002880. PubMed PMID: 24029635.
66 Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, et al. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke. 2013;44(2):505–11. Epub 2013/01/05. doi: 10.1161/STROKEAHA.112.679043. PubMed PMID: 23287783; PubMed Central PMCID: PMC3586809.
67 Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci U S A. 2014;111(51):18315–20. Epub 2014/12/10. doi: 10.1073/pnas.1416166111. PubMed PMID: 25489101; PubMed Central PMCID: PMC4280578.
68 Fu Y, Hao J, Zhang N, Ren L, Sun N, Li YJ, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71(9):1092–101. Epub 2014/07/09. doi: 10.1001/jamaneurol.2014.1065. PubMed PMID: 25003359.
69 Langhauser F, Kraft P, Gob E, Leinweber J, Schuhmann MK, Lorenz K, et al. Blocking of alpha4 integrin does not protect from acute ischemic stroke in mice. Stroke. 2014;45(6):1799–806. Epub 2014/04/20. doi: 10.1161/STROKEAHA.114.005000. PubMed PMID: 24743435.
70 Llovera G, Hofmann K, Roth S, Salas-Perdomo A, Ferrer-Ferrer M, Perego C, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): Anti-CD49d treatment for acute brain ischemia. Sci Transl Med. 2015;7(299):299ra121. Epub 2015/08/08. doi: 10.1126/scitranslmed.aaa9853. PubMed PMID: 26246166.
71 Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003;25(8):801–10. Epub 2003/12/13. doi: 10.1179/016164103771953880. PubMed PMID: 14669522.
72 Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–9. Epub 2005/01/07. doi: 10.1093/brain/awh369. PubMed PMID: 15634731.
73 Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–8. Epub 2005/08/10. doi: 10.1212/01.wnl.0000173067.84247.36. PubMed PMID: 16087918.
74 Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681–6. Epub 2005/10/29. doi: 10.1161/01.STR.0000189658.51972.34. PubMed PMID: 16254224.
75 Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. NeuroImage. 2010;50(1):233–42. Epub 2009/12/17. doi: 10.1016/j.neuroimage.2009.12.029. PubMed PMID: 20005962.
76 Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–104. Epub 2006/11/02. doi: 10.1001/jama.296.17.2095. PubMed PMID: 17077374.
77 Lo AC, Guarino PD, Richards LG, Haselkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362(19):1772–83. Epub 2010/04/20. doi: 10.1056/NEJMoa0911341. PubMed PMID: 20400552.

