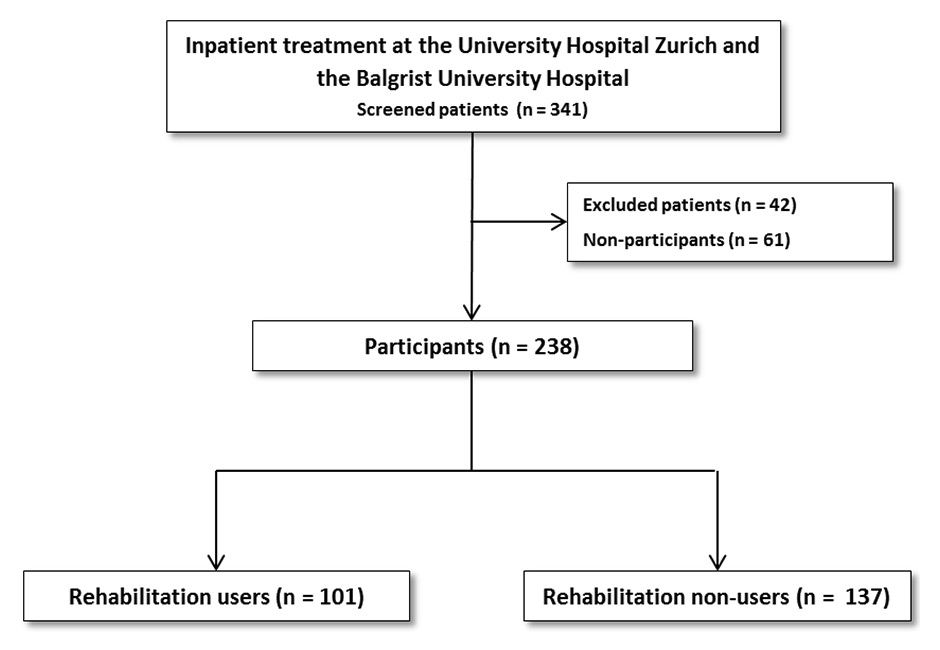
Figure 1
Flowchart of study participants vs nonparticipants, rehabilitation users vs rehabilitation nonusers.
DOI: https://doi.org/10.4414/smw.2015.14214
Cancer is one of the major issues in healthcare. It is the second most frequent cause of death overall and the most frequent cause of death in persons between 45 and 64 years of age in Switzerland [1]. Over 38 000 new cancer cases are diagnosed and about 16 500 patients die in Switzerland each year [2]. The 5-year survival rate after an initial diagnosis is 57% in women and 49% in men [3]. Around 4% of the population in Switzerland live with a past cancer diagnosis [4]. As a result of early diagnosis, prevention, and progress in medical therapies, the number of long-term cancer survivors has increased steadily over time. Nowadays, cancer may be considered a chronic disease rather than a lethal disease [1, 5].
Cancer patients frequently experience physical impairment and psychological distress, which is associated with poor quality of life (QoL) [6–15]. After exhaustive, acute medical treatment, patients return to their former environments without the energy and abilities that they had before. They often experience difficulties in resuming their previous social and work roles. Physical and emotional burdens can impair social functioning, which includes maintenance of friendships and relationships with family members [16]. Rehabilitation aims to reduce disabilities resulting from the disease and its treatment by improving physical, social, psychological and vocational functioning in order to return to a premorbid condition [17, 18]. Rehabilitation also helps patients to deal with possible residual disabilities so as to maintain and regain independence, social integration and participation in everyday life [19]. Available studies suggest that cancer rehabilitation improves QoL, physical health and psychological well-being [20–24].
Cancer rehabilitation programmes vary across countries depending on the public health system and social security legislation [25]. In the Anglo-Saxon countries and Nordic European countries, outpatient rehabilitation is more common. In Switzerland, inpatient rehabilitation is the primary pattern, as is the case in Germany [25–27]. Many studies have focused on the benefits of specific rehabilitation intervention strategies, such as exercise and aerobic programmes designed in outpatient settings [28–31]. Other studies have reported the benefits of inpatient rehabilitation in general [32–36]. However, many of these studies used a pre-post design without control groups in evaluation studies [37], which limits their findings about the effectiveness of rehabilitation.
Rehabilitation for cancer patients is often underused compared with that for patients with other diseases, such as cardiovascular diseases [38–40]. Cheville speculated that the public perception of cancer as an unavoidable progressive and terminal disease might have slowed the development of cancer rehabilitation services in the past [39]. Some studies have shown that many cancer patients were not referred to rehabilitation after hospital discharge [12, 41, 42]. Patients were usually referred to rehabilitation at later stages of the disease, when QoL and physical health was already low. Hewitt suggested that more effective assessment and structured procedures are required [10]. In Germany, the use of cancer rehabilitation in inpatient and outpatient settings has increased in recent years, because it is considered an inherent part of treatment [43].
In Switzerland, cancer rehabilitation usually consists of physiotherapeutic treatment and other specific treatments (e. g. nutrition counselling, lymphatic drainage, psychotherapy). Inpatient rehabilitation usually lasts 2 to 3 weeks and can be prolonged on request. Cancer rehabilitation can also be performed in an outpatient setting. Bachmann et al. found that case management in outpatient rehabilitation can improve QoL of cancer patients after acute therapy in Switzerland [44].
All Swiss residents must purchase basic health insurance, which covers a standardised basic benefit package including several health services. The basic health insurance can be supplemented by semiprivate or private insurance policies that give access to more extensive coverage than basic health insurance, including options such as better levels of accommodation or choice of physicians in hospitals [45].
Referral to rehabilitation requires several decisions, which results in a rather complicated procedure. First, patients might have to express their wish to attend rehabilitation, and physicians have to estimate the potential for rehabilitation for a specific patient. However, this is rarely based on a standardised procedure. Second, even after such a positive decision the rehabilitation must be reimbursed by health insurance companies. Third, hospital social services have to find a rehabilitation clinic with sufficient capacity. The overall procedure presents several challenges. Good linkage of acute care and rehabilitation is required.
Limited data are available for German-speaking countries on differences in sociodemographic or medical characteristics between cancer patients using (users) and not using (nonusers) rehabilitation. Studies from Germany report no differences in age, occupational position or marital status [46–49]. Other studies have reported lower use of rehabilitation in breast cancer patients who are self-employed, are cohabiting with partners [27, 50–53] and have a shorter duration of chemotherapy [53]. Geyer et al. found that breast cancer patients with lower education were more likely to undergo inpatient cancer rehabilitation [47]. This is in contrast with more general findings that patients with a lower socioeconomic status were less likely to undergo rehabilitation [54]. Studies from Germany have shown that breast cancer patients and younger patients with intestinal tumours are more likely to undergo rehabilitation than patients with other cancer diagnoses. In terms of gender, data also suggest that women are more likely to undergo cancer inpatient rehabilitation than men [43].
In Switzerland, 18 rehabilitation clinics offer inpatient cancer rehabilitation. Most clinics are located in the German-speaking part of Switzerland. About 14 000 patients use inpatient rehabilitation, including internal/cancer rehabilitation, per year, with a mean duration of 24 days in the clinic [55–57]. Only about 1% of cancer patients undergo inpatient rehabilitation after hospitalisation [58]. About 90% of them are referred after acute inpatient treatment [58].
To date, no empirical data are available about the sociodemographic and medical characteristics of patients undergoing inpatient cancer rehabilitation in Switzerland. More data would help to inform policy makers and practitioners about the use of rehabilitation among cancer patients. Therefore, the purposes of this study were (1) to describe differences in sociodemographic and medical characteristics between patients who undergo inpatient cancer rehabilitation and patients who do not; and (2) to analyse data stratified for women and men, since clinical characteristics differ between these populations.
Patients aged at least 18 years with acute inpatient treatment for cancer or for benign or malignant brain tumours were recruited at the University Hospital Zurich and the Balgrist University Hospital (Switzerland) between April 2013 and November 2014. Both the University Hospital Zurich and the Balgrist University Hospital exclusively provide acute health care and no rehabilitation for cancer patients. Some 18.3% of all cancer cases in the area of Zurich are treated at these hospitals [59]. We only included patients from these two hospitals to ensure a similar referral organisation procedure throughout the entire sample. As some tumour sites are associated with cognitive impairment, which might be more severe in older subjects, we screened patients aged ≥50 years for cognitive impairment (Mini Mental State Examination [MMSE], cut-off of at least 25 points) to ensure their cognitive ability to complete the questionnaires [60]. The cut-off indicates a high chance for mild to severe cognitive impairment. Furthermore, sufficient German knowledge was required. These patients were referred to six rehabilitation clinics in several regions of Switzerland. Rehabilitation programmes are adapted to the abilities and needs of the patient, in compliance with the International Classification of Functioning, Disability and Health (ICF) [61]. The study was approved by the Cantonal Ethics Committee of Zurich (KEK-ZH Nr. 2012-0563).
Sociodemographic and medical data were obtained from the patients’ clinical records. Additional sociodemographic information regarding employment status, highest education attained, marital/cohabitating status, children in household, income and treatments after hospitalisation was obtained during hospitalisation with a standardised questionnaire, which was developed especially for this study. Completing the questionnaire took about 5 minutes. Referral allocation to rehabilitation was decided by the physician responsible for acute management in agreement with the patient. Patients’ health insurance companies decided whether inpatient rehabilitation was reimbursed or not.
Tumour stage was classified according to the Tumour-Node-Metastasis (TNM) Classification of Malignant Tumours [62]. Thus, brain tumours and haematological malignancies were not included in the analysis of tumour stage. The cancer sites were categorised according to the World Health Organization (WHO) / International Agency for Research on Cancer (IARC) Classification of Tumours, fourth edition [63].
First, participants and nonparticipants were compared. Second, the participating patients were divided into users and nonusers. We compared sociodemographic and medical characteristics. Since cancer sites differed substantially between male and female patients, gender was included in further analyses. Descriptive and medical data for women and men with regard to use of inpatient rehabilitation are provided in table 3 in numbers and percentages.
All statistical analyses were performed using the Statistical Package for the Social Sciences version 21.0. As a result of non-normal distribution, a Mann-Whitney-U test and a Pearson chi-square test or a Fisher’s exact test as univariate analysis were conducted to compare nonparticipants and participants in order to determine biases in age, sex, nationality, employment status, insurance type, cancer stage (excluding brain tumours and haematological malignancies), cancer type, cancer site, treatment and duration of hospitalisation. The sociodemographic and medical data of the nonparticipants are noted in appendix 1. The same statistical analysis procedures were conducted to compare differences between inpatient rehabilitation users and nonusers and for gender. All tests were two-tailed, and the level of statistical significance was set at 95% confidence level (p <0.05). We included age, sex, marital/cohabitating status, children in household, insurance type, employment status, cancer stage (excluding brain tumours and haematological malignancies), cancer type, cancer site, treatment and duration of hospitalisation in the evaluation.
| Table 1: Sociodemographic characteristics. | |||||||
| Sociodemographic characteristics | Total | Rehabilitation users(n = 101) | Rehabilitation nonusers (n = 137) | p-value | |||
| n | % | n | % | n | % | ||
| Age in years (median, IQR, range) | 61.0 (±17.0, 20–88) | 62.0 (±14.0, 20–84) | 59.0 (±20.0, 20–88) | 0.227 * | |||
| Age <61 ≥61 | 124 114 | 52.1 47.9 | 49 52 | 48.5 51.5 | 75 62 | 54.7 45.3 | 0.361 ¶ |
| Sex Male Female | 123 115 | 51.7 48.3 | 58 43 | 57.4 42.6 | 65 72 | 47.4 52.6 | 0.149 ¶ |
| Marital/cohabitating status Marital/Cohabiting Living alone | 175 63 | 73.5 26.5 | 72 29 | 71.3 28.7 | 103 34 | 75.2 24.8 | 0.553 ¶ |
| Children ≤18 years living at home No children Children | 37 201 | 15.5 84.5 | 13 88 | 12.9 87.1 | 24 113 | 17.5 82.5 | 0.369 ¶ |
| Nationality Swiss Other | 210 28 | 88.2 11.8 | 92 9 | 91.1 8.9 | 118 19 | 86.1 13.9 | 0.310 ¶ |
| Level of education †,§ Obligatory school Apprenticeship High school University | 24 117 34 62 | 10.1 49.4 14.3 26.2 | 10 50 18 23 | 9.9 49.5 17.8 22.8 | 14 67 16 39 | 10.3 49.3 11.8 28.7 | 0.770 ‡ |
| Employment status Employed Unemployed, IV compensation Retired | 116 27 95 | 48.7 11.3 39.9 | 40 16 45 | 39.6 15.8 44.6 | 76 11 50 | 55.5 8.0 36.5 | 0.029 ‡ |
| n = 238 IQR = interquartile range; IV = invalidity benefit * Mann-Whitney U-test ¶ Fisher’s exact test † missing n = 1 § compulsory school (aged ≥6 years); apprenticeship (aged ≥15 years); high school (aged ≥15 years); university (aged ≥19 years) ‡ Pearson chi-square test | |||||||
A total of 341 patients were assessed for eligibility using a consecutive sampling approach between April 2013 and November 2014 (fig. 1 shows the patient flow). Forty-two patients were excluded from completing the questionnaires because of cognitive or physical impairment (n = 19, 45.2% of the excluded patients) or limited knowledge of German (n = 23, 55.8% of the excluded patients). Of the 299 eligible patients, 61 (20.4%) refused to participate. Finally, 238 (79.6% of the total assessed) patients were included in the study. No significant differences were found between participants and nonparticipants in age, nationality, health insurance type, tumour stage, cancer type or cancer site. Participants did not differ in treatment and use of inpatient rehabilitation from nonparticipants. However, study participants stayed in hospital longer than nonparticipants (p = 0.014) (appendix 1).

Figure 1
Flowchart of study participants vs nonparticipants, rehabilitation users vs rehabilitation nonusers.
Of the 238 cancer patients, 101 (42.4%) patients used inpatient rehabilitation and 137 (57.6%) did not. Descriptive information on users and nonusers is presented in table 1. For the total sample of all patients,the median age was 61 years, with 52.1% (n = 124) aged below 61 years. The sample consisted of 51.7% (n = 123) male patients. Three quarters of the patients were married or cohabiting with a partner (73.5%, n = 175), 15.5% (n = 37) had minors in their household, and 48.7% (n = 116) were employed. Half of the patients (n = 117) had completed apprenticeships, 10.1% (n = 24) basic school level, 14.3% (n = 34) high school, and 22.2% (n = 62) university. No significant differences in sociodemographic characteristics were found between rehabilitation users and rehabilitation nonusers excepting employment status (p = 0.029): rehabilitation users were less likely to be employed than rehabilitation nonusers.
As table 2 shows, rehabilitation users stayed longer in hospital (M = 20.7; SD = 10.5) than rehabilitation non-users (M = 13.9; SD = 10.4) (p <0.001). The majority of patients had basic insurance (71.8%, n = 171). Patients differed in insurance type, since the percentage of patients with semiprivate or private supplementary health insurance was higher for rehabilitation users (p = 0.030). All tumour stages were represented: 17.0% (n = 33) in stage I, 24.7% (n = 48) in II, 27.3% (n = 53) in III and 30.9% (n = 60) IV. The majority of patients underwent surgery (85.7%, n = 204) and suffered from carcinoma (63.9%, n = 152). There were no differences between rehabilitation users and rehabilitation nonusers regarding tumour stage, cancer type or type of treatment. The most frequent diagnoses were tumours of the head and neck (23.9%, n = 57), of the digestive organs (14.7%, n = 35) and haematological malignancies (12.6%, n = 30). Rehabilitation users and rehabilitation nonusers differed in their cancer site (p = 0.001). Patients with tumours of the digestive organs (p = 0.003) or of the thoracic organs (p = 0.013) used rehabilitation more often, whereas breast cancer patients were less likely to use rehabilitation (p = 0.013).
Women who underwent rehabilitation were longer in acute treatment (M = 20.4; SD = 12) than those who did not use rehabilitation (M = 12.3; SD = 10.3; p <0.001) (table 3). Similar results were found for men (rehabilitation users M = 21.0; SD = 9.1; rehabilitation nonusers M = 15.5; SD = 10.2; p <0.001). Neither female nor male patients differed by age in the use of rehabilitation. Men who underwent rehabilitation differed in the distribution of level of education (p = 0.039) from those who did not. Employed men were less likely to undergo rehabilitation (p = 0.051). Male patients with semiprivate or private supplementary health insurance used rehabilitation more often than men with basic health insurance (p = 0.037). Women with an advanced tumour stage used inpatient rehabilitation more often (p = 0.012). Women with tumours of the female genital organs or with breast cancer were less likely to use rehabilitation (p = 0.023). Differences in cancer site were found in both sexes (women p = 0.023; men p = 0.046). Men with tumours of the digestive or of the thoracic organs were more likely to undergo inpatient rehabilitation, while men with haematological malignancies were less likely to do so (trend).
| Table 2:Medical characteristics. | ||||||||
| Medical characteristics | Total | Rehabilitation users(n = 101) | Rehabilitation nonusers (n = 137) | p-value | ||||
| n | % | n | % | n | % | |||
| Duration of hospitalisation (median, IQR, range) | 15.0 (±11.0, 2–81) | 19.0 (±8.0, 7–81) | 12.0 (±10.0, 2–78) | <0.001* | ||||
| Insurance type Basic Semiprivate, private supplementary | 171 67 | 71.8 28.2 | 65 36 | 64.4 35.6 | 106 31 | 77.4 22.6 | 0.030 ¶ | |
| Tumour stage † I II III IV | 33 48 53 60 | 17.0 24.7 27.3 30.9 | 10 22 23 30 | 11.8 25.9 27.1 35.3 | 23 26 30 30 | 21.1 23.9 27.5 27.5 | 0.326 § | |
| Cancer site Head and neck Digestive organs Female genital organs Breast Haematological malignancies Thoracic organs Sarcoma extremities Brain Other ‡ | 57 35 25 23 30 22 21 14 11 | 23.9 14.7 10.5 9.7 12.6 9.2 8.8 5.9 4.6 | 26 23 8 4 12 15 5 4 4 | 25.7 22.8 7.9 4.0 11.9 14.9 5.0 4.0 4.0 | 31 12 17 19 18 7 16 10 7 | 22.6 8.8 12.4 13.9 13.1 5.1 11.7 7.3 5.1 | 0.001 § | |
| Cancer type Carcinoma Sarcoma Brain tumour Lymphoma Multiple myeloma Other ** | 152 32 14 16 14 10 | 63.9 13.4 5.9 6.7 5.9 4.2 | 67 13 4 6 6 5 | 66.3 12.9 4.0 5.9 5.9 5.0 | 85 19 10 10 8 5 | 62.0 13.9 7.3 7.3 5.8 3.6 | 0.888 § | |
| Type of treatment Surgery Stem cell transplantation Other ¶¶ | 204 20 14 | 85.7 8.4 5.9 | 88 7 6 | 87.1 6.9 5.9 | 116 13 8 | 84.7 9.5 5.8 | 0.781 § | |
| n = 238. IQR = interquartile range * Mann-Whitney U-test ¶ Fisher’s exact test † n = 194, brain tumour and haematological malignancies excluded (missing n = 44) § Pearson chi-square test ‡ melanoma, endocrine tumour, urinary tract ** mesothelioma, melanoma, endocrine tumour ¶¶ radiotherapy, chemotherapy, photo-dynamic therapy, other inpatient care | ||||||||
| Table 3: Characteristics for women and men with regard to use of inpatient rehabilitation. | |||||||
| Characteristics | Total | Rehabilitation users | Rehabilitation nonusers | p-value | |||
| n | % | n | % | n | % | ||
| Women | |||||||
| Duration of hospitalisation (median, IQR, range) | 13.0 (±11.0, 2–81) | 19.0 (±9.0, 7–81) | 11.0 (±9.0, 2–78) | <0.001 * | |||
| Age <61 ≥61 | 65 50 | 56.5 43.5 | 23 20 | 53.5 46.5 | 42 30 | 58.3 41.7 | 0.698 ¶ |
| Marital/cohabitating status Married/cohabiting Living alone | 73 42 | 63.5 36.5 | 24 19 | 55.8 44.2 | 49 23 | 68.1 31.9 | 0.231 ¶ |
| Level of education † Obligatory school Apprenticeship High school University | 16 57 16 25 | 14.0 50.0 14.0 21.9 | 9 19 6 9 | 20.9 44.2 14.0 20.9 | 7 38 10 16 | 9.9 53.5 14.1 22.5 | 0.417 § |
| Employment status Employed Unemployed, IV compensation Retired | 53 19 43 | 46.1 16.5 37.4 | 17 11 15 | 39.5 25.6 34.9 | 36 8 28 | 50.0 11.1 38.9 | 0.124 § |
| Insurance type Basic Semiprivate, private supplementary | 79 36 | 68.7 31.3 | 27 16 | 62.8 37.2 | 52 20 | 72.2 27.8 | 0.306 ¶ |
| Tumour stage ‡ I II III IV | 18 20 28 29 | 18.9 21.1 29.5 30.5 | 1 9 10 15 | 2.9 25.7 28.6 42.9 | 17 11 18 14 | 28.3 18.3 30.0 23.3 | 0.012 § |
| Cancer site Head and neck Digestive organs Female genital organs Breast Haematological malignancies Thoracic organs Sarcoma extremities Brain Other ** | 16 13 25 23 9 6 10 11 2 | 13.9 11.3 21.7 20.0 7.8 5.2 8.7 9.6 1.7 | 6 8 8 4 4 5 2 4 2 | 14.0 18.6 18.6 9.3 9.3 11.6 4.7 9.3 4.7 | 10 5 17 19 5 1 8 7 0 | 13.9 6.9 23.6 26.4 6.9 1.4 11.1 9.7 0 | 0.023 § |
| Men | |||||||
| Duration (median/IQR) of hospitalisation | 17.0 (±11.0, 2 – 65) | 18.5 (±7.0, 10 – 65) | 14.0 (±12.0, 2 – 53) | <0.001 * | |||
| Age <61 ≥61 | 59 64 | 48.0 52.0 | 26 32 | 44.8 55.2 | 33 32 | 50.8 49.2 | 0.589 ¶ |
| Marital/cohabitating status Married/cohabiting Living alone | 102 21 | 82.9 17.1 | 48 10 | 82.8 17.2 | 54 11 | 83.1 16.9 | 1.000 ¶ |
| Level of education Obligatory school Apprenticeship High school University | 8 60 18 37 | 6.5 48.8 14.6 30.1 | 1 31 12 14 | 1.7 53.4 20.7 24.1 | 7 29 6 23 | 10.8 44.6 9.2 35.4 | 0.039 § |
| Employment status Employed Unemployed, IV compensation Retired | 63 8 52 | 51.2 6.5 42.3 | 23 5 30 | 39.7 8.6 51.7 | 40 3 22 | 61.5 4.6 33.8 | 0.051 § |
| Insurance type Basic Semiprivate, private supplementary | 92 31 | 74.8 25.2 | 38 20 | 65.5 34.5 | 54 11 | 83.1 16.9 | 0.037 ¶ |
| Tumour stage ¶ I II III IV | 15 28 25 31 | 15.2 28.3 25.3 31.3 | 9 13 13 15 | 18.0 26.0 26.0 30.0 | 6 15 12 16 | 12.2 30.6 24.5 32.7 | 0.848 § |
| Cancer site Head and neck Digestive organs Haematological malignancies Thoracic organs Sarcoma extremities Brain Other †† | 41 22 21 16 11 3 9 | 33.3 17.9 17.1 13.0 8.9 2.4 7.3 | 20 15 8 10 3 0 2 | 34.5 25.9 13.8 17.2 5.2 0 3.4 | 21 7 13 6 8 3 7 | 32.3 10.8 20.0 9.2 12.3 4.6 10.8 | 0.046 § |
| Women n = 115; men n = 123 IQR =interquartile range; IV = invalidity benefit * Mann-Whitney U-test. ¶ Fisher’s exact test † missing n = 1 § Pearson chi-square test ‡ n = 95, brain tumour and haematological malignancies excluded (missing n = 20) ** Melanoma, endocrine tumour. ¶¶ n = 99, brain tumour and haematological malignancies excluded (missing n = 24) †† Melanoma, endocrine tumour, urinary tract | |||||||
To our best knowledge, this is the first study that has analysed differences in sociodemographic and medical characteristics between users and nonusers of inpatient cancer rehabilitation in Switzerland. We found that rehabilitation users and nonusers differed in medical and in sociodemographic characteristics. Users stayed longer in hospital, and inpatient rehabilitation was more likely to be used by patients with tumours of the digestive organs or of the thoracic organs and less likely by breast cancer patients. Interestingly, inpatient rehabilitation users were more likely to have semiprivate or private supplementary health insurance and were less likely to be employed than nonusers. This is an important finding that needs further empirical investigation, since it indicates that social factors might facilitate rehabilitation use or be obstacles for rehabilitation use.
Our study is in line with reports in Switzerland that patients with semiprivate or private supplementary health insurance are more likely to use inpatient rehabilitation [57, 64, 65]. This finding might be explained by the higher chance of such patients obtaining the rehabilitation more promptly. This finding does not coincide with better education of users, since education did not predict rehabilitation use in the current study. Indeed, the opposite was the case: Similarly to Geyer et al., we found that rehabilitation users had a lower education than nonusers, especially among men [47]. This finding might be explained by the higher motivation of more educated patients to return to work [66–68]. According to Lehmann et al., our study reveals that employed patients, especially men, were less likely to undergo cancer inpatient rehabilitation [48]. Returning to work, and therefore to normal, as soon as possible might be a strategy for coping with cancer among employed cancer patients [69, 70]. Mehnert has shown that men with cancer in particular often return to work and show a shorter period of occupational disability [71]. Women might tend to make their decision in relation to family or children [72], a possibility which unfortunately was not assessed in our study. Another possible explanation is that nonusers were less impaired than users before rehabilitation and consequently more often in employment.
Our results suggest that women with more advanced cancer more often use rehabilitation. One explanation might be that women with less impairment prefer to stay at home with their relatives to reassume their social role as soon as possible [72]. However, they might recognise the need for rehabilitation later. In our study, women with breast or female genital organs cancers did not often use inpatient rehabilitation, which is in contrast to findings from Germany [26, 47, 53]. In Germany, cancer patients might use rehabilitation even after acute outpatient treatment (e. g. chemotherapy, radiotherapy) with less severe impairment [58]. In our study, women with breast or female genital organ cancers might not meet criteria for referral as these conditions might lead to less impairment than other cancers. This explanation appears quite plausible, since women with breast cancer stayed less time in hospital than patients with other cancer diagnoses.
Our study has some limitations that should be considered in the interpretation of our findings. The broad inclusion criteria increase the external validity of our results, but such an approach limits the interpretation for specific diagnoses and patient groups. We were only able to run stratified analyses for gender, but other variables such as education or age could provide meaningful results in a larger sample. In addition, the value of our dataset could have been increased by inclusion of measures of psychopathology and quality of life, as well as stress tests such as the 6-minute walk test or the sit-to-stand test. Unfortunately, limited funding did not allow for a more comprehensive data collection in this study.
A strength of our study is the design, which facilitated the collection of data on nonusers of rehabilitation. The balanced number of users and nonusers made the comparison of both groups feasible. Another strength of our study was the data assessment within different healthcare settings. Acute care and rehabilitation should be well connected, but such studies are rarely done, since the transfer of patients to another system increases problems in data collection. Studies often focus either on acute care or rehabilitation, so this study fills an important gap.
This study provides a basis for further research on this issue. Studies focused on specific diagnoses or with a larger sample are required.
This is the first study that examined the use of inpatient rehabilitation among cancer patients in Switzerland. Our study revealed that referral to inpatient rehabilitation is highly associated with more severe illness, but also with sociodemographic factors such as health insurance and employment, particularly in men. However, the latter indicates that decisions for referrals to inpatient rehabilitation may not be regularly based on medical factors, so cancer patients with regular work and no semiprivate or private supplementary health insurance might underuse rehabilitation. Additionally, our findings provide evidence that gender might influence the use of inpatient cancer rehabilitation. Consequently, more specific and standardised tools assessing medical and psychosocial factors are needed to better and more reliably identify patients who need inpatient cancer rehabilitation.
| Table: Sociodemographic and medical characteristics | |||||||
| Characteristics | Total | Participants (n = 238) | Nonparticipants (n =61) | p-value | |||
| n | % | n | % | n | % | ||
| Age in years (median, IQR, range) | 61.0 (±17.0, 19–88) | 61.0 (±17.0, 20–88) | 63.0 (±22.0, 19–86) | 0.266 * | |||
| Duration of hospitalisation (median, IQR, range) | 15.0 (±11.0, 2–81) | 15.0 (±11.0, 2–81) | 12.0 (±10.0, 3–45) | 0.014 * | |||
| Rehabilitation Users Nonusers | 121 178 | 40.5 59.5 | 101 137 | 42.4 57.6 | 20 41 | 32.8 67.2 | 0.190 ¶ |
| Sex Male Female | 146 153 | 48.8 51.2 | 123 115 | 51.7 48.3 | 23 38 | 37.7 62.3 | 0.062 ¶ |
| Age <61 years ≥61 years | 150 149 | 50.2 49.8 | 124 114 | 52.1 47.9 | 26 35 | 42.6 57.4 | 0.199 ¶ |
| Nationality Swiss Other | 264 35 | 88.3 11.7 | 210 28 | 88.2 11.8 | 54 7 | 88.5 11.5 | 1.000 ¶ |
| Employment status † Employed Unemployed, IV compensation Retired | 137 38 122 | 46.1 12.8 41.1 | 116 27 95 | 48.7 11.3 39.9 | 21 11 27 | 35.6 18.6 45.8 | 0.127 § |
| Insurance type Basic Semiprivate, private | 218 81 | 72.9 27.1 | 171 67 | 71.8 28.2 | 47 14 | 77.0 23.0 | 0.519 ¶ |
| Tumour stage ‡ I II III IV | 47 54 67 73 | 19.5 22.4 27.8 30.3 | 33 48 53 61 | 16.9 24.6 27.2 31.3 | 14 6 14 12 | 30.4 14.6 30.4 29.3 | 0.103 § |
| Cancer Site Head and Neck Digestive organs Female genital organs Breast Haematological malignancies Thoracic organs Sarcoma extremities Brain Other ** | 69 43 35 29 36 30 23 23 11 | 23.1 14.4 11.7 9.7 12.0 10.0 7.7 7.7 3.7 | 57 35 25 23 30 22 21 14 11 | 23.9 14.7 10.5 9.7 12.6 9.2 8.8 5.9 4.6 | 12 8 10 6 6 8 2 9 0 | 19.7 13.1 16.4 9.8 9.8 13.1 3.3 14.8 0 | 0.124 § |
| Cancer type Carcinoma Sarcoma Brain tumour Lymphoma Multiple myeloma Other ¶¶ | 197 34 22 17 18 11 | 65.9 11.4 7.4 5.7 6.0 3.7 | 152 32 14 16 14 10 | 63.9 13.4 5.9 6.7 5.9 4.2 | 45 2 8 1 4 1 | 73.8 3.3 13.1 1.6 6.6 1.6 | 0.039 § |
| Type of treatment Surgery Stem cell transplantation Other †† | 258 27 14 | 86.3 9.0 4.7 | 204 20 14 | 85.7 8.4 5.9 | 54 7 0 | 88.5 11.5 0 | 0.126 § |
| n = 299. IQR = interquartile range; IV = invalidity benefit * Mann-Whitney-U test ¶ Fisher’s exact test † missing n = 2 § Pearson chi-square test. ‡ n = 240, brain tumour and haematological malignancies excluded (missing n =59) ** melanoma, endocrine tumour, urinary tract ¶¶ mesothelioma, melanoma, endocrine tumour †† radiotherapy, chemotherapy, photodynamic therapy, other inpatient care | |||||||
Acknowledgement: This study was supported by the Zurzach Rehabilitation Foundation SPA, Bad Zurzach Switzerland and the University of Zurich. We thank all patients in this study for completing the sets of questionnaires. We are grateful to the participating hospitals departments of the University Hospital Zurich (Department of Cranio-Maxillo-facial and Oral Surgery, Department of Gynaecology, Department of Oncology, Department of Otorhinolaryngology and Head and Neck Surgery, Department of Radiation of Oncology, Department of Thoracic Surgery, Department of Visceral and Transplant Surgery), the Balgrist Hospital Zurich, the Oncology Centre Hirslanden Zurich, the Susenberg Clinic, the Zürcher Höhenklinik Davos, and the RehaClinic Bad Zurzach.
1 Oncosuisse. Nationales Krebsprogramm für die Schweiz 2011–2015. Bern: Rub Graf-Lehmann AG; 2011.
2 NICER. Statistics 2014 [Internet]. [cited 2015 Mar 2]. Available from: http://www.nicer.org/NicerReportFiles2015/EN/report/atlas.html?&geog=0
3 EUROCARE-5. Survival Analysis 2000-2007 [Internet]. [cited 2015 Mar 2]. Available from: https://w3.iss.it/site/eu5results/forms/SA0007.aspx
4 Lorez M, Heusser R, Arndt V. Prevalence of cancer survivors in Switzerland. Schweizer Krebsbulletin. 2014;4:285–9.
5 McCorkle R, Ercolano E, Lazenby M, Schulman-green D. Self-Management : Enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin. 2011;50–62.
6 Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol. 2003;13(3):248–66.
7 Dalton SO, Laursen TM, Ross L, Mortensen PB, Johansen C. Risk for hospitalization with depression after a cancer diagnosis: A nationwide, population-based study of cancer patients in Denmark from 1973 to 2003. J Clin Oncol. 2009;27(9):1440–5.
8 Fialka-Moser V, Crevenna R, Korpan M, Quittan M. Cancer rehabilitation. Particularly with aspects on physical impairments. J Rehabil Med. 2003;35(4):153–62.
9 Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–92.
10 Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington DC: National Academies Press; 2006.
11 Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: age, health, and disability. J Gerontol A Biol Sci Med Sci. 2003;58(1):82–91.
12 Lehmann JF, DeLisa JA, Warren CG, DeLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development, and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410–9.
13 Poppelreuter M, Weis J, Kulz AK, Tucha O, Lange KW, Bartsch HH. Cognitive dysfunction and subjective complaints of cancer patients: a cross-sectional study in a cancer rehabilitation centre. Eur J Cancer. 2004;40(1):43–9.
14 Adler NE, Institute of Medicin. Cancer care for the whole patient: meeting psychosocial health needs. Washington DC: National Academies Press; 2008.
15 Van Harten WH, van Noort O, Warmerdam R, Hendricks H, Seidel E. Assessment of rehabilitation needs in cancer patients. Int J Rehabil Res. 1998;21(3):247–57.
16 Goodman A. Organic unity theory: the mind-body problem revisited. Am J Psychiatry. 1991;148(5):553–63.
17 Cromes GF. Implementation of interdisciplinary rehabilitation. Rehab Couns Bull. 1978;21:230–7.
18 Cheville AL. Cancer rehabilitation. Semin Oncol. 2005;32(2):219–24.
19 World Health Organization. Disability prevention and rehabilitation: report of the WHO expert committee on disability prevention and rehabilitation. Technical Report Series 668. Geneva: WHO; 1981.
20 Edwards AG, Hulbert-Williams N, Neal RD. Psychological interventions for women with metastatic breast cancer. Cochrane Database Syst Rev. 2008;(3):CD004253.
21 Mewes JC, Steuten LMG, Ijzerman MJ, van Harten WH. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist. 2012;17(12):1581–93.
22 Riesenberg H, Lübbe AS. In-patient rehabilitation of lung cancer patients – a prospective study. Support Care Cancer. 2010;18(7):877–82.
23 Teichmann J V. Oncological rehabilitation: evaluation of the efficiency of inpatient rehabilitation. Rehabil. 2002;41(1):53–63.
24 Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100.
25 Weis J, Giesler JM. Rehabilitation for cancer patients. Recent Results Cancer Res. 2014;197:87–101.
26 Bartsch HH, Zeiss T. Rehabilitation of patients with breast cancer. Rehabil. 2014;53(4):268–78.
27 Koch U. Current developments in rehabilitation. Rehabil. 2000;39(6):315–6.
28 Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660–8.
29 Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–21.
30 De Backer IC, van Breda E, Vreugdenhil A, Nijziel MR, Kester AD, Schep G. High-intensity strength training improves quality of life in cancer survivors. Acta Oncol. 2007;46(8):1143–51.
31 Dimeo F, Schwartz S, Wesel N, Voigt a., Thiel E. Effects of an endurance and resistance exercise program on persistent cancer-related fatigue after treatment. Ann Oncol. 2008;19(8):1495–9.
32 Cole RP, Scialla SJ, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623–7.
33 Guo Y, Shin KY, Hainley S, Bruera E, Palmer JL. Inpatient rehabilitation improved functional status in asthenic patients with solid and hematologic malignancies. Am J Phys Med Rehabil. 2011;90(4):265–71.
34 Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 Suppl 1):S63–8.
35 Huang ME, Sliwa JA. Inpatient rehabilitation of patients with cancer: efficacy and treatment considerations. PM R. 2011;3(8):746–57.
36 Spruit M a, Janssen PP, Willemsen SCP, Hochstenbag MMH, Wouters EFM. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. 2006;52(2):257–60.
37 Haaf H-G. Findings on the Effectiveness of Rehabilitation. Rehabil. 2005;44(5):e1–20.
38 Paul K, Buschbacher R. Cancer rehabilitation: increasing awareness and removing barriers. Am J Phys Med Rehabil. 2011;90(5 Suppl 1):S1–4.
39 Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med RehabilJ Phys Med Rehabil. 2011;90(5 Suppl 1):S27–37.
40 Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: A retrospective chart review. J Appl Gerontol. 2013;32(4):443–56.
41 Alfano CM, Ganz P a., Rowland JH, Hahn EE. Cancer survivorship and cancer rehabilitation: Revitalizing the link. J Clin Oncol. 2012;30(9):904–6.
42 Movsas SB, Chang VT, Tunkel RS, Shah V V, Ryan LS, Millis SR. Rehabilitation needs of an inpatient medical oncology unit. Arch Phys Med Rehabil. 2003;84(11):1642–6.
43 Deutsche Rentenversicherung. Reha-Bericht 2013 [Internet]. [cited 2015 Mar 24]. Available from: http://www.deutsche-rentenversicherung.de/Allgemein/de/Inhalt/6_Wir_ueber_uns/03_fakten_und_zahlen/04_reha_jahresberichte/downloads_reha_jahresberichte/rehabericht_2013.html
44 Bachmann-Mettler I, Rosemann T. Ambulante Onkologische Rehabilitation Case Management in onkologischer Rehabilitation [Internet]. [cited 2015 Apr 2]. Available from: https://assets.krebsliga.ch/downloads/schlussbericht_camon_kls_11112013.pdf
45 Busato A, Widmer M, Matter P. Variation in incidence of orthopaedic surgery between populations with basic or basic plus supplementary health insurance in Switzerland. Swiss Med Wkly. 2011;141:1–7.
46 Härter M, Reuter K, Aschenbrenner A, Schretzmann B, Marschner N, Hasenburg A, Weis J. Psychiatric disorders and associated factors in cancer: results of an interview study with patients in inpatient , rehabilitation and outpatient treatment. Eur J Cancer. 2001;37(11):1385–93.
47 Geyer S, Schlamstedt-Jahn U. Are there social inequalities in the utilisation of oncological rehabilitation by breast cancer patients. Gesundheitswesen. 2012;74(2):71–8.
48 Lehmann C, Beierlein V, Hagen-Aukamp C, Kerschgens C, Rhee M, Frühauf S, Otto J, Graefen M, Krüll A, Berger D, Koch U, Bergelt C. Psychosocial predictors of utilization of medical rehabilitation services among prostate cancer patients. Rehabil. 2012;51(3):160–70.
49 Weis J, Moser MT BH. Goal-orientated evaluation of inpatient rehabilitation programs for women with breast cancer (ZESOR-study). In: Jäckel WH, Bengel J, Herdt J E, editor. Research in rehabilitation: Results from a research network in southwest Germany. Stuttgart: Schattauer; 2006.
50 Deutsche Rentenversicherung Bund. Rehabilitation 2009. Statistik der Deutschen Rentenversicherung. Band 179. Berlin: Deutsche Rentenversicherung Bund; 2010.
51 Husmann G, Kaatsch P, Katalinic A. Krebs in Deutschland. 2005/2006. Häufigkeiten und Trends. Berlin: Robert-Koch-Institut; 2010.
52 Waldmann a, Lautz E, Hampe J, Schreiber S, Schafmayer C, Katalinic A. Utilization of inpatient rehabilitation of younger patients with colorectal neoplasms – results of the project “Popgen-colorectal cancer.” Rehabil. 2007;46(6):349–55.
53 Weis J, Moser MT, Bartsch HH. Zielorientierte Evaluation stationärer onkologischer Rehabilitationsmaßnahmen ZESOR -Studie [Internet]. [cited 2015 Mar 24]. Available from: http://forschung.deutsche-rentenversicherung.de/ForschPortalWeb/rehaDoc.pdf?rehaid=5f5eb3b5f78bf136c1256e9b002f82b6
54 Bürger W, Morfeld M. Are there class-specific disadvantages in utilization of medical rehabilitation? Rehabilitation. 1999;38(Suppl 2):S134–41.
55 ANQ-Vorstand. Nationaler Messplan Rehabilitation: Umsetzungskonzept [Internet]. [cited 2015 Mar 26]. Available from: http://www.anq.ch/fileadmin/redaktion/deutsch/20121024_Reha_Umsetzungskonzeption_20_Kurzversion_D_OF.pdf
56 Bundesamt für Statistik. Medienmitteilung [Internet]. 2012 [cited 2015 Mar 19]. Available from: http://www.bfs.admin.ch/bfs/portal/de/index.html
57 Klinik Susenberg. Zahlen [Internet]. [cited 2015 Mar 19]. Available from: http://www.susenbergklinik.ch/ueber-uns/zahlen/
58 Gesundheitsdepartement Kanton St. Gallen. Spitalplanung Rehabilitation 2014. Versorgungsbericht [Internet]. [cited 2015 Mar 19]. Available from: http://www.sg.ch/home/gesundheit/gesundheitsversorgung/spitalliste/_jcr_content/Par/downloadlist/DownloadListPar/download.ocFile/Versorgungsbericht_Rehabilitation.pdf
59 Krebsregister der Kantone Zürich und Zug. Jahresbericht 2012 [Internet]. [cited 2015 Aug 3]. Available from: http://www.krebsregister.usz.ch/fachwissen/Documents/Krebsregister_Jahresbericht_2012.pdf
60 Kessler, J., Markowitsch, H. J., Denzler P. Mini-Mental-Status-Test (MMST). Göttingen: Beltz Test GmbH; 2000.
61 International Classification of Functioning, Disability and Health (ICF) [Internet]. [cited 2015 Aug 3]. Available from: http://www.who.int/classifications/icf/en/
62 Sobin LH, Gospodarowicz M, Wittekind C. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009.
63 WHO/IARC Classification of Tumours – Fourth Edition [Internet]. [cited 2015 Mar 24]. Available from: http://www.iarc.fr/en/publications/list/bb/
64 Klinik Schloss Mammern. Zahlen & Fakten [Internet]. [cited 2015 Mar 22]. Available from: http://www.klinik-schloss-mammern.ch/de/zahlen_fakten.61
65 Reha Chrischona. Jahresbericht Reha Chrischona 2013 [Internet]. [cited 2015 Mar 19]. Available from: http://www.reha.buespi.ch/files/G8KZCEI/Jahresbericht-Reha-Chrischona-2013
66 Hensel M, Egerer G, Schneeweiss a., Goldschmidt H, Ho a. D. Quality of life and rehabilitation in social and professional life after autologous stem cell transplantation. Ann Oncol. 2002;13(2):209–17.
67 Gudbergsson SB, Fossa SD, Dahl AA. A study of work changes due to cancer in tumor-free primary-treated cancer patients. A NOCWO study. Support Care Cancer. 2008;16(10):1163–71.
68 Peteet JR. Cancer and the meaning of work. Gen Hosp Psychiatry. 2000;22(3):200–5.
69 Gray RE, Fitch M, Phillips C, Labrecque M, Fergus K. To tell or not to tell: Patterns of disclosure among men with prostate cancer. Psychooncology. 2000;9(4):273–82.
70 Kinsinger DP, Penedo FJ, Antoni MH, Dahn JR, Lechner SC, Schneiderman N. Psychosocial and sociodemographic correlates of benefit-finding in men treated for loclized prostate cancer. Psychooncology. 2006;15(11):954–61.
71 Mehnert A. Employment and work-related issues in cancer survivors. Crit Rev Oncol Hematol. 2011;77(2):109–30.
72 Bürger W. Gibt es geschlechtsspezifische Benachteiligungen in der Inanspruchnahme von medizinischen Rehabilitationsmaßnahmen? In: Worringten U, Zwingmann C, editors. Rehabilitation weiblich – männlich Geschlechtsspezifische Rehabilitationsforschung. Weinheim: Juventa; 2001. p. 55–71.
73 Lehmann C, Bergelt C, Welk H, Hagen-Aukamp C, Berger D, Koch U. Do outpatient and inpatient rehabilitation programs differ in applied interventions and success? An analysis of medical discharge summaries. Phys Medizin Rehabil Kurortmedizin. 2008;18(2):59–68.
74 Bundesamt für Statistik. Bildungsstatistik 2013 [Internet]. [cited 2015 Mar 24]. Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/15/01/key/blank/01.html
Disclosure statement: This study was supported by the Zurzach Rehabilitation Foundation SPA, Bad Zurzach Switzerland and the University of Zurich. The authors declare no conflict of interest.