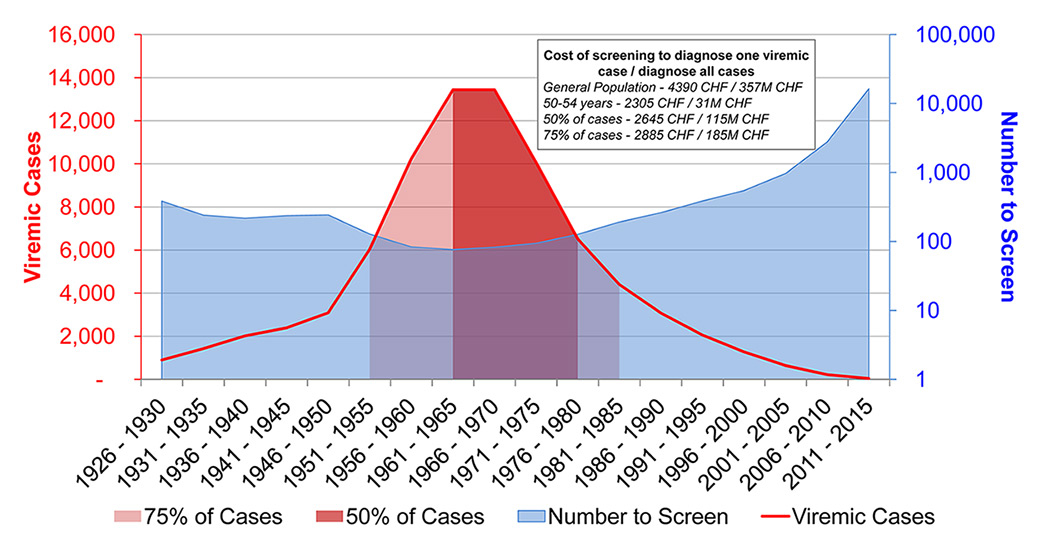
Figure 1
Percent of cases by 5-year birth cohort, the number of individuals needed to test in order to diagnose one viraemic case, and the associated cost to diagnose one viraemic case by birth cohort of interest.
DOI: https://doi.org/10.4414/smw.2015.14221
Globally, there is a growing need for effective diagnosis and screening strategies targeting patients with hepatitis C virus (HCV) infection. Despite frequent use, the success of risk based screening falls short of its potential [1]. This may be due in part to a patient’s hesitation to disclose their current or prior risk status, or to a lack of knowledge of risk status [2]. In 2013 the US Preventive Services Task Force recommended birth cohort screening to compliment current screening efforts among high risk populations [3]. A recent study conducted by the Swiss Federal Office of Public Health analysed the birth year distribution of all reported HCV cases and concluded that 60% were born between 1955 and 1974 [4]. The present analysis sought to identify which birth cohort would require the least screening in order to diagnose one new viraemic HCV infection. In addition, it compared the direct cost of screening several different birth cohorts.
Historical estimates for anti-HCV prevalence (1.6% [range: 0.8–1.8%]) [5, 6], age distribution, annual number of newly diagnosed cases (1 310 anti-HCV) and total number of diagnosed cases alive (41 300 anti-HCV) [7] were compiled from the literature and government sources. HCV has been a notifiable disease since 1988, and the Swiss reporting systems have been previously well described [4]. The scope of the analysis was limited to HCV viraemic cases, with a viraemia rate of 79.7% applied to anti-HCV estimates [8]. A previously described disease progression model was used to age the HCV-infected population to 2015, taking into account mortality and cure rates [9, 10]. Each year, the population was moved from one single-year age cohort to the next to simulate aging. Results were aggregated to 5-year cohorts for reporting. Additionally, the model tracked diagnosed cases, assuming a constant number of new diagnoses each year after 2011. By 2015, approximately 39% of patients were estimated to be diagnosed.
For the analysis of the number needed to be screened to diagnose one viraemic case (NNS), the median age in each five-year age cohort was selected and converted to a birth year. The NNS was calculated by taking the inverse of the viraemic HCV prevalence in each cohort, after accounting for the diagnosis rate, as follows:
The NNS to identify 50 or 75% of the HCV-infected population was calculated by aggregating five-year birth cohorts to achieve the desired cohort size, and then using the formula above.
Costs associated with diagnosing (anti-HCV serology = CHF 25 and HCV RNA = CHF 180) and informing treatment (genotype testing = CHF 180) were collected by use of official reimbursement rates [11].
Uncertainty analysis was completed using Crystal Ball, an Excel add-in by Oracle. Beta-PERT distributions were used to model uncertainty associated with all inputs. Monte-Carlo simulation was used to determine the 95% uncertainty intervals (UIs).
The median age of the viraemic HCV-infected population in 2015 was 49 years. Approximately 50% of this population was born during 1961–1980 and over 75% were born during 1951–1985 (fig. 1).

Figure 1
Percent of cases by 5-year birth cohort, the number of individuals needed to test in order to diagnose one viraemic case, and the associated cost to diagnose one viraemic case by birth cohort of interest.
Screening of the general population at random could identify one case per 159 (95% UI 140–361) persons screened. By focusing screening on the population born between 1961 and 1965 (17% of total cases), it is estimated that one new case could be found for every 76 (95% UI 66–181) persons screened (fig. 1).
Within the 1961–1980 cohort one new case could be found per 90 (95% UI 78–201) persons screened, and within the 1951–1985 cohort one new case could be found per 99 (95% UI 87–226) persons screened.
The cost of diagnosing one new viraemic case was highest when screening the general population (CHF 4 390, 95% UI 3 905–9 435) and lowest when screening persons aged 50–54 years (CHF 2 305, 95% UI 2 065–4 935). When screening the 50% cohort, costs amounted to CHF 2 645 (95% UI 2 355–5 430), and within the 75% cohort, costs amounted to CHF 2 885 (95% UI 2 570–6 065).
This analysis presents a variety of birth cohort screening strategies alongside the NNS and associated cost of diagnosis. The strategy with the largest impact would involve the 1951–1985 cohort, which captures 75% of all infections and would require screening of 99 persons to identify one new case. By contrast, screening within the 1961–1965 cohort could identify one new case for every 76 persons screened; however, this cohort only captures 17% of all viraemic cases.
A unique feature of this analysis is the focus on viraemic cases and the exclusion of the previously diagnosed population; however, this also results in a higher NNS. Nonetheless, focusing on the undiagnosed viraemic population provides a more realistic estimate of the costs associated with identifying a truly new case, as this population will be the focus for treatment intervention.
Considering the direct cost of diagnosis, these results suggest that it is more cost effective to test populations with a relatively high HCV prevalence as compared with the general population. The same conclusion could also be applied to high-risk populations (persons who inject drugs and men who have sex with men), which is in line with previous publications [4, 6].
One limitation of this study is the assumption of a uniform diagnosis rate across all ages. In reality, the diagnosis rate among older adults may be higher as a result of increased health awareness and attendance at health visits [12], disease progression or the presentation of symptoms. Diagnosis rates among a younger population may be higher owing to risk-based screening.
Enhancing HCV detection rates is possible through targeted screening of high-prevalence birth cohorts. Considering only the direct cost of screening and treatment informing tests, screening within these birth cohorts is also more cost effective than screening within the general population. Screening within a larger birth cohort (for example, the 75% cohort versus the 50% cohort) has the additional benefit of identifying a large number of new cases, with marginally higher cost (CHF 2 885 vs 2 645).
1 Tomaszewski KJ, Deniz B, Tomanovich P, Graham CS. Comparison of current US risk strategy to screen for hepatitis C virus with a hypothetical targeted birth cohort strategy. Am J Public Health. 2012;102(11):e101–e106.
2 Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349–57.
3 Rein DB, Smith BD, Wittenborn JS, Lesesne SB, Wagner LD, Roblin DW, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156(4):263–70.
4 Bruggmann P, Richard JL. Birth year distribution in reported hepatitis C cases in Switzerland. Eur J Public Health. 2014.
5 Sagmeister M, Renner EL, Mullhaupt B, Wong JB. Simulation of hepatitis C based on a mandatory reporting system. Eur J Gastroenterol Hepatol. 2002;14(1):25–34.
6 Fretz R, Negro F, Bruggmann P, Lavanchy D, De Gottardi A, Pache I, et al. Hepatitis B and C in Switzerland-healthcare provider initiated testing for chronic hepatitis B and C infection. Swiss Med Wkly. 2013;143: w13793 .
7 Swiss Federal Office of Public Health. Number of hepatitis C cases reported in Switzerland between 1988 and 2012 by year of birth (mandatory notification of laboratory confirmed cases): FOPH/ID/EPI/RIC. 2013.
8 Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293–300.
9 Mullhaupt B, Bruggmann P, Bihl F, Blach S, Lavanchy D, Razavi H, et al. Modeling the Health and Economic Burden of Hepatitis C Virus in Switzerland. PLoS One 2015;10(6):e0125214.
10 Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(Suppl 1):34–59.
11 Swiss Federal Office of Public Health. [Analysis List]. 2015 Jan 1.
12 Deeks A, Lombard C, Michelmore J, Teede H. The effects of gender and age on health related behaviors. BMC Public Health. 2009;9:213.
Disclosure statement:P.B. has served as advisor and speaker for, and has received project and research grants from Roche, MSD, Janssen, AbbVie, Gilead, Viif and BMS. F.N. has served as advisor for MSD, Gilead, Novartis, Bristol Myers Squibb, AbbVie and Janssen and has received unrestricted research grants from Roche and Gilead. S. B. and H.R. are employees of the Center for Disease Analysis (CDA), Louisville, Colorado, USA. B.M. has served as an advisory board member for Roche, MSD, Janssen Therapeutics, AbbVie, Boehringer Ingelheim, Gilead and BMS; as a consultant for Gilead and AbbVie; and has received research grants from Roche and Gilead. D.S. has served as a consultant for Gilead, an advisor for Roche, MSD, Gilead, Novartis, Janssen and Boehringer Ingelheim and has received an unrestricted research grant from Roche. F.B. and D.L. have no conflicts of interest to declare.
Statement of human rights and the welfare of animals:This article does not contain any studies with human participants or animals performed by any of the authors.
Funding:This work was supported by Gilead Sciences. Gilead Sciences had no input on the content, the study design, data selection, decision to publish or preparation of the manuscript.