Figure 1
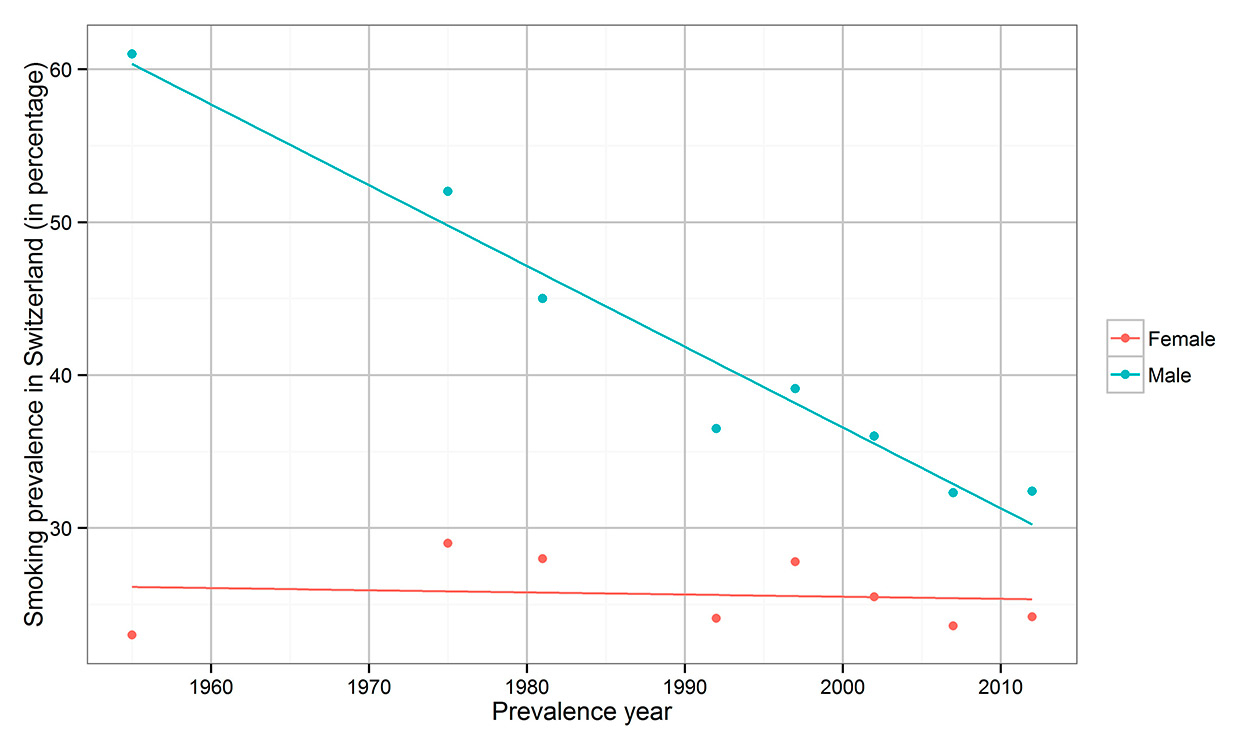
Smoking prevalence in Switzerland, 1955–2012. Source: 1955 [14], 1975–1981 [15], 1992–2012 [16].
DOI: https://doi.org/10.4414/smw.2016.14327
Lung cancer still causes the largest proportion of cancer deaths in many countries worldwide [1, 2]. In Switzerland, lung cancer is the leading cause of cancer death in men and the second most common cause of cancer death in women, accounting for 40.3/100 000 deaths in men and 18.7/100 000 deaths in women (mean mortality rate 2008–2012, age-standardised, European standard) [3]. Changes in histological subtypes of lung carcinoma and concurrent sex-specific shifts within the last decades have been reported globally [4]. Of the five main histomorphological lung carcinoma subtypes, adenocarcinoma (ADC) and squamous cell carcinoma (SCC) are proportionately the most important [5]. A shift from SCC to ADC has been observed in men with a coincident increase of SCC and ADC in women [5]. Less studied is the phenomenon of quantitative susceptibility of one side of the lung. As the right and left lung are different in volume, it has been hypothesised that lung cancer incidence coincides with this asymmetry [6].
Although there are several risk factors for lung carcinoma, such as environmental and occupational causes, genetic and sex-specific factors, nutrition, overweight, and maybe even human papilloma virus infection, tobacco smoking is considered the main risk factor [7–9]. Smoking accounts for up to 90% of lung carcinoma, with a delay of occurrence of around 20–30 years [10, 11]. Therefore, lung cancer incidence reflects the smoking behaviour of a population [12], while international lung cancer incidence reflects the stage of the tobacco epidemic [13]. Figure 1 depicts the tobacco epidemic in Switzerland [14–16], where smoking prevalence in women was 24.1% in 1992 and 25.5% in 2002, and in men was 36.5% in 1992 and 36% in 2002 [16]. In the USA, smoking prevalence reached high levels in the 1950s in men (about 55%) and in the 1960s in women (about 35%) [17–19], whereas in Switzerland smoking prevalence peaked in the 1950s in men (about 61%) and in the 1970s in women (about 29%) [14–16]. A coincident increase of lung cancer incidence was observed in the 1970s in Europe [13] and in the USA [12]. Lung cancer incidence in the USA has decreased in men since the 1980s and in women after 2000, reflecting changes in the smoking epidemic [20, 21]. Because of different smoking behaviour among women compared with men, i.e. lower smoking prevalence and lower numbers of cigarettes smoked per day, lung cancer in women occurred later and will likely not reach as high levels as the epidemic did among men [11].
To provide information about changes in trends over the last three decades, data of the population-based Cancer Registry of the Canton of Zurich were analysed with a focus on sex-specific changes as well as histomorphological and topographic characteristics.
The canton of Zurich is, with respect to population, the largest Swiss canton with 1 359 712 inhabitants in 2010. Anonymised data of the Cancer Registry of the Canton of Zurich were analysed for changes in trends in lung cancer incidence by histological subtype, age, sex and laterality. Epidemiological data are available since 1980. The Cancer Registry provides general demographic data about the patient, data about the histological type, date and type of diagnosis, the site of the tumour, and data about treatment and survival status.

Figure 1
Smoking prevalence in Switzerland, 1955–2012. Source: 1955 [14], 1975–1981 [15], 1992–2012 [16].
The analytical cohort consisted of 16 985 primary incident cases of lung cancer in the Canton of Zurich from 1980 to 2010. One hundred eighty-seven of the 16 985 cases were excluded from the study because of missing age information.
Cancer data of the Swiss Canton of Zurich were filtered using International Classification of Diseases (ICD) codes (C33 and C34, 7 cases C809 with cytological or histological confirmation). Lung cancer was grouped using the ICD for Oncology (ICD-O-3) [22] – five histologic subtypes and one group with others and not otherwise specified types (see appendix). Of all cases, 74.5% were histologically confirmed, and 18% were cytologically confirmed. In 2% of the cases, the only information in the registry was from a death certificate.
Incidence rates (IRs, cases per 100 000 person-years) were calculated using a weighted average of rates in 5-year age groups according to the European standard [23]. IRs for ten-year age cohorts (40–49, 50–59, 60–69, 70–79 and ≥80 years) were calculated for the major histological subtypes ADC, SCC and small cell carcinoma (SCLC). Confidence intervals were calculated for all IRs using the delta method (confidence intervals of the log rates and back transformation to rates).
Male-to-female incidence-rate ratio (M/F-IRR) and left-to-right lung incidence-rate ratio (L/R-IRR) were calculated to compare sex- or site-specific susceptibility. To compare the histological types shown in table 1, the following tests were used: analysis of variance for continuous data, chi-square for categorical data, and Poisson regression for IR and L/R-IRR. To study the effect of sex, incidence year, and age for each major histological subtype we performed a multivariable Poisson regression. Poisson regression was only applied for the three major histological types owing to larger sample size and clinical relevance.
| Table 1: Characteristics of primary incident lung cancer cases diagnosed in the Canton of Zurich 1980–2010. | ||||||||
| All cases | Adenocarcinoma | Squamous cell carcinoma | Small cell carcinoma | Carcinoid tumour | Large cell carcinoma | Others and NOS | p-value | |
| Variable | n = 16 798 (100%) | n = 5357 (31.9%) | n = 4895 (29.1%) | n = 2592 (15.4%) | n = 250 (1.5%) | n = 1065 (6.3%) | n = 2639 (15.7%) | |
| Age (years) | <0.001 | |||||||
| Mean ± SD | 67.5 ± 11.2 | 66.2 ± 11.3 | 68.1 ± 9.9 | 66.0 ± 10.6 | 62.1 ± 16.4 | 65.2 ± 11.4 | 71.9 ± 11.5 | |
| Median | 68.2 | 66.5 | 68.8 | 66.5 | 65.7 | 65.4 | 72.9 | |
| Quartile 25%; 75% | 60.0; 75.8 | 58.3; 74.7 | 61.4; 75.5 | 58.8; 73.9 | 51.3; 74.6 | 57.6; 73.5 | 64.5; 80.7 | |
| Sex, n (%) | <0.001 | |||||||
| Women | 4652 (100%) | 2034 (43.7%) | 720 (15.5%) | 701 (15.1%) | 141 (3.0%) | 305 (6.6%) | 751 (16.1%) | |
| Men | 12 146 (100%) | 3323 (27.4%) | 4175 (34.4%) | 1891 (15.6%) | 109 (0.9%) | 760 (6.3%) | 1888 (15.6%) | |
| Men/women ratio | 2.61 | 1.63 | 5.80 | 2.70 | 0.77 | 2.49 | 2.51 | |
| Period of diagnosis, n (%) | <0.001 | |||||||
| 1980–1989 | 4796 (100%) | 1088 (22.7%) | 1962 (40.9%) | 889 (18.5%) | 49 (1.0%) | 228 (4.8%) | 580 (12.1%) | |
| 1990–1999 | 5342 (100%) | 1623 (30.4%) | 1594 (29.8%) | 829 (15.5%) | 93 (1.7%) | 386 (7.2%) | 817 (15.3%) | |
| 2000–2010 | 6660 (100%) | 2646 (39.7%) | 1339 (20.1%) | 874 (13.1%) | 108 (1.6%) | 451 (6.8%) | 1242 (18.6%) | |
| Laterality unknown, n (%) | ||||||||
| Women | 584 (12.6%) | 185 (9.1%) | 40 (5.6%) | 97 13.8%) | 17 (12.1%) | 42 (13.8%) | 203 (27.0%) | <0.001 |
| Men | 1284 (10.6%) | 301 (9.1%) | 178 (4.3%) | 197 (10.4%) | 6 (5.5%) | 75 (9.9%) | 527 (27.9%) | <0.001 |
| IR women | <0.001 | |||||||
| 1980–1989, mean IR (95% CI) | 18.5 (16.7–20.2) | 5.1 (4.5–5.8) | 3.4 (2.7–4.0) | 3.3 (2.7–4.0) | 2.0 (1.0–3.0) | 1.7 (1.1–2.2) | 2.9 (2.2–3.7) | |
| 1990–1999, mean IR (95% CI) | 23.5 (21.9–25.0) | 8.3 (7.5–9.1) | 3.6 (3.1–4.1) | 3.9 (3.3–4.5) | 1.9 (1.2–2.5) | 2.6 (2.1–3.2) | 3.2 (2.7–3.7) | |
| 2000–2010 mean IR (95% CI) | 29.5 (27.9–31.0) | 12.6 (11.8–13.4) | 4.0 (3.4–4.5) | 3.8 (3.3–4.3) | 2.2 (1.4–3.0) | 2.4 (1.9–2.8) | 4.5 (3.9–5.2) | |
| IR men | <0.001 | |||||||
| 1980–1989 mean, IR (95% CI) | 80.0 (77.1–82.8) | 15.1 (14.0–16.3) | 34.2 (32.5–35.9) | 14.1 (13.0–15.2) | 2.3 (1.3–3.3) | 4.7 (3.8–5.5) | 9.5 (8.5–10.5) | |
| 1990–1999 mean, IR (95% CI) | 69.0 (66.6–71.3) | 17.8 (16.7–19.0) | 22.9 (21.7–24.2) | 10.4 (9.5–11.2) | 2.2 (1.5–3.0) | 5.4 (4.7–6.0) | 10.2 (9.3–11.1) | |
| 2000–2010 mean, IR (95% CI) | 56.3 (54.5–58.2) | 19.4 (18.4–20.4) | 12.8 (12.0–13.6) | 7.4 (6.8–8.0) | 2.1 (1.3–2.8) | 4.6 (4.0–5.1) | 10.2 (9.4–10.9) | |
| L/R-IRR (IR left / IR right) | <0.001 | |||||||
| Women | 0.72 | 0.65 | 0.81 | 0.81 | 0.78 | 0.79 | 0.72 | |
| Men | 0.83 | 0.79 | 0.86 | 0.82 | 1.00 | 0.76 | 0.86 | |
| IR = incidence rate, standardised (European standard); L/R-IRR = left-to-right lung incidence-rate ratio; NOS = not otherwise specified (n = 1983) including non-small cell carcinoma (n = 455) | ||||||||
Of all cases, 72.3% were male and 27.7% were female, with a mean age of 67.5 (SD ± 11.2; table 1). The absolute number of lung cancer cases in the Canton of Zurich increased from 1980 to 2010. In the decade 1980–1989, 4796 cases were reported, 5342 cases in 1990–1999 (+11.4%) and 6660 cases in 2000–2010 (+24.7%). Over the whole period, ADC was most common (31.9%), followed by SCC (29.1%), others and not otherwise specified (15.7%), SCLC (15.4%), large cell carcinoma (6.3%), and carcinoid tumour (1.5%).
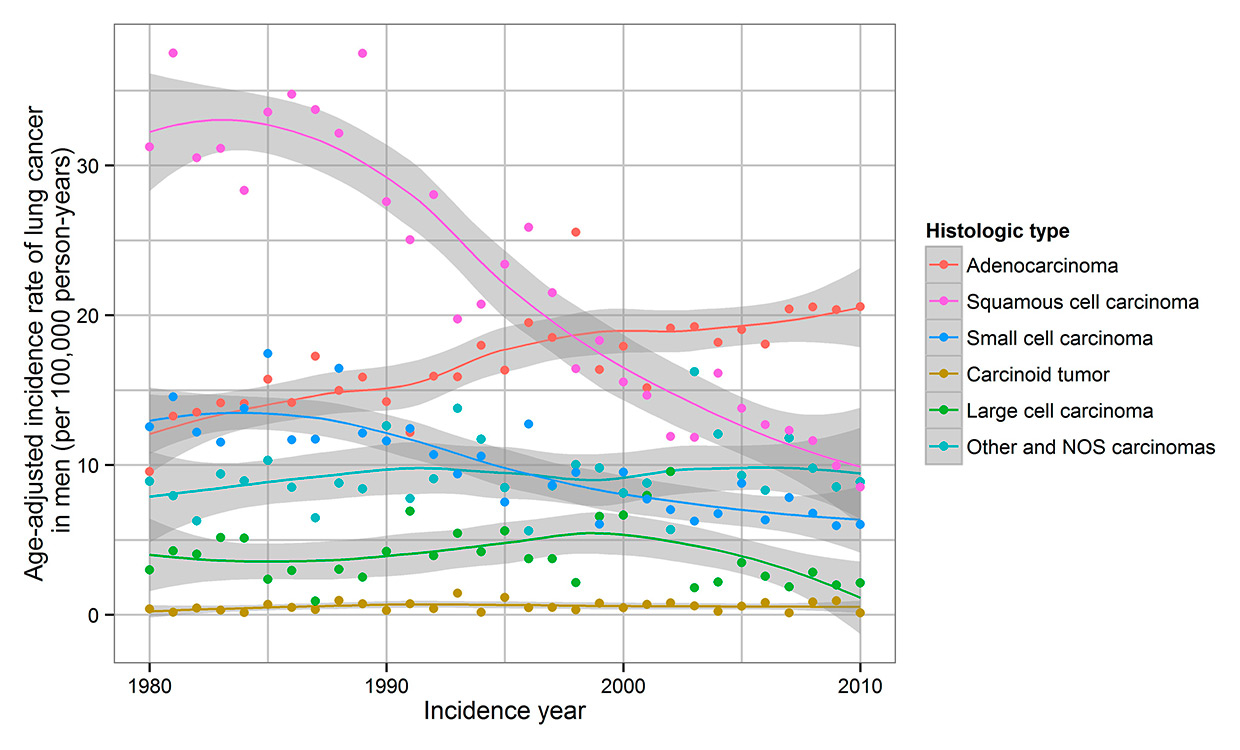
Figure 2
Trends in lung cancer incidence in men by histological type in the Canton of Zurich, Switzerland, 1980–2010. Rates are age adjusted to the standard European population.
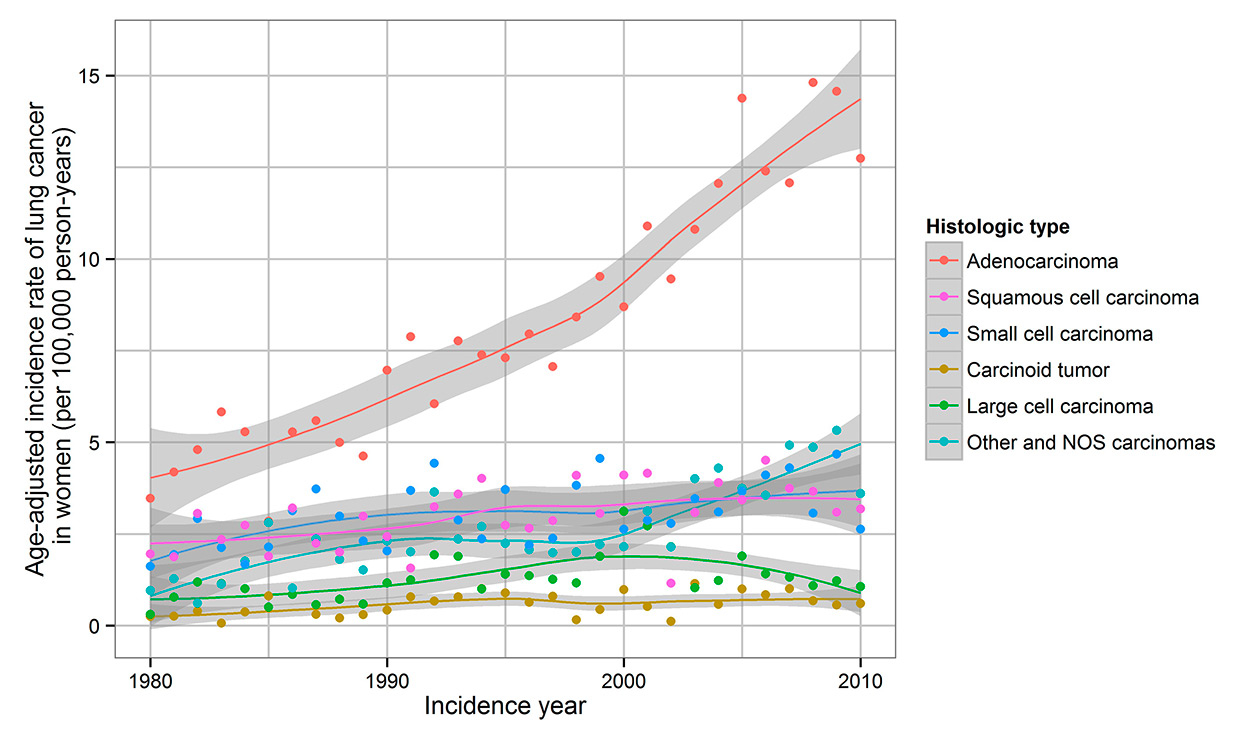
Figure 3
Trends in lung cancer incidence in women by histological type in the Canton of Zurich, Switzerland, 1980–2010. Rates are age adjusted to the standard European population. NOS = not otherwise specified.
The mean age-standardised IR in men decreased from 80.0 (1980–1989) to 56.3 (2000–2010), while there was an increase in IR per decade from 18.5 (1980–1989) to 29.5 (2000–2010) in women. The M/F-IRR was 2.6 for all lung cancer sites, with the highest ratio for SCC (5.8) and the lowest for carcinoid tumour (0.77).
From 1980 to 1989, SCC was the predominant histological subtype in men. From 1998 to 2010, ADC became the major subtype (fig. 2). In women, ADC was the predominant histological subtype from 1980 onward (fig. 3). For ADC, there was an increase of IR in women (5.1 to 12.6) and in men (15.1 to 19.4). A decrease in IR of SCC in men (34.2 to 12.8) occurred over the study period, whereas IR slightly increased in women (3.4 to 4.0). SCC IR in men peaked in 1989, but the IR in women showed constant levels with a discrete peak in 2006.
In women, IR of all histological subtypes increased, but in men an increase occurred only in ADC. ADC accounted for a larger proportion of lung carcinomas in women (43.7%) than in men (27.4%). However, IR for every histological subtype was significantly lower for women than for men. Sex differences in IR were most evident in SCC and least in carcinoid tumour.
IR of ADC for age cohorts of over 50-year-old women and men increased from 1980 until 2010 (fig. 4). The highest IR in the last decade was observed in men aged 70–79 and ≥80, but a decade earlier in women. IR of SCC showed a decrease over the years, especially in men aged 70–79 and ≥80 years. The only increase was seen in the women’s age cohorts of 60–69 and 70–79. IR of SCLC for women was constant apart from a slight increase in age cohorts 60–69 and 70–79. IR of SCLC of men decreased except for men ≥80 years old.
Results of the Poisson regression confirmed the observation that the incidence of all three major histological types was higher among men than women (table 2), and that the incidence of ADC increased over the observation period, while the incidence of SCC and SCLC decreased. The incidence of ADC increased with age, whereas the IRRs for SCC decreased with age; the IRRs for SCLC did not differ statistically significantly between the age groups. We also applied a Poisson regression model with interaction terms between sex and incidence year, as well as sex and age. Statistically significant interactions between sex and incidence year and between sex and age were observed for all histological types combined and partly also for histological subtypes, such that changes in IR over time depended on both sex and age group (figs 2–4). For example, the increase in ADC incidence over time was stronger in men than in women and particularly in men of the two upper age categories (fig. 4).
For all histological subtypes, there was a higher susceptibility of the right lung, which was more distinct in women than in men (table 1). In 11.1% of the cases, information about laterality was not available. L/R-IRR showed statistically significant variations according to histological subtype, with the lowest L/R-IRR seen for ADC.
| Table 2: Associations of sex, incidence year and age with lung cancer subtypes in the Canton of Zurich, 1980–2010. | |||||||
| Adenocarcinoma | Squamous cell carcinoma | Small cell carcinoma | |||||
| IRR* | CI | IRR* | CI | IRR* | CI | ||
| Sex | Female | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| Male | 1.94 | (1.83–2.06) | 7.42 | (6.80–8.09) | 2.96 | (3.39–4.16) | |
| Incidence year | 1980 | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) |
| 1990 | 1.29 | (1.19–1.39) | 0.73 | (0.68–0.78) | 0.90 | (0.77–0.94) | |
| 2000 | 1.65 | (1.53–1.77) | 0.50 | (0.46–0.54) | 0.84 | (0.60–0.73) | |
| 2010 | 1.91 | (1.66–2.20) | 0.30 | (0.24–0.36) | 0.64 | (0.30–0.51) | |
| Age (years) | 30–39 | 0.50 | (0.42–0.60) | 0.46 | (0.40–0.54) | 0.00 | – |
| 40–49 | 0.62 | (0.57–0.68) | 0.70 | (0.56–0.86) | 0.95 | (0.50–0.79) | |
| 50–59 | 0.70 | (0.65–0.77) | 0.61 | (0.56–0.66) | 1.13 | (0.87–1.19) | |
| 60–69 | 1.00 | (reference) | 1.00 | (reference) | 1.00 | (reference) | |
| 70–79 | 1.32 | (1.22–1.43) | 1.29 | (1.19–1.39) | 1.06 | (1.37–1.65) | |
| ≥80 | 2.99 | (2.72–3.28) | 2.53 | (2.22–2.87) | 1.30 | (2.74–3.76) | |
| * Incidence-rate ratios (IRR) are mutually adjusted for sex, incidence year, and age | |||||||
From our analysis three major findings have emerged. First, this study reports an increase of ADC incidence in the Canton of Zurich in men and women. Second, IR of SCC decreased in men but not in women. Third, there were changes in M/F-IRR and L/R-IRR depending on the histological subtype and the time period.
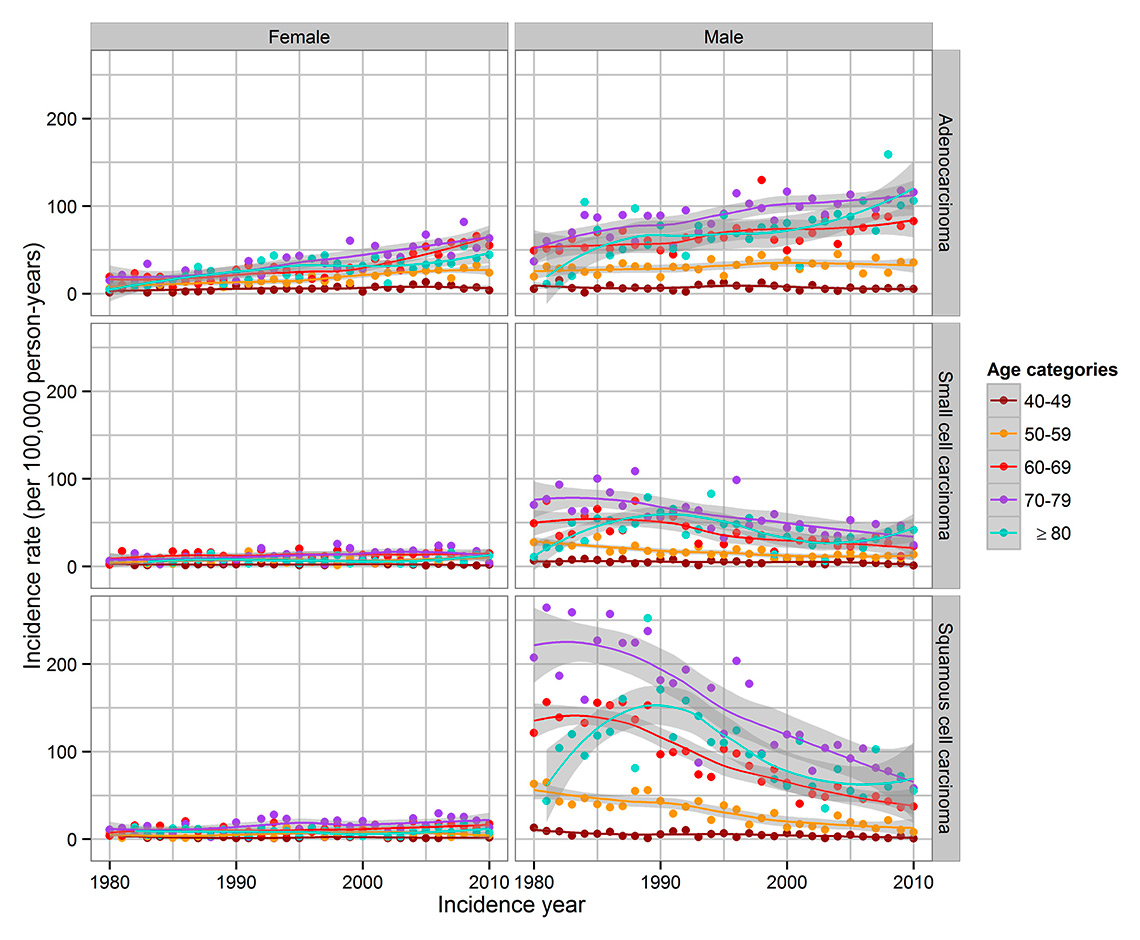
Figure 4
Trends in lung cancer incidence by sex, major histological type and age categories in the Canton of Zurich, Switzerland, 1980–2010. NOS = not otherwise specified.
IR of lung cancer in the Canton of Zurich was much lower than in the USA over the whole observed period, and will probably never reach such high levels (peak IR in men: USA 102.1/100,000 (1984), Canton of Zurich 80.1/100,000 (1985); in women: USA 53.6/100,000 (2007), Canton of Zurich 29.4/100,000 (2009)) [12, 21, 24]. There are few studies of lung cancer incidence in Switzerland, but more on lung cancer mortality. However, lung cancer mortality rates closely follow IR owing to the high fatality rate of lung cancer (lung cancer 5-year survival <20% [25]). Rising lung cancer mortality in Switzerland was observed from 1950 to the early 1970s among men [26] and an ongoing increase in women [13, 27], although still lower than in the USA and in many European countries. The lung cancer mortality rate in Switzerland is one of the lowest in Europe, especially among men [13, 28]. In Switzerland and many European countries lung cancer mortality rate among women increased over the last decade [13, 27]. We observe the same trend in the Canton of Zurich: IR in women increased over the observation period.
As in other European countries [4], ADC is the predominant histological subtype in the Canton of Zurich, with a rising ADC incidence. Our data show an ongoing increase of ADC incidence and an increase of ADC among men and women projecting the future mortality burden in the Canton of Zurich. In the USA an increase of ADC incidence has been observed since 1980 [8, 29–31] with a temporary decrease of ADC in men up to 2005 [31]. An earlier study in the Swiss Cantons of Vaud and Neuchâtel from 1974 to 1994 also reported an increase of ADC incidence among men and women [5]. Even though some studies showed a higher rate of ADC IR in women than in men [29, 31, 32], our data show similar results as other studies, with significantly higher IR of ADC in men than in women [8, 33, 34]. As for SCC IR, we observed declines during 1989 to 2010 in men and a slight increase from 1980 to 2010 in women. The same trends were visible in the Cantons of Vaud and Neuchâtel from 1974 to 1994 [5]. SCC IR in the Canton of Zurich in men reached a peak in 1989, but there no peak in SCC IR in women was visible. Similarly, a peak in the mid-1980s and a decrease of SCC IR have been reported in the USA [31].There is an association with smoking for all histological subtypes, but for SCLS and SCC this association is much stronger than it is for ADC [31, 35, 36]. Deeper inhalation of cigarette smoke, changes in the composition of cigarettes, and the fact that lung cancer risk after smoking cessation decreases faster for SCC and SCLC might have led to the shift towards ADC [12, 18, 36–39]. The fastest decrease of risk after smoking cessation is seen in SCC [38]. Environmental or dietary factors probably have minor effects on changes of histological subtypes in countries with high smoking prevalence rates. Air pollution in the USA had little impact on the increase of ADC, as in the USA only 3% of cases are environmentally caused [37, 40].
The composition of cigarettes has changed remarkably over time. Therefore, the epidemic data are strongly influenced by different cigarette types [11]. At first, cigarettes were unfiltered; however, starting in 1950 the proportion of filtered cigarettes increased in the USA reaching 97.5% in 1992. In Switzerland, this process advanced even quicker [18]. A reduction of SCC but not of ADC risk for lifetime filtered cigarette smoking compared with unfiltered cigarettes was observed [39]. Tobacco types have different characteristics. In Switzerland, the predominant type is air-cured Maryland, a non-blended tobacco [18]. Smoking behaviour changed owing to the introduction of low-yield cigarettes and the increase of nitrosamines, which are found in blended cigarettes in high doses and mainly cause ADC [31, 37, 39]. The lower nicotine content of low-yield cigarettes increases the puff volume and therefore the tar, nicotine and nitrosamine levels in the lung [18, 36, 39]. The invention of filtered cigarettes encouraged deep inhalation, supporting a shift from tracheal and bronchial exposure to a more peripheral smoke exposure in the lung [36], which might have led to an increase in peripheral ADC [41, 42]. In general, there was a decrease in the smoking prevalence in Switzerland in men from 61% in 1955 to 32.4% in 2012 (fig. 1), and a mainly constant smoking prevalence in women from 23% in 1955 to 24.2% in 2012 [14–16]. Nevertheless, an increase of smoking prevalence among men and women was reported in 1997 (fig. 1). Therefore, antismoking campaigns increased in Switzerland between 1997 and 2007, which generated a reduction of smoking prevalence from 33% to 28% [16, 43]. Since 2006, restaurants in Switzerland have gradually become smoke-free zones [44]. To improve the future and combat the increasing trends in ADC IR, further campaigns and restrictions are needed.
Several [45–50], but not all [29, 41, 51–55] studies reported a higher susceptibility of women than men to develop lung cancer. However, a recent meta-analysis indicates a higher risk of diagnosis attributable to cigarette smoking in men [56]. As in the USA [31], our study shows the highest rates in M/F-IRR in SCC, intermediate rates in SCC, and lower rates in ADC. This might be explained by the higher incidence of ADC in nonsmoking women globally [12, 29, 37] and different smoking behaviour. Women started smoking later and generally consume lower-tar products [57]; men tend to smoke more cigarettes per day and inhale more deeply [48, 58]. Other explanations might include sex-related differences in nicotine metabolism or interactions of smoking with hormone use in women [12], but further research is clearly needed to address these issues.
Our data showed a significantly lower L/R-IRR in ADC than in SCLC or SCC. One study also reports a higher susceptibility of the right lung in women and men (L/R-IRR for women 0.88, L/R-IRR for men 0.86) [6]. Possible explanations include different volumes of the right and left lung, and unequal smoke exposure according to different tidal volumes [6] resulting from anatomical characteristics. The right lung represents 55% of the volume and the left lung 45%. Furthermore, the right bronchus is shorter and the branching angle between the trachea and the bronchus is less than it is on the left side [59].
The advantages of this study are the large sample size and the continuous data that document changes over three decades. Our data reflect global and European changes, and there is great importance in analysing histological subtypes, since aetiology, identification, diagnosis and therapeutic approaches change over the years. However, there are several limitations of this study. Shifts in diagnostic procedures and changes in classification may lead to a diagnostic artefact. With the introduction of ICD-O-3 the code non-small cell carcinoma was added in 2001 and used by the Cancer Registry of Zurich since 2003. After 2003 the IR of not otherwise specified large cell carcinoma in the Canton of Zurich decreased drastically. Non-small cell carcinoma and not otherwise specified carcinoma were not differentiated in our study (further information in the Methods section). With the invention of new diagnostic methods, such as immunostaining for FTT-1, a marker for ADC, a decrease of unspecified carcinoma occurred with a coincident increase of ADC [34]. Additionally, complete individual data about smoking behaviour and other risk factors of lung carcinoma for analysis are missing.
In conclusion, there is an ongoing increase of ADC incidence in the Canton of Zurich, despite a declining trend in SCC and general lung cancer incidence. In all histological types, there is a significantly higher susceptibility of the right lung. Despite a distinct decrease of lung cancer incidence in men (apart from ADC), we do see a continuing increase of lung cancer incidence in women in the Canton of Zurich. A critical point to further decrease prevalence rates of smoking, and thus decrease the burden of lung cancer, is to prevent uptake of smoking among adolescents and young adults, but also efforts to increase smoking cessation rates among current smokers. However, further efforts are needed to improve survival of lung cancer patients. Improvement in early detection in particular among high-risk individuals and accuracy of discrimination of histological types and specific therapy will hopefully increase lung cancer survival.
Adenocarcinoma (M: 8015, 8050, 8140, 8141, 8190, 8200, 8211, 8230, 8250-8255, 8260, 8290, 8310, 8430, 8480, 8481, 8490, 8550, 8560), SCC (M : 8051-8053, 8060, 8070, 8071-8078, 8080-8084)
Small cell carcinoma (M : 8041-8045, 8002)
Large cell carcinoma (M : 8012-8014)
Carcinoid tumor (M : 8240, 8244, 8246, 8249)
Other carcinomas and not otherwise specified types (M : 8000, 8001, 8003-8005, 8010, 8011, 8015, 8020-8022, 8030-8035, 8040, 8046, 8570, 8572, 8573, 8720, 8800, 8801, 8804, 8810, 8815, 8830, 8840, 8852, 8890, 8940, 8972, 8980, 9040, 9041, 9120, 9130, 9240, 9540, 8033)
1 Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
2 Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403.
3 Bundesamt für Statistik. Krebsmortalität: Todesfälle, Raten, Entwicklung, Medianalter, Risiko, pro Krebslokalisation. Neuchâtel: Bundesamt für Statistik Schweiz. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/02/05/key/01/02.html (accessed: 06. February 2016)
4 Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84(1):13–22.
5 Levi F, Franceschi S, La Vecchia C, Randimbison L, Te VC. Lung carcinoma trends by histologic type in Vaud and Neuchâtel, Switzerland, 1974–1994. Cancer. 1997;79(5):906–14.
6 Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control. 2006;17(5):655–62.
7 McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7(2):418–9.
8 Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–94.
9 Li YJ, Tsai YC, Chen YC, Christiani DC. Human papilloma virus and female lung adenocarcinoma. Semin Oncol. 2009;36(6):542–52.
10 Peto R, Lopez AD, Boreham J, Thun M, Heath C. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339(8804):1268–78.
11 Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1 Suppl):21S–49S.
12 Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32(4):605–44.
13 Bray FI, Weiderpass E. Lung cancer mortality trends in 36 European countries: secular trends and birth cohort patterns by sex and region 1970–2007. Int J Cancer. 2010;126(6):1454–66.
14 Gsell O. Rauchergewohnheiten der Ärzteschaft der Schweiz. Schweize Med Wochenschr.1956;23:669–75
15 Abelin Th, Müller R. Trend der Rauchgewohnheiten in der Schweiz 1975–1981. Int J Public Health. 1983;28(3):185–95.
16 Schweizerische Gesundheitsbefragung (SGB). Tabakkonsum nach Alter, Geschlecht, Sprachgebiet, Bildungsniveau,1992–2012. Neuchâtel: Bundesamt für Statistik. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/14/02/02/key/03.html (accessed: 05. January 2016)
17 Roemer E, Schorp MK, Piadé JJ, Seeman JI, Leyden DE, Haussmann HJ. Scientific assessment of the use of sugars as cigarette tobacco ingredients: a review of published and other publicly available studies. Crit Rev Toxicol. 2012;42(3):244–78.
18 Hoffmann D, Djordjevic MV, Hoffmann I. The changing cigarette. Prev Med. 1997;26(4):427–34.
19 Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4(4):327–38.
20 Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29.
21 Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER cancer statistics review, 1975-2013. Bethesda, MD: National Cancer Institute. Available from: http://seer.cancer.gov/csr/1975_2013/ (accessed: 25. April 2016)
22 Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin LH, Parkin DM, et al. International Classification of Diseases for Oncology. 3 ed. Geneva, Switzerland: World Health Organization; 2000.
23 Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer. 1967;2(3):269–79.
24 American Cancer Society. Cancer facts & figures 2014. Atlanta: American Cancer Society; 2014.
25 Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010.
26 La Vecchia C, Levi F, Decarli A, Wietlisbach V, Negri E, Gutzwiller F. Trends in smoking and lung cancer mortality in Switzerland. Prev Med. 1988;17(6):712–24.
27 Bosetti C, Malvezzi M, Rosso T, Bertuccio P, Gallus S, Chatenoud L, et al. Lung cancer mortality in European women: trends and predictions. Lung Cancer. 2012;78(3):171–8.
28 Brenner H, Francisci S, de Angelis R, Marcos-Gragera R, Verdecchia A, Gatta G, et al. Long-term survival expectations of cancer patients in Europe in 2000–2002. Eur J Cancer. 2009;45(6):1028–41.
29 Jemal A, Travis WD, Tarone RE, Travis L, Devesa SS. Lung cancer rates convergence in young men and women in the United States: analysis by birth cohort and histologic type. Int J Cancer. 2003;105(1):101–7.
30 Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014.
31 Lewis DR, Check DP, Caporaso NE, Travis WD, Devesa SS. US lung cancer trends by histologic type. Cancer. 2014;120(18):2883–92.
32 Houston KA, Henley SJ, Li J, White MC, Richards TB. Patterns in lung cancer incidence rates and trends by histologic type in the United States, 2004–2009. Lung Cancer. 2014;86(1):22–8.
33 Devesa SS, Shaw GL, Blot WJ. Changing patterns of lung cancer incidence by histological type. Cancer Epidemiol Biomarkers Prev. 1991;1(1):29–34.
34 Travis WD, Lubin J, Ries L, Devesa S. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer. 1996;77(12):2464–70.
35 Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: a meta-analysis. Lung Cancer. 2001;31(2-3):139–48.
36 U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress. A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014.
37 Burns DM, Anderson CM, Gray N. Do changes in cigarette design influence the rise in adenocarcinoma of the lung? Cancer Causes Control. 2011;22(1):13–22.
38 Kenfield SA, Wei EK, Stampfer MJ, Rosner BA, Colditz GA. Comparison of aspects of smoking among the four histological types of lung cancer. Tob Control. 2008;17(3):198–204.
39 Stellman SD, Muscat JE, Thompson S, Hoffmann D, Wynder EL. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80(3):382–8.
40 Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M, (Cancers) CRAcg. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–93.
41 Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368(4):351–64.
42 Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89(21):1580–6.
43 Marti J. The impact of tobacco control expenditures on smoking initiation and cessation. Health Econ. 2014;23(12):1397–410.
44 Otto B. Die Geschichte des Rauchens – wie Nichtrauchen wieder gesellschaftsfähig wird. Berne: Verlag Hans Huber, Hogrefe AG; 2010.
45 Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88(3-4):183–92.
46 Begg CB, Zhang ZF, Sun M, Herr HW, Schantz SP. Methodology for evaluating the incidence of second primary cancers with application to smoking-related cancers from the Surveillance, Epidemiology, and End Results (SEER) program. Am J Epidemiol. 1995;142(6):653–65.
47 Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993;138(5):281–93.
48 Harris RE, Zang EA, Anderson JI, Wynder EL. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol. 1993;22(4):592–9.
49 Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect. 1995;103(Suppl 8):143–8.
50 Hebert JR, Kabat GC. Distribution of smoking and its association with lung cancer: implications for studies on the association of fat with cancer. J Natl Cancer Inst. 1991;83(12):872–4.
51 Halpern MT, Gillespie BW, Warner KE. Patterns of absolute risk of lung cancer mortality in former smokers. J Natl Cancer Inst. 1993;85(6):457–64.
52 Doll R, Peto R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J. 1976;2(6051):1525–36.
53 Doll R, Gray R, Hafner B, Peto R. Mortality in relation to smoking: 22 years’ observations on female British doctors. Br Med J. 1980;280(6219):967–71.
54 Kreuzer M, Boffetta P, Whitley E, Ahrens W, Gaborieau V, Heinrich J, et al. Gender differences in lung cancer risk by smoking: a multicentre case-control study in Germany and Italy. Br J Cancer. 2000;82(1):227–33.
55 De Matteis S, Consonni D, Pesatori AC, Bergen AW, Bertazzi PA, Caporaso NE, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol. 2013;177(7):601–12.
56 Yu Y, Liu H, Zheng S, Ding Z, Chen Z, Jin W, et al. Gender susceptibility for cigarette smoking-attributable lung cancer: A systematic review and meta-analysis. Lung Cancer. 2014;85(3):351–60.
57 Gray N. The consequences of the unregulated cigarette. Tob Control. 2006;15(5):405–8.
58 Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20561 cases. Ann Oncol. 2002;13(7):1087–93.
59 Clarke SW. Aerosols and the Lung: Clinical and Experimental Aspects. Butterworth & Co; 1984.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.