Role of the odorant receptor in neuronal connectivity in the olfactory bulb
DOI: https://doi.org/10.4414/smw.2015.14228
Nelly
Redolfi, Claudia
Lodovichi
Summary
Olfaction is a highly sophisticated sensory modality able to detect and discriminate thousands of different odours, even at very low concentration. How such a challenging task is achieved remains to be fully understood. A unique feature of the olfactory system is the dual role of the odorant receptor: it does detect odours in the olfactory epithelium but it also contributes to neuronal circuit formation in the olfactory bulb. The odorant receptors are indeed expressed on the cilia that protrude in the nasal cavity, where they bind odorants, and at the axon termini, where they could act as axon guidance molecules. In this review we discuss findings that show how the odorant receptor contributes in regulating neuronal connectivity.
Representation of the external world in sensory systems: the topographical maps
Specificity of connectivity in the central nervous system is essential for normal brain function. In the sensory systems neurons in the peripheral organs/areas project their axons in specific loci in the central nervous system to create an internal representation of the external world. The spatial segregation of sensory afferents provides topographic maps that encode the quality and the location of sensory stimuli.
The topographic organisation of the olfactory system (OS) differs in many ways from that of other sensory modalities such as the visual or the auditory systems. Olfactory sensory neurons (OSNs) regenerate constantly throughout the life of the individual and they project with exquisite precision in the same loci in the olfactory bulb (OB), the first retransmission area of the OS, to maintain the sensory map. How the specificity of synaptic connections is achieved and maintained remains to be completely understood. Although several aspects have been elucidated, some critical points remain to be addressed.
This review is limited to the mammalian OS and the contribution of the odorant receptor (OR) in neuronal circuit formation in the OB, with selective coverage reflecting the authors’ research interests. More extensive studies on axon guidance molecules and electrical activity involved in neuronal wiring in the OB can be found in several excellent recent reviews [1–4].
Organisation of the olfactory epithelium and the olfactory bulb
The olfactory epithelium exhibits a coarse topographic organisation. Each OSN expresses only one in a repertoire of more than 1 000 odorant receptor genes, described as the “one neuron, one receptor” rule [5–10]. OSNs expressing the same OR are located within a broad but circumscribed area of the olfactory epithelium, where they are intermingled with OSNs bearing different ORs. According to the distribution of OSNs expressing a specific OR within a given area [11–13], the olfactory epithelium (OE) has been subdivided in different zones, along the dorsoventral axis (fig. 1).
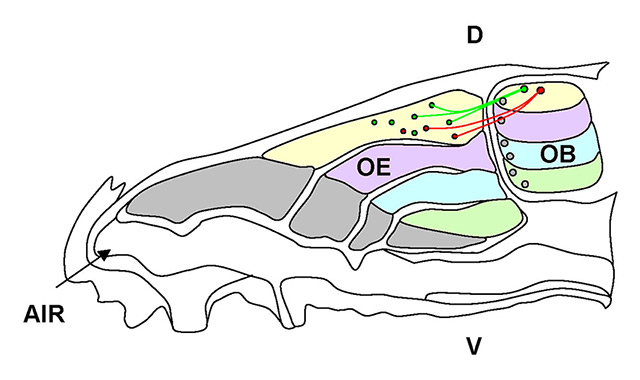
Figure 1
Schematic of the olfactory sensory neuron (OSN) projections to the olfactory bulb (OB). The olfactory epithelium (OE) is divided in zones along the dorsoventral axis, which are defined by the expression of the odorant receptors (ORs). OSNs expressing the same receptor (red or green) are confined within a zone and project their axons to a corresponding area (along the dorsoventral axis) of the olfactory bulb.
D = dorsal; V = ventral.
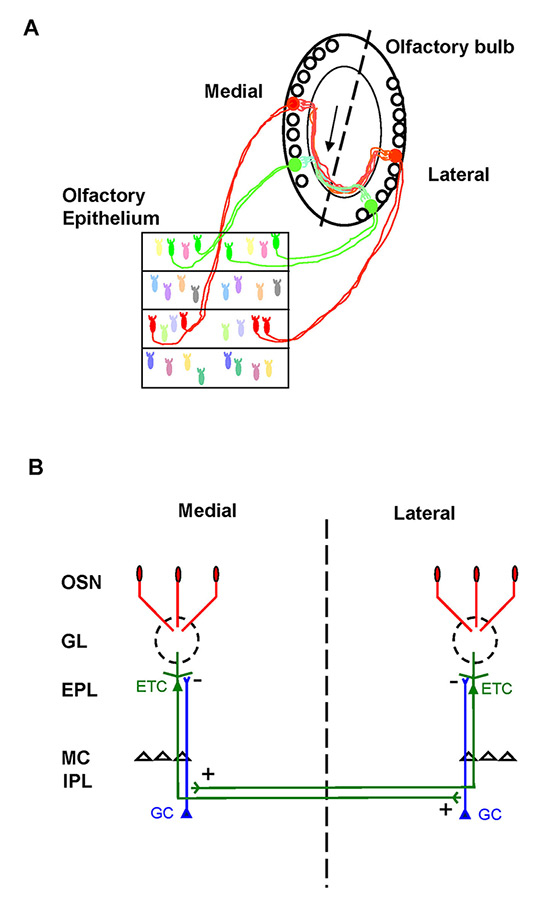
Figure 2
Schematic of neuronal wiring in the olfactory bulb. A. Olfactory sensory neurons (OSNs) expressing the same odorant receptor (green and red) converge to form glomeruli on the medial and on the lateral side of each olfactory bulb (OB). Glomeruli receiving axons expressing the same odorant receptor (OR) (i.e. homologous glomeruli) are specifically and reciprocally connected by the intrabulbar link, related to external tufted cells (arrow). Dashed line indicates the line of symmetry between the two mirror-symmetric maps in horizontal sections (Lodovichi et al., 2003 [27]). B. Schematic of the intrabulbar connections between homologous glomeruli. Briefly, external tufted cells (ETC) connected to a given glomerulus form an axonal projection in the internal plexiform layer (IPL) precisely underneath the homologous glomerulus, on the opposite side of the OB. In the IPL, ETC form excitatory synapses with the granule cells, the major inhibitory interneurons in the OB. This connection is reciprocal.
EPL = external plexiform layer; GC = granule cells; GL = glomeruli; MC = mitral cells
Spatial order is achieved in the OB, where axons of OSNs expressing the same OR converge to form glomeruli in specific loci on the medial and on the lateral side of each OB (fig. 2A) [14–16]. A hallmark of mature glomeruli is that they are formed exclusively by axons bearing the same OR (i.e. homogeneous glomeruli), defined as the “one glomerulus, one receptor” rule. A glomerulus is a spherical structure of neuropil formed by the OSN axon terminals that make synapses with the postsynaptic cells, namely the mitral and tufted cells, along with the periglomerular and the granule cells, connected to them. Notably mitral and tufted cells extend their single apical dendrite into a given glomerulus. Each glomerulus defines, therefore, a module that processes the sensory information related to a given OR [17]. Cells within the same glomerular unit could, however, respond to odours in a nonhomogeneous fashion. Differences in odour responses can be related to the cell types, for instance mitral cells are more narrowly tuned than periglomerular cells [18, 19]. The modulatory inputs from interneurons and/or centrifugal afferents can also modulate differently the response of principal neurons connected to the same glomerulus [18–20].
As a consequence of the spatial segregation of the sensory afferents that gives rise to the sensory (or glomerular) map of the OB [1], an odour is encoded by a spatial pattern of activated glomeruli [21–24]. The specificity of the topographical organisation in the OB is therefore crucial for normal sensory information processing.
The presence in each OB of a medial and a lateral glomerulus receiving OSN axons expressing the same OR (i.e. homologous glomeruli) provides each OB with two mirror-symmetric maps of homologous glomeruli (fig. 2). This feature distinguishes the spatial organisation of sensory afferents in the OB from other sensory modalities, in which sensory neurons form, mostly, a single continuous representation. Whether homologous glomeruli form independent structures or are symmetric elements of an integrated system remained unknown. Schoenfeld (1985) [25] described in the hamster olfactory bulb an intrabulbar associational system related to external tufted cells that reciprocally connected large areas of the medial and the lateral side of each OB. Because Schoenfeld’s study was performed prior to the discovery of odorant receptors and their representation in symmetric maps, it was unknown what this system was connecting. By targeting tracer injections to identified glomeruli in transgenic mice, it was found that external tufted cells form an axonal projection, confined to a single glomerulus precisely underneath the homologous glomerulus (fig. 2B) on the opposite side of the OB [26, 27]. The connection is reciprocal between homologous glomeruli throughout the bulb, as demonstrated by injections targeted to the medial or the lateral glomerulus, and analysis of the corresponding external tufted cell projections on the opposite side of the OB [27]. These studies revealed a precise topographically organised linkage between the mirror-symmetric maps of homologous glomeruli in rodent OB [27].
The OB therefore exhibits two levels of topographic organisation: (1) the sensory or glomerular map, resulting from the convergence of like-axons to form glomeruli in specific loci of the OB and (2) the intrabulbar link that connects homologous glomeruli.
Sensory map in the olfactory bulb: role of the odorant receptor
The development of a sensory map is regulated by the complex interactions between axon guidance molecules expressed in a specific spatio-temporal pattern and electrical activity.
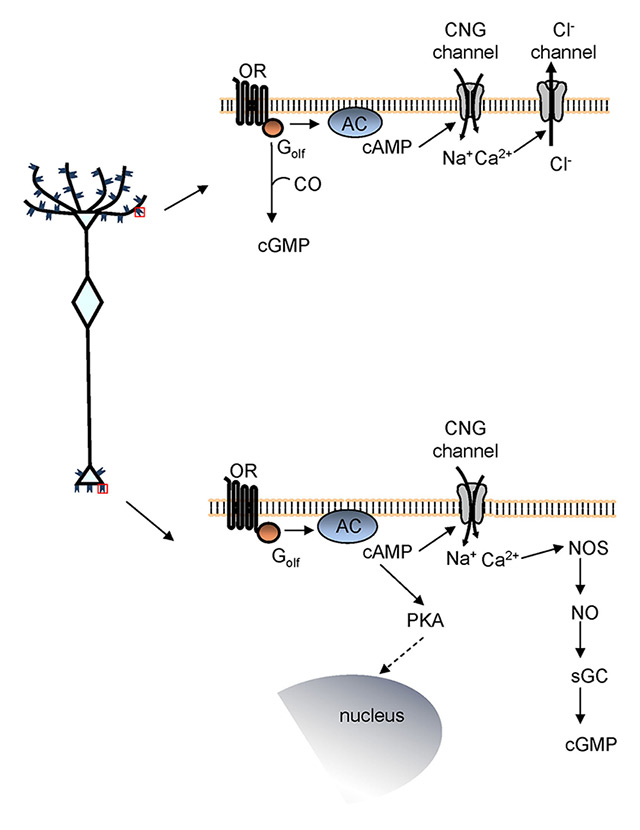
Figure 3
Signalling pathway coupled to the odorant receptor (OR). Left, schematic of an olfactory sensory neuron (OSN). Right, signalling pathway coupled to the OR expressed at the cilia (top) and at the axon terminal (bottom).
AC = adenylyl cyclase; CNG channel = cyclic nucleotide-gated channel; CO = carbon monoxide; Golf= olfaction-specific G protein; NO = nitric oxide; NOS = nitric oxide synthase; PKA = protein kinase A; sGC = soluble guanylyl cyclase.
The tight connection between the OR identity and the spatial organisation of the sensory afferents in the OB prompted the hypothesis that the OR was involved not only in odour detection in the olfactory epithelium, but also in axonal convergence in the OB. This hypothesis was corroborated by elegant genetic experiments that demonstrated how mutations in the OR gene sequence resulted in mistargeting of the OSNs expressing the mutated OR and, consequently, in an altered sensory map [28–30]. These studies demonstrated that by swapping the sequence of a given OR in the locus of another OR, the axon expressing the swapped OR targeted a glomerulus that was not the original one, nor the one of the swapped OR. The mistargeted glomerulus could be more or less close to the original or to the swapped one, according to the homology between the swapped OR gene sequences and the zones of the OE where they were expressed. All together these works demonstrated that the OR plays an instructive role in the axonal convergence, although it is not the only determinant (see below).
The role of the OR in OSN axon targeting was strengthened by the evidence that the OR is specifically expressed not only at the cilia but also at the axon terminal. The soma and the proximal part of the axon appear devoid of OR expression [31, 32]. Furthermore, the OR is locally translated in the distal portion of the axon [33]. Such a discrete and spatially compartmentalised expression of the OR at the axon termini pointed to a specific function of the OR in that location, where it could act as a axon guidance molecule.
To affirm that the OR at the axon terminal could act as an axon guidance molecule, it was critical to demonstrate that the receptor in that location is functional and coupled to a signalling pathway. The OR is a G protein-coupled receptor. At the cilia, upon binding odour molecules, the OR activates a specific G protein, Golf,that in turn induces the synthesis of cyclic adenosine monophosphate (cAMP) via activation of adenylyl cyclase III. Cyclic AMP then binds to cyclic nucleotide gated (CNG) channels allowing an influx of Na+and Ca2+ [34]. The rise in Ca2+ level opens Ca2+-gated Cl– channels [35], resulting in an efflux of Cl– that contributes to depolarising the OSNs leading, ultimately, to the action potentials (fig. 3) [36–38]. Whether the same signalling pathway was coupled to the OR expressed at the axon terminal remained unknown. By studying the spatiotemporal dynamics of cAMP and Ca2+ in single OSNs in real time imaging experiments, Maritan et al. (2009) demonstrated that focal application of odours (the only known ligands of the ORs, until now) with a glass pipette at the axon terminal elicited a prompt rise in cAMP and Ca2+, via CNG channel activation, at the axon terminal of OSNs. A prompt rise in Ca2+ was observed also in glomeruli in response to odours focally applied with a pipette to the olfactory bulb [39]. Altogether, these data showed that the OR at the axon terminal is functional and coupled to local increases of cAMP and Ca2+ (fig. 3).
Cyclic AMP appears to exert its action locally at the growth cone, modulating Ca2+ level and cytoskeleton rearrangements, but also at the nucleus where it can regulate the expression of genes coding for axon guidance molecules. The latter hypothesis was corroborated by the evidence that axonal OR activation was coupled not only to the local increase of cAMP but also to the translocation of protein kinase A, the major target of cAMP, to the nucleus [39, 40]. At the nuclear level protein kinase A can modulate, via cAMP response element-binding protein (CREB) phosphorylation, the expression of molecules involved in axon targeting.
Although cAMP is the primary second messenger generated upon OR activation, cyclic guanosine monophosphate (cGMP) is also synthesized. The slow kinetics of cGMP suggests that it is not involved in the initial odour detection events but rather in long-term cellular responses, such as adaptation and developmental processes. The mechanism regulating cGMP production and its interaction with cAMP remain unknown. Pietrobon et al. (2011), by studying cGMP dynamics in vivo in OSNs, demonstrated that cGMP is generated in the entire OSN, including the axon terminal, upon OR activation. The odour-dependent rise in cGMP is due to soluble guanylyl cyclase activation by nitric oxide (NO) and required an increase in cAMP. The link between cAMP rise and the synthesis of cGMP is an increase in cytosolic Ca2+ (fig. 3). As observed for cAMP, cGMP exerts its action locally at the cilia and at the axon terminal growth cone, but also at the nuclear level, where it has been shown to regulate CREB phosphorylation [41, 40]. Pietrobon et al. thoroughly dissected the mechanism underlying cGMP generation and its strict interplay with cAMP and Ca2+. This connection is of relevance for the OSN axon targeting, since these second messengers have been shown to modulate axon elongation and targeting in several systems [42]. The levels of cAMP regulate the turning behaviour of the axon in response to a gradient of the same molecules [43]. The ratio of cAMP to cGMP contributes in setting the polarity (attraction or repulsion) of the axon response to guidance cues [44]. All together these data provide compelling evidence that the OR expressed at the axon terminal contributes to providing OSNs with instructions to reach the proper target.
The expression and the local translation of the OR at the axon terminal is in line with a growing body of evidence that indicates the axon terminus is an autonomous compartment [45], where molecules involved in axon targeting are locally translated and expressed. The major advantage of the axon as an autonomous compartment that regulates local protein synthesis and expression is to endow the axon with the ability to respond promptly to cues encountered in the environment along the path to the proper target.
One critical open question is the mechanism underpinning the generation of the OR-derived cAMP involved in the axon targeting process. In a recent work in transgenic mice that express activity mutants of the beta-adrenergic receptor (a G protein coupled receptor like the OR) in OSNs, it was found that mutants with altered agonist-independent activity exhibit different levels of axon guidance molecules such as neuropilin 1 and plexin1. The authors hypothesized that a similar agonist-independent activity mechanism could apply to the OR. The different levels of agonist-independent activity could then be responsible for regulating expression of axon guidance molecules, via the OR-derived cAMP signals [46]. An alternative or a complementary hypothesis envisions that a few molecules expressed in gradients in the OB can bind and activate the OR at the axon termini, providing the OSN with information to reach the proper target [28, 31, 39, 40]. The presence of a functional OR at the axon termini corroborates this hypothesis. However, the identity of these molecules remains, at the moment, unclear. The two mechanisms could coexist and regulate different phases of the development of the sensory map.
Axon guidance molecules involved in the sensory map formation in the olfactory bulb
Although the OR identity plays an instructive role in the OSN axonal convergence, it is not the only determinant. The location of a glomerulus in the OB can be defined by coordinates along different axes: dorso-ventral, medio-lateral, antero-posterior. Several molecules that regulate the targeting of OSNs along these axis, contribute to the coalescence of like-axons to form glomeruli in specific loci of the OB.
A coarse correspondence between the location of OSNs in zones of the olfactory epithelium and the location of glomeruli along the dorso-ventral axis of the OB has been observed. OSNs located in the most dorsal area of the OE project to the most dorsal region of the OB, while the ones located more ventrally form glomeruli in the more ventral region of the OB (fig. 1) [13]. Factors associated with the location of the OSNs in a given zone of the OE are likely to dictate the target of the OSN axons along the dorso-ventral axis. However the mechanism underpinning the location of a given OR in a zone of the epithelium and how this association may bear on the targeting to the OB remains to be understood. Two sets of repulsive ligand/receptor molecules seem to modulate the axonal project along this axis: Slit/Robo [47, 48] and Sema 3F / neuropilin 2 [49–52].
The antero-posterior axis appears to be regulated by the OR derived cAMP [53], whose levels are thought to regulate expression of neuropilin 1 and its ligand semaphorin 3A [53–56]. Other molecules involved in the antero-posterior localization of glomeruli are Ephrins, namely Ephrin A3 and A5 and their receptor Eph A [57]. Deletion or overexpression of Ephrin A3 or A5 determine a shift in the location of glomeruli along the antero-posterior axis. Mice deficient in Sema 3A exhibited more significant defects in the sensory map, including alterations in the coalescence of like axons in the proper target and aberrant targeting.
The spatial segregation of sensory axons along the mediolateral axis is poorly understood. Insulin-like growth factor 1 appears to be required for innervation of the lateral side of the OB [58].
During embryonic and postnatal life, the convergence of OSN axons to form glomeruli in the OB is a process that develops in subsequent steps ([59], NR and CL unpublished data). OSN axons project to a coarse area of the OB and then, progressively, they coalesce to form glomeruli that at first can be heterogeneous (i.e. glomeruli that receive OSN axons expressing different OR). Through a refinement process, glomeruli reach their mature organisation of homogeneous glomeruli (for a detailed description see [59]). In the final targeting process, homophilic interactions among axons are likely to take place. Several molecules have been associated with this process, such as Kirrel 2 and 3 [60], Big 2 [61], Ephrin A and Eph A [57].
Role of electrical activity in the development of the topographic organisation of the olfactory bulb
In most sensory systems, both spontaneous and stimulus-evoked electrical activity exert a prominent role in sculpting the specificity of neuronal connectivity. Whether and how electrical activity contribute in circuit formation in the olfactory bulb remains a matter of significant debate.
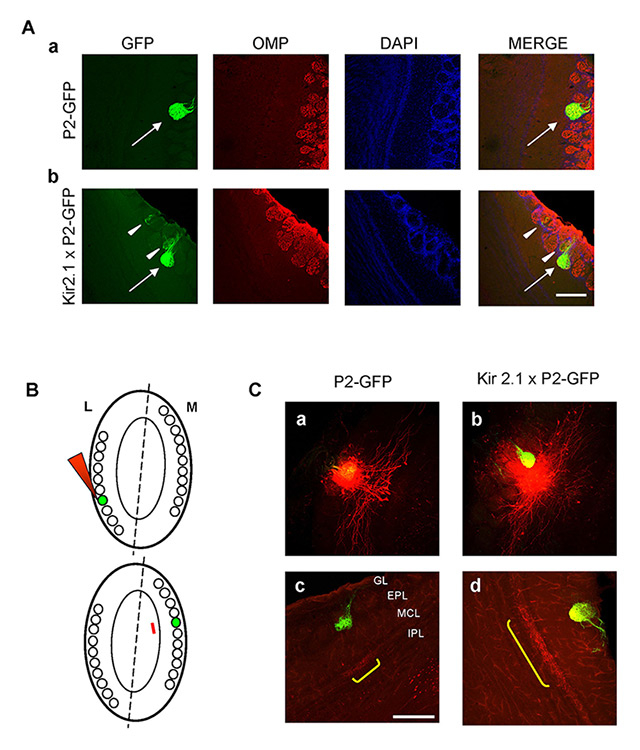
Figure 4
Neuronal wiring in the olfactory bulb of Kir2.1 mice. A. Organisation of glomeruli in control and Kir2.1 mice. Organisation of P2 glomeruli was revealed by immunolabelling horizontal sections of the olfactory bulb (OB) with antibodies against olfactory marker protein (OMP, in red). GFP expressed in P2 axons (green). Nuclei stained by the nuclear marker, DAPI (blue). Aa, Ab, P2-axons coalesce to form glomeruli formed by fibres expressing OMP and GFP, i.e. bearing P2 receptor, in P2-GFP and in Kir2.1 X P2-GFP mice (arrows). Ab, P2-axons innervate also additional adjacent glomeruli formed by fibres expressing GFP and OMP, but also only OMP (i.e. expressing a different receptor than P2, arrowheads) in Kir2.1 X P2-GFP mice. B. Schematic of the experimental procedure. The dashed line indicates line of symmetry between glomerular maps, within each olfactory bulb. Homologous glomeruli are indicated by green labels. The red arrowhead indicates the tracer injection targeted to the lateral glomerulus (top). The red line refers to external tufted cell (ETC) projection just underneath the medial homologous glomerulus on the opposite side of the OB (bottom). C. Intrabulbar circuitry in control and Kir2.1 mice. Examples of focal tracer injections (red spots) targeted to the medial P2-GFP glomerulus in control (a) and Kir2.1 mice (b). ETC axons labelled in (a) and (b) give rise to anterograde projections (yellow brackets) underneath the homologous P2-GFP glomeruli on the opposite side of the olfactory bulb in control (c) and Kir2.1 (d) mice. In Kir2.1 mice the anterograde projection is not confined to the homologous glomerulus, as in controls, but larger (d) (Lorenzon et al., 2015 [76]). Scale bar = 200 µm.
EPL = external plexiform layer; GL = glomerular layer; IPL = internal plexiform layer ;MCL = mitral cell layer.
Mutant mice lacking functional CNG channels, a critical component in the signalling pathway coupled to the OR, failed to respond to a wide array of odours. However the convergence of sensory axons to form glomeruli was only slightly altered in these mutant mice [62, 63]. The intrabulbar links between homologous glomeruli were present, but appeared unrefined in the CNG ko mice [64]. Analogous results were observed in mice knockout for Golf, the G protein coupled to the OR. Despite a significant reduction in response to odours, the sensory map was not perturbed in Golf knockout mice [65].
Electrical activity can also regulate the expression of molecules involved in the axon guidance process such as Kirrel and Ephrin As – Eph A. In CNG knockout mice the expression of Kirrel 2 and Eph A5 is downregulated while the expression of Kirrel 3 and Eprhin A5 is upregulated [60]. Despite the different levels of these proteins in absence of activity, the sensory map was not perturbed in CNG knockout mice, suggesting that different mechanisms regulate the coalescence of like axon to form glomeruli.
When the odour evoked activity was reduced by naris occlusion, the glomerular map was perturbed by the persistence of additional heterogeneous glomeruli [66], indicating that the refinement process was altered.
A completely different scenario was observed in mice knockout for adenylyl cyclase III. In these mutant mice, odour-evoked responses were abolished and the sensory map was deeply perturbed [67–69]. OSN axons targeted the glomerular layer but did not coalesce to form distinct glomeruli. A similar alteration of the sensory map was observed in mutant mice in which the cytoplasmic end of the transmembrane domain III of the OR, the one responsible for coupling to G protein, was mutated. As a consequence of this genetic modification, the OR was not able to couple to G protein [53]. In both cases (adenylyl cyclase III knockout mice and OR mutated mice), the genetic modifications hamper the synthesis of cAMP associated with the OR activation. These results corroborate the function of the OR-derived cAMP in axon targeting. In Golf knockout mice, cAMP synthesis is likely assured by the expression of Gα, which is expressed during embryonic development and might be re-expressed upon elimination of Golf expression.
Odorant exposure, per se, did not seem to affect circuit formation and/or function in the OB [70, 71]. However when odour exposure was associated with aversive conditioning in the early neonatal period, it was found to influence OSNs axon wiring accelerating glomeruli refinement [70]. In adult mice, odorant exposure associated to a foot shock significantly increased OSN response to the conditioned odour [71].
Altogether these data indicate that odour-evoked activity does not play an instructive role in the convergence of OSNs to form the glomerular map or in the formation of the intrabulbar circuitry. However the refinement of the sensory map appeared to be affected when odour-evoked activity was reduced by naris occlusion, but not in genetically modified mice (see above).
Odour exposure, per se, did not affect the plasticity of the system, which was modified when odour exposure was associated with a conditional stimulus, suggesting a role of centrifugal afferents in this form of plasticity and learning [70–72].
The OR modulates not only odour-evoked activity but also spontaneous firing in OSNs. In Drosophila [73] and in the mouse [74] it has been shown that OSNs expressing different ORs exhibit different spontaneous firing rates. OSNs expressing a mutant inactive OR lack spontaneous firing, although they are able to fire action potentials in response to current injections [75]. These results indicate that the spontaneous firing in OSNs originates from spontaneous activation of the OR.
What is the role of afferent spontaneous activity in the topographic organisation of the OB?
Spontaneous activity is known to play a crucial role in the early development in many sensory modalities such as vision. The role of basal activity in the OS remained to be understood. To address this question, Yu et al. (2004) generated a mutant line of mice in which OSNs overexpressed the Kir 2.1 channels. As a consequence of this genetic manipulation, OSNs are hyperpolarized and the basal firing is dramatically reduced, while the odour evoked responses are superimposable between Kir 2.1 and control mice [76]. This line of mice therefore represents a unique useful model to dissect the role of spontaneous afferent activity in the wiring of the OB. Yu et al (2004) found that in Kir 2.1 overexpressing mice the sensory map is altered by the presence of additional glomeruli. Whether these glomeruli were homogenous or heterogeneous, how they affected the functional map and olfactory behaviour remains unknown. Whether the lack of spontaneous afferent activity perturbed the intrabulbar connections, the second level of the topographic organisation of the olfactory bulb remained to be investigated.
Combining electrophysiological recording, functional imaging, anatomical tracing and behavioural analysis, Lorenzon and colleagues (2015) found that the olfactory bulb of Kir 2.1 over-expressing mice exhibited unrefined connectivity, which in turn affected the functional maps and olfactory behaviour. To analyze the organisation of glomeruli, Kir 2.1 mice were crossed with P2 - GFP mice. In the latter lines of mice, the OSNs expressing the odorant receptor P2 co-express green fluorescent protein (GFP). As a result of this genetic manipulation, the OSNs and the corresponding glomeruli can be easily identified. Sections of Kir 2.1 X P2-GFP mice were immunolabelled with antibodies against the olfactory marker protein (OMP), a protein expressed by all mature sensory neurons. In Kir 2.1 mutant mice and in controls, the main glomeruli were formed by fibres expressing GFP and OMP (i.e., axons expressing P2). However, in Kir 2.1 mutant mice, P2 axons targeted also additional glomeruli that were formed by axons expressing OMP and GFP, but also by fibres expressing only OMP (i.e. expressing a different OR, fig. 4A). These data indicate that in Kir2.1 mice the sensory map was disrupted by the presence of additional heterogeneous glomeruli. The functional maps were more blurred than in controls, reflecting the anatomical alterations [76]. Although preserved, the intrabulbar connections between homologous glomeruli were not confined to a single glomerulus, as in controls, but coarser (i.e. larger, fig. 4C). These results indicated that the absence of afferent spontaneous activity hampers the refinement of the wiring in the OB, which also maintains in adults a coarser structure, appropriate to early stages of development.
What are the functional consequences of the altered connectivity of Kir 2.1 mice? As stated in the first sentence of this review, specificity of connectivity is essential for normal brain function. Kir 2.1 over-expressing mice exhibited unrefined connectivity in the OB that resulted in the inability to discriminate odours that give rise to similar functional maps such as enantiomers, i.e. mirror symmetric pairs of molecules that differ only in their optical activity. Kir 2.1 mice retain, however, the ability to differentiate odours that elicit a very different spatial pattern of activated glomeruli, such as 2-methylbutyric acid and 2-cyclobutanecarboxylic acid [76].
Notably, overexpression of Kir2.1 in adults induced regression of the already refined connectivity of the sensory map and of the intrabulbar connections to a coarser status [76]. These data clearly demonstrate that spontaneous afferent activity plays a crucial role not only in the development but also in the maintenance of neuronal circuits in the OB.
To further dissect the role of the OR in the sensory map formation and plasticity, a given OR was expressed in most OSNs [77]. In this scenario, the sensory map was altered by the presence of additional heterogeneous glomeruli. As observed by Lorenzon et al. (2015), abolishing the expression of the transgene during embryonic and early postnatal life restored the stereotypical pattern of OSN convergence. However if the sensory map developed abnormally, the suppression of the transgene expression in a subsequent phase of life was unable to restore the typical pattern of OSNs convergence, as if a memory of the bad map persisted in the system [77]. A similar persistence of the perturbed map, (when it developed in a wrong way) was observed also in Kir 2.1 mice [78]. The mechanism underpinning these data, as well as their relationship with the intrabulbar connection remain to be understood, in a system with such a high degree of plasticity.
Conclusions
The development of a sensory map is a complex phenomenon regulated by molecules expressed in a specific spatio-temporal pattern and by electrical activity.
The topography of the OB hinges on the OR identity. In this review we discussed findings that show that the OR expressed at the axon terminus is functional and can regulate neuronal connectivity. Furthermore, we discussed the complex role of electrical activity in the OB topography. Recent data indicated that the OR can mediate not only odour-evoked activity but also spontaneous firing in olfactory sensory neurons. Over the years, several studies analyzed the role of odour-evoked activity, reaching the conclusion that it does not play an instructive role in the topographic organisation of the OB. It could, however, contribute to the refinement and plasticity of the OB circuitry. The role of spontaneous activity has recently been thoroughly investigated. It was found that although spontaneous afferent activity does not instruct OSNs axon targeting, it exerts a crucial role in the refinement and in the maintenance of the glomerular map and the intrabulbar link between homologous glomeruli.
References
1 Murthy VN. Olfactory maps in the brain. Annu Rev Neurosci. 2011;34:233–58.
2 Cho JH, Prince JE, Cloutier JF. Axon guidance events in the wiring of the mammalian olfactory system. Mol Neurobiol. 2009;39(1):1–9.
3 de Castro F. Wiring Olfaction: The Cellular and Molecular Mechanisms that Guide the Development of Synaptic Connections from the Nose to the Cortex. Front Neurosci. 2009;3:52.
4 Nishizumi H, Sakano H. Developmental regulation of neural map formation in the mouse olfactory system. Dev Neurobiol. 2015;75(6):594–607.
5 Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–87.
6 Chess A, et al. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78(5):823–34.
7 Serizawa S, et al. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302(5653):2088–94.
8 Shykind BM, et al. Gene switching and the stability of odorant receptor gene choice. Cell. 2004;117(6):801–15.
9 Lewcock JW, Reed RR. A feedback mechanism regulates monoallelic odorant receptor expression. Proc Natl Acad Sci U S A. 2004;101(4):1069–74.
10 Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20(12):648–53.
11 Ressler KJ, Sullivan SL, Buck LB. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73(3):597–609.
12 Vassar R, Ngai J, Axel R. Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell. 1993;74(2):309–18.
13 Miyamichi K, et al. Continuous and overlapping expression domains of odorant receptor genes in the olfactory epithelium determine the dorsal/ventral positioning of glomeruli in the olfactory bulb. J Neurosci. 2005;25(14):3586–92.
14 Ressler KJ, Sullivan SL, Buck LB. A molecular dissection of spatial patterning in the olfactory system. Curr Opin Neurobiol. 1994;4(4):588–96.
15 Vassar R, et al. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79(6):981–91.
16 Mombaerts P, et al. Visualizing an olfactory sensory map. Cell. 1996;87(4):675–86.
17 Shepherd GM. The synaptic organization of the brain. 2004, Oxford: Oxford University Press.
18 Tan J, et al. Odor information processing by the olfactory bulb analyzed in gene-targeted mice. Neuron. 2010;65(6):912–26.
19 Kikuta S, et al. Odorant response properties of individual neurons in an olfactory glomerular module. Neuron. 2013;77(6):1122–35.
20 Dhawale AK, et al. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci. 2010;13(11):1404–12.
21 Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23(3):499–511.
22 Uchida N, et al. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3(10):1035–43.
23 Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32(4):723–35.
24 Soucy ER, et al. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12(2):210–20.
25 Schoenfeld TA, Marchand JE, Macrides F. Topographic organization of tufted cell axonal projections in the hamster main olfactory bulb: an intrabulbar associational system. J Comp Neurol. 1985;235:503–18.
26 Belluscio L, et al. Odorant receptors instruct functional circuitry in the mouse olfactory bulb. Nature. 2002;419(6904):296–300.
27 Lodovichi C, Belluscio L, Katz LC. Functional topography of connections linking mirror-symmetric maps in the mouse olfactory bulb. Neuron. 2003;38(2):265–76.
28 Wang F, et al. Odorant receptors govern the formation of a precise topographic map. Cell. 1998;93(1):47–60.
29 Feinstein P, et al. Axon guidance of mouse olfactory sensory neurons by odorant receptors and the beta2 adrenergic receptor. Cell. 2004;117(6):833–46.
30 Feinstein P, Mombaerts P. A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell. 2004;117(6):817–31.
31 Barnea G, et al. Odorant receptors on axon termini in the brain. Science. 2004;304(5676):1468.
32 Strotmann J, et al. Olfactory receptor proteins in axonal processes of chemosensory neurons. J Neurosci. 2004;24(35):7754–61.
33 Dubacq C, Jamet S, Trembleau A. Evidence for developmentally regulated local translation of odorant receptor mRNAs in the axons of olfactory sensory neurons. J Neurosci. 2009;29(33):10184–90.
34 Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005;15(3):343–9.
35 Reisert J, et al. Mechanism of the excitatory Cl-response in mouse olfactory receptor neurons. Neuron. 2005;45(4):553–61.
36 Menini A. Calcium signalling and regulation in olfactory neurons. Curr Opin Neurobiol. 1999;9(4):419–26.
37 Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11(3):188–200.
38 Kleene SJ. The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses. 2008;33(9):839–59.
39 Maritan M, et al. Odorant receptors at the growth cone are coupled to localized cAMP and Ca2+ increases. Proc Natl Acad Sci U S A. 2009;106(9):3537–42.
40 Lodovichi C, Belluscio L. Odorant receptors in the formation of the olfactory bulb circuitry. Physiology (Bethesda). 2012;27(4):200–12.
41 Pietrobon M, et al. Interplay among cGMP, cAMP, and Ca2+ in living olfactory sensory neurons in vitro and in vivo. J Neurosci. 2011;31(23):8395–405.
42 Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annu Rev Cell Dev Biol. 2007;23:375–404.
43 Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388(6639):275–9.
44 Nishiyma M, et al. Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature. 2003;423(6943):990–5.
45 Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13(5):308–24.
46 Nakashima A, et al. Agonist-independent GPCR activity regulates anterior-posterior targeting of olfactory sensory neurons. Cell. 2013;154(6):1314–25.
47 Cloutier JF, et al. Differential requirements for semaphorin 3F and Slit-1 in axonal targeting, fasciculation, and segregation of olfactory sensory neuron projections. J Neurosci. 2004;24(41):9087–96.
48 Nguyen-Ba-Charvet KT, et al. Robos and slits control the pathfinding and targeting of mouse olfactory sensory axons. J Neurosci. 2008;28(16):4244–9.
49 Cloutier JF, et al. Neuropilin-2 mediates axonal fasciculation, zonal segregation, but not axonal convergence, of primary accessory olfactory neurons. Neuron. 2002;33(6):877–92.
50 Takeuchi H, et al. Sequential arrival and graded secretion of Sema3F by olfactory neuron axons specify map topography at the bulb. Cell. 2010;141(6):1056–67.
51 Walz A, Rodriguez I, Mombaerts P. Aberrant sensory innervation of the olfactory bulb in neuropilin-2 mutant mice. J Neurosci. 2002;22(10):4025–35.
52 Cho JH, et al. Requirement for Slit-1 and Robo-2 in zonal segregation of olfactory sensory neuron axons in the main olfactory bulb. J Neurosci. 2007;27(34):9094–104.
53 Imai T, Suzuki M, Sakano H. Odorant receptor-derived cAMP signals direct axonal targeting. Science. 2006;314(5799):657–61.
54 Imai T, et al. Pre-target axon sorting establishes the neural map topography. Science. 2009;325(5940):585–90.
55 Schwarting GA, et al. Semaphorin 3A is required for guidance of olfactory axons in mice. J Neurosci. 2000;20(20):7691–7.
56 Schwarting GA, et al. Semaphorin 3A-mediated axon guidance regulates convergence and targeting of P2 odorant receptor axons. Eur J Neurosci. 2004;19(7):1800–10.
57 Cutforth T, et al. Axonal ephrin-As and odorant receptors: coordinate determination of the olfactory sensory map. Cell. 2003;114(3):311–22.
58 Scolnick JA, et al. Role of IGF signaling in olfactory sensory map formation and axon guidance. Neuron. 2008;57(6):847–57.
59 Royal SJ, Key B. Development of P2 olfactory glomeruli in P2-internal ribosome entry site-tau-LacZ transgenic mice. J Neurosci. 1999;19(22):9856–64.
60 Serizawa S, et al. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127(5):1057–69.
61 Kaneko-Goto T, et al. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 2008;57(6):834–46.
62 Lin DM, et al. Formation of precise connections in the olfactory bulb occurs in the absence of odorant-evoked neuronal activity. Neuron. 2000;26(1):69–80.
63 Zheng C, et al. Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit. Neuron. 2000;26(1):81–91.
64 Marks CA, et al. Activity-dependent plasticity in the olfactory intrabulbar map. J Neurosci. 2006;26(44):11257–66.
65 Belluscio L, et al. Mice deficient in G(olf) are anosmic. Neuron. 1998;20(1):69–81.
66 Zou DJ, et al. Postnatal refinement of peripheral olfactory projections. Science. 2004;304(5679):1976–9.
67 Trinh K, Storm DR. Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium. Nat Neurosci. 2003;6(5):519–25.
68 Col JA, et al. Adenylyl cyclase-dependent axonal targeting in the olfactory system. Development. 2007;134(13):2481–9.
69 Zou DJ, et al. Absence of adenylyl cyclase 3 perturbs peripheral olfactory projections in mice. J Neurosci. 2007;27(25):6675–83.
70 Kerr MA, Belluscio L. Olfactory experience accelerates glomerular refinement in the mammalian olfactory bulb. Nat Neurosci. 2006;9(4):484–6.
71 Kass MD, et al. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 2013;342(6164):1389–92.
72 Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12(6):784–91.
73 Hallem EA, Carlson JR. The odor coding system of Drosophila. Trends Genet. 2004;20(9):453–9.
74 Reisert J. Origin of basal activity in mammalian olfactory receptor neurons. J Gen Physiol. 2010;136(5):529–40.
75 Connelly T, Savigner A, Ma M. Spontaneous and sensory-evoked activity in mouse olfactory sensory neurons with defined odorant receptors. J Neurophysiol. 2013;110(1):55–62.
76 Lorenzon P, et al. Circuit formation and function in the olfactory bulb of mice with reduced spontaneous afferent activity. J Neurosci. 2015;35(1):146–60.
77 Tsai L, Barnea G. A critical period defined by axon-targeting mechanisms in the murine olfactory bulb. Science. 2014;344(6180):197–200.
78 Ma L, et al. A developmental switch of axon targeting in the continuously regenerating mouse olfactory system. Science. 2014;344(6180):194–7.



