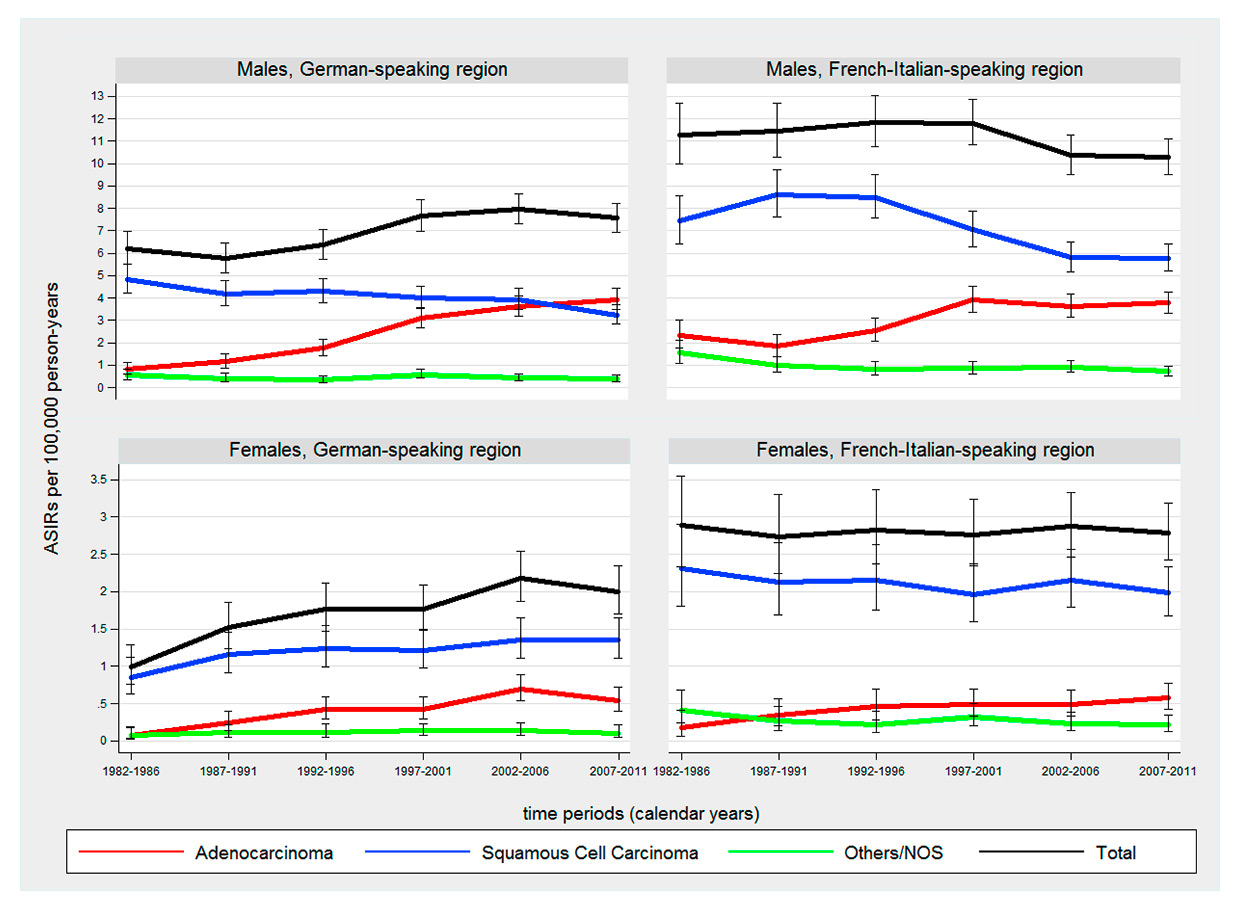
Figure 1
Time trends in age-standardised incidence rates (ASIRs) (European standard population) of oesophageal cancer by sex, histological subtype and language region from 1982 to 2011.
NOS = not otherwise specified
DOI: https://doi.org/10.4414/smw.2015.14245
Abbreviations
95% CI 95% confidence interval
APC annual percentage change
ASIR age-standardized incidence rates
BS/BL Basel
FR Fribourg
GE Geneva
GR/GL Glarus/Graubünden
JU/NE Jura/Neuchâtel
PY person-years
SG/AR/AI Sankt Gallen/Appenzell Ausserhoden/Appenzell Innerhoden
TI Ticino
US Unites States
VD Vaud
VS Valais
ZH Zurich
In Switzerland, each year approximately 500 new cases of oesophageal carcinoma are diagnosed and over 800 cases of gastric carcinoma. Owing to their high lethality, oesophageal and gastric cancers have a substantial public health impact in Switzerland [1, 2].
Studies in developed countries have reported substantial increases in the incidence of adenocarcinoma of the oesophagus and/or gastric cardia accompanied by stabilising or declining rates of oesophageal squamous cell carcinoma. In the United States (US), the incidence of oesophageal adenocarcinoma has risen rapidly since the mid-1970s, especially among white males, surpassing oesophageal squamous cell carcinoma [3]. During the same time period, the incidence of gastric cardia adenocarcinoma in the US also increased [3]. Similar trends have been observed in in several European countries (e.g. the Nordic countries [4], Germany [5], Spain [6], United Kingdom [7–11]). However, there are substantial variations in both incidence rates and trends between countries and within regions [4, 12–15].
Data on the incidence of oesophageal adenocarcinoma in Switzerland have been mixed, were reported for varying timeframes and were regionally limited [4, 12, 13, 16–19]. An international study reported an increase in the incidence of oesophageal squamous cell carcinoma in women but no significant trends for oesophageal adenocarcinoma or cancer of the gastric cardia for Basel and Geneva (1973–1995) [4]. In other studies, no increase was found for the combined incidence rates of oesophageal and gastric cardia adenocarcinoma in Basel (1981–1992) [12] or for oesophageal adenocarcinoma in Eastern Switzerland (1989–1999) [17]. In contrast, increasing incidence of oesophageal adenocarcinoma has been reported for the canton of Vaud (1976–1994) [18], and for central Switzerland (1982–2007) [19].
This study aimed to evaluate trends in the incidence of oesophageal cancer (by both major histological subtypes and anatomical location) and gastric cancer (by anatomical location) using nationally representative data over a 30-year period.
Data on primary oesophageal and gastric cancer diagnoses were obtained from Swiss cantonal cancer registries that held at least five years of consecutive data between 1982 and 2011. The study population included cases from Basel-Stadt/Basel-Landschaft (BS/BL), Fribourg (FR), Geneva (GE), Grison/Glarus (GR/GL), Neuchâtel/Jura (NE/JU), St. Gallen-Appenzell (SG/AR/AI), Ticino (TI), Valais (VS), Vaud (VD) and Zurich (ZH). The definition of primary cancers followed the rules defined by the International Association of Cancer Registries (IACR) and International Association for Research on Cancer (IARC) [20]. With the exception of cases from TI diagnosed prior to 2003, all cancer cases were coded according to the third edition of the International Classification of Diseases for Oncology (ICD-O-3). Cases from TI diagnosed prior to 2003 were coded according to the second revision (ICD-O-2) and were automatically recoded to ICD-O-3 following international standards [21]. Proportions of death certificate only cases decreased from 4.9% in 1982 to 0.9% in 2011 (2.0% 1982–2011). In some cancer registries, a complete and systematic matching of registered incidence cases with data from death certificates was not carried out for all years [22]. The proportion of death certificate only cases with confirmed death certificate matching was 2.6% during 1982–2011. Overall, 94.3% of all cases were histologically verified ranging from 90.4% in 1982 to 95.6% in 2011. The Swiss Federal Statistical Office provided cantonal mid-year population estimates for all persons with permanent residence status within each canton.
Switzerland has three major language regions (German, French, Italian) which adjoin countries sharing the same mother tongue. There are regional cultural differences between the language regions reflecting those of the countries bordering Switzerland. To enable a separate group inspection, we followed the approach of the Swiss Federal Statistical Office (SFO) and the National Institute for Cancer Epidemiology and Registration (NICER) and classified all cases into the following language regions: German-speaking (BS/BL, GR/GL, SG/AR/AI, ZH) and French-Italian-speaking (FR, GE, JU/NE, TI, VS, VD) [22, 23]. The language regions were formed by grouping the cantons according to the language spoken by the majority of the population. Overall, 85% of the permanent resident population of the cantons grouped to the German-speaking region indicated German as their main language. In the cantons of the French-Italian-speaking region, 68% of the population declared French and 17% Italian as their main language. Because of the gradual introduction of the cantonal cancer registries, the population covered by cancer registration in the French-Italian-speaking region ranged from 58.3% in 1982 to 100% since 2006. Coverage in the German-speaking region varied between 43.4% in 1982 and 46.9% in 2009. The coverage of the years 2010 and 2011 dropped to 38.9% because data from the cancer registry of BS/BL were not available at the time when the present statistical analysis was carried out.
Anatomical locations of oesophageal cancer were defined as: (1) upper and middle third (ICD-O-3: C15.0, C15.1, C15.3, C15.4), (2) lower third (ICD-O-3: C15.2, C15.5), and (3) overlapping / not otherwise specified (NOS) (ICD-O-3: C15.8, C15.9). Anatomical locations of gastric cancers were categorised into (1) cardia (ICD-O-3: C16.0), (2) noncardia and (3) overlapping/NOS (ICD-O-3: C16.8, C16.9). The proportion of NOS cases (ICD-O-3: Cx.9) varied substantially by cancer registry from 0.9% to 39.2% for oesophageal, and 11.2% to 26.9% for gastric cancer.
Histological categories were classified as: (1) adenocarcinoma (ICD-O-3: M814-M857), (2) squamous cell carcinoma (ICD-O-3: M8050-M8082), (3) other histology / NOS (ICD-O-3: M800-M804, M809-M813, M858-M994). Overall, proportions of cases with NOS histological subtypes (ICD-O-3: M800-M804) were 7.6% in oesophageal cancer and 6.4% in gastric cancer.
To assess time trends, annual and 5-year age-standardised incidence rates (ASIR; direct method, European standard [24]) per 100 000 person-years (PY) were calculated using mid-year population estimates for each canton. Estimated annual percentage changes (APC) with 95 percent confidence intervals (95% CIs) were obtained by fitting linear regression models using the natural log of annual ASIRs as target variable and the year of incidence as predictor.
Time trends were analysed by cancer site (oesophageal cancer, gastric cancer), histological subtype and anatomical location. Analyses stratified by anatomical location were restricted to cancer registries providing specified topography information for more than 80% of their cases (ICD-O-3: C15.0-C15.8, C16.0-C16.8), which led to exclusion of TI and ZH. All analyses were stratified by sex and language region.
Between 1982 and 2011, 7 280 oesophageal and 15 484 gastric cancers were diagnosed in the study population. The contributions of the regional cancer registries are shown in table 1. APCs for oesophageal and gastric cancers by sex, histology, anatomical location and language region are presented in table 2.

Figure 1
Time trends in age-standardised incidence rates (ASIRs) (European standard population) of oesophageal cancer by sex, histological subtype and language region from 1982 to 2011.
NOS = not otherwise specified
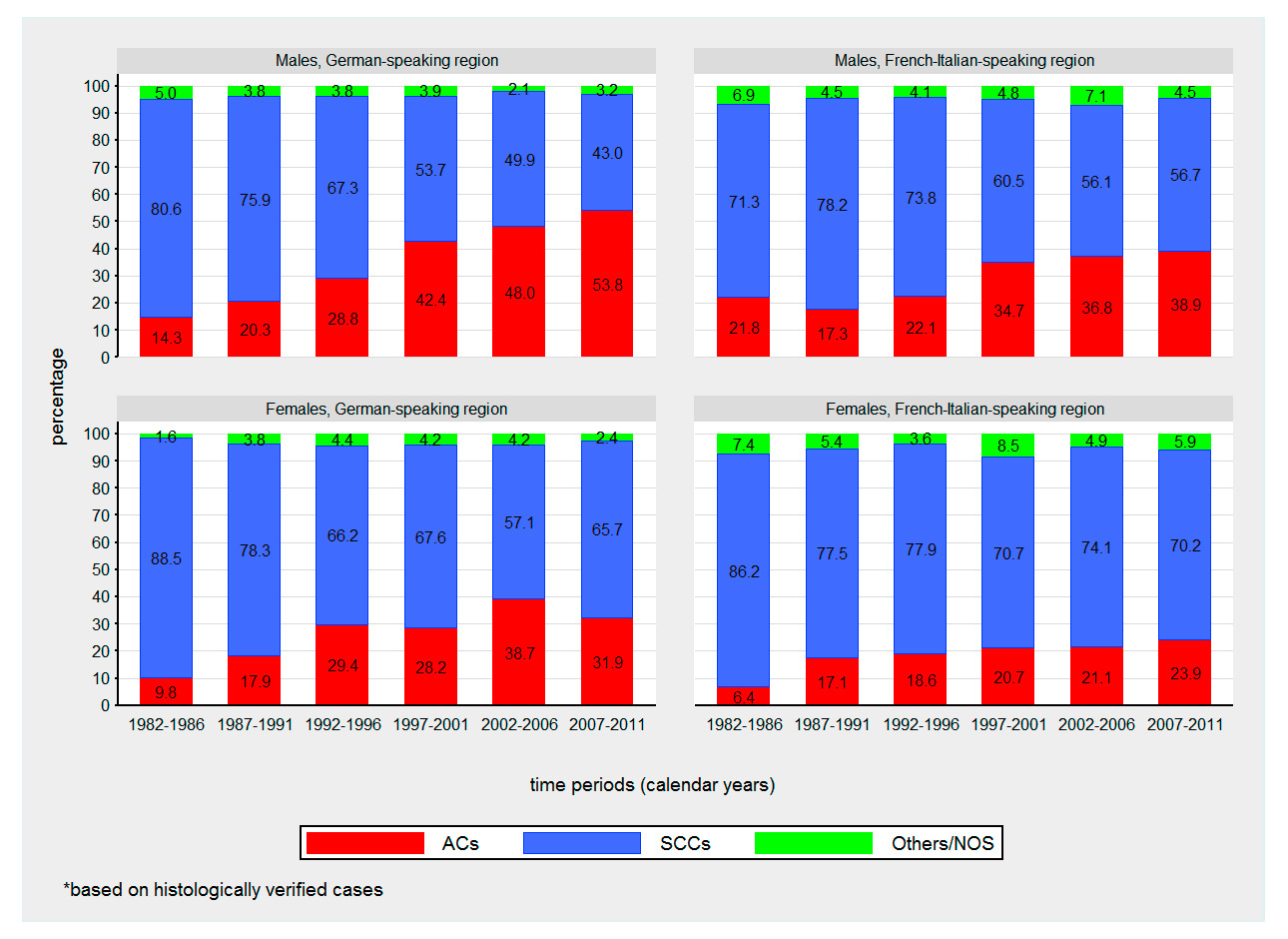
Figure 2
Time trends in proportions of oesophageal cancer cases by sex, histologic subtype and language region from 1982 to 2011.
ACC = adenocarcinoma; SCC = squamous cell carcinoma, Others/NOS = other histologic subtype / not otherwise specified
For both sexes and all time periods under investigation, the incidence of oesophageal cancer was higher in the French-Italian-speaking region than the German-speaking region owing to distinctly higher rates of oesophageal squamous cell carcinoma (p <.001 for all time periods). For example, the overall male ASIR in the French-Italian-speaking region ranged from 10.3–11.8 per 100 000 PY versus 5.8–8.0 per 100 000 PY in the German-speaking region. Overall, the incidence of oesophageal cancer was stable in the French-Italian-speaking region (male APC –0.5 [95% CI –1.0, 0.0], female APC –0.1 [95% CI –0.7, 0.6]), but increased in the German-speaking region (male APC 1.2 [95% CI 0.6, 1.8], female APC 2.5 [95% CI 1.4, 3.5]). Oesophageal cancer was much more frequent in males than in females (85% and 75% of oesophageal cancer occurring in males in the German and French-Italian regions, respectively). Squamous cell carcinoma represented more than 60% of all histological types of oesophageal cancer.
The incidence of oesophageal adenocarcinoma increased significantly in both sexes and language regions during 1982–2011. The steepest increase was observed in males of the German-speaking region (APC 6.8% [95% CI 5.8, 7.8]) with ASIRs of 0.8 per 100 000 PY in 1982–1987 and 3.9 per 100 000 PY in 2007–2011. In males of the French-Italian-speaking region, incidence of oesophageal adenocarcinoma rose substantially between 1987 and 2001 (APC 6.7% [95%CI 4.3, 9.1]) followed by a plateau of approximately 4.0 per 100 000 PY in subsequent years (fig. 1). In 2008–2011, both language regions showed similar ASIRs for oesophageal adenocarcinoma in both sexes (p >0.05). The male to female ratio for oesophageal adenocarcinoma was 10:1 in the German-speaking region and 6.5:1 in the French-Italian-speaking region for the latest time period.
The incidence of oesophageal squamous cell carcinoma decreased significantly in males of both language regions by around –1.5% per year. In contrast, a slight but significant increase of oesophageal squamous cell carcinoma was observed in females of the German-speaking region with an ASIR of 0.8 per 100 000 PY in 1982–1987 and 1.4 per 100 000 PY in 2007–2011 (APC 1.4% [95% CI 0.3, 2.4) (table 2, fig. 1). The male to female ratio of oesophageal squamous cell carcinoma was 2.4:1 in the German-speaking region and 2.9:1 in the French-Italian-speaking region.
Squamous cell carcinoma was the predominant histological subtype of oesophageal cancer during the study period for females of both language regions and males of the French-Italian-speaking region. Overall, the proportion of oesophageal adenocarcinoma rose substantially from 1982–2011 and became the predominant histological subtype (53.8%) in males of the German-speaking region, surpassing squamous cell carcinoma in 2007–2011 (fig. 2).
Most cancers of the upper/middle oesophagus were squamous cell carcinomas (86.6%), whereas adenocarcinoma was the more common subtype of the lower part (48.1%). In males of both language regions, the increasing incidence trends of oesophageal adenocarcinoma were accompanied by an increase of cancers in the lower part of the oesophagus. However, in females this relationship was less clear (table 2).
The German-speaking region showed significantly higher ASIRs of gastric cancer compared with the French-Italian-speaking region in 1982–1987 (1.2-fold higher in males and 1.4-fold higher in females, p <.001), but more similar rates until the end of the observation period (p >0.05 in both sexes) (fig. 3). The incidence of gastric cancer decreased significantly in both sexes and language regions with APCs ranging from –1.5% (95% CI –2.0, –1.1) in females of the French-Italian-speaking region to –3.2% (95% CI –3.7, –2.8) in females of the German-speaking region. For cancer of the gastric cardia, no significant APCs were observed (table 2). The male to female ratio of cancer of the gastric cardia was 4.2:1 in the German-speaking region and 5.5:1 in the French-Italian-speaking region. The corresponding ratio for noncardia gastric cancer was 1.6:1 in both language regions. Cancer of the gastric cardia showed stable or slightly decreasing ASIRs (p >0.05) whereas the incidence of noncardia gastric cancer decreased significantly (p <0.001) in both sexes and language regions with APCs from –2.1% (95% CI –2.9, –1.3) in females from the French-Italian-speaking region to –4.2% (95% CI –5.0, –3.3) in males in German-speaking region. However, due to high proportions of NOS gastric cancer (13–20% per time period) these results should be interpreted with caution.
| Table 1:Oesophageal and gastric cancer cases in Swiss cancer registries from 1982 to 2011. | |||||
| Cancer registry | Diagnosis period | Oesophageal cancer n (%) | Gastric cancer n (%) | ||
| Basel Stadt / Basel Landschaft1 | 1982–2009 | 684 | (9.4) | 1 820 | (11.8) |
| Fribourg2 | 2006–2011 | 117 | (1.6) | 140 | (0.9) |
| Geneva2 | 1982–2011 | 866 | (11.9) | 1 424 | (9.2) |
| Grison/ Glarus1 | 1989–2011 1992–2011 | 285 | (3.9) | 696 | (4.5) |
| Neuchâtel/ Jura2 | 1982–2011 2005–2011 | 356 38 | (4.9) (0.5) | 646 34 | (4.2) (0.2) |
| St. Gallen–Appenzell1 | 1982–2011 | 725 | (10.0) | 2 096 | (13.5) |
| Ticino2,3 | 1996–2011 | 383 | (5.3) | 1 009 | (6.5) |
| Vaud2 | 1982–2011 | 1 644 | (22.6) | 2 036 | (13.2) |
| Valais2 | 1989–2011 | 456 | (6.3) | 939 | (6.1) |
| Zurich1,3 | 1982–2011 | 1 726 | (23.7) | 4 644 | (30.0) |
| 1 German-speaking region 2 French-Italian-speaking region 3 Data from Zurich and Ticino were excluded for all analyses stratified by location (proportion of cases with unspecified location >20%). | |||||
| Table 2:Estimated annual percentage change for oesophageal and gastric cancer by sex, histology, anatomical location and language region from 1982 to 2011. | ||||||||
| German-speaking region | French-Italian-speaking region | |||||||
| n | APC | 95% CI | p-value | n | APC | 95% CI | p-value | |
| MALES | ||||||||
| Oesophageal cancers | 2 564 | 1.2 | 0.6, 1.8 | <0.001 | 2 854 | –0.5 | –1.0, 0.0 | 0.058 |
| Location* | ||||||||
| Upper/middle third | 463 | –1.4 | –2.6, –1.7 | 0.027 | 1 216 | –0.6 | –1.5, 0.2 | 0.136 |
| Lower third | 676 | 2.7 | 1.1, 4.3 | 0.002 | 1 073 | 0.8 | 0.1, 1.6 | 0.024 |
| Overlapping/NOS | 190 | 1.8 | –0.4, –4.0 | 0.101 | 269 | –2.8 | –4.8, –0.8 | 0.008 |
| Histology | ||||||||
| Adenocarcinoma | 949 | 6.8 | 5.8, 7.8 | <0.001 | 853 | 2.8 | 1.8, 3.8 | <0.001 |
| Squamous cell carcinoma | 1 449 | –1.4 | –2.0, –0.7 | <0.001 | 1 755 | –1.5 | –2.3, –0.8 | <0.001 |
| Others/NOS | 166 | –0.1 | –2.2, 2.0 | 0.929 | 246 | –2.4 | –3.9, –0.8 | 0.004 |
| Gastric cancers | 5 485 | –3.1 | –3.6, –2.8 | <0.001 | 3 763 | –2.3 | –2.9, –1.8 | <0.001 |
| Location* | ||||||||
| Cardia | 775 | –0.5 | –1.3, 0.4 | <0.258 | 893 | –0.7 | –1.9, 0.3 | 0.169 |
| Noncardia | 1 295 | –4.2 | –5.0, –3.3 | <0.001 | 1 507 | –3.4 | –4.1, –2.6 | <0.001 |
| Overlapping/NOS | 623 | –4.3 | –5.4, –3.3 | <0.001 | 807 | –3.7 | –4.7, –2.7 | <0.001 |
| FEMALES | ||||||||
| Oesophageal cancers | 856 | 2.5 | 1.4, 3.5 | <0.001 | 1 006 | –0.1 | –0.7, 0.6 | 0.856 |
| Location* | ||||||||
| Upper/middle third | 292 | 4.4 | 1.7, 7.1 | 0.002 | 524 | 0.2 | –1.0, 1.4 | 0.719 |
| Lower third | 292 | 1.0 | –1.0, 3.0 | 0.326 | 332 | 0.9 | –0.4, 2.3 | 0.170 |
| Overlapping/NOS | 23 | –0.9 | –5.4, 3.4 | 0.647 | 53 | –0.4 | –3.4, 2.7 | 0.809 |
| Histology | ||||||||
| Adenocarcinoma | 235 | 5.5 | 3.3, 7.8 | <0.001 | 183 | 4.1 | 2.6, 5.6 | <0.001 |
| Squamous cell carcinoma | 553 | 1.4 | 0.3, 2.4 | 0.011 | 704 | –0.5 | –1.4, 0.3 | 0.219 |
| Others/NOS | 68 | –1.6 | –4.9, 1.7 | 0.315 | 119 | –1.3 | –3.7, 1.1 | 0.286 |
| Gastric cancers | 3 771 | –3.2 | –3.7, –2.8 | <0.001 | 2 465 | –1.5 | –2.0, –1.1 | <0.001 |
| Location* | ||||||||
| Cardia | 273 | –1.5 | –3.0, 0.1 | 0.065 | 267 | 0.0 | –1.5, 1.4 | 0.962 |
| Noncardia | 1 124 | –3.9 | –4.8, –3.0 | <0.001 | 1 163 | –2.1 | –2.9, –1.3 | <0.001 |
| Overlapping/NOS | 522 | –3.9 | –5.5, –2.3 | <0.001 | 582 | –3.3 | –4.8, –1.8 | <0.001 |
| 95% CI = 95% confidence interval; APC = estimated annual percentage change; NOS = not otherwise specified * Data from Zurich and Ticino were excluded for all analyses stratified by location (proportion of cases with unspecified location >20%) | ||||||||
This study reports trends on oesophageal and gastric cancer incidence by location and histology in Switzerland from 1982 to 2011 by combining data from ten Swiss regional cancer registries.
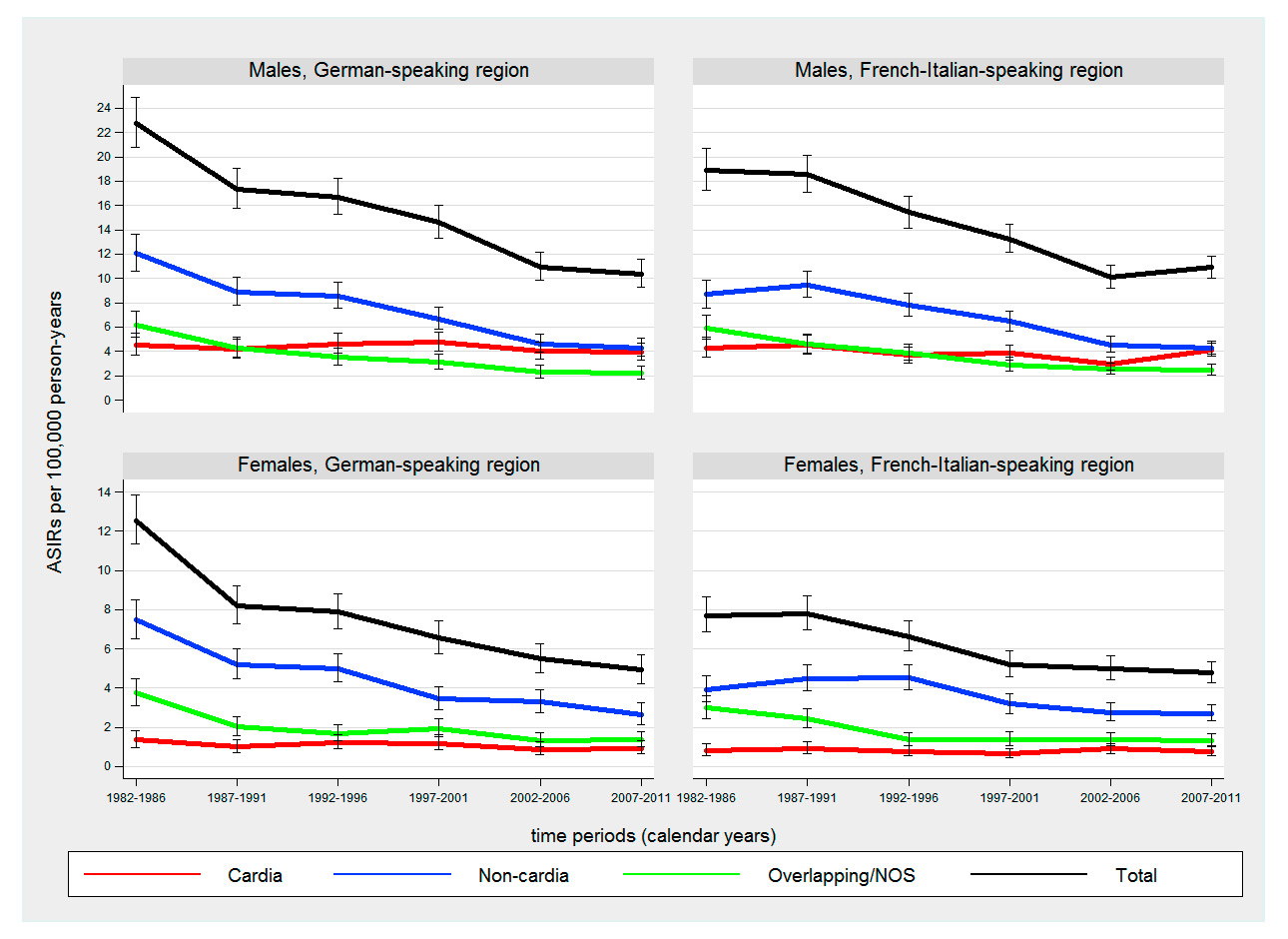
Figure 3
Time trends in age-standardised incidence rates (ASIRs) (European standard population) of gastric cancer by sex, anatomical location and language region from 1982 to 2011.
NOS = not otherwise specified
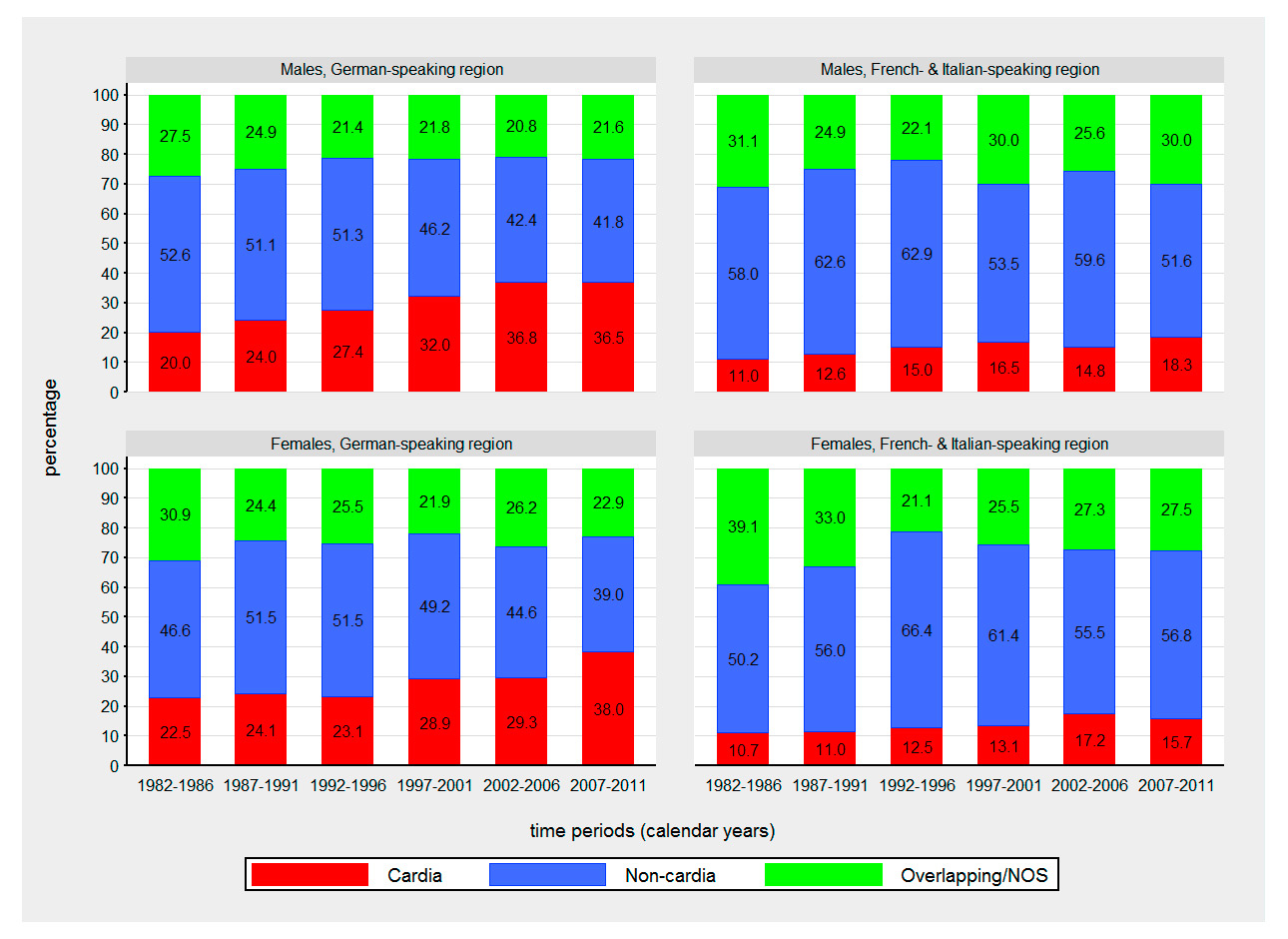
Figure 4
Time trends in proportions of gastric cancer cases by sex, anatomical location and language region from 1982 to 2011.
NOS = not otherwise specified
We observed a substantial increase of oesophageal adenocarcinoma in Switzerland, as in other developed countries [3–11, 25–29]. In the German-speaking region of Switzerland, a continuous increase of oesophageal adenocarcinoma was observed throughout the study period, whereas a plateau was reached in males of the French-Italian-speaking region from 2001 onwards after a previous increase. In males of the German-speaking region oesophageal adenocarcinoma has become the predominant histology since 2007, accompanied by a corresponding decline of oesophageal squamous cell carcinoma.
Tobacco smoking and alcohol consumption have repeatedly been shown to be the major risk factors of oesophageal squamous cell carcinoma in developed countries, accounting for the large majority of all observed cases [30–32]. As in other European countries, smoking-related cancers and smoking prevalence in Swiss males continues to decrease [33]. In addition, levels of alcohol consumption in Switzerland have been falling continuously in the last 30 years [34], especially among Swiss men [33]. However, alcohol consumption is distinctly more frequent in the French- and Italian-speaking region than in the German-speaking region and smoking prevalence is slightly higher in the French-speaking region compared with the German- and Italian-speaking regions of Switzerland [35]. Decreasing trends in male smoking prevalence and alcohol consumption are likely reflected in our results showing a substantial decline in squamous cell carcinoma of the oesophagus. Among Swiss women overall, increases in smoking prevalence have preceded substantial increases in lung cancer incidence (+16%) and mortality (+15%) (1998–2002 to 2003–2007) [36]. However, in this study an increasing trend of oesophageal squamous cell carcinoma was observed only in females of the German-speaking region.
Albeit less pronounced, tobacco smoking is also associated with an increased risk of adenocarcinoma of the oesophagus and gastric cardia [37, 38]. As previously mentioned, smoking prevalence in Swiss males continues to decline, which serves as an argument against smoking as a major contributing factor for the increase of oesophageal adenocarcinoma in Switzerland [36, 39]. The major factors discussed in the context of the rising incidence of oesophageal adenocarcinoma are increasing prevalence of gastro-oesophageal reflux disease (GORD) and obesity, and decreasing prevalence of Helicobacter pylori infection. Frequent symptoms of GORD have been shown to be associated with oesophageal adenocarcinoma. A 2010 meta-analysis reported a 5-fold increased risk of oesophageal adenocarcinoma among persons reporting at least weekly GORD symptoms and a 7-fold increase for those with daily symptoms, compared with individuals with less frequent or no symptoms [40]. A recent study reported an 11% increased risk of oesophageal adenocarcinoma for each 5 kg/m2 increment of body mass index (BMI) [41]. Other studies suggest a synergistic interaction between BMI and GORD [42]. Based on a pooled analysis of data from the US, Australia and European countries, Hoyo et al. estimated that around 20% of oesophageal adenocarcinoma are attributable to synergistic effects of high BMI (≥27.5 kg/m2) and the presence of GORD [42]. Like in other developed countries, an increasing prevalence of overweight and obesity has been observed in Switzerland. Between 1992 and 2012, the total overweight population (BMI ≥25 kg/m2) increased from 30.4% to 41.1% and in the obese segment (BMI ≥30 kg/m2) from 5.4% to 10.3% [33]. The prevalence of GORD in Swiss adults was 17.6% in 2004 [43]. The disease was more frequent in the French-speaking region of Switzerland but equally distributed among the sexes. A meta-analysis of H. pylori infection, an important risk factor for noncardia gastric cancer [44, 45], showed a nearly 40% reduced risk of oesophageal adenocarcinoma in people infected with H. pylori [46]. Investigating the prevalence of H. pylori infection in Swiss adolescents in 1999–2002, Heuberger et al. found one of the lowest prevalence of H. pylori infection in Europe (9.7%) [47]. However, observed trends in known risk factors can only partly explain the rising incidence and the striking predominance of oesophageal adenocarcinoma in males [38].
For cancer of the gastric cardia, we observed stable rates in both sexes and language regions. Similar results have been reported from other developed countries (Australia 1982–1993 [26], Finland 1976–1995 [48], Netherlands 1973–2011 [49], Spain 1980–2004 [6]). In contrast, an increase of both oesophageal adenocarcinoma and cancer of the gastric cardia has been observed in Scotland (1978–1997) [7], eastern-England (1995–2006) [8] and the US (1974–1994) [3]. Since our analyses were based on data with high proportions of cases with unspecified anatomical location they should be interpreted with caution.
When adenocarcinomas of the (lower) oesophagus and gastric cardia have been examined, they have often been analysed together, on the assumption that both malignancies share several common features and have similar risk factors [50, 51]. However, substantial epidemiological differences between oesophageal and gastric adenocarcinoma have been described, suggesting that these two cancer sites may be distinct cancer types with partly different risk factors [52]. A recent review investigating risk factors for oesophageal and gastric cardia adenocarcinoma reported an association for GORD and oesophageal adenocarcinoma, but not for GORD and adenocarcinoma of the gastric cardia [53]. Furthermore, it remains unclear whether obesity is associated with an increased risk for adenocarcinoma of the gastric cardia, an established risk factor for oesophageal adenocarcinoma [53–55]
Similar to other developed countries, the incidence of noncardia gastric cancer decreased substantially in Switzerland [56]. The observed decline is presumably due to decreases in the prevalence of H. pylori infection, the main identified risk factor of noncardia gastric cancer [44, 45, 57]. In addition, reduction in the prevalence of tobacco smoking in males along with improvements in food preservation and nutrition may have also contributed to the decrease of noncardia gastric cancer incidence [57].
This is the first Swiss population-based investigation on oesophageal and gastric cancer trends providing the most nationwide representative findings to date. Although the cantonal cancer registries included do not cover the entire Swiss population, the large study sample representing the major language regions allows generalisability of these findings to the national level. Overall, the study population had less than 5% of death certificate only cases for each year under investigation indicating a high completeness of case ascertainment. Furthermore, the collection of cancer cases showed a high proportion of histologically verified cases (>90% for each time period).
Population coverage of the German-speaking region was below 50% for all time periods under investigation. Therefore, incidence figures for the German-speaking region might be less precise than for the French-Italian speaking region. Another weakness of this study is the relatively large proportion of cancer cases with unspecified anatomical location. This was particularly true for gastric cancer, with approximately 16% of all analysed cases in this group. Because of high proportions of cases with unspecified location (>20%), cases from ZH and TI had been excluded for all analyses stratified by anatomical location. Population coverage for these analyses dropped by around 25% and 14% for the German-speaking region and French-Italian-speaking regions, respectively. Therefore these results should be interpreted with caution. Further, the formation of the language regions based on cantonal borders is just an approximation of the natural language regions of Switzerland. Some cantons are part of more than one language region. However, as cantonal information was the most detailed geographical information available, no more accurate allocation was possible within this work.
In Switzerland, healthcare and cancer registration is organised at the cantonal level (detailed descriptions of the Swiss health care system and the organisation of cancer registration can be found elsewhere [58–61]). Therefore it cannot be ruled out that regional differences in the provision of healthcare services and/or regional differences in the completeness of cancer registration might have influenced the results.
A major weakness of this study is the lack of information on prevalence trends in risk and protective factors covering the whole study period and/or periods preceding the observed incidence trends. Another limitation is the difficulty of distinguishing the organ of origin in cancers arising at the gastro-oesophageal junction [55, 62]. To circumvent this problem, several other studies have combined adenocarcinoma of the oesophagus and gastric cardia for their analyses. However, due to the high proportion of cases with unspecified anatomical location, this solution was not feasible for our study. Moreover, there is evidence that adenocarcinomas of the oesophagus and gastric cardia may be distinct cancer types, although they share some common risk factors [52, 53]. Last but not least, the rising incidence of oesophageal adenocarcinoma may be at least in part affected by an increase in the standard use of endoscopic procedures and advances in endoscopic technology, leading to more sensitive detection [63].
In Switzerland the incidence of oesophageal adenocarcinoma has risen, whereas incidence of noncardia gastric cancer has substantially decreased during the study period 1982–2011. Reasons for the rising incidence in oesophageal adenocarcinoma may include increases in the prevalence of GORD and obesity. For oesophageal squamous cell carcinoma, mixed trends were observed, possibly related to sex and region-specific lifestyle changes. We observed stable rates for cancers of the gastric cardia. However, as a result of high proportions of these cases with unspecified location, this finding should be interpreted with caution. Further investigations are needed to identify specific risk factors that may be responsible for the observed trends.
1 Spitale A, Feller A, Lorez M. Trends in stomach cancer survival in Switzerland. Swiss Cancer Bulletin. 2013;3:241–6.
2 Ruhstaller T, Arndt V, Lorez M. Trends in survival from oesophageal cancer in Switzerland Swiss Cancer Bulletin. 2014;3:227–31.
3 Devesa SS, Blot WJ, Fraumeni JF, Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83(10):2049–53.
4 Vizcaino AP, Moreno V, Lambert R, Parkin DM. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973–1995. Int J Cancer. 2002;99(6):860–8.
5 Bollschweiler E, Holscher AH. Carcinoma of the esophagus – actual epidemiology in Germany. Onkologie. 2001;24(2):180–4.
6 Aragones N, Izarzugaza MI, Ramos M, Chirlaque MD, Almar E, Martinez C, Oesophago-gastric Cancer Working Group. Trends in oesophago-gastric cancer incidence in Spain: analysis by subsite and histology. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 3):iii69–75.
7 Brewster DH, Fraser LA, McKinney PA, Black RJ. Socioeconomic status and risk of adenocarcinoma of the oesophagus and cancer of the gastric cardia in Scotland. Br J Cancer. 2000;83(3):387–90.
8 Gajperia C, Barbiere JM, Greenberg D, Wright K, Lyratzopoulos G. Recent incidence trends and sociodemographic features of oesophageal and gastric cancer types in an English region. Alimentary pharmacology & therapeutics. 2009;30(8):873–80.
9 Lepage C, Rachet B, Jooste V, Faivre J, Coleman MP. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103(11):2694–9.
10 Newnham A, Quinn MJ, Babb P, Kang JY, Majeed A. Trends in the subsite and morphology of oesophageal and gastric cancer in England and Wales 1971–1998. Alimentary pharmacology & therapeutics. 2003;17(5):665–76.
11 Powell J, McConkey CC. The rising trend in oesophageal adenocarcinoma and gastric cardia. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 1992;1(3):265–9.
12 Botterweck AA, Schouten LJ, Volovics A, Dorant E, van Den Brandt PA. Trends in incidence of adenocarcinoma of the oesophagus and gastric cardia in ten European countries. Int J Epidemiol. 2000;29(4):645–54.
13 Castro C, Bosetti C, Malvezzi M, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25(1):283–90.
14 Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30(6):1415–25.
15 Drahos J, Wu M, Anderson WF, Trivers KF, King J, Rosenberg PS, et al. Regional variations in esophageal cancer rates by census region in the United States, 1999–2008. PloS One. 2013;8(7):e67913.
16 Bosetti C, Levi F, Ferlay J, Garavello W, Lucchini F, Bertuccio P, et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer. 2008;122(5):1118–29.
17 Hurschler D, Borovicka J, Neuweiler J, Oehlschlegel C, Sagmeister M, Meyenberger C, Schmid U. Increased detection rates of Barrett’s oesophagus without rise in incidence of oesophageal adenocarcinoma. Swiss Med Wkly. 2003;133(37-38):507–14.
18 Levi F, Randimbison R, La Vecchia C. Esophageal and gastric carcinoma in Vaud, Switzerland, 1976-1994. Int J Cancer Journal. 1998;75(1):160–1.
19 Schmassmann A, Oldendorf MG, Gebbers JO. Changing incidence of gastric and oesophageal cancer subtypes in central Switzerland between 1982 and 2007. Eur J Epidemiol. 2009;24(10):603–9.
20 International rules for multiple primary cancers (ICD-O Third Edition). Lyon: International Agency for Research on Cancer (IARC); 2004.
21 IARCcrgTools – check and conversion programs for cancer registries. 2.05 ed: International Association of Cancer Registries (IACR); 2005.
22 Data and methods – Cancer Incidence and Mortality in Switzerland by NICER: National Institute for Cancer Epidemiology and Registration (NICER); [09/12/2014]. Available from: http://www.nicer.org/NicerReportFiles2014/methods_file/methods.htm.
23 Surveys, Sources – Cancer epidemiology – Data and methods Neuchâtel: Swiss Federal Statistical Office (SFO); [23/10/2015]. Available from: http://www.bfs.admin.ch/bfs/portal/en/index/infothek/erhebungen__quellen/blank/blank/kbs/02.html.
24 Doll R, Cook P. Summarizing indices for comparison of cancer incidence data. Int J Cancer. 1967;2(3):269–79.
25 Armstrong RW, Borman B. Trends in incidence rates of adenocarcinoma of the oesophagus and gastric cardia in New Zealand, 1978–1992. Int J Epidemiol. 1996;25(5):941–7.
26 Lord RV, Law MG, Ward RL, Giles GG, Thomas RJ, Thursfield V. Rising incidence of oesophageal adenocarcinoma in men in Australia. J Gastroenterol Hepatol. 1998;13(4):356–62.
27 Otterstatter MC, Brierley JD, De P, Ellison LF, Macintyre M, Marrett LD, Semenciw R, Weir HK. Esophageal cancer in Canada: trends according to morphology and anatomical location. Can J Gastroenterol. 2012;26(10):723–7.
28 Thomas RJ, Lade S, Giles GG, Thursfield V. Incidence trends in oesophageal and proximal gastric carcinoma in Victoria. Aust N Z J Surg. 1996;66(5):271–5.
29 Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2012;23(12):3155–62.
30 Brown LM, Hoover R, Silverman D, Baris D, Hayes R, Swanson GM, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol. 2001;153(2):114–22.
31 Pandeya N, Olsen CM, Whiteman DC. Sex differences in the proportion of esophageal squamous cell carcinoma cases attributable to tobacco smoking and alcohol consumption. Cancer Epidemiol. 2013;37(5):579–84.
32 Ribeiro U, Jr., Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83(9):1174–85.
33 Schweizerische Gesundheitsbefragung 2012. Neuchâtel: Federal Statistical Office (FSO), 2013.
34 Tackling harmful alcohol use – country note Switzerland: Organisation for Economic Cooperation and Development (OECD); [23/07/2015]. Available from: http://www.oecd.org/switzerland/Tackling-Harmful-Alcohol-Use-Switzerland-en.pdf.
35 Gmel G, Kuendig H, Notari L, Gmel C. Suchtmonitoring Schweiz – Konsum von Alkohol, Tabak und illegalen Drogen in der Schweiz im Jahr 2013. Lausanne, Schweiz: 2014.
36 Cancer in Switzerland – development from 1983–2007. Neuchâtel: Federal Statistical Office (FSO), National Institute for Cancer Epidemiology and Registration (NICER), Swiss Childhood Cancer Registry (SCCR), 2011.
37 Cook MB, Kamangar F, Whiteman DC, Freedman ND, Gammon MD, Bernstein L, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102(17):1344–53.
38 Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA: a cancer journal for clinicians. 2013;63(4):232–48.
39 Swiss Health Survey 2012 – Overview. Neuchâtel: Federal Statical Office (FSO), 2013.
40 Rubenstein JH, Taylor JB. Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Alimentary pharmacology & therapeutics. 2010;32(10):1222–7.
41 Turati F, Tramacere I, La Vecchia C, Negri E. A meta-analysis of body mass index and esophageal and gastric cardia adenocarcinoma. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2013;24(3):609–17.
42 Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the International BEACON Consortium. Int J Epidemiol. 2012;41(6):1706–18.
43 Schwenkglenks M, Marbet UA, Szucs TD. Epidemiology and costs of gastroesophageal reflux disease in Switzerland: a population-based study. Sozial- und Praventivmedizin. 2004;49(1):51–61.
44 Brenner H, Arndt V, Stegmaier C, Ziegler H, Rothenbacher D. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159(3):252–8.
45 Webb PM, Law M, Varghese C, Forman D, Yuan JM, Yu M, et al. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347–53.
46 Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, et al. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World journal of gastroenterology: WJG. 2013;19(36):6098–107.
47 Heuberger F, Pantoflickova D, Gassner M, Oneta C, Grehn M, Blum AL, Dorta G. Helicobacter pylori infection in Swiss adolescents: prevalence and risk factors. Eur J Gastroenterol Hepatol. 2003;15(2):179–83.
48 Sihvo EI, Salminen JT, Ramo OJ, Salo JA. The epidemiology of oesophageal adenocarcinoma: has the cancer of gastric cardia an influence on the rising incidence of oesophageal adenocarcinoma? Scand J Gastroenterol. 2000;35(10):1082–6.
49 Holster IL, Aarts MJ, Tjwa ET, Lemmens VE, Kuipers EJ. Trend breaks in incidence of non-cardia gastric cancer in the Netherlands. Cancer Epidemiology. 2014;38(1):9–15.
50 Blot WJ, Devesa SS, Kneller RW, Fraumeni JF, Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265(10):1287–9.
51 Wang HH, Antonioli DA, Goldman H. Comparative features of esophageal and gastric adenocarcinomas: recent changes in type and frequency. Human Pathology. 1986;17(5):482–7.
52 El-Serag HB, Mason AC, Petersen N, Key CR. Epidemiological differences between adenocarcinoma of the oesophagus and adenocarcinoma of the gastric cardia in the USA. Gut. 2002;50(3):368–72.
53 Carr JS, Zafar SF, Saba N, Khuri FR, El-Rayes BF. Risk factors for rising incidence of esophageal and gastric cardia adenocarcinoma. J Gastrointest Cancer. 2013;44(2):143–51.
54 Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(5):872–8.
55 Olefson S, Moss SF. Obesity and related risk factors in gastric cardia adenocarcinoma. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18(1):23–32.
56 Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, et al. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50(7):1330–44.
57 Peleteiro B, La Vecchia C, Lunet N. The role of Helicobacter pylori infection in the web of gastric cancer causation. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 2012;21(2):118–25.
58 Heusser R, Noseda G. Rahmenbedingungen für ein wirksames Krebsmonitoring in der Schweiz. Schweizer Krebsbulletin. 2012;32(2):143–8.
59 Heusser R, Lorez M, Bosshard D, Noseda G. Aufbau eines wirksamen nationalen Krebsmonitorings in der Schweiz: eine Aufgabe von NICER und den kantonalen Krebsregistern. Schweizer Krebsbulletin. 2011;31(3):237–41.
60 Wegmuller B, Bienlein M. The Swiss health care system. World hospitals and health services: the official journal of the International Hospital Federation. 2007;43(1):10–1.
61 Frei A, Huntsche E. The Swiss health care system. Eur J Health Econ. 2001;2:76–78.
62 Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–6.
63 Li-Qing Y. New endoscopic diagnosis and treatment options for early esophageal cancer. Journal of Gastrointestinal & Digestive System. 2012.
Disclosure Statement: The work received no funding. None of the authors declared any conflict of interest relevant to this article. No ethical approval was required.