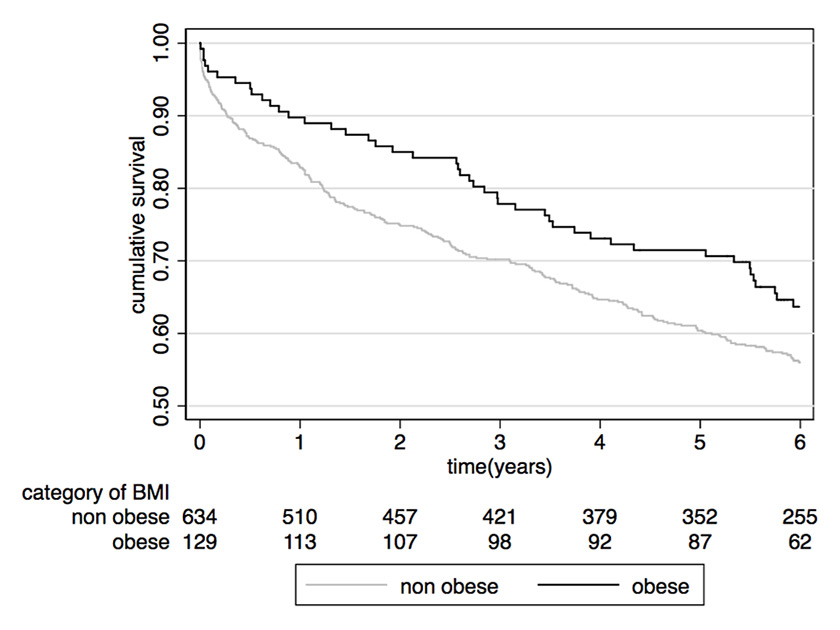
Figure 1
Association between obesity and survival in a cohort of patients with community-acquired pneumonia.
nonobese = body mass index (BMI) <30 kg/m2; obese = BMI ≥30 kg/m2
DOI: https://doi.org/10.4414/smw.2015.14265
Imagine two patients presenting at the emergency room, both suffering from a community-acquired pneumonia and with similar age, ethnicity and comorbidities. However, one of them has a normal body mass index (BMI) of 18.5–25 kg/m2, while the other one meets the criteria for mild obesity (BMI 30–35 kg/m2). Which patient is at higher risk for mortality over the next few years? Most people would choose the mildly obese patient. Empirical evidence, however, shows that the mildly obese patient with pneumonia, compared with the normal weight patient, has a more favourable prognosis in regard to mortality – a phenomenon that has been named the “obesity paradox”. Similar data are available for patients suffering from heart failure [1], acute coronary syndrome [2], diabetes type 2 [3], cancer [4], kidney disease [5], stroke [6], chronic obstructive lung disease [7] or community-acquired pneumonia [8] and the elderly population [9].
Obesity and the metabolic syndrome are associated with negative health outcomes. Obesity is a risk factor for diabetes type 2, hypertension, dyslipidaemia, cardiovascular diseases and several malignancies. In a healthy population, the BMI-associated mortality curve is U-shaped with lowest mortality at a BMI between 22.5–25 kg/m2 [10]. Thus, it seems paradoxical that epidemiological studies in the above-mentioned patient populations reported a survival benefit for overweight and obese patients. Several reasons have been discussed that could explain these paradoxical associations, which are outlined in more detail below. Furthermore, several open questions remain regarding the obesity paradox. Whether the obesity paradox is only true for mild obesity (BMI 30–35 kg/m2) or if patients with more severe obesity also have survival benefits is unclear, and findings of the existing studies are inconsistent. The type of obesity (i.e., hedonic or metabolic) may also play an important role. A recent study looking at >15 000 adults from the National Health and Nutrition Examination Survey (NHANES) found central obesity to be a risk factor for mortality even among individuals with a normal BMI [11]. Multivariate analyses demonstrated that normal-weight men and women with central obesity were more likely to die during a mean follow-up of 14 years than were other participants. In their analysis waist-to-hip ratio was the better outcome predictor compared with BMI. Also, from the “SOS study” we have learned that weight loss caused by bariatric surgery improves survival in morbidly obese patients (≥34 kg/m2 in men and ≥38 kg/m2 in women) [12].
Although clinical data have repeatedly shown improved survival in obese patients, underlying pathophysiological mechanisms remain largely unclear. One often discussed argument for a survival advantage in obesity is the increased metabolic reserve during cachectic states – such as disease-associated cachexia [13, 14]. Loss of appetite and the consequent unintentional weight loss is a frequent finding associated with acute disease and may lead to malnutrition with negative impact on immune function. In this context, the fat reserves of obese patients may make them more resistant to the catabolic progression of wasting diseases [15]. Aquilani and colleagues found that, in patients with chronic heart failure, only obese individuals had balanced muscle protein catabolism, and it is known that an unbalanced protein catabolism is associated with reduced immunological capacity and tissue integrity [16]. Fat tissue also has other relevant beneficial effects, for instance, in the protection against bone fractures [17], infectious complications and pressure sores [18].

Figure 1
Association between obesity and survival in a cohort of patients with community-acquired pneumonia.
nonobese = body mass index (BMI) <30 kg/m2; obese = BMI ≥30 kg/m2
Another argument is a possible difference in immune and inflammatory response. Obesity is known to be a state of chronic inflammation and proinflammatory adipokines, released by adipocytes, are thought to play a crucial role in the pathogenesis of obesity and related adverse outcomes [19–21]. A more pronounced inflammatory response, evidenced by higher body temperature and a higher increase in C-reactive protein levels was associated with favourable long-term prognosis in one study looking at a large cohort of patients with community-acquired pneumonia [22]. Thus, one may speculate that a stronger inflammatory and immune response may help the body to overcome an infectious episode with favourable long-term effects. A recent study investigated associations of obesity and inflammation biomarkers in a cohort of 763 patients with community-acquired pneumonia [23]. In this cohort, we observed that obese patients had higher survival rates when compared with the nonobese population, providing again empirical evidence in favour of the obesity paradox (fig. 1). However, there was no evidence of a different immune response in obese patients, which could explain these findings [23]. Inflammation was measured by means of different biomarkers (C-reactive protein, white cell count, procalcitonin, proadrenomedullin), but other markers such as cytokines and interleukins were not available in this cohort [24]. Thus the study still does not preclude inflammation from being the missing link in the obesity paradox.
There are several pro- and anti-inflammatory adipokines, which play an important role in immune function. For example the proinflammatory tumour necrosis factor-alpha (TNF-α) and its soluble anti-inflammatory antibodies are both produced by adipocytes [25, 26]. The latter are supposed to be beneficial in the overwhelming TNFα production observed in lethal sepsis [25]. Leptin is another adipokine, which is important for host response by modulating T-cell response [27]. Adiponectin has anti-inflammatory effects and may suppress inflammation in the lungs [21]. Also, the inflammatory response per se has in turn effects on lipids and obesity [28]. Clearly, further studies are needed to investigate the relation of immune-biomarkers and outcome in the obese population.
There are important methodological shortcomings in epidemiological studies that have analysed the association between BMI and mortality, which could explain the “obesity paradox” (fig. 2). First, because all studies were observational, they do not prove causality and are prone to confounding. Smoking and heavy alcohol consumption is more frequent in lean persons and is associated with increased mortality [29, 30]. Cancer and other severe chronic illnesses cause unintentional weight loss and cachexia. Mild obesity may therefore rather be mirror of a good health condition with absence of chronic diseases. This effect is called “reverse causation” because obesity is rather a consequence than a cause of a good health condition with absence of chronic diseases [31]. Although many studies used statistical techniques such as multivariable adjustment to overcome this bias, residual confounding is possible [32]. Stokes and Preston recently published a study in which the obesity paradox disappeared after restricting the reference group to patients who never experienced weight loss. Mortality was even increased in the overweight/obese group after limiting the analysis to never-smokers [33]. Importantly, not only unintentional weight loss is a possible confounder, but also intentional weight loss or diet. Two individuals may have the same BMI, one without a history of dieting and the other with multiple attempts to lose weight. This difference could have an impact on the immune system and consequently on the ability to handle any illness.
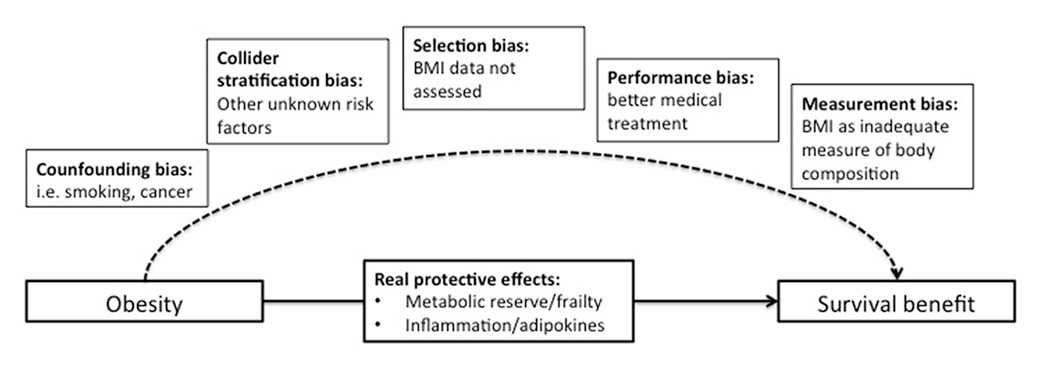
Figure 2
Possible explanations of the obesity paradox.
Another recently discussed methodological issue similar to confounding is the “collider stratification bias” [31, 32, 34–36]. This bias occurs if several medical conditions, which interact with each other, are risk factors for the disease under investigation. For example, obese people have a higher risk of acquiring pneumonia and thus obesity is a pneumonia risk factor [8]. Other risk factors include alcoholism [37] or smoking, which are negatively associated with mortality. Yet when the impact of weight on pneumonia outcomes is investigated, the proportion of patients with pneumonia due to alcoholism or smoking may be less in the obese group because obesity itself is a risk factor. This results in a biased group of patients in regard to their BMI.
Also, selection bias may be an important issue in previous studies. For example, the obesity paradox has been described in multiple studies with stroke patients, conducted in different countries, including first and recurrent strokes, ischaemic and haemorrhagic strokes. The results are consistent and show a protective effect of being overweight and/or obese (when compared with the lean counterparts) on short- and long-term mortality, functional outcomes and even recurrent strokes. These results were proved to be independent of age, cause of death and severity of stroke [6, 38]. However, it should be noted that the majority of these studies are either a subanalysis of clinical trials or a retrospective analysis of observational studies, where a significant proportion of patients was not included in the analysis because of lack of a record of BMI (e.g. 45% [39]). This may represent an important selection bias and it is not possible to determine whether the study represents a rather selected stroke population.
Also, it is possible that obese individuals receive better medical treatment (e.g. statins), which could have a favourable impact on outcomes [8]. This phenomenon is called “performance bias”. Nonetheless, a recent large cohort of patients with cardiovascular disease showed that the paradoxical association between BMI and outcome persisted when only patients with optimal medical therapy were studied, suggesting that the obesity paradox is independent of the intensity of the medical therapy received [40].
Finally, there is also the possibility of a “measurement bias”. The use of BMI for body composition assessment is controversial because it does not differentiate between adipose tissue and lean muscle mass. The results in trials with other body composition measures (waist-to-hip ratio or waist circumference) are not consistent [41].
Current evidence suggests that the optimal BMI for the healthy population is different from the optimal BMI for the population with chronic/wasting diseases and the elderly population. If the obesity paradox is true and a higher BMI has beneficial effects on long-term survival, the concept of optimal BMI may need to be revised and individualised, where factors such as quality of the diet (vs pure calorie restriction), general fitness and metabolic state should be taken in consideration. In the population of chronic heart failure patients, the obesity paradox was only prevalent in the patients with low fitness, while patients with high fitness had a better survival independent of their BMI [42]. Also a differentiation between “unhealthy” and “healthy” metabolic states (defined by parameters of the metabolic syndrome) in the obese population has been suggested [43]. Padwal et al. proposed the Edmonton obesity staging system for better classification of obesity; obesity-related comorbidities and functional status are part of this staging system, which shows a high correlation with mortality [44]. However, this assessment is more complex and time consuming, questioning its applicability in daily routine.
Another unresolved issue is whether age is an effect modifier on the relationship of obesity and mortality, i.e. if obesity is protective in older age, but less so in younger persons. Most studies including patients with chronic medical diseases had a high mean age of participants – and data on younger patients are lacking. Even though most studies adjusted their results for age, the question of effect modification cannot be answered conclusively. One study that stratified patients by age found the obesity paradox only in patients >65 years of age [45].
Because the underlying mechanisms of the obesity paradox remain unclear, further good quality studies are warranted to understand this paradoxical relationship and to define the optimal BMI for populations with chronic and wasting diseases. Whenever possible, the design of observational studies should take into consideration the limitations and biases described above. It is also important to analyse more patient-centred outcomes other than mortality, such as quality of life, infectious complications and functional capacity. Ideally, randomised trials should assess the long-term effect of intentional weight loss of obese patients in these patient populations.
1 Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22.
2 Niedziela J, Hudzik B, Niedziela N, Gasior M, Gierlotka M, Wasilewski J, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol. 2014;29:801–12.
3 Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162:20–6.
4 Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99:999–1005.
5 Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415–25.
6 Kim BJ, Lee S-H, Jung K-H, Yu K-H, Lee B-C, Roh J-K, For Korean Stroke Registry i: Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. 2012;79:856–63.
7 Yamauchi Y, Hasegawa W, Yasunaga H, Sunohara M, Jo T, Takami K, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. 2014;9:1337–46.
8 Nie W, Zhang Y, Jee SH, Jung KJ, Li B, Xiu Q. Obesity survival paradox in pneumonia: a meta-analysis. BMC Med. 2014;12:61.
9 Landi F, Onder G, Gambassi G, Pedone C, Carbonin P, Bernabei R, Grp Italiano FA. Body mass index and mortality among hospitalized patients. Arch Intern Med. 2000;160:2641–4.
10 Prospective Studies C, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96.
11 Sahakyan KR, Somers VK, Rodriguez-Escudero JP, Hodge DO, Carter RE, Sochor O, et al. Normal-Weight Central Obesity: Implications for Total and Cardiovascular Mortality. Ann Intern Med. 2015.
12 Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52.
13 Schuetz P. “Eat your lunch!” – controversies in the nutrition of the acutely, non-critically ill medical inpatient. Swiss Med Wkly. 2015;145:w14132.
14 Schutz P, Bally M, Stanga Z, Keller U. Loss of appetite in acutely ill medical inpatients: physiological response or therapeutic target? Swiss Med Wkly. 2014;144:w13957.
15 Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H, Anker SD, Morley JE. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–42.
16 Aquilani R, La Rovere MT, Febo O, Boschi F, Iadarola P, Corbellini D, et al. Preserved muscle protein metabolism in obese patients with chronic heart failure. Int J Cardiol. 2012;160:102–8.
17 Laet C, Kanis JA, Odén A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int. 2005;16:1330–8.
18 Bouillanne O, Dupont-Belmont C, Hay P, Hamon-Vilcot B, Cynober L, Aussel C. Fat mass protects hospitalized elderly persons against morbidity and mortality. Am J Clin Nutr. 2009;90:505–10.
19 Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7.
20 Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801.
21 Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
22 Alan M, Grolimund E, Kutz A, Christ-Crain M, Thomann R, Falconnier C, et al. Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: a 6-year prospective follow-up study. J Intern Med. 2015;278:174–84.
23 Braun N, Hoess C, Kutz A, Christ-Crain M, Thomann R. CHWZBMaPS: Obesity paradox in patients with community-acquired pneumonia: is inflammation the missing link? submitted.
24 Schuetz P, Mueller B. The role of immune and metabolic biomarkers for improved management of sepsis patients. Expert Rev Clin Immunol. 2014;10:1255–62.
25 Van Zee KJ, Kohno T, Fischer E, Rock CS, Moldawer LL, Lowry SF. Tumor necrosis factor soluble receptors circulate during experimental and clinical inflammation and can protect against excessive tumor necrosis factor alpha in vitro and in vivo. Proc Natl Acad Sci U S A. 1992;89:4845–9.
26 Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–975.
27 Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901.
28 Gruber M, Christ-Crain M, Stolz D, Keller U, Muller C, Bingisser R, et al. Prognostic impact of plasma lipids in patients with lower respiratory tract infections – an observational study. Swiss Med Wkly. 2009;139:166–72.
29 Albanes D, Jones DY, Micozzi MS, Mattson ME. Associations between smoking and body weight in the US population: analysis of NHANES II. Am J Public Health. 1987;77:439–44.
30 Andersen KK, Olsen TS. The obesity paradox in stroke: Lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2013:n/a-n/a.
31 Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med. 2014;62:96–102.
32 Preston SH, Stokes A. Obesity paradox: conditioning on disease enhances biases in estimating the mortality risks of obesity. Epidemiology. 2014;25:454–61.
33 Stokes A, Preston SH. Smoking and reverse causation create an obesity paradox in cardiovascular disease. Obesity (Silver Spring) 2015.
34 Lajous M, Bijon A, Fagherazzi G, Boutron-Ruault MC, Balkau B, Clavel-Chapelon F, Hernan MA. Body mass index, diabetes, and mortality in French women: explaining away a “paradox”. Epidemiology. 2014;25:10–4.
35 Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med. 2014.
36 Banack HR, Kaufman JS. From bad to worse: collider stratification amplifies confounding bias in the “obesity paradox”. Eur J Epidemiol. 2015.
37 Almirall J, Bolibar I, Balanzo X, Gonzalez CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J. 1999;13:349–55.
38 Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J. 2012.
39 Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body Mass Index and Poststroke Mortality. Neuroepidemiology. 2008;30:93–100.
40 Hansel B, Roussel R, Elbez Y, Marre M, Krempf M, Ikeda Y, et al. Cardiovascular risk in relation to body mass index and use of evidence-based preventive medications in patients with or at risk of atherothrombosis. Eur Heart J. 2015.
41 Chrysant SG, Chrysant GS. New insights into the true nature of the obesity paradox and the lower cardiovascular risk. J Am Soc Hypertens. 2013;7:85–94.
42 McAuley PA, Artero EG, Sui X, Lee DC, Church TS, Lavie CJ, et al. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clin Proc. 2012;87:443–51.
43 Lavie CJ, De Schutter A, Milani RV. Healthy obese versus unhealthy lean: the obesity paradox. Nat Rev Endocrinol. 2015;11:55–62.
44 Padwal RS, Pajewski NM, Allison DB, Sharma AM. Using the Edmonton obesity staging system to predict mortality in a population-representative cohort of people with overweight and obesity. CMAJ. 2011;183:E1059–66.
45 Calabia J, Arcos E, Carrero JJ, Comas J, Valles M. Does the obesity survival paradox of dialysis patients differ with age? Blood Purif. 2015;39:193–9.
Disclosure statement:PS is supported in part by the Swiss National Science Foundation (SNSF Professorship, PP00P3_150531 / 1), the Swiss Academy for Medical Sciences (Schweizerische Akademie der Medizinischen Wissenschaften [SAMW]), and the Research Council of the Kantonsspital Aarau (1410.000.044). No other potential conflict of interest relevant to this article was reported.