Figure 1
Analysis of the 67 cases with methotrexate administration intervals shorter than a week at the University Hospital, Zurich.
DOI: https://doi.org/10.4414/smw.2015.14218
Methotrexate is used in the treatment of many medical conditions such as cancer as well as autoimmune diseases. In addition, scheduling of doses varies widely, from cyclical protocols for cancer chemotherapy (including high methotrexate doses) to weekly administration for autoimmune diseases such as rheumatoid arthritis and psoriasis (usually as low-dose methotrexate). Methotrexate is a high-risk drug with a narrow therapeutic window, difficult dosing regimens and dose adjustments to renal or hepatic organ insufficiency and the patient’s condition. As a result of this complexity, misunderstandings sporadically lead to serious incidents for patients, e.g. when methotrexate is administered once every morning (mo) instead of once every Monday (Mo) [1]. As a consequence, this overdosing may result in serious adverse drug events from a broad range of toxicities or, even death [2–4].
Methotrexate tops the list of high-risk drugs in the hospital setting causing fatal medication errors, life-threatening conditions, new or prolonged hospitalisations [5]. Frequent incidents involving methotrexate prescriptions and even fatal outcomes have been reported [2]. However, publications are mainly limited to case reports [1, 6–8] and, to the best of our knowledge, no comprehensive studies about the incidence of too frequent methotrexate prescriptions have been published. In addition, the thorough retrospective analysis of methotrexate prescription errors is very laborious because of the large number of prescriptions to be analysed and the heterogeneity of regimens applicable.
Various initiatives have been undertaken to reduce erroneous prescriptions. In England, the Department of Health defined the daily application of oral methotrexate as a “never event” (a medication error that should never occur) [9]. Multiple organisations have released recommendations to prevent future incidents with methotrexate [10–12]. However, as far as we know, no study has been published about the impact of methotrexate alerts before and after implementation, or about strategies to realise methotrexate alerts.
Two cases of too frequent methotrexate administrations were reported in 2014 to the Critical Incident Reporting System (CIRS) at the University Hospital Zurich (USZ) and one of these – a patient with febrile neutropenia – was reported to the regional pharmacovigilance centre of Zurich as a severe adverse drug reaction. Triggered by these cases we conducted a comprehensive retrospective analysis of the incidence of prescriptions for too frequent methotrexate dosing in nononcological therapies in the computerised physician order entry (CPOE) system at the USZ. For the purpose of comparison, an equivalent analysis has been performed at the Hospital Simmental-Thun-Saanenland AG (STS AG). To avoid too frequent methotrexate administrations a different quality assurance measure was implemented at each hospital. Finally, both concepts were assessed for their impact on patient safety.
We included all inpatients admitted to the USZ between December 2nd 2009 and June 30th 2014 (55 months) and retrospectively analysed all methotrexate medication orders and administrations in the electronic health record. Medication data from intermediate care, intensive care, emergency room and operating room were not electronically available and were therefore excluded.
The USZ uses KISIM (CISTEC AG, Zurich, Switzerland) as its CPOE system.
KISIM features a chemotherapy regimen calculation and prescription tool which labels the generated methotrexate prescription as chemotherapy. Those labelled orders were also excluded from the analysis.
To detect too frequent methotrexate administrations we computed the timespan of all consecutive planned administrations for each patient. Timespans of less than a week were considered as too frequent. Finally, the identified cases were processed manually to determine erroneous prescriptions.
As a result of our retrospective analysis a quality assurance programme was implemented at the USZ starting January 2015. This programme consists of a query for a daily list of all new methotrexate prescriptions to be checked by clinical pharmacologists. Methotrexate prescriptions in nononcological patients were reviewed for daily dosing. If too frequent dosing was observed, the attending physician was directly contacted by the clinical pharmacologist by phone. The data of the first 8 months were evaluated for a preliminary assessment of the programme.
We included all inpatients admitted to the STS between December 2nd 2009 and June 30th 2014 (55 months). The retrospective analysis was performed manually and involved all methotrexate medication orders and administrations in the electronic health record. Methotrexate chemotherapy prescriptions as well as medication data from intermediate care, intensive care, emergency room and operation room were electronically available but excluded from this analysis.
The STS uses CGM PHOENIX (CompuGroup Medical Schweiz AG, Niederwangen, Switzerland) as its CPOE system.
At the STS a methotrexate alert has been implemented since August 12th 2011 after there were several queries about how to properly apply methotrexate in nononcological patients. The retrospective analysis compared the period without alert (20 months) and the period with alert (35 months) to assess the impact of the alert on reducing too frequent prescription errors.
Chemotherapies and nononcological treatments are ordered within different working areas of the CPOE system. The implemented alert is triggered whenever methotrexate is prescribed for nononcological patients. The alert is interruptive and informs the user that methotrexate is, as a rule, ordered once a week. However, a too frequent prescription is still possible without further notice.
A medication error was defined as a failure in the treatment process that leads to, or has the potential to lead to, harm to the patient [13]. A prescription error is a failure in the treatment process on the level of the prescription and an administration error is an error on the level of the administration. A medication error is often, but not necessarily always, the result of an erroneous prescription, i.e. the error occurred on the administration level. A prescription error that was subsequently corrected and therefore did not reach the patient was defined as a near miss.
At both hospitals, the critical incident reporting system (CIRS) aims to learn from errors and near misses and to improve healthcare services. Thus, employees may anonymously report critical incidents with a standardised form on a third party server via a secured internet connection. For privacy reasons the office of quality management and patient safety anonymises name, time references and case specific details prior to publishing the case on the intranet. Cases to be reported are (1) near misses, (2) reversible harmful incidents and adverse events, as well as (3) no-harm incidents [14]. Cases with irreversible harm to the patient must not be reported in the CIRS system. At this time cases potentially relevant for pharmacovigilance are not automatically forwarded to a designated registration office [15].
In Switzerland professionals and consumers may send reports of adverse reactions to one of six regional centres in Switzerland. These reports are forwarded to the Swissmedic national pharmacovigilance centre, which collaborates with the international centre for drug safety run by the World Health Organization. In accordance with the Law on Therapeutic Products implemented on January 1st 2002, all serious adverse reactions must be reported. Adverse reactions are considered serious if they: (1) result in death, (2) are life-threatening, (3) lead to or prolong hospitalisation, (4) involve a persistent disability or incapacity, or, (5) are otherwise to be considered medically significant. The mere suspicion of causality between a reaction and a medicine suffice to report an event; no proof is needed [16].
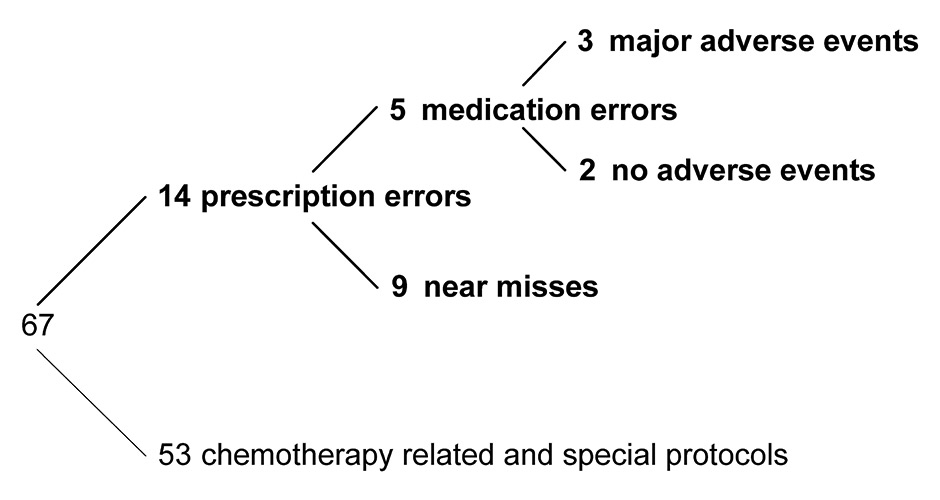
In the 55-month period of our study, a total of 4 007 903 medication prescriptions were ordered for 172 709 inpatients at the USZ, including 2 341 methotrexate prescriptions ordered for 1 561 patients. From those methotrexate prescriptions, a subset of 1 093 prescriptions was excluded from further analysis because they were linked to chemotherapy. Among the remaining 1 248 prescriptions for 888 patients, we identified 67 cases with more than one planned methotrexate administration per week. Next, clinicians and clinical pharmacologists scrutinised the medical record for each of the cases and identified 14 incidents that erroneously prescribed methotrexate too frequently, representing an incidence of 1.6% (14 prescription errors / 888 inpatients). Finally, in five incidents the erroneous prescriptions were not corrected and eventually led to medication errors – i.e. methotrexate was administered too frequently – including three incidents where major adverse events were observed (fig. 1). In two of the major adverse events the erroneous methotrexate prescription has been identified as the pancytopenia inducing drug, resulting in a prolonged stay of 7 days for one patient and a prolonged stay of 51 days including a 13 day intensive care unit admissions for another patient. In the third major adverse event methotrexate was not identified or suspected to be the causing drug in a multimorbid patient. The remaining two medication errors did not lead to harm to the patient and no adverse events were observed.

Figure 1
Analysis of the 67 cases with methotrexate administration intervals shorter than a week at the University Hospital, Zurich.
Regarding prescription errors, daily methotrexate prescriptions were the main source of errors (11/14). In two incidents more than one prescription was involved and in one incident a prescription with three administrations per week (Mon, Wed, Fri) remained uncorrected.
In the first 8 months after the quality assurance programme at the USZ started, clinical pharmacologists checked 652 methotrexate prescriptions ordered for oncological and nononcological inpatients and outpatients. So far, two prescriptions of too frequent methotrexate administrations have been intercepted by the clinical pharmacologist (on average one daily methotrexate prescription per 4 months). In addition, eight prescriptions with flawed unit entries (e.g. 1 mg instead of 1 tablet) and three double entries (e.g. 10 mg and 15 mg weekly) have been identified. However, the dosing of these prescriptions was rather low and none of them posed a genuine short-term patient risk.
The number of anonymised incidents reported to the CIRS System at the USZ and to the pharmacovigilance systems show that two medication errors with adverse events had been reported to the CIRS system and one case with adverse reactions was reported to the pharmacovigilance system. Medication errors without adverse events and near misses were not reported.
In the 55-month period of our study, 1 619 245 medication prescriptions were ordered for 99 872 inpatients including 217 methotrexate prescriptions ordered for 202 inpatients. Too frequent prescriptions were observed in three cases; two cases occurred before (79 inpatients, 20-month period) and one case occurred after (123 inpatients, 35-month period) the alert was installed. This implies a drop of the incidence (errors/inpatient) from 2.5% (2/79) to 0.8% (1/123). Further, in none of these cases was methotrexate administered too often, i.e. the prescription errors did not reach the patients.
In this quality control study all nononcological methotrexate prescriptions were analysed in respect of too frequent prescription in two Swiss hospitals: one for tertiary and one for secondary care. The initial incidences of too frequent low-dose methotrexate prescriptions were considered unacceptable at both hospitals.
Both quality assurance measures implemented improved patient safety. At the STS an interruptive alert was implemented which appears at order entry for every nononcological methotrexate prescription. The alert was implemented in August 2011 after several, predominantly young, physicians inquired about the proper application of methotrexate in nononcological patients. This measure resulted in a drop of the incidence of too frequent methotrexate prescriptions from 2.5% to 0.8%.
At the USZ the incidence (errors/inpatients) of 1.6% triggered a programme for quality checks by clinical pharmacologists based on a daily list of all new methotrexate prescriptions. The programme has been running for 8 months now and has successfully intercepted two cases where methotrexate was prescribed too frequently. Besides, the programme revealed that not only too frequent prescriptions but also flawed dosing or unit entries were an issue. Consequently, 8 prescriptions with incorrect dosing were identified and subsequently corrected in cooperation with the attending physicians. On average four prescriptions (with a maximum of 12) were reviewed per day, resulting in a significant increase in patient safety.
Several recommendations for safer methotrexate prescriptions in CPOE systems have been published [10–12]. However, the implementation of these recommendations is slow in hospital and pharmacy systems [17, 18], and, we know of no report that compares the situation before and after a methotrexate alert has been implemented. On the one hand, methotrexate prescription regimens are very heterogeneous, which makes prescriptions prone to error and the development of specific and effective automated alerts very challenging. On the other hand methotrexate displays a narrow therapeutic range, with a potential for severe toxicity or even fatal outcomes in cases of accidental overdosing [5]. Displaying information at every methotrexate order entry may be criticised as being unspecific and therefore inducing alert fatigue and systematic overrides. However, methotrexate prescriptions are quite rare compared with other drug prescriptions; young physicians especially are often not familiar with the drug and could benefit from additional decision support. More elaborate algorithms may differentiate between oncological and nononcological therapies and alert only when the time between two planed administrations is less than 7 days. Additionally, such alerts would also need to be able to deal with dosages split across different days and dosages composed of tablets of different strengths. In contrast to automated alerts, clinical pharmacologists’ interventions display higher sensitivity and specificity. Timely review of medications and punctual communications with direct contact to clinicians decrease medication errors and rates of adverse drug reactions [19, 20].
We realised that only a fraction of prescription errors were reported to the CIRS at the USZ and one incident with an adverse event was independently reported to the regional pharmacovigilance centre. The direct flow of information from CIRS to the regional pharmacovigilance centre might deter professionals from reporting adverse events because of fear of involvement in litigation. Near misses and critical incidents are usually part of CIRS and not reporting them misses an opportunity for the organisation to learn from errors before they reach the patient. Correcting prescription errors is common practice in the healthcare process and therefore health professionals may prefer to focus on urgent priorities instead of reporting near misses [21]. Furthermore, spontaneous reporting systems are well-known for their significant and widespread underreporting. Reporting rates and quality are dependent on the initiative and motivation of the reporters. However, the value of reported incidents lies in signal detection and identification of hazards on which to focus quality improvement activities [22].
Some limitations of the study need to be addressed. Too frequent low-dose methotrexate administrations are not only a problem in the hospital but also in the ambulatory setting. Prescriptions of ambulatory patients are not entered in our CPOE and were therefore not included in this study. Further, this study focused on analysing and preventing prescription and medication errors at the hospital. Factors leading to such errors, e.g. misinterpretation of prescriptions at hospital admission, were not addressed and could be a field of interest in further studies. The reduction of the incidence of prescriptions with too frequent administrations could not be documented with statistical significance and longer follow-up periods would be needed; i.e. despite the fact that the number of incidents was considered much too high for the hospitals and their patients, these numbers were too low for inferential statistics. Both hospitals had introduced CPOE long before the start of the study period. Therefore, it was not possible to compare the incidences before (ordering on paper) and after the implementation of CPOE. No data exist regarding the incidence of methotrexate prescription errors in the paper-ordering era. However, CPOE with clinical decision support systems prevent medication errors, as shown in several studies [23–25].
In conclusion, the initial incidences of too frequent low-dose methotrexate prescriptions observed were considered as unacceptable at both hospitals, since any accidental overdosing of methotrexate has the potential for life-threatening consequences. As a result, each hospital introduced different quality assurance measures. Preliminary data available indicate that both approaches implemented may have an impact on patient safety. Clinical pharmacologists’ interventions were able to avoid two potentially severe methotrexate prescription errors within 8 months. To assess and compare different concepts of quality assurance, however, long-term studies are needed for reasons of statistical and medical significance.
1 Arnet I, Bernhardt V, Hersberger KE. Methotrexate intoxication: the pharmaceutical care process reveals a critical error. J Clin Pharm Ther. 2012;37(2):242–4.
2 Moore TJ, Walsh CS, Cohen MR. Reported medication errors associated with methotrexate. Am J Health Syst Pharm. 2004;61(13):1380–4.
3 Weinblatt ME. Toxicity of low dose methotrexate in rheumatoid arthritis. J Rheumatol Suppl. 1985;12(Suppl 12):35–9.
4 Psoriasis-liver-methotrexate interactions. Arch Dermatol. 1973;108(1):36-42.
5 Saedder EA, Brock B, Nielsen LP, Bonnerup DK, Lisby M. Identifying high-risk medication: a systematic literature review. Eur J Clin Pharmacol. 2014;70(6):637–45.
6 Sinicina I, Mayr B, Mall G, Keil W. Deaths following methotrexate overdoses by medical staff. J Rheumatol. 2005;32(10):2009–11.
7 Karch AM, Karch FE. A weekly dosage taken daily. Am J Nurs. 2003;103(4):64.
8 Fässler EMI, Galeazzi RL. “Low dose”-Methotrexat: Nebenwirkungen und Toxizität. Schweiz Med Forum. 2003(49):1211–15.
9 Department of Health/ Patient Safety and Investigations. The “never events” list 2012/13. 2012 [cited 2015 Feb 3]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/215206/dh_132352.pdf.
10 Institute for Safe Medication Practices. Still Outside the Bull’s Eye: 2014-2015 Targeted Medication Safety Best Practices (Baseline Survey Results). 2014 [cited 2015 Feb 2]. Available from: http://www.ismp.org/newsletters/acutecare/showarticle.aspx?id=76.
11 National Patient Safety Agency. Oral methotrexate tablets - IT requirement specification. 2006 [cited 2015 Jan 29]. Available from: http://www.nrls.npsa.nhs.uk/EasySiteWeb/getresource.axd?AssetID=60036&type=full&servicetype=Attachment.
12 Patient Safety Foundation. Quick-Alert Nr. 28 Methotrexat-Intoxikation -> Vermeidung von oralen Überdosierungen. 2012 [cited 2015 Feb 3]. Available from: http://www.patientensicherheit.ch/dms/de/themen/quick-alerts/3328_Quick-Alert_Nr-28_Methotrexat_20121010_dt/3328_Quick-Alert_Nr.28_Methotrexat_20121010_dt.pdf.
13 Ferner RE, Aronson JK. Clarification of terminology in medication errors: definitions and classification. Drug Saf. 2006;29(11):1011–22.
14 Hewitt TA, Chreim S. Fix and forget or fix and report: a qualitative study of tensions at the front line of incident reporting. BMJ Qual Saf. 2015;24(5):303–10.
15 Meier M, Giuliani F, Schneemann M. Bedeutung von CIRS für Spitäler und Hausarztpraxen. Praxis. 2015;104(5):219–26.
16 Swissmedic Pharmacovigilance Centre. Pharmacovigilance. [cited 2015 May 5]. Available from: https://www.swissmedic.ch/marktueberwachung/00135/00160/index.html?lang=en.
17 Institute for Safe Medication Practices. 2014-15 Targeted Medication Safety Best Practices for Hospitals (Baseline Survey Results 2014). 2014 [cited 2015 Feb 3]. Available from: http://www.ismp.org/Tools/BestPractices/TMS%20Results-040214.pdf http://www.ismp.org/Tools/BestPractices/TMS Results-040214.pdf .
18 Ojeleye O, Avery AJ, Boyd MJ. Assessing the safety features of electronic patient medication record systems used in community pharmacies in England. Br J Clin Pharmacol. 2014;78(2):401–9.
19 Kaushal R, Bates DW, Abramson EL, Soukup JR, Goldmann DA. Unit-based clinical pharmacists’ prevention of serious medication errors in pediatric inpatients. Am J Health Syst Pharm. 2008;65(13):1254–60.
20 Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf. 2011;20(9):738–46.
21 Reason J. Human error: models and management. West J Med. 2000;172(6):393–6.
22 Westbrook JI, Li L, Lehnbom EC, Baysari MT, Braithwaite J, Burke R, et al. What are incident reports telling us? A comparative study at two Australian hospitals of medication errors identified at audit, detected by staff and reported to an incident system. Int J Qual Health Care. 2015;27(1):1–9.
23 van Doormaal JE, van den Bemt PM, Zaal RJ, Egberts AC, Lenderink BW, Kosterink JG, et al. The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. J Am Med Inform Assoc. 2009;16(6):816–25.
24 Bates DW, Leape LL, Cullen DJ, Laird N, Petersen LA, Teich JM, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280(15):1311–6.
25 Chertow GM, Lee J, Kuperman GJ, Burdick E, Horsky J, Seger DL, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286(22):2839–44.
Authors’ contribution:S. Karlen: designed and performed the research, assessed patient history, analysed and interpreted data, and wrote the manuscript.
M. Oertle: performed the research, assessed patient history, analysed and interpreted data, and edited the manuscript.
S. Weiler: contributed to the research, assessed patient history, performed quality assurance measures, interpreted data, and edited the manuscript.
M. Schneemann: contributed to the research, assessed patient history, interpreted data, and edited the manuscript.
E. Eschmann: contributed to the research, interpreted data, and edited the manuscript.
G.A. Kullak-Ublick: contributed to the research, interpreted data, and edited the manuscript.
J. Blaser: designed the research, interpreted data, and edited the manuscript.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.