Does airway intervention before primary nonsurgical therapy for T3/T4 laryngeal squamous cell carcinoma impact on oncological or functional outcomes?
DOI: https://doi.org/10.4414/smw.2015.14213
René
Schariatzadeh, Thomas
Pezier, Gabriela
Studer, Stephan
Schmid, Gerhard
Huber
Summary
QUESTIONS UNDER STUDY: Even today, some patients with laryngeal cancer present with airway obstruction necessitating an intervention in the form of either a tracheostomy or transoral laser debulking (TOL). Controversy exists as to whether such an intervention is a risk factor for poor oncological or functional outcome in patients who then undergo primary (chemo)radiotherapy.
METHODS: Retrospective chart review of all patients undergoing primary curative nonsurgical treatment for T3/T4 laryngeal squamous cell cancer at the University Hospital Zurich between 1981 and 2011.
RESULTS: A total of 29/114 patients had an airway intervention before initiation of (chemo)radiotherapy (21/29 tracheostomies, 8/29 TOL). Kaplan-Meier analysis showed no statistical difference in oncological outcomes between the groups with and without intervention (5 year overall survival: 52% vs 70%, disease specific survival: 73% vs 79%, recurrence free survival: 53% vs 63%). In functional terms, we report an overall functional larynx rate of 60%.
CONCLUSIONS: Airway intervention was not found to be a risk factor for poor oncological or functional outcome in this patient group.
Introduction
In the western world, laryngeal cancer was until recently the most common noncutaneous cancer of the head and neck [1]. It is now the second most common after the recent precipitous increase in oropharyngeal cancers.
Traditionally, treatment for advanced laryngeal cancer was a total laryngectomy. However, after the landmark Department of Veterans Affairs study [2] in 1991 and subsequent studies [3], many centres worldwide now treat all but the most advanced tumours with primary (chemo)radiotherapy, saving surgical treatment in the form of total laryngectomy for residual or recurrent disease or only the most bulky primary disease. This “organ preserving” approach is believed to achieve similar oncological outcomes to a primary surgical approach, with the benefit of retained laryngeal function for breathing, speaking and swallowing.
Selection of patients for either primary (chemo)radiotherapy or primary total laryngectomy is normally made in a tumour board setting and depends on local protocols, expertise and, of course, patient choice. One factor which is weighed in the balance is whether the patient has had to have an airway intervention prior to definitive treatment. Even with good access to medical care, some patients’ first presentation of their laryngeal cancer will be with acute airway obstruction, necessitating an emergency/urgent airway intervention. This airway intervention is life-saving in the short term and often performed outside of head and neck cancer centres by noncancer specialists. Patients are then referred on for definitive treatment of their laryngeal cancer. The Tumour Board must then decide if the airway intervention has disrupted/seeded the primary cancer and decide how this would influence the decision as the primary treatment modality.
For patients who undergo primary (chemo)radiotherapy, there also exists the possibility of requiring airway intervention either during or after their (chemo)radiotherapy. For example, tumour oedema from (chemo)radiotherapy may compromise the airway during therapy. There are also a few patients who, despite a satisfactory oncological outcome, have a nonfunctioning larynx and may require permanent tracheostomy or even laryngectomy potentially months or even years after the (chemo)radiation was completed.
The aim of this study was to retrospectively analyse a cohort of patients undergoing primary nonsurgical treatment for laryngeal cancer and see how many patients required an airway intervention, when it was performed, and what form this took (tracheostomy or transoral laser debulking [TOL]). Special emphasis is placed on patients who underwent airway intervention prior to definitive treatment and results are expressed in terms of both oncological and functional outcomes.
Materials and methods
A retrospective case note review was performed of all patients who underwent primary curative non-surgical treatment for T3/T4 laryngeal squamous cell cancer at the University Hospital Zurich between 1981 and 2011. Patients with less advanced disease, treated primarily with another modality or treated with palliative intent from the beginning, were excluded.
Patients’ demographic, staging, treatment and outcome data were collected with use of electronic patient records. Staging was based on both a clinical and a radiological examination of the patient and both versions 6 and 7 of the American Joint Committee on Cancer (AJCC) manual [4] were used as the study period straddled the change in 2009. Airway interventions in the form of tracheostomy or TOL were recorded, as was the date of eventual reversal of the tracheostomy if this was done. Patients who suffered from residual or recurrent disease were also recorded, along with their management.
All patients were discussed in our tumour board meeting and underwent primary nonsurgical treatment. Over such a long time-period one can imagine that the tumour board’s attitude to surgery or nonsurgical treatment changed. Anecdotally, it is probably fair to say that the more recent patients were more likely to have (chemo)radiotherapy than surgery, even those with advanced disease. Ultimately, the patients also have the final decision as to which treatment they will choose. Also of note, for patients with T4 disease and cartilage involvement, surgical treatment was recommended. The radiotherapy protocols evolved during the study period, notably with the advent of intensity modulated radiation therapy (IMRT) in March 2002 (consecutively since June 2003). All patients after this date were treated with IMRT, all those before underwent traditional multiplanar external beam radiotherapy. Details of the exact regimen have been published elsewhere [5].
Patients undergoing tracheostomy had this performed in the traditional open manner in all cases. No percutaneous tracheostomies were performed. Transoral debulking was performed using a CO2 laser. The indication for a tracheostomy was the same regardless of whether it was pre-, during or post- definitive treatment, i.e., a compromised airway with either inadequate ventilation or recurrent aspiration.
In terms of oncological outcomes, local recurrence (LR) was defined as either residual or recurrent squamous cell cancer (SCC) of the larynx or level 6. Regional recurrence (RR) was defined as either residual or new disease in the lateral neck, levels 1–5. Distant recurrence (DR) was diagnosed on imaging studies unless with possibility for cytological/histological confirmation existed.
Statistical analysis of overall survival (OS), disease specific survival (DSS), local recurrence-free survival (LRFS) and regional recurrence-free survival (RRFS) was calculated using the Kaplan-Meier method. The log-rank test was used for univariate analysis (SPSS, Chicago, Illinois, v21) and Pearson’s chi-squared or Fisher’s exact test for comparison of subgroups, as appropriate.
Functional outcomes are expressed as descriptive statistics of how many patients were able to be weaned off their tracheostomies, how many patients had permanent tracheostomies and how many progressed to total laryngectomy.
Results
A total of 114 patients (106 men, 8 women) were included in this study. Median age at diagnosis was 62 years. A more detailed description of patients and extent of disease can be found in table 1, which also compares the nonairway intervention group with the airway intervention group.
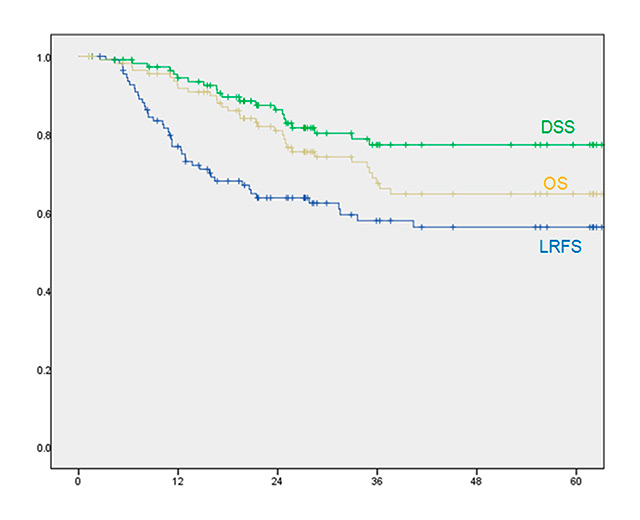
Figure 1
Five-year (60-month) comparison of overall survival (OS), disease specific survival (DSS) and local recurrence-free survival (LRFS). Note that the LRFS lies under the other two curves, indicating that despite local recurrence, patients can often be successfully salvaged.
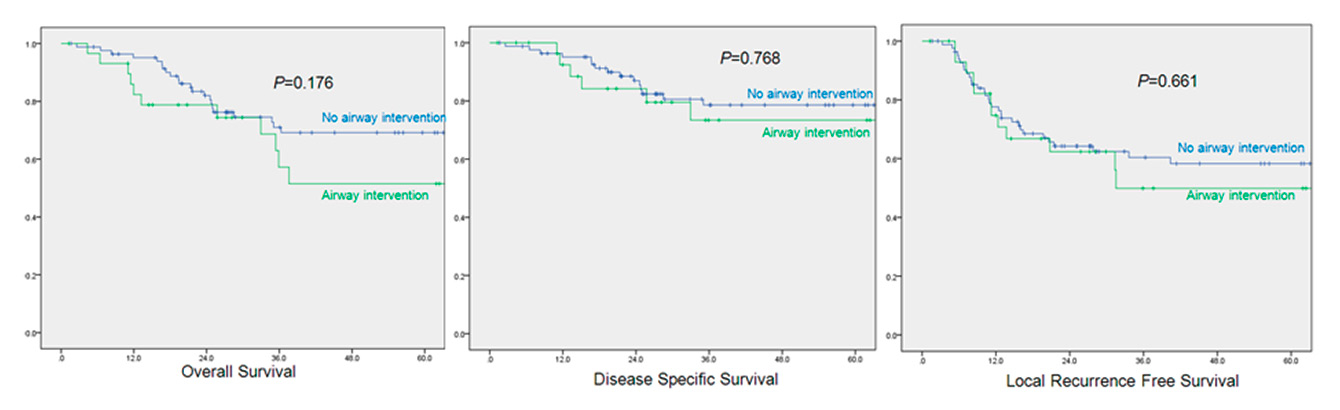
Figure 2
Pretreatment airway intervention vs no airway intervention: 5-year survival.
Oncological outcomes and timing of airway intervention
For the cohort as a whole, 5 year OS, DSS, LRFS were 65%, 78% and 56% respectively (fig. 1). Only 4/114 patients had evidence of disease less than 4 months after the end of primary therapy, implying that they had residual tumours that had been incompletely treated (persistent disease). A further 43/114 patients had recurrence of disease >6 months after end of therapy. Forty of 47 recurrences occurred within the first 2 years after initial therapy. Recurrences were treated with total laryngectomy (33/47 patients), neck dissection (1/47 patient) or palliative therapies (13/47).
Twenty-nine of the 114 patients had an airway intervention before commencement of radiotherapy (21/29 tracheostomies, 8/29 laser debulking [TOL]). One of these patients initially had an unsuccessful TOL which needed to be converted to a tracheostomy. During treatment, a further 3/114 patients had tracheostomy performed. After completion of treatment 19 patients underwent tracheostomy, and 5 patients had TOL.
No intervention vs predefinitive therapy airway intervention
To ensure that our comparison between the intervention and nonintervention groups was unbiased, we analysed the demographics and disease burden of the two groups to ensure comparability. Where 2x2 tables could be constructed, a two-tailed Fischer’s exact test was used, otherwise Pearson’s chi-squared test (table 1). This showed no significant differences in terms of age, gender or disease burden between the two groups.
Kaplan Meier survival analysis was then performed to compare the oncological outcomes in the two groups. No statistically significant differences in 5 year OS (70% vs 52%, p = 0.176), DSS (79% vs 73%, p = 0.768) and LRFS (58% vs 50%, p = 0.661) between no airway intervention vs pre-therapy airway intervention was found (fig. 2).
Pretreatment tracheostomy vs no tracheostomy / TOL
In the predefinitive therapy setting, it might be argued that there are fundamental differences between endoscopic approaches to securing the airway such as TOL and the intuitively more “risky” tracheostomy. The latter might cut directly into the tumour, seeding the tract and also runs the risk of disrupting lymphatics. Furthermore, the AJCC staging does not necessarily capture the subtleties of tumour burden and perhaps tracheostomies are still performed on the more severely affected patients, despite our negative chi-squared and Fischer’s tests outlined above. We therefore performed an analysis of the 21 patients undergoing pretherapy tracheostomy against the remaining 93 patients (i.e. those with TOL or no intervention). Once again, no statistically difference in disease burden or demographics was found between the two groups. Five-year LRFS was 51.3% vs 54.5% for the no-tracheostomy vs tracheostomy groups respectively (p = 0.633).
Functional outcomes
In total, 43/114 of patients had a tracheostomy at some stage of their treatment (21 pretreatment, 3 during treatment and 19 after). Thirteen of the 43 patients were able to be successfully weaned from their tracheostomies, 19/43 ultimately had a laryngectomy and 11/43 can be deemed to have a permanent tracheostomy despite an anatomical intact larynx (table 2).
Despite the initially “organ-preserving” approach, 46/114 (40%) patients ultimately lost their laryngeal function, resulting in 35 laryngectomies and 11 permanent tracheostomies. Whilst 33/35 laryngectomies were performed for oncological reasons, 2/35 were performed for purely functional reasons without any sign of cancer. For the cohort as a whole we therefore have a “functional larynx” rate of 68/114 (60%). Of the patients who needed pretherapy tracheostomy, this “functional larynx” rate was 12/21 (57%) and for patients undergoing no pretherapy intervention of 56/93 (60%).
|
Table 1: Comparison of patients and disease characteristics between intervention and non-intervention groups. |
| |
|
No airway intervention
|
Pretherapy airway intervention
|
Chi-squared/
Fisher’s exact
|
Total
|
|
Gender
|
Female |
6 |
2 |
1 |
8 |
| Male |
79 |
27 |
|
106 |
| Age
|
<60 |
37 |
10 |
0.389 |
47 |
| >60 |
47 |
20 |
|
67 |
|
cT-stage |
T3 |
58 |
19 |
0.678 |
77 |
| T4 |
25 |
10 |
|
35 |
| T4b |
1 |
1 |
|
2 |
|
cN-stage
|
N0 |
54 |
22 |
0.358 |
76 |
| N1 |
9 |
4 |
|
13 |
| N2a |
2 |
0 |
|
2 |
| N2b |
4 |
3 |
|
7 |
| N2c |
12 |
1 |
|
13 |
| N3 |
3 |
0 |
|
3 |
|
Stage |
III |
47 |
19 |
0.772 |
66 |
| IVa |
33 |
10 |
|
43 |
| IVb |
4 |
1 |
|
5 |
|
Site
|
Glottic |
28 |
10 |
0.649 |
38 |
| Supraglottic |
41 |
17 |
|
58 |
| Subglottic |
2 |
1 |
|
3 |
| Transglottic |
13 |
2 |
|
15 |
|
Table 2:Overview Any Recurrence free survival and functional larynx rate. |
|
Airway intervention
|
Tracheostomy
|
Debulking
|
Recurrence-free survival (%)
|
Functional larynx rate (%)
|
| None
n = 58 (51%) |
|
|
63 |
61 |
| Pretherapy airway intervention
n = 29 (25%) |
21 |
8 |
53 |
54 |
| During therapy airway intervention
n = 3 (3%) |
3 |
0 |
26 |
26 |
| Post-therapy airway intervention
n = 24 (21%) |
19 |
5 |
| Total
n = 114 (100%) |
43 |
13 |
61 |
60 |
Discussion
Laryngeal cancer is the second most common noncutaneous cancer of the head and neck. Cure rates are excellent for early disease, but problematic in more advanced disease. Indeed, laryngeal cancer is the only cancer for which survival outcomes have worsened in the last 20 years [1]. Many reasons have been put forward to explain this, including the increasing use of primary nonsurgical therapy [6].
Organ-preserving treatment of laryngeal cancer aims to achieve the same oncological outcomes as surgical approaches with the benefit of maintaining laryngeal function (speech and swallow). Laser ablation for T1 tumours or partial laryngectomies for more advanced tumours are surgical possibilities, but (chemo)radiotherapy is currently the most widespread treatment modality, especially since the advent of IMRT. Advances continue apace with dose painting being the latest innovation leading many units and patients to prefer a primarily nonsurgical approach to laryngeal cancer in all but the most advanced cases. In a few centres worldwide, organ-preserving surgery in the form of transoral robotic surgery (TORS) is once again being preferred to (chemo)radiotherapy.
Before deciding which treatment to recommend a patient, the tumour board must carefully decide the oncological risks and the functional risks. These questions must be answered not only before the start of treatment but throughout the patient’s management. For example, one key question is how to identify those patients who are nonresponders to (chemo)radiotherapy as early as possible. Our unit, in common with many others, use a positron emission tomography / computed tomography (PET-CT) scan at 3 months post-treatment, but ideally, some form of test during or even before the treatment would be far better (e.g. diffusion-magnetic resonance imaging [MRI], PET-CT).
One factor which might push tumour boards towards a surgical management plan is an antecedent airway intervention. It seems plausible that patients who have presented with airway obstruction and needed some form of surgical intervention might have sub-optimal outcomes with (chemo)radiotherapy and might therefore be more appropriately treated with a total laryngectomy with adjuvant radiotherapy. A recent publication showed that an antecedent airway intervention followed by primary total laryngectomy with adjuvant radiotherapy was not associated with adverse outcomes [7]. So if antecedent airway intervention was associated with increased risk in primary (chemo)radiotherapy patients then the message would be clear: patients with antecedent airway interventions should be counselled towards a primary surgical treatment.
Overall the literature in this area is confusing. Many articles have discussed the possibility of tumour inoculation [8] or seeding [9] during intubation [10], tracheostomy [11] or other operative manipulation of a laryngeal tumour. Some articles find that tracheostomy is a risk factor [12–14] whilst others find no increased risk [7, 15, 16]. The picture is further confused as studies often suffer from heterogeneous patient groups (for example mixing primary and salvage laryngectomies together) or from small sample size (for example fewer than 100 patients).
Furthermore, the key question, namely, if the patient has an antecedent airway intervention, what should the definitive management plan be, is not addressed. The airway intervention is often a “given” and may have been performed in non-head and neck units outside of normal hours as a life-saving procedure. To simply tell the patients that they are possibly at increased oncological risk does not seem particularly helpful.
We purposively picked a highly homogeneous patient group in order to answer a very specific question. Do patients with antecedent airway intervention undergoing primary nonsurgical treatment have worse outcomes than those without the antecedent airway intervention? Our data would suggest that there is no increased risk, though we accept the limitations of the retrospective nature of our data and small sample size. It is however in keeping with the largest series looking at this problem from Zhao et al. [15] (548 patients) and Petrovic et al. [16] (402 patients), who could also find no increased risk.
During treatment, some patients whose airway was initially deemed safe, suffer radiation-induced swelling which further reduces the diameter of the already compromised airway and necessitates airway intervention. This was the situation for three of our patients and, unfortunately, our data show that such circumstances are fore-bears of a dismal outcome.
The last group of patients who need tracheostomy after completing radiotherapy often require it because of an aspiration risk as much as for ventilation. These patients can sometimes be helped with intensive swallow physiotherapy and develop tricks to minimise their aspiration risk. Some however will find their voice next to useless and their aspiration risk so high as to justify a total laryngectomy for functional reasons. This was the case for 2/114 patients.
Having answered our primary oncological question, we have also looked at functional results associated with antecedent airway intervention. Overall, we report a functional larynx rate of 60% for the cohort as a whole is fairly similar to published figures (which often include less advanced cancers) [2, 3].
Conclusions
Our data would suggest that pre(chemo)radiotherapy airway intervention, whether in the form of tracheostomy or TOL does not negatively impact on oncological outcome. This adds to the considerable debate on this topic as many surgeons instinctively feel that it must increase risk.
In terms of functional outcomes, we report that of 114 patients initially undergoing primary nonsurgical treatment, 33 needed laryngectomy for oncological control, 2 for frozen larynx and a further 11 will remain tracheostomy dependent. This latter group may have a preserved larynx, but it is unfortunately, nonfunctional. This gives a “functional” larynx rate of 68/114 (60%) which is similar to other published figures [2, 3].
References
1 Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277–300.
2 Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324(24):1685–90.
3 Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–8.
4 Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
5 Studer G, Peponi E, Kloeck S, Dossenbach T, Huber G, Glanzmann C. Surviving hypopharynx-larynx carcinoma in the era of IMRT. Int J Radiat Oncol Biol Phys. 2010;77(5):1391–6.
6 Olsen KD. Reexamining the treatment of advanced laryngeal cancer. Head & neck. 2010;32(1):1–7.
7 Pezier TF, Nixon IJ, Joshi A, et al. Pre-operative tracheostomy does not impact on stomal recurrence and overall survival in patients undergoing primary laryngectomy. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2013;270(5):1729–35.
8 Gilson SD, Stone EA. Surgically induced tumor seeding in eight dogs and two cats. J Am Vet Med Assoc. 1990;196(11):1811–5.
9 Halfpenny W, McGurk M. Stomal recurrence following temporary tracheostomy. J Laryngol Otol. 2001;115(3):202–4.
10 Glaninger J. Problem of implantation metastasis by intubation anesthesia in surgery of cancer of the larynx. Monatsschr Ohrenheilkd Laryngorhinol. 1959;93(3):170–8.
11 Stell PM, van den Broek P. Stomal recurrence after laryngectomy: aetiology and management. J Laryngol Otol. 1971;85(2):131–40.
12 Esteban F, Moreno JA, Delgado-Rodriguez M, Mochon A. Risk factors involved in stomal recurrence following laryngectomy. J Laryngol Otol. 1993;107(6):527–31.
13 MacKenzie R, Franssen E, Balogh J, Birt D, Gilbert R. The prognostic significance of tracheostomy in carcinoma of the larynx treated with radiotherapy and surgery for salvage. Int J Radiat Oncol Biol Phys. 1998;41(1):43–51.
14 Basheeth N, O’Leary G, Khan H, Sheahan P. Oncologic outcomes of total laryngectomy: impact of margins and preoperative tracheostomy. Head & neck. 2015;37(6):862–9.
15 Zhao H, Ren J, Zhuo X, Ye H, Zou J, Liu S. Stomal recurrence after total laryngectomy: a clinicopathological multivariate analysis. Am J Clin Oncol. 2009;32(2):154–7.
16 Petrovic Z, Djordjevic V. Stomal recurrence after primary total laryngectomy. Clin Otolaryngol Allied Sci. 2004;29(3):270–3.

