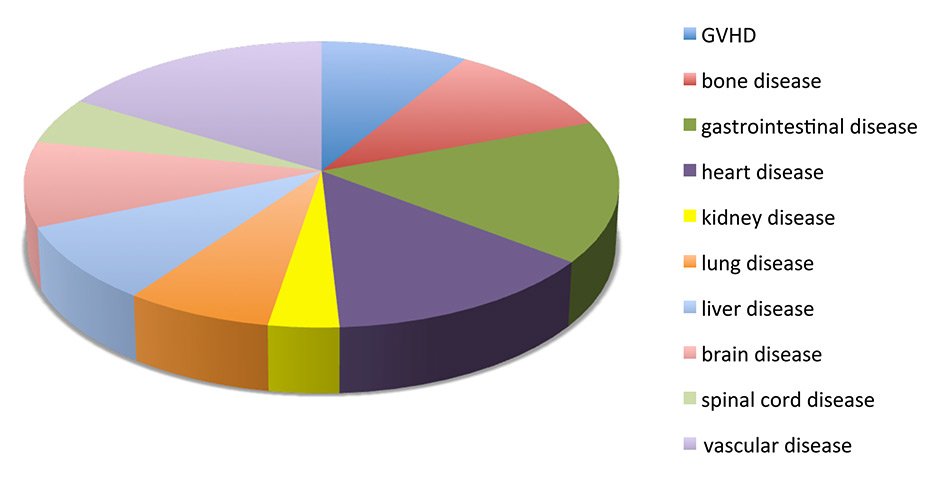
Figure 1
Summary of the main categories of disease present in the clinical trials that are completed, active or waiting for activation (registered as of July 15th, 2015 at http://clinicaltrial.gov).
GVHD = graft versus host disease
DOI: https://doi.org/10.4414/smw.2015.14229
Mesenchymal stem cells were first described in the 1960s as a rare population of plastic-adherent, nonhaematopoietic stromal cells in the bone marrow with osteogenic potential [1, 2]. These cells, initially called colony-forming-unit-fibroblast (CFU-F), were renamed mesenchymal stem cells because of their potential for differentiating into adipocytes, osteoblasts or chondrocytes [3]. Since then, it has been reported that MSCs can also be induced to differentiate into cells of ectodermal (epithelia, neurons) and endodermal (lung cells, muscle cells, gut epithelial cells) tissues [4, 5], although the physiological relevance of this remains to be determined.
More recently, MSCs have been isolated from many other tissues including adipose tissue [6], umbilical cord blood [7], umbilical cord Wharton’s jelly [8], synovial membrane [9] and tooth pulp [10]. This diversity in terms of tissue origin, together with the use of different culture media and protocols, has added to the complexity of defining and comparing culture-expanded MSCs that in fact consist of a heterogeneous population of cells and display a spectrum of phenotypic and functional properties. This led to the proposal to establish minimum criteria for MSCs that include plastic adherence, trilineage (adipogenic, chondrogenic, osteogenic) differentiation in vitro, cell surface expression of CD90, CD73 and CD105, and absence of haematopoietic markers such as CD45 [11]. To date, the lack of specific markers to define MSCs poses an additional challenge in the field, and the use of more advanced molecular criteria such as cell transcriptome, proteome and secretome has been proposed [12].
Barely more than 10 years ago, the biomedical community discovered that, rather surprisingly, MSCs were not only poorly immunogenic (thus, potentially attractive candidates for tissue regeneration therapies, given their supposed stem cell capacities) but also displayed remarkable immunomodulatory capacities (making them attractive candidates in the treatment of inflammation-associated diseases). Since then, these cells have captured an enormous amount of attention from biomedical researchers. PubMed identifies almost 35 000 references for “mesenchymal stem cells”. As often happens in rapidly expanding fields, there has also been a great number of preclinical and even clinical studies performed with poorly defined MSC populations and inappropriate experimental settings or read-outs, leading to controversial interpretation of results.
In this review we will identify and discuss what in our opinion remain the main open questions in MSC biology and will attempt to distinguish between myths and facts concerning the cell regeneration potential and the immune modulatory capacities of endogenous and therapeutic MSC.
The origin, identity and function of MSCs in vivo remain enigmatic. Several studies show that MSCs lie adjacent to blood vessels and are localised in almost every perivascular space of the body (rev in [13]). MSCs co-express many markers in common with pericytes, a poorly defined cell type that resides on the abluminal surface of endothelial cells in the microvasculature of connective tissue. The current consensus holds that perivascular cells (that include pericytes and adventitial cells) form mesenchymal stem cells in most tissues (reviewed in [14]). A combination of markers such as NG2, CD146, and PDGFRβ was reported to label specifically pericytes in a range of human organs, including foetal and adult skin, pancreas, heart, brain, lungs, bone marrow and placenta. Long-term cultures of these isolated cells displayed similar morphological features to those of cultured mesenchymal stromal cells, as well as trilineage potential in vitro and osteogenic potential in vivo [15]. Furthermore, human perivascular stem cells displayed greater healing capacity in a mouse bone injury model, as compared to the total stromal vascular fraction [16]. These results have led to suggest that it would be more appropriate to refer to MSCs as “multipotent perivascular-derived cells” [17]. However, despite their being perivascular, not all MSCs can be referred to as pericytes, and not all pericytes exhibit MSC-specific properties [13]. Furthermore, two recent studies show that MSCs may originate from other cells in addition to pericytes. Using genetic lineage tracing and a mouse model of tooth damage, Feng and colleagues showed that pericytes do not account for all of the odontoblasts, suggesting mobilisation of another source of MSCs of nonpericyte origin [18]. Another recent study using permanent genetic labelling of Schwann precursor cells showed that peripheral nerve-associated glial cells generate multipotent MSCs that produce pulp cells and odontoblasts during tooth development, renewal and repair [19]. Altogether, these results show that MSCs in a single tissue may have more than one origin and suggest that the contribution of pericytes may depend on the extent of vascularisation.
The functional characterisation of the MSC has been mainly deduced from in-vitro studies with culture-expanded cells. Thus, many open questions remain concerning the physiological activities of these cells in vivo, particularly regarding endogenous MSCs. MSCs were originally isolated from bone marrow, where they appear to play a key role in constructing and maintaining the haematopoietic stem cell (HSC) microenvironment [20]. MSCs expressing the neural stem cell marker Nestin have been shown to associate physically with HSCs and adrenergic nerve fibres, and to express high levels of HSC maintenance factors such as CXCL-12, c-kit ligand, angiopoietin-1, interleukin-7 (IL7), vascular cell adhesion molecule-1 (VCAM-1), and osteopontin [21]. In the bone marrrow, MSCs are thought to exhibit a homoeostatic default immunosuppressive phenotype, via the expression of molecules such as Gal-1, angiopoietin-1, osteopontin and thrombospondin, in order to inhibit inappropriate HSC differentiation [22]. Altogether, these data support the notion that MSC may play a direct role in the support of haematopoietic cells inside the bone marrow stem cell niche.
In addition to the haematopoietic niche, MSCs are localised in almost every perivascular space of the body. It is believed that this allows them to detect local or distant tissue damage and respond by migrating to such sites and promoting tissue repair and healing. However, there is little evidence supporting in-vivo mobilisation of endogenous MSCs. The most solid data may come from a couple of studies analysing foetal stem cell mobilisation. One study showed that microchimerism was detected in human maternal bone marrow decades after pregnancy [23]. Furthermore, experiments with mice suggest that luciferase-positive foetal MSCs that colonise the maternal bone marrow can then migrate to wounds inflicted on post-partum wild-type mothers [24]. However, induction of MSC mobilisation upon injury is not supported by studies in heart [25] or lung [26] transplant patients, where the MSCs present in the transplanted organ were all of donor origin, even several years after transplantation, suggesting that MSCs do not migrate between tissues, even under inflammatory conditions such as those found in the transplanted organs.
It has been hypothesised, at least for umbilical cord MSCs, that their primary role in situ is to maintain stromal tissue by differentiating into myofibroblasts that elaborate the extracellular matrix, and that the disturbance of the perivascular niche (for example by growth or injury) provides the specific cues required for MSC regeneration capacities [27].
An interesting perspective on the role of endogenous MSCs has recently been put forth by Caplan and colleagues, who propose that local MSCs function by managing the repair and regeneration activities of the body in a site-specific manner: when sensing damage, pericytes become MSCs and provide the appropriate microenvironment for local tissue regeneration by secreting immunomodulatory, angiogenic and trophic factors, after which they can then revert to their pericyte phenotype [28].
Independently of their origin, the stem cell nature of MSCs has been a widely accepted notion. However, very few studies have demonstrated the existence of stem cells among human cultured MSCs at the clonal level. Human bone marrow stromal cell clones gave rise to chondrogenic or adipogenic progenitors [29] and experiments combining the use of green fluorescent protein-tractable cells from human umbilical cord with rigorous cell seeding showed the existence of a small subpopulation of multipotent cells that give rise to more restricted self-renewing progenitors with a hierarchical process of differentiation into five distinct mesenchymal lineages – adipogenic, chondrogenic, osteogenic, myogenic and fibroblastic [27]. This study also showed that the greater the potential of a cell for differentiation, the rarer it is within the mesenchymal compartment. Therefore, only a very small fraction of MSCs are in fact, bona fide stem cells. This may explain why, although MSCs were first heralded for their therapeutic potential in tissue regeneration based on a “cell replacement” concept, there has been scarce evidence for in-vivo differentiation of MSCs into different cell types. In addition, the evidence is controversial, since bone marrow-derived mesenchymal stromal cell cultures have been shown to contribute to many tissues upon transplantation through heterotypic fusion with endogenous cells. In this sense, it has been proposed that therapeutic recovery of myocardial function observed upon MSC administration may be due, at least partially, to fusion of MSCs with resident cardiomyocytes leading to nuclear reprogramming [30, 31]. However, the low frequency of this biological process is unlikely to account for the improvements in preclinical models of injured tissue. These are more likely a result of the secretion by the MSC of paracrine factors that can favour angiogenesis or inhibit inflammation. In fact, the MSC secretome contains numerous pro-angiogenic factors such as growth factors (e.g. vascular endothelial growth factor and macrophage colony stimulating factor), chemokines/cytokines (e.g. monocyte chemoattractant protein-1 and interleukin-6 [IL6]) and angiopoietin 1 and 2, indicating that they may represent a promising therapeutic strategy in disorders characterised by insufficient angiogenesis such as chronic wounds, stroke and myocardial infarction (reviewed in [32]). It should be noted, however, that they can also secrete antiangiogenic factors such as tissue inhibitor of metalloproteinase-1 [33, 34] (Zanotti, manuscript in preparation) whose relevance in vivo remains to be determined. Thus, beyond their angiogenic potential, MSCs seem to contribute to tissue regeneration mainly by modulating inflammation (reviewed in [35]). Accordingly, the clinical value of MSCs is derived from their immunomodulatory activity rather than from their stem cell properties.
The immunosuppressive capacity of the MSC was first documented in reports that showed that human allogeneic MSCs suppressed T cell proliferation in a mixed lymphocyte reaction assay in vitro via the secretion of soluble factors [36, 37] and that baboon MSCs prolonged skin graft survival in vivo [38]. Since then, there is a considerable amount of literature showing that MSCs can produce a multitude of cytokines and growth factors, soluble or in extracellular vesicles, that can suppress immune responses by inhibiting B and T cell proliferation, dendritic cell and monocyte maturation, and by promoting generation of regulatory T cells and anti-inflammatory (M2-like) macrophages (review in [17, 39, 40] (table 1).
There are a number of excellent recent reviews on the mechanisms by which MSCs modulate innate and adaptive immune responses [39, 41–43]. However, a recurring problem in the field of MSC research is that the mechanisms by which these cells modulate immune responses has mostly been characterised in vitro. It is unclear to what degree these mechanisms are relevant to transplanted or endogenous MSCs in vivo. Here, we will focus on discussing certain concepts that have been proposed and accepted on the basis of in vitro, and sometimes in vivo, experiments but that are not supported by recent evidence.
Culture-expanded MSCs express low levels of major histocompatibility complex (MHC) class I and practically no MHC class II or costimulatory molecules, and therefore it is widely accepted that soluble factors mediate their immune suppressive activity. Many factors released by MSC, such as transforming growth factor-β (TGFβ), hepatocyte growth factor, prostaglandin E2 (PGE2), indolamine 2,3-dioxygenase (IDO) (for human MSCs), NO (for mouse MSCs), IL6 and possibly IL10 have been shown to contribute to their modulatory effects on innate and adaptive cells, at least in vitro (reviewed in [44, 45].
It has been assumed that, in order to exert their immunomodulatory or regenerative effect in vivo, MSCs must migrate to damaged tissue sites, where they release a cocktail of soluble factors in response to the inflammatory microenvironment [35, 46, 47]. However, there is accumulating experimental evidence that does not support this notion and that indicates that MSCs may induce immune and regenerative effects in the host via endocrine signalling.
First, although very few studies have successfully addressed the fate of systemically administered MSCs in vivo, the number of MSCs reaching the target tissue seems to be extremely low [48, 49]. In fact, it has been shown that the vast majority of intravenously infused MSCs are trapped in the lung where they die shortly after [50, 51]. So, how can the effect of intravenously administered MSCs be explained? Interestingly, in a mouse myocardial infarction model, infused human MSCs were shown to exert their protective function by secreting the anti-inflammatory factor tumour necrosis factor-α (TNF-α)-induced protein 6 (TSG-6) upon their trapping and activation in the lung [52]. In addition, phagocytosis of dead MSCs in the lung could eventually result in the generation of macrophages with a regulatory phenotype [53]. It is possible, though unlikely, that some MSCs may escape elimination and account for the therapeutic effect.
Second, a number of studies, where MSCs have been administered via other routes that prolong their survival but limit their migration, showed that these cells can exert an effect on distant tissue. For example, MSCs administered intraperitoneally have been shown to reduce joint inflammation, independently of MSC viability and migration to the joints [54]. A recent study using a mouse model of colitis shows that intraperitoneal administration of MSC ameliorates colitis in mice via the release of TSG-6 and independently of their capacity to localise to the intestine [55]. Furthermore, by administering subcutaneously mouse MSCs encapsulated in alginate microcapsules, we have conclusively shown that MSCs do not need to migrate to inflamed tissue in order to modulate local, antigen-specific immune responses in vivo[56]. In our model we observed a decrease in recruitment of all immune cells that correlated with a decrease in endothelial cell activation and angiogenesis (Zanotti, manuscript in preparation). This is in line with studies showing that intravenously administered MSCs decrease dendritic cell maturation and migration towards reactive lymph nodes [57].
Evidently, the question remains as to the precise soluble factors underlying the endocrine function of MSCs. Cytokine-activated MSCs secrete a wide range of bioactive molecules such as cytokines, antioxidants, pro- and anti-angiogenic molecules and growth factors [58], and injection of MSC-conditioned medium into mice was reported to mimic the beneficial effect of transplanted MSCs in terms of wound healing and arteriogenesis [59, 60]. In this context, the role of MSC microvesicles has received much attention during recent years. MSC derived microvesicles have been shown to protect against kidney injury [61]. In another study, microvesicles derived from MSCs could inhibit proliferation of lymphocytes isolated from mice with experimental autoimmune encephalomyelitis and were shown to express programmed death ligand 1 (PD-L1), galecin-1 and membrane-bound TGF-β [62], and infusion of MSC exosomes was reported to enhance the survival of allogeneic skin grafts in mice and the induction of regulatory T cells (Tregs) [63]. Although the idea of replacing cellular therapies based on MSC with MSC-derived microvesicles is attractive and presents a series of advantages, their immunomodulatory capacity in vitro seems to be lower as compared with their cellular counterparts [64]. Thus, the role of these vesicles in vivo and their capacity to suppress ongoing immune responses remains to be confirmed. It is likely that the endocrine effect of MSCs may involve the secretion of soluble factors that promote the induction of resident cells with regulatory phenotypes, such as M2-type macrophages [25].
MSCs have been reported to modulate the immune system through a broad panel of mechanisms. They secrete anti-inflammatory factors such as TGFβ, hepatocyte growth factor and PGE2 [36, 65] and express inhibitory molecules such as PD-L1, FasL (Augello et al. 2005 [67]; Gu et al. 2013 [68]) or human leucocyte antigen-G (Selmani et al. 2008 [106]) that directly inhibit T cell proliferation. By producing TGFβ and other factors such as PGE2, they may also promote the induction of cells with regulatory functions, such as Tregs [66], regulatory B cells [67, 68], and anti-inflammatory macrophages [69] and have recently been shown to induce regulatory dendritic cells via Notch signalling [70].
The question is whether all these effects are relevant in vivo and in all settings. Several studies indicate that MSCs are not constitutively inhibitory and that their immunoregulatory effect is activated by an inflammatory environment (reviewed in [39, 71]). Accordingly, MSCs have been shown to be most effective when administered after the onset of the inflammatory response [72].
MSCs may sense the local environment via different receptors, including Toll-like receptors (TLRs) and cytokine receptors. Human and mouse MSCs express several TLRs and, depending on their tissue origin, their expression can be up- or downregulated by environmental cues such as hypoxia and inflammatory cytokines [73]. Based on studies showing that TLR4 stimulation led to the upregulation of proinflammatory cytokines such as IL6, while TLR3 priming led to the production of anti-inflammatory molecules such as IL4 and PGE2, it was proposed that MSCs may adopt a MSC1 or MSC2 phenotype [74]. However, the effect of TLR3 and TLR4 stimulation remains controversial, with some studies showing that both receptors enhance immunosuppresion in vitro through IDO induction [75] and others showing upregulation of proinflammatory cytokines [76]. In addition, priming through other TLRs may also upregulate the expression of immunomodulatory proteins [77, 78]. It is clear that more investigation is needed to understand if and how different damage-associated molecular pattern molecules play a role in determining MSC function in vivo.
Cytokine signalling prior to TLR stimulation seems to be important for inducing immunoregulatory MSCs. For example, MSCs were shown to ameliorate sepsis in mice upon stimulation by lipopolysaccharide and TNFα. This led to nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signalling, cyclooxygenase-2 expression and synthesis of PGE2 by MSC, which binds to EP2/EP4 receptors on macrophages and leads to IL10 production [79]. The amount of inflammatory cytokines may also be important and only high levels of interferon-γ (IFNγ) and IL1 or TNFα were able to induce upregulation of inducible nitric oxide synthase (iNOS) by MSCs at sufficient levels to inhibit T cell proliferation [80]. In fact, it has been proposed that iNOS for murine cells or IDO for human cells may represent the “molecular switch” for immune-suppressive MSCs. Injection of iNOS-deficient MSCs into the footpad of mice led to an aggravated ovalbumin-induced delayed-type hypersensitivity response in terms of footpad thickness and enhanced leucocyte infiltration, while wild-type MSCs were protective [80, 81].
Overall, these results suggest that MSCs are receptive to environmental cues that play a key role in determining their activation and function. However, the precise mechanisms underlying these findings remain to be determined in vivo, particularly in experimental settings where MSCs do not migrate to inflamed tissues. It is highly likely that the paracrine/endocrine function of MSCs that are encapsulated [56], injected subcutaneously (our unpublished results) or intraperitoneally [55, 82] is also dependent on the environment. These cells could be activated by circulating inflammatory factors, or simply as a result of their dynamic compaction into spheres, which has been shown to activate caspase-dependent IL1 signalling and the secretion of inflammation modulators such as TSG-6 and PGE2 [82]. Intravenously infused MSCs, which have a very short life and limited distribution to inflamed tissues, have in fact been shown to induce a systemic inflammatory response within hours after infusion [48]. This could contribute to the documented transient T cell apoptosis observed, leading to TGFβ production by macrophages and enhanced Treg generation [83]. One could speculate that the effect of infused MSCs depends on a short-lived but dynamic regulatory feedback between MSCs and macrophages. This would agree with recent studies that indicate that the ability of MSCs to suppress T cell proliferation is monocyte dependent and involves polarisation of monocytes to anti-inflammatory macrophages (reviewed in [39]).
| Table 1: soluble factor that mediates anti-inflammatory mechanism of mesenchymal stem cells. | |||
| Target cell | Mediator | Action(s) | Reference |
| T cell | Cleaved CCL2 | Inhibits CD4+ Th17 cells | [102] |
| CXCL9/CXCL10 | Promotes T cell chemotaxis | [81] | |
| sGalectin1 and 3 | Inhibits T cell proliferation | [103] | |
| Haem oxygenase-1 | Inhibits T cell response | [104] | |
| induces IL-10+ Tr1 and TGF-β + Treg | [105] | ||
| HGF | inhibits T cell proliferation | [36] | |
| sHLA-G5 | suppresses T cell function | [106, 107] | |
| Induces Treg | [106] | ||
| IDO* | Inhibits T cell proliferation | [108] | |
| IL6 | inhibits T cell proliferation | [109] | |
| IL10 | Inhibits T cell responses | [110] | |
| Decreases Th17 cell differentiation | [111] | ||
| iNOS/NO* | Inhibits T cell proliferation | [81] | |
| LIF | Inhibits T cell proliferation | [112] | |
| PGE2 | Inhibits T cell proliferation | [113] | |
| Modulates Th1, Th2 cytokine production | [113] | ||
| TGFb | Induces Treg | [114] | |
| B cell | Cleaved CCL2 | Suppression of immunoglobulin production | [115] |
| IDO | Induces Breg | [116] | |
| Natural killer cell | IDO | Impairs NK cell proliferation and function | [117] |
| sHLA-G5 | Inhibits NK cell function | [106] | |
| PGE2 | Inhibits NK cell function | [118] | |
| TGFb | Inhibits NK cell activation and function | [118] | |
| Dendritic cell | IL6 | Modulation of DC maturation | [119] |
| PGE2 | Modulation of DC maturation | [120,121] | |
| Modulates mature DC cytokine production | [65] | ||
| Macrophage | CCL2/CCL3/CCL12 | Promotes macrophage chemotaxis | [60] |
| IDO | Promotes type II macrophage differentiation | [122] | |
| PGE2 | Promotes type II macrophage differentiation | [79,123] | |
| TSG6 | Regulates macrophage (downregulation of NF-κB) | [124] | |
| HLA-G = human leucocyte antigen G; IDO = indolamine 2,3 dioxygenase;IL-6, interleukin-6; iNOS = inducible NO synthase; MSC = mesenchymal stem cell; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B-cells; NO = nitric oxide; LIF = leukaemia inhibitory factor; TGF-β = transforming growth factor-β; PGE2 = prostaglandin E2; TSG6 = tumour necrosis factor-α-induced protein. | |||
Based on the ability of MSCs to expand in culture and differentiate into multiple cellular phenotypes, they were considered as potentially useful therapeutic agents for tissue repair [3]. Although animal experiments suggested that MSCs were capable of homing to and proliferating within injured tissues, it soon became apparent that their effect did not require significant engraftment or differentiation [84]. In fact, it was shown that in response to injury, MSCs secrete large quantities of bioactive molecules able to mediate tissue repair by limiting apoptosis and stress response, and by recruiting immune cells that enhance wound repair [52, 84, 85]. For these reasons, we will focus on the clinical value of MSC based on their immunoregulatory capacity rather than on their stem cell properties.

Figure 1
Summary of the main categories of disease present in the clinical trials that are completed, active or waiting for activation (registered as of July 15th, 2015 at http://clinicaltrial.gov).
GVHD = graft versus host disease
In one of the first clinical applications of MSCs, Le Blanc and colleagues used maternal-derived haploidentical MSCs to treat a boy suffering from treatment-resistant graft versus host disease (GvHD) and reported recovery and survival beyond 1 year after two administrations of MSCs [86]. Since then, the number of clinical trials using MSC has soared (nearly 350 registered trials, most of them in phase I or II) and are addressing their efficacy in the prevention or treatment of a broad spectrum of diseases, mostly of autoimmune origin but also in solid organ transplantation, neurological disorders and even autism [58, 78, 87, 88] (fig. 1). Relatively small trials have been conducted by academic institutions with unmatched and haploidentical MSCs from early passages, for example in the treatment of acute GvHD [89], with relative success. However, MSC-based therapies have been predominately developed by companies that, by extensive culture expansion, generate therapeutic doses to treat entire cohorts of patients, and products developed by companies such as FBC-Pharmicell’s Hearticellgram®-AMI and Mesoblasts’ Prochymal® have already received regulatory approval in certain countries [90].
MSCs are currently being tested in a wide range of clinical settings, mainly in autoimmune diseases (multiple sclerosis, rheumatoid arthritis, Crohn’s disease, etc.), GvHD, wound repair, ischaemia/stroke, liver diseases and HSC engraftment. Despite the large number of ongoing clinical trials, the demonstration of a beneficial effect from MSCs in large placebo-controlled trials remains elusive. In some cases, MSCs have even been reported to lead to the exacerbation of disease symptoms [91, 92]. This heterogeneity in response to MSC administration is most likely due to the fact that their function may vary according to factors such as the administration route, the timing, and their tissue and/or donor origin. In clinical settings, systemic intravenous delivery route for MSCs has shown clinical efficacy despite entrapment in nontarget tissues (reviewed in [93]). However, local administration, although less explored, may prove to be highly effective. For example, intrafistular administration of MSCs in patients with Crohn’s disease led to amelioration of mucosal healing and was associated with an increase in circulating and mucosal Tregs [94]. In a preclinical model of GvHD we have shown that encapsulated MSCs were more efficient in modulating antigen-specific responses than intravenously administered MSCs [56]. Not all MSCs may work the same: while human umbilical cord (UBC) MSCs were capable of preventing GvHD in a humanised NOD/SCID model, murine MSCs had no effect. However, even the same UBC-MSCs were not protective when administered at onset or late in the course of the disease [95]. Similarly, mouse MSCs were shown to protect from GvHD only at timepoints when the animals had high levels of IFNγ [72], supporting the notion that MSCs are immunosuppressive only when activated by inflammatory cytokines. This would help explain why MSCs improved overall survival in patients with acute GvHD [89] but not in patients that received MSCs at the time of HSCT [96]. Furthermore, Ball et al. showed that the administration of MSCs early after the diagnosis of steroid refractory GvHD resulted in higher complete response rates and better overall survival [97]. This suggested the use of MSCs as second-line treatment in subsequent paediatric European clinical trials in order to prevent massive damage to gut and liver (HOVON113).
Therefore, a better understanding of the patient inflammatory status at the time of MSC infusion could allow the development of relevant biomarkers to decide the best timing and route of MSC administration [39]. Concerning the donor origin of MSCs, the assumption that they are immunologically privileged has encouraged the use of large-scale allogeneic MSC preparations as a “one-size fits all” therapy [17]. However, recent data indicate that allogeneic MSCs can provoke an immune response resulting in rejection. MSCs exposed to IFNγ upregulate MHC class I and class II [86] and it has been shown that intraperitoneal injections of allo-MSCs can result in elevated titres of allo-reactive antibodies and rejection of same-party skin grafts [98]. Transplantation of allogeneic but not syngeneic transgene-expressing MSCs has been shown to result in humoral and cellular responses to the neoantigen [99, 100]. Thus, while MSCs are poorly immunogenic, they cannot be considered as immune-privileged. Furthermore, these results indicate that, while both auto- and allo-MSCs are being used in clinical trials, direct efficacy comparisons between both cell sources are urgently required. In any case, the immune memory generated by allo-MSCs calls for caution about their sequential administration [17].
Another issue for concern is the potential ability of MSCs to transform upon culture expansion. Since it was reported that sarcoma developed following transplantation of MSCs into animals [101], determination of their therapeutic efficacy and safety is now required for clinical applications. However, human MSCs are less susceptible to transformation upon long-term in-vitro culture [39] and, so far, no malignant transformation has been reported in clinical trials.
During the last two decades, the amount of scientific literature on MSCs has increased exponentially. However, there has also been an overwhelming number of poorly reproducible, confusing and/or controversial reports, in great part due to the use of poorly defined cell populations and/or experimental models. Recent evidence, obtained mainly from experiments in vivo, has helped to start distinguishing facts from myths surrounding MSC biology. It is now quite clear that only a small population of MSCs are in fact pluripotent stem cells, and that their clinical potential relies on their capacity to modulate inflammation and tissue repair rather than on their cell differentiation capacity. It is also becoming evident that MSCs do not need to migrate to the inflamed tissue in order to exert their function but rather, in response to inflammatory cues, release a series of soluble factors that act in a paracrine and even endocrine manner. Further characterisation of the human and mouse MSC secretome is therefore an attractive research avenue.
For MSC to fulfil their potential in the prevention and/or treatment of immune-mediated human diseases, further investigation into several issues is urgently needed. Foremost, unifying mechanisms of MSC function in vivo need to be identified. Second, standardised protocols for the culture and expansion of therapeutic MSCs, including their tissue and donor origin, need to be established. Third, relevant biomarkers that may guide clinicians as to the best route and timing of MSC administration and/or help them evaluate the efficacy of the therapy are needed. Finally, large placebo-controlled trials are required and long-term safety data on MSC-based therapies have yet to be obtained.
Disclosure statement:The laboratory of A.V. is supported by the EC FP7 project MERLIN (grant agreement no 602363). LZ is supported by Fondazione Umberto Veronesi per la Ricerca. No financial support and no other potential conflict of interest relevant to this article was reported.
1 Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–47.
2 Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403.
3 Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50.
4 Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
5 Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–9.
6 Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95.
7 Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109(1):235–42.
8 Wang H-S, Hung S-C, Peng S-T, Huang C-C, Wei H-M, Guo Y-J, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–7.
9 De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–42.
10 Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97(25):13625–30.
11 Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
12 Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10(3):244–58.
13 Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol. 2011;12(2):126–31.
14 Crisan M, Corselli M, Chen WCW, Péault B. Perivascular cells for regenerative medicine. J Cell Mol Med. 2012;16(12):2851–60.
15 Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13.
16 James AW, Zara JN, Corselli M, Askarinam A, Zhou AM, Hourfar A, et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. 2012;1(9):673–84.
17 Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60.
18 Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proceedings of the National Academy of Sciences. 2011;108(16):6503–8.
19 Kaukua N, Shahidi MK, Konstantinidou C, Dyachuk V, Kaucka M, Furlan A, et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature. Nature Publishing Group; 2014;513(7519):551–4.
20 Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36.
21 Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34.
22 Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36(10):2566–73.
23 O’Donoghue K, Chan J, la Fuente de J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364(9429):179–82.
24 Seppanen E, Roy E, Ellis R, Bou-Gharios G, Fisk NM, Khosrotehrani K. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: evidence from a murine fetal microchimerism model. PLoS ONE. 2013;8(5):e62662.
25 Hoogduijn MJ, Crop MJ, Peeters AMA, Korevaar SS, Eijken M, Drabbels JJ, et al. Donor-derived mesenchymal stem cells remain present and functional in the transplanted human heart. Am J Transplant. 2009;9(1):222–30.
26 Lama VN, Smith L, Badri L, Flint A, Andrei A-C, Murray S, et al. Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest. 2007;117(4):989–96.
27 Sarugaser R, Hanoun L, Keating A, Stanford WL, Davies JE. Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy. PLoS ONE. 2009;4(8):e6498.
28 Caplan AI, Hariri R. Body Management: Mesenchymal Stem Cells Control the Internal Regenerator. Stem Cells Transl Med. 2015;4(7):695–701.
29 Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113 (Pt 7):1161–6.
30 Kouris NA, Schaefer JA, Hatta M, Freeman BT, Kamp TJ, Kawaoka Y, et al. Directed Fusion of Mesenchymal Stem Cells with Cardiomyocytes via VSV-G Facilitates Stem Cell Programming. Stem Cells International. 2012;2012:414038.
31 Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14(6):840–50.
32 Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–96.
33 Lee J-K, Park S-R, Jung B-K, Jeon Y-K, Lee Y-S, Kim M-K, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE. 2013;8(12):e84256.
34 Burlacu A, Grigorescu G, Rosca A-M, Preda MB, Simionescu M. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev. 2013;22(4):643–53.
35 Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009–16.
36 Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43.
37 Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–97.
38 Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8.
39 Bernardo ME, Fibbe WE. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Stem Cell. Elsevier Inc; 2013;13(4):392–402.
40 Prockop DJ. Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells. 2013;31(10):2042–6.
41 Keating A. Perspective. Stem Cell. Elsevier Inc; 2012;10(6):709–16.
42 Wang J, Lu Z-H, Gabius H-J, Rohowsky-Kochan C, Ledeen RW, Wu G. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 2009;182(7):4036–45.
43 Uccelli A, Mancardi G, Chiesa S. Is there a role for mesenchymal stem cells in autoimmune diseases? Autoimmunity. 2008;41(8):592–5.
44 Yagi H. The role of mesenchymal stem cells in cancer development. 2013:1–6.
45 Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr Mol Med. 2013;13(5):856–67.
46 Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72(4):430–41.
47 Assis ACM, Carvalho JL, Jacoby BA, Ferreira RLB, Castanheira P, Diniz SOF, et al. Time-dependent migration of systemically delivered bone marrow mesenchymal stem cells to the infarcted heart. Cell Transplant. 2010;19(2):219–30.
48 Hoogduijn MJ. The life and fate of mesenchymal stem cells. 2014:1–6.
49 Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27(10):2624–35.
50 Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–92.
51 Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297.
52 Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63.
53 Lu W, Fu C, Song L, Yao Y, Zhang X, Chen Z, et al. Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int J Cardiol. 2013;165(2):333–40.
54 Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56(4):1175–86.
55 Sala E, Genua M, Petti L, Anselmo A, Arena V, Cibella J, et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology. 2015;149(1):163–176.e20.
56 Zanotti L, Sarukhan A, Dander E, Castor M, Cibella J, Soldani C, et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia. Nature Publishing Group; 2012;27(2):500–3.
57 Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, et al. Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proceedings of the National Academy of Sciences. 2011;108(42):17384–9.
58 Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–78.
59 Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–85.
60 Chen L, Tredget EE, Wu PYG, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE. 2008;3(4):e1886.
61 Bruno S, Collino F, Iavello A, Camussi G. Effects of mesenchymal stromal cell-derived extracellular vesicles on tumor growth. Front Immunol. 2014;5:382.
62 Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid A-A, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147(1-2):47–54.
63 Zhang B, Yin Y, Lai RC, Tan SS, Choo ABH, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23(11):1233–44.
64 Conforti A, Scarsella M, Starc N, Giorda E, Biagini S, Proia A, et al. Microvescicles derived from mesenchymal stromal cells are not as effective as their cellular counterpart in the ability to modulate immune responses in vitro. Stem Cells Dev. 2014;23(21):2591–9.
65 Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–22.
66 Engela AU, Hoogduijn MJ, Boer K, Litjens NHR, Betjes MGH, Weimar W, et al. Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol. 2013;173(2):343–54.
67 Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, et al. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35(5):1482–90.
68 Gu Y-Z, Xue Q, Chen Y-J, Yu G-H, Qing M-D, Shen Y, et al. Different roles of PD-L1 and FasL in immunomodulation mediated by human placenta-derived mesenchymal stem cells. Hum Immunol. 2013;74(3):267–76.
69 Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS ONE. 2010;5(2):e9252.
70 Liu X, Ren S, Ge C, Cheng K, Zenke M, Keating A, et al. Sca-1+Lin-CD117- Mesenchymal Stem/Stromal Cells Induce the Generation of Novel IRF8-Controlled Regulatory Dendritic Cells through Notch-RBP-J Signaling. J Immunol. 2015;194(9):4298–308.
71 Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia. 2011;25(9):1408–14.
72 Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38(6):1745–55.
73 Delarosa O, Dalemans W, Lombardo E. Toll-like receptors as modulators of mesenchymal stem cells. Front Immunol. 2012;3:182.
74 Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4):e10088.
75 Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Köppel A, et al. Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells. 2009;27(4):909–19.
76 Romieu-Mourez R, François M, Abate A, Boivin M-N, Birman E, Bailey D, et al. Mesenchymal stromal cells expressing ErbB-2/neu elicit protective antibreast tumor immunity in vivo, which is paradoxically suppressed by IFN-gamma and tumor necrosis factor-alpha priming. Cancer Res. 2010;70(20):7742–7.
77 Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Evidence for the involvement of galectin-3 in mesenchymal stem cell suppression of allogeneic T-cell proliferation. Scand J Immunol. 2010;71(4):267–74.
78 English K, Wood KJ. Mesenchymal stromal cells in transplantation rejection and tolerance. Cold Spring Harb Perspect Med. 2013;3(5):a015560.
79 Nemeth K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
80 Li W, Ren G, Huang Y, Su J, Han Y, Li J, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19(9):1505–13.
81 Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–50.
82 Bartosh TJ, Ylöstalo JH, Bazhanov N, Kuhlman J, Prockop DJ. Dynamic compaction of human mesenchymal stem/precursor cells into spheres self-activates caspase-dependent IL1 signaling to enhance secretion of modulators of inflammation and immunity (PGE2, TSG6, and STC1). Stem Cells. 2013;31(11):2443–56.
83 Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10(5):544–55.
84 Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17(6):939–46.
85 Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, et al. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27(3):670–81.
86 Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41.
87 Knaän-Shanzer S. Concise review: the immune status of mesenchymal stem cells and its relevance for therapeutic application. Stem Cells. 2014;32(3):603–8.
88 Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54.
89 Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86.
90 Cyranoski D. Canada approves stem cell product. Nat Biotechnol. 2012;30(7):571–1.
91 Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends in Molecular Medicine. 2010;16(5):203–9.
92 Kishk NA, Abokrysha NT, Gabr H. Possible induction of acute disseminated encephalomyelitis (ADEM)-like demyelinating illness by intrathecal mesenchymal stem cell injection. J Clin Neurosci. 2013;20(2):310–2.
93 Kurtz A. Mesenchymal stem cell delivery routes and fate. Int J Stem Cells. 2008;1(1):1–7.
94 Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60(6):788–98.
95 Tisato V, Naresh K, Girdlestone J, Navarrete C, Dazzi F. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia. 2007;21(9):1992–9.
96 Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–98.
97 Ball LM, Bernardo ME, Roelofs H, van Tol MJD, Contoli B, Zwaginga JJ, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013;163(4):501–9.
98 Badillo AT, Beggs KJ, Javazon EH, Tebbets JC, Flake AW. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol Blood Marrow Transplant. 2007;13(4):412–22.
99 Campeau PM, Rafei M, François M, Birman E, Forner K-A, Galipeau J. Mesenchymal stromal cells engineered to express erythropoietin induce anti-erythropoietin antibodies and anemia in allorecipients. Mol Ther. 2009;17(2):369–72.
100 Zangi L, Margalit R, Reich-Zeliger S, Bachar-Lustig E, Beilhack A, Negrin R, et al. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells. 2009;27(11):2865–74.
101 Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371–9.
102 Rafei M, Campeau PM, Aguilar-Mahecha A, Buchanan M, Williams P, Birman E, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182(10):5994–6002.
103 Sioud M, Mobergslien A, Boudabous A, Fløisand Y. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011;38(2):385–90.
104 Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–4.
105 Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117(18):4826–35.
106 Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–22.
107 Rizzo R, Campioni D, Stignani M, Melchiorri L, Bagnara GP, Bonsi L, et al. A functional role for soluble HLA-G antigens in immune modulation mediated by mesenchymal stromal cells. Cytotherapy. 2008;10(4):364–75.
108 Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–62.
109 Najar M, Rouas R, Raicevic G, Boufker HI, Lewalle P, Meuleman N, et al. Mesenchymal stromal cells promote or suppress the proliferation of T lymphocytes from cord blood and peripheral blood: the importance of low cell ratio and role of interleukin-6. Cytotherapy. 2009;11(5):570–83.
110 Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–9.
111 Qu X, Liu X, Cheng K, Yang R, Zhao RCH. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp Hematol. 2012;40(9):761–70.
112 Nasef A, Mazurier C, Bouchet S, François S, Chapel A, Thierry D, et al. Leukemia inhibitory factor: Role in human mesenchymal stem cells mediated immunosuppression. Cell Immunol. 2008;253(1-2):16–22.
113 Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, et al. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol. 2008;181(6):4389–96.
114 Nemeth K, Keane-Myers A, Brown JM, Metcalfe DD, Gorham JD, Gorham JD, et al. Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proceedings of the National Academy of Sciences. 2010;107(12):5652–7.
115 Rafei M, Hsieh J, Fortier S, Li M, Yuan S, Birman E, et al. Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood. 2008;112(13):4991–8.
116 Peng Y, Chen X, Liu Q, Zhang X, Huang K, Liu L, et al. Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia. 2015;29(3):636–46.
117 Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111(3):1327–33.
118 Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24(1):74–85.
119 Djouad F, Charbonnier L-M, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–32.
120 Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26):6576–83.
121 Chen L, Zhang W, Yue H, Han Q, Chen B, Shi M, et al. Effects of human mesenchymal stem cells on the differentiation of dendritic cells from CD34+ cells. Stem Cells Dev. 2007;16(5):719–31.
122 François M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–95.
123 Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30(10):2283–96.
124 Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118(2):330–8.