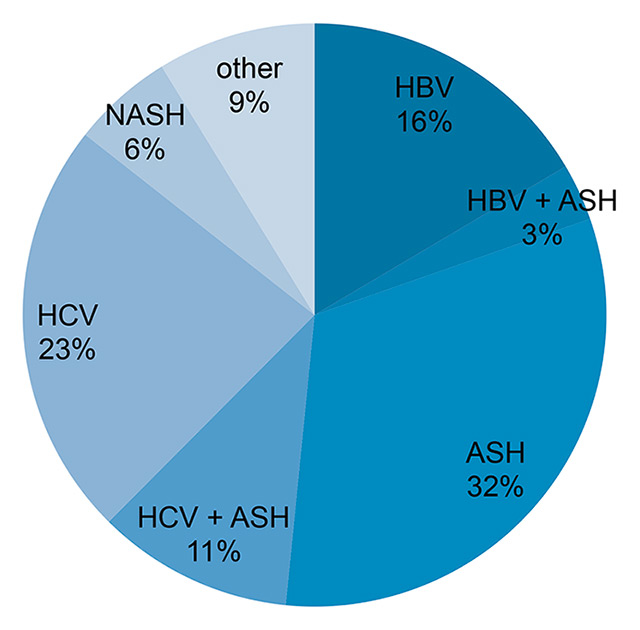
Figure 1
Frequency of underlying liver disease of the patients included in the surveillance cohort.
ASH = alcoholic steatohepatitis; HBV = chronic hepatitis B; HCV = chronic hepatitis C; NASH = nonalcoholic steatohepatitis.
DOI: https://doi.org/10.4414/smw.2015.14200
Abbreviations
AASLD American Association for the Study of Liver Diseases
AFP alpha-foetoprotein
APASL Asian Pacific Association for the Study of the Liver
ASH alcoholic steatohepatitis
BCLC Barcelona clinic liver cancer
CEUS contrast-enhanced ultrasound
CT computed tomography
EASL European Association for the Study of the Liver
EORTC European Organisation for Research and Treatment of Cancer
HCC hepatocellular carcinoma
HBV hepatitis B virus
HCV hepatitis C virus
MRI magnetic resonance imaging
NASH nonalcoholic steatohepatitis
NNS number needed to screen
RFA radiofrequency ablation
SIRT selective internal radiotherapy
TACE transarterial chemoembolisation
US ultrasound
Liver cancer is the fifth most common cancer in men worldwide and seventhmost common in women [1]. Because the diagnosis often is delayed, hepatocellular carcinoma (HCC) has the reputation of a rapidly progressive cancer with a bad prognosis. However, HCC usually develops on the basis of liver cirrhosis and has a prolonged subclinical growth period during which it can be diagnosed in earlier stages that are amenable to curative treatments. It has long been recognised that patients with liver cirrhosis have an increased risk for HCC. Moreover, chronic viral hepatitis B has been found to increase HCC risk even in the precirrhotic stage of the disease, especially in patients with Asian and African ancestry. The potential benefit of detection of HCC at earlier stages has motivated several expert groups and professional associations to recommend HCC surveillance with 6 monthly liver ultrasound examinations for patients at risk for HCC [2–4]. These recommendations are based on limited evidence. Well-designed prospective randomised trials that compare surveillance versus no surveillance are lacking, and most experts agree that such studies are unlikely to be performed in the future [5, 6]. A recent meta-analysis concluded that current clinical policy recommendations for HCC screening can neither be supported nor refuted on the basis of published studies, because there was only very-low-strength evidence for efficacy of HCC screening, and because data about harms caused by screening are completely missing [7].
In Switzerland, HCC screening policies vary widely between regions, hospitals and practitioners. HCC screening programmes are implemented in the hepatology speciality clinics of University Hospitals and major regional referral hospitals, but are largely absent outside this setting. Given the controversies about the benefits of HCC screening, we analysed the performance of a long-standing surveillance programme performed at the University Hospital Basel, Switzerland.
The HCC surveillance programme at the Clinic for Gastroenterology and Hepatology of the University Hospital Basel follows the American Association for the Study of Liver Diseases (AASLD) Practice Guidelines [3] and includes patients with cirrhosis and hepatitis B virus (HBV) carriers without cirrhotic liver but increased risk for HCC (Asian males ≥40 years, Asian women ≥50 years, Africans ≥20 years, family history of HCC). Surveillance patients are investigated every 6 months by means of abdominal ultrasound (US). We performed a systematic review of clinical charts, US reports and reports of additional examinations such as computed tomography (CT), magnetic resonance imaging (MRI), contrast-enhanced ultrasound (CEUS) and liver biopsy. All these examinations and analyses were performed or directly supervised by board-certified gastroenterologists/hepatologists, radiologists and pathologists.
Patients were classified as adherent to the surveillance programme if the interval between US examinations did not exceed 8 months. Nonadherent patients were subdivided into further participation in the surveillance with intervals >8 months or nonadherence for unknown reason. They were classified as lost to follow-up if they left Switzerland or did not react to more than three calls.
Determination of serum alpha-foetoprotein (AFP) levels is not mandatory in the Basel surveillance programme, but AFP levels were nevertheless determined in more than two-thirds of the visits, and were collected and analysed as well.
In all cases with focal lesion detected by US, the results of the following examinations, their congruency and the ability to make a definitive diagnosis were determined by reviewing all reports.
Diagnosed HCCs were staged according to the Barcelona Clinic Liver Cancer (BCLC) staging system [2].
In the years 2011 and 2012, 42 patients with a diagnosis or the suspicion of an HCC were referred to our clinic for further staging and treatment from other hospitals or practitioners. Seven of them were in an external US surveillance programme, 35 had no surveillance. The characteristics of these patients are shown in table 2. The BCLC stages and the treatments of those 42 patients were used for comparison with the 9 patients who were detected in the internal surveillance programme.
The study was based on regular patient charts. There were no additional examinations, interviews or questionnaires for study purposes. All data presented in this report are anonymised. According to the regulations of the University of Basel, no ethical approval was required for this retrospective study.
The number needed to screen (NNS) was calculated by dividing the total number of patients in the surveillance programme who had at least one US in the 2-year study period and the total number of surveillance US examinations carried out during the study period, by the number of patients with newly diagnosed HCCs detected through a screening US. For these calculations, all patients were included irrespective of their adherence to the surveillance programme. The costs per HCC detected were calculated by dividing the sum of the costs of all surveillance US examinations (consultation, US and laboratory tests) plus all further procedures such as CT, MRI, CEUS and liver biopsies by the number of newly diagnosed HCCs. For this cost calculation we used representative bills for surveillance US examinations, CT, MRI, CEUS and liver biopsies. Patients are charged according to the Tarmed tariff, the official reimbursement system for Switzerland (Tarmed Suisse [8]). The mean effective cost per surveyed patient per year was calculated by dividing the overall costs by the number of surveyed patients and the number of study years.
| Table 1: Modalities used for diagnosis of HCC, staging and treatment in 9 patients with newly diagnosed HCC. | ||||||
| HCC patients | Biopsy | CT | CEUS | BCLC | Within Milan | Therapy |
| Patient A | Yes | Yes | No | A | Yes | Liver transplantation |
| Patient B | Yes | Yes | No | Yes | RFA | |
| Patient C | Yes | Yes | Not done | A | Yes | Laparoscopic resection |
| Patient D | Yes | Yes | Not done | A | Yes | RFA |
| Patient E | Yes | Yes | Not done | A | Yes | Resection |
| Patient F | Yes | No | Not done | Yes | Laparoscopic resection | |
| Patient G | Not done | Yes | Yes | A | Yes | Resection |
| Patient H | Not done | Yes | No | A | Yes | Resection |
| Patient I | Not done | Yes | Not done | A | No (4 nodules, all <11 mm) | Liver transplantation |
| Accuracy | 6 / 6 (100%) | 8 / 9 (89%) | 1 / 4 (25%) | |||
| BCLC = Barcelona Clinic Liver Cancer stage; CEUS = contrast enhanced ultrasound; CT = computed tomography; HCC = hepatocellular carcinoma; RFA = radio frequency ablation | ||||||
In the 2-year period from 2011 to 2012, 696 surveillance US examinations were carried out in 285 patients, of whom 248 (87.0%) had liver cirrhosis. Of these, 63% were male and 37% female patients. The age ranged from 22 to 83 years (median = 55). The underlying liver disease was hepatitis C virus (HCV) infection alone in 23%, HCV and alcoholic steatohepatitis (ASH) in 11%, hepatitis B virus (HBV) infection alone in 16%, HBV and ASH in 3%, ASH alone in 32%, nonalcoholic steatohepatitis (NASH) in 6% and other liver diseases in 9% (eight primary biliary cirrhosis, four autoimmune hepatitis, two combination of primary biliary cirrhosis / autoimmune hepatitis, two primary sclerosing cholangitis, two haemochromatosis, one porphyria, one Carney syndrome, two cystic fibrosis, one drug-induced/toxic hepatitis, one nodular reactive hyperplasia, one multifactorial hepatitis) (fig. 1). A total of 145 (51%) patients had had surveillance US examinations already before 2011 (pre-existing HCC surveillance cohort), 140 (49%) patients entered the surveillance programme during the study period from 2011–2012. During the 2-year observation period, 63 (22%) of the patients had four or more, 81 (28%) had three, 56 (20%) had two and 85 (30%) had one surveillance US examination(s).

Figure 1
Frequency of underlying liver disease of the patients included in the surveillance cohort.
ASH = alcoholic steatohepatitis; HBV = chronic hepatitis B; HCV = chronic hepatitis C; NASH = nonalcoholic steatohepatitis.
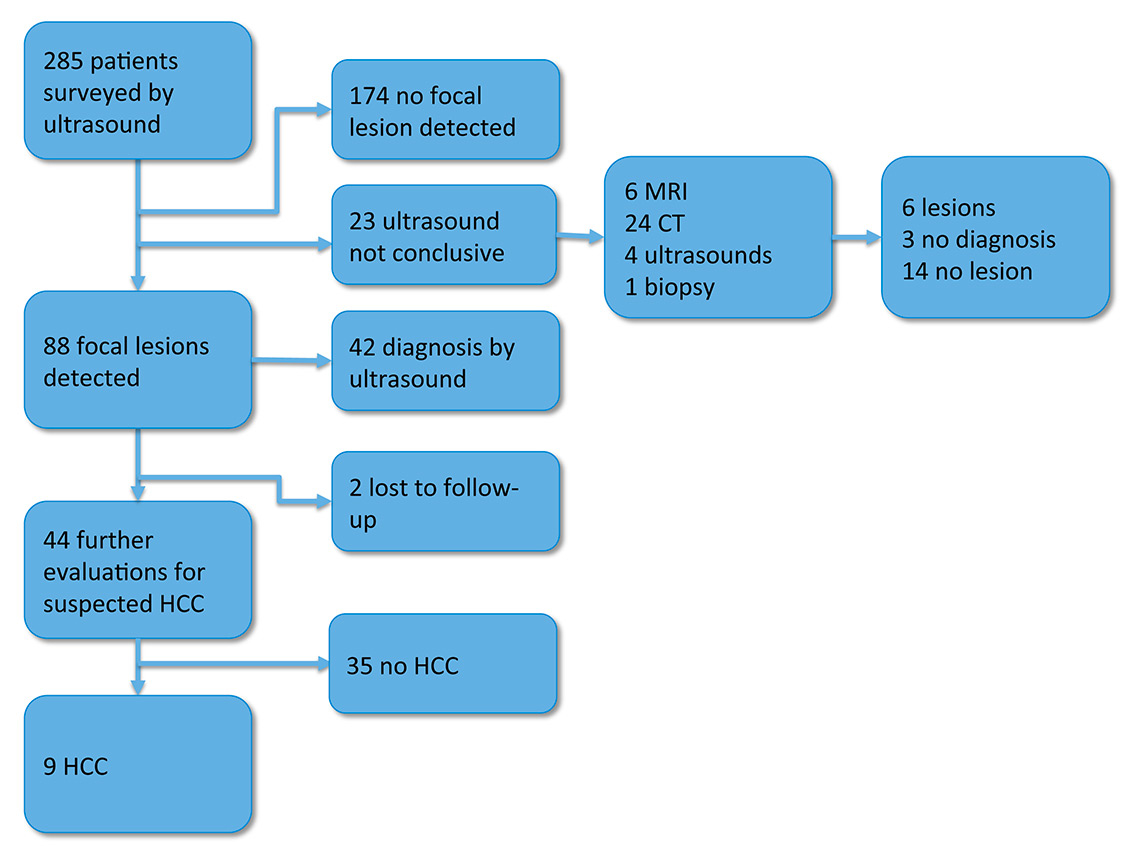
Figure 2
Diagnostic work-up and results in the 285 patients surveyed at the University Hospital Basel in the years 2011 and 2012.
CT = computed tomography; HCC = hepatocellular cancer; MRI = magnetic resonance imaging
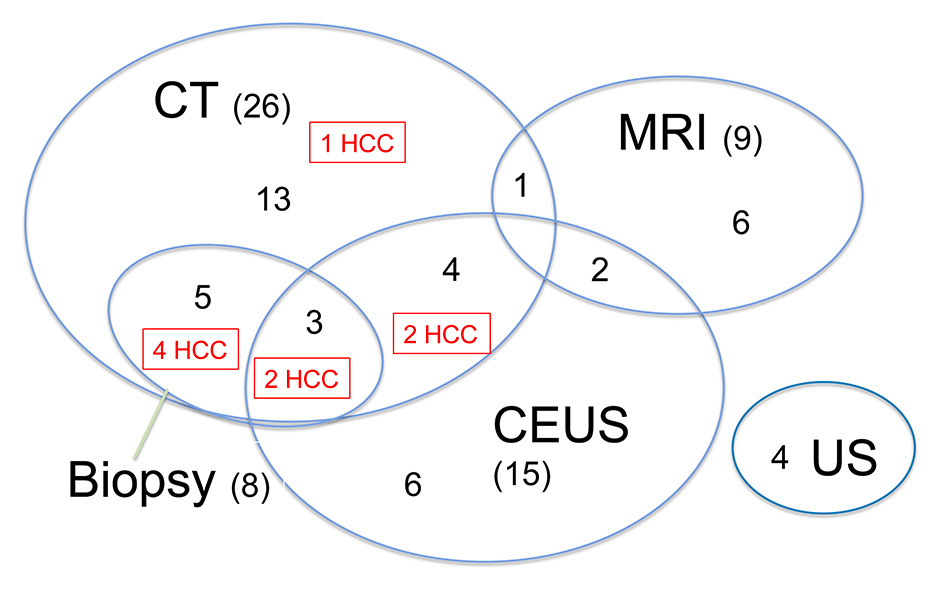
Figure 3
Number and combination of the examinations carried out in order to evaluate a suspicious lesion detected by surveillance ultrasound. Numbers in parenthesis show the total examination number for the different modalities. Regular numbers show the number of patients with any given combination of examinations. The red boxes highlight the examinations used in patients diagnosed with HCC.
CT = computed tomography; CEUS = contrast enhanced ultrasound; HCC = hepatocellular cancer; MRI = magnetic resonance imaging
Overall, 221 (77.5%) of the patients attended all planned screening visits and were classified as adherent. Sixty-four (22.5%) of the patients were nonadherent: 21 had surveillance intervals that exceeded 8 months and 43 patients did not attend further screening visits for unknown reasons and were considered lost to follow-up.
A focal lesion was detected with US in 88 (31%) of the 285 patients in the surveillance programme (fig. 2). The US examination allowed a definitive diagnosis in 42 of these patients (12 simple cysts, 8 haemangiomas, 6 focal fat sparing, 8 calcifications, 2 arteriovenous shunts, 2 regenerative nodules, 3 cicatrices, 1 periportal connective tissue). Two patients were lost to follow-up. The other 44 patients had further examinations with CT (26), MRI (9), native (4) and contrast-enhanced US (15), needle biopsy (8) or a combinations of several of these (fig. 3). Twenty-three of them had one, seven patients had two, seven had three, and seven had more than three additional examinations. In nine of them a HCC was diagnosed, most often through combination of two imaging techniques and/or needle biopsies (fig. 2 and 3, table 1). In the other 35 of the 44 patients with suspicious or unclear focal lesions who underwent further diagnostic evaluations, 12 definitive diagnosis were made (one metastasis, one echinococcal cyst, three focal fat sparing, two regenerative nodules and five haemangiomas), eight cases had no confirmation of a focal lesion by use of other imaging modalities, three patients were lost to follow-up and 12 cases remained without diagnosis. In all cases without diagnosis the lesion was <10 mm. All lesions were confirmed in additional imaging studies (CT or MRI), but no diagnosis could be obtained. The lesions did not progress in size during follow-up. Of note, in 2 of the 12 patients the lesions were found to increase in size on follow-up US after the end of the study period (2011–2012), and were then diagnosed as HCCs.
No focal lesion was seen in 174 (61%) of the patients. No further examinations were made in these patients because the US examinations were of high quality and allowed the exclusion of an HCC with high confidence.
In the remaining 23 (8%) patients surveillance US was inconclusive (i.e. could not detect or rule out a focal lesion with sufficient certainty) (fig. 2). These ultrasounds were not conclusive as a result of difficult ultrasonic conditions such as obesity, ascites, meteorism or an uncooperative patient. All the patients had further evaluations (24 CT, 6 MRI, 4 US and 1 biopsy). Six patients were found to have a focal lesion (four cysts, one regenerative nodule, one cicatrix), three patients had a “small arterial enhancing nodule” (SAEN), in 14 patients a focal lesion could be definitely ruled out.
In summary, in the surveillance cohort of 285 patients, 9 (3%) HCCs were detected in a 2-year period. Focal lesions with other aetiologies were diagnosed in 60 (21%) patients. In 174 patients, focal lesions were ruled out with high confidence in the surveillance US. In the remaining 42 patients, further diagnostic work-up ruled out a focal lesion in 22 patients, remained inconclusive in 15, and 5 patients were lost to follow-up.
Of the nine newly diagnosed HCCs, two were at BCLC stage 0 and seven at BCLC stage A. Six of the seven patients with BCLC stage A were within Milan Criteria (single nodule ≤5 cm or three nodules ≤3 cm of diameter [2, 9]) (table 1). Two patients were treated with liver transplantation, five with surgical resection and two with radiofrequency ablation (RFA) (table 1).
The number needed to screen in order to detect one HCC in a surveillance period of 2 years is 32 patients or 78 surveillance US examinations. In the 2 years, 2011 and 2012, of the surveillance programme, the following examinations were performed: 696 surveillance US, 55 CT, 18 MRI, 16 CEUS, 17 further US and 10 biopsies. The surveillance US caused total costs of 197 977 Swiss francs (CHF). Indirect costs generated by further examinations (CT, MRI, CEUS, US, biopsies and laboratory analysis) amounted to CHF 69 331. The combined total costs of the surveillance programme were CHF 267 308. The calculated costs per newly detected HCC were CHF 29 701. The annual costs per patient add up to CHF 469.
In eight of the nine patients with HCC, the diagnosis could be made using the noninvasive imaging criteria defined by the European Association for the Study of the Liver (EASL) – European Organisation for Research and Treatment of Cancer (EORTC) guidelines [2]. In one patient who did not fulfil the noninvasive imaging criteria, the diagnosis was made by use of US-guided needle biopsy and histology (table 1). Biopsies were performed in six of the nine patients with HCC, and confirmed the diagnosis in all of them. In the remaining three patients, a biopsy was not performed because of subcapsular localisation of the focal lesion. These three patients were treated with laparoscopic atypical resection (“surgical biopsy”) in two, and liver transplantation in one patient. In all cases, HCC was confirmed by histopathology in the resected specimen or the explanted liver.
AFP measurements were not an integral part of the surveillance programme. Therefore, we cannot address the question as to whether AFP measurements improved detection rates of HCCs in our programme. However, since AFP measurements were nevertheless ordered in 69.3% of all surveillance US examinations (482 of 696) by the US examiner, we wanted to investigate if AFP measurements caused additional unnecessary further examinations. In total, AFP was increased in 68 patients (23.9%). The value was between upper limit of normal (5.8 ng/ml) and 50 ng/ml in 60 patients, between 50 ng/ml and 400 ng/ml in 8 patients, and never above 400 ng/ml. Increased AFP values did not lead to further examinations in the absence of a focal lesion detected by US. In patients with focal lesions on US suspicious of HCC, increased AFP values did not change the diagnostic pathway. Likewise, in patients with nonconclusive US examinations, AFP values did not influence the time-point or the choice of additional examinations such as CT or MRI.
In the years 2011 and 2012, a total of 51 newly diagnosed HCCs were seen in the Clinic for Gastroenterology and Hepatology of the University Hospital Basel. As described above, nine of them were detected in the context of the institutional HCC surveillance programme. The other 42 patients were referred from other hospitals or practitioners. Seven of them had regular HCC screening US examinations, and were therefore also detected in screening programmes. The other 35 patients were not screened. Table 2 summarises patient characteristics of these three different groups of HCC patients.
As expected, HCCs detected in a screening programme were at an earlier stage. Of the nine patients detected in the University Hospital Basel surveillance cohort, two had BCLC stage 0 and seven had BCLC stage A. Of the seven externally screened patients, one was at BCLC 0, two were at BCLC A, and four at BCLC stage B. Eighteen of the 35 nonsurveyed patients also had early stage HCC and were at BCLC 0 or A, but the remaining patients were at BCLC B (nine), BCLC C (three) or BCLC D (five) (fig. 4A).
As a consequence of the frequently advanced stage in nonsurveyed patients, only 12 of the 35 patients in this group were treated with curative intent (one liver transplant, seven resections, four RFA). In contrast, all patients from the University Hospital Basel surveillance cohort had curative treatments (two liver transplants, five resections and two RFA). Treatment of externally screened patients was once with liver transplantation, one resection and five with transarterial chemoembolisation (fig. 4B).
| Table 2: Patient characteristics (age, sex, cirrhosis and underlying liver disease) of internally surveyed, externally surveyed and not surveyed HCC patients. | ||||
| Patient characteristics HCC patients | Age in years (median and range) | Sex | Cirrhosis | Underlying liver disease |
| Internal surveillance n = 9 | 58.4 (50–74) | 78% male | 100% | 11% HCV (1), 33% HCV + ASH (3), 33% ASH (3), 11% HBV + ASH (1), 11% HBV (1) |
| External surveillance n = 7 | 64.7 (24–78) | 86% male | 86% | 43% HCV (3), 43% ASH (3), 14% HBV (1) |
| No surveillance n = 35 | 68.1 (45–87) | 80% male | 77% | 20% HCV (7), 11% HCV + ASH (4), 34% ASH (12), 9% HBV + ASH (3), 3% HBV (1), 11% NASH (4), 3% PBC (1) and 9% no liver disease (3) |
| All HCC patients n = 51 | 65.9 (24–87) | 80% male | 82% | 22% HCV (11), 14% HCV + ASH (7), 35% ASH (18), 8% HBV + ASH (4), 6% HBV (3), 8% NASH (4), 2% PBC (1), 6% no liver disease (3) |
In this HCC surveillance programme of a clinic for Gastroenterology and Hepatology of a University Hospital in Switzerland the yearly HCC detection rate was 1.6%. The number needed to screen was 32 patients or 78 surveillance US examinations. All of the patients from the surveillance cohort could undergo curative treatment. The estimated costs per detected HCC were CHF 29 701 in this surveillance programme. We conclude that HCC surveillance is highly effective in detecting HCCs in the early stages and that the costs are reasonably low.
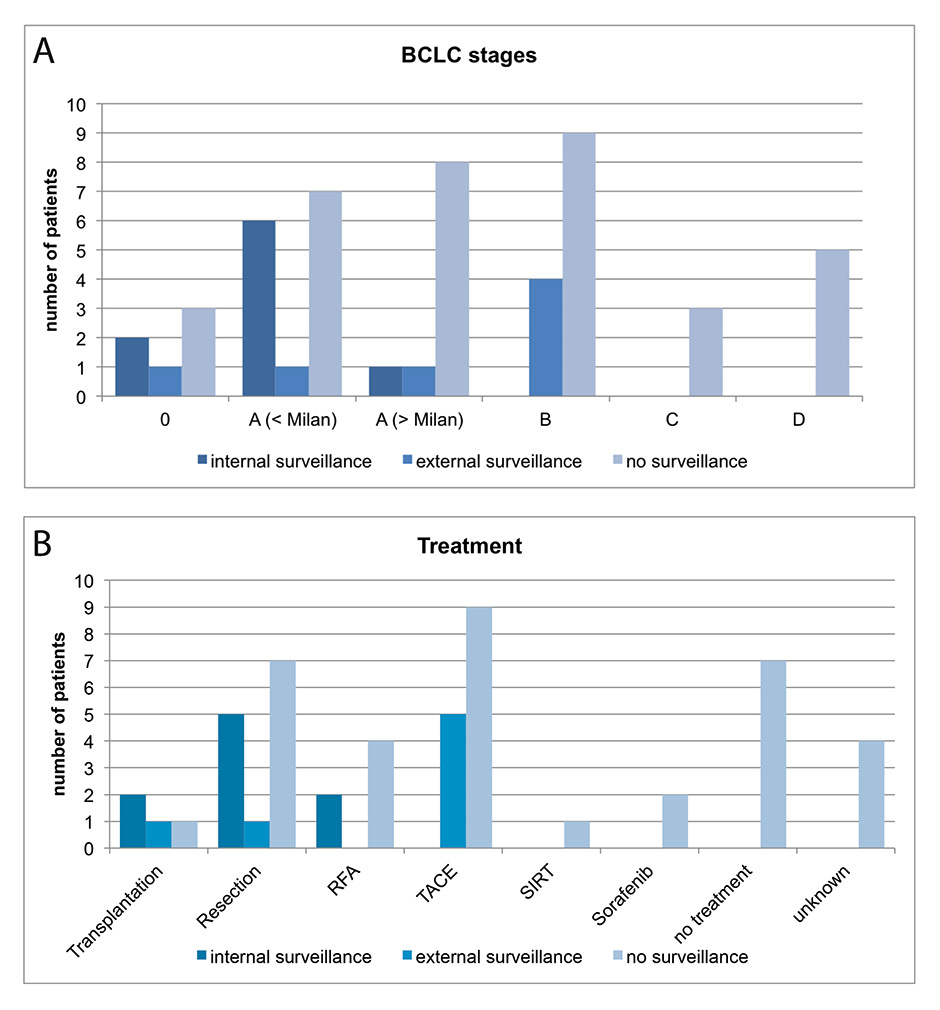
Figure 4
(A) Barcelona Clinic Liver Cancer (BCLC) staging of internal surveillance (dark blue), external surveillance (blue), no surveillance (light blue). Stages 0 and A are considered as very early and early stages. BCLC stage A patients were subdivided in those within the Milan criteria, and those with tumours outside the Milan criteria.
(B) Applied treatments for HCCs detected in internal surveillance (dark blue), external surveillance (blue), or in patients without surveillance (light blue).
HCC = hepatocellular cancer; RFA = radiofrequency ablation; SIRT = selective internal radiotherapy; TACE = transarterial chemoembolisation
None of the patients in the surveillance programme was physically harmed. There were several additional examinations performed to clarify further US findings. For instance, 32 CT scans, 7 MRI scans, 4 CEUS examinations and 1 liver biopsy were done because of suspicious lesions detected with US or because the quality of the US examination was insufficient to rule out a focal lesion. None of these additional examinations resulted in the detection of an HCC, and can therefore be considered as “unnecessary”. The total estimated costs of these examinations were CHF 24 778. More subjective adverse effects of these additional examinations such as patient anxiety and emotional stress were not assessed in our study. This is a largely neglected aspect of HCC surveillance, which should be addressed with a carefully designed prospective study using validated questionnaires such as the Hospital Anxiety and Depression Scale (HADS) [10].
The present study has several limitations. First, mortality was not assessed, and the study therefore cannot contribute to the on-going controversy about whether HCC surveillance programmes are effectively reducing HCC-related mortality in patients at risk for HCC. Second, the study was retrospective and not randomised. The control group consisted of 35 patients referred to the Clinic for Gastroenterology and Hepatology who were not in an HCC surveillance programme. We cannot exclude other factors beside the HCC screening intervention that significantly influenced the HCC stage at diagnosis. For instance, only 77% of the nonsurveyed patients with HCCs had cirrhosis, whereas all nine patients from the internal surveillance cohort were cirrhotic. However, the fact that nine patients from the surveillance cohort could undergo curative treatments strongly suggests a real advantage for patients undergoing HCC surveillance. Third, the study was not designed to compare the efficiency of HCC surveillance in a referral centre (University Hospital) versus surveillance in private practice or peripheral hospitals. Therefore, we cannot explain the differences in HCC stages and treatment modalities between patients from these two surveillance settings. Forth, the cost estimates are based on the sum of all costs generated directly (US surveillance) and indirectly (follow-up examinations) during the 2-year study period divided by the number of patients in the cohort. This calculation underestimates the costs per patients in a surveillance programme, because 49% of the patients entered our cohort during the study period and had fewer than four US examinations and consequently fewer follow-up examinations. Therefore, the calculated costs are a lower estimate of the real costs of an HCC surveillance programme in Switzerland.
Despite these limitations, our study provides strong evidence that HCC surveillance performs very favourably in real-life and at reasonably low costs. The study results support the current recommendations from the AASLD guidelines [2, 3], the EASL-EORTC guidelines [2] and the Asian Pacific Association for the Study of the Liver (APASL) guidelines [4] that all recommend US surveillance for patients at risk of HCC.
1 El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–73 e1261.
2 European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43.
3 Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2.
4 Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74.
5 Sherman M. Surveillance for hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28:783–93.
6 Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54:1998–2004.
7 Kansagara D, Papak J, Pasha AS, O'Neil M, Freeman M, Relevo R, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161:261–9.
8 TARMED Suisse. 2015 cited; Available from: http://www.tarmedsuisse.ch
9 Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9.
10 Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70.
11 Bond M, Pavey T, Welch K, Cooper C, Garside R, Dean S, et al. Systematic review of the psychological consequences of false-positive screening mammograms. Health Technol Assess. 2013;17:1–170, v-vi.
12 Carter SM, Williams J, Parker L, Pickles K, Jacklyn G, Rychetnik L, et al. Screening for Cervical, Prostate, and Breast Cancer: Interpreting the Evidence. Am J Prev Med. 2015.
Disclosure statement: The study was funded by financial resources of the Hospital. No other potential conflict of interest relevant to this article was reported.