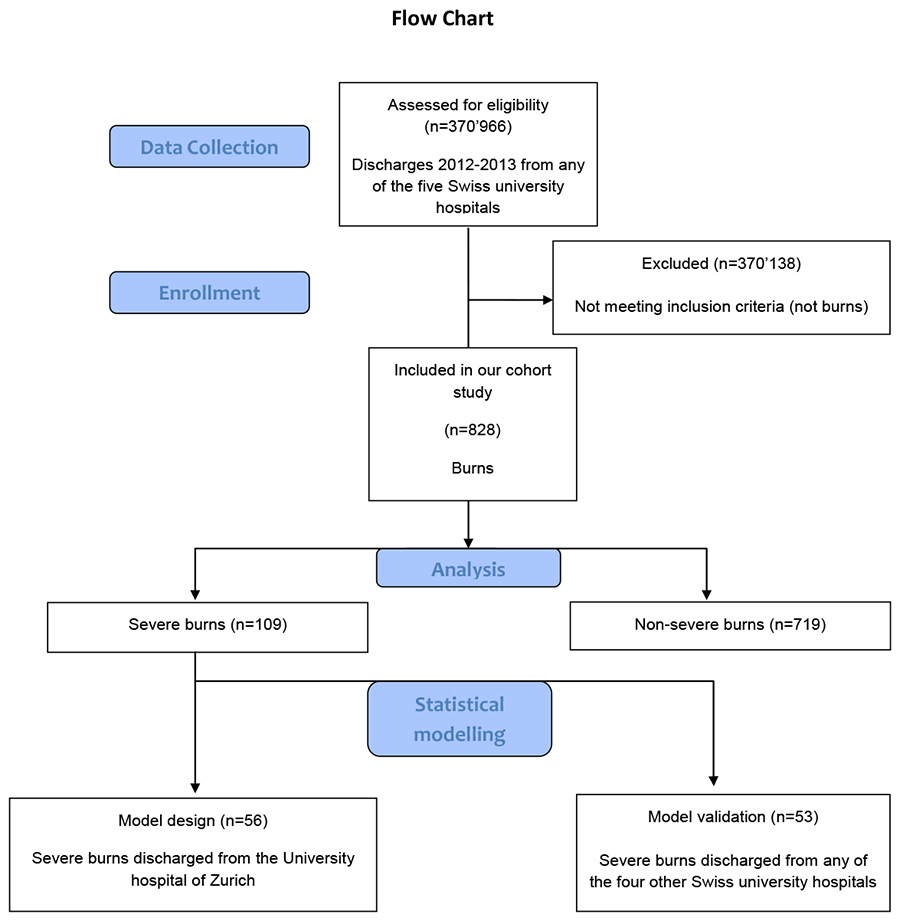
Figure 1
Flow-chart of the study design and enrollment.
DOI: https://doi.org/10.4414/smw.2015.14217
Specialised burn care is developed to meet the complex needs of burn patients from the time of the injury to the rehabilitation phase [1]. Centralising the treatment for severely burned individuals in specialised burn care has resulted in steadily improved outcomes, decreased mortality and improvement of quality of life after severe burn injury [2–6]. Specialised burn care is expensive, with the severity of injury as well as the complexity of institutional care infrastructure influencing treatment costs [7].
Diagnosis-related group (DRG) systems categorise hospital cases into clusters which, in view of resource utilisation and treatment costs, are expected to be similar. DRGs were initially established to measure hospital output. [8, 9] Nonetheless, they are currently used as a basis for inpatient reimbursement in numerous countries [9, 10]. The main advantage of a DRG system is an increase in transparency, which is supposed to lead to an increase in efficiency as well as in quality of care [10]. In Switzerland, since 2012 inpatient care including hospital treatment for burns is reimbursed with a DRG-specific flat rate. Certain procedures or medications receive supplemental funding [11]. Days of inpatient care exceeding the high trim point for the DRG increase the reimbursement premium. However, the increase is insufficient to cover the costs for the additional length of stay, so as to not provide a financial incentive for inefficient patient management or medical complications.
In a DRG-based reimbursement system, optimal reimbursement should reflect the calculated average cost of cases for a particular DRG. On the whole, the mean costs should be covered. Assuming an equal distribution of patient morbidity within one DRG over all care providers nationwide, the reimbursement system can be deemed fair [12]. However, the accuracy of funding patient care strongly depends on the capability of the reimbursement system to establish case clusters with homogeneous actual treatment costs. This can be problematic for medical conditions with a low incidence and a wide spread of treatment costs, as small case numbers and large differences in costs cause imprecise and erratic calculation of reimbursement premiums.
Switzerland has a population of 8 million inhabitants, a demographic structure and economic development similar to neighbouring European countries [13, 14]. There are two specialised burn centres in Switzerland, one at the Centre Hospitalier Universitaire Vaudois (Lausanne) (CHUV) and one at our centre, the University Hospital of Zurich (USZ), providing specialized burn care nationwide.
The aim of this study was to develop a simple and accurate model predicting total treatment costs, applicable as a reimbursement scheme for severe burn care within a prospective payment system of an industrialised country.
We designed a retrospective multicentre, cross-sectional cohort study with a 2-year timeframe, January 1st 2012 to December 31st 2013, from a healthcare provider perspective.

Figure 1
Flow-chart of the study design and enrollment.
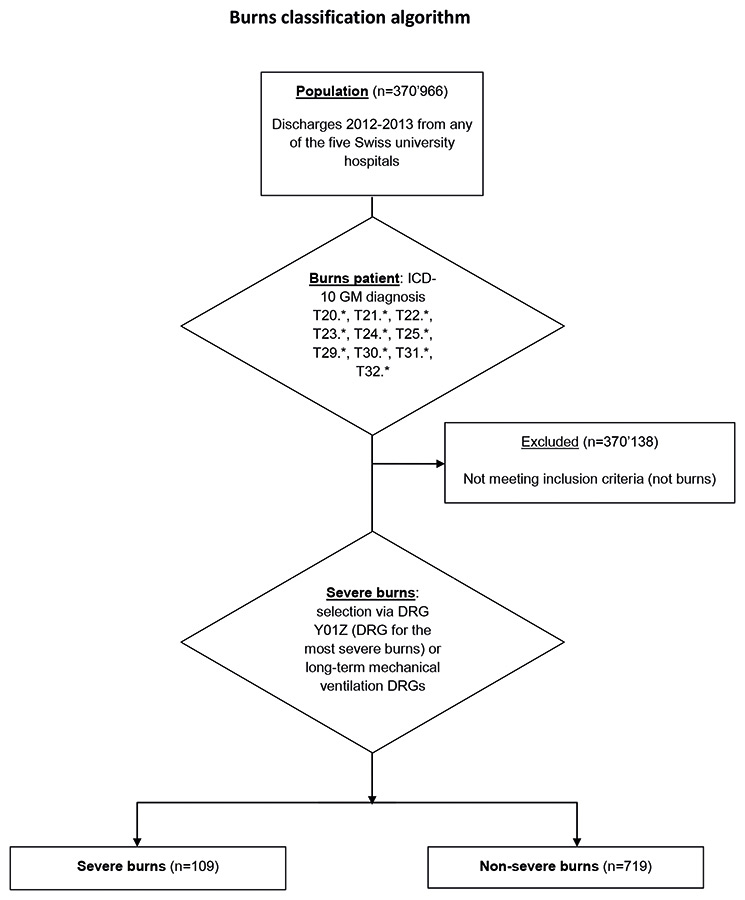
Figure 2
Burns classification algorithm.
ICD10-GM = International Classification of Diseases, 10th revision, German modification
Approval of the study from the cantonal ethics board of Zurich was obtained prior to the analysis.
In a first phase, we described the study population from University Hospital of Zurich, focusing on cost predictors, in a univariate analysis. In the second phase, we designed a linear regression model to predict the cost of care for severe burns. Here, we firstly designed a model with the cases of severe burns from our burn centre, then validated our model with the discharges of the four other university hospitals, CHUV Lausanne, Hopital Universitaire de Geneve, Inselspital Bern and University Hospital of Basel. Finally, we applied our model to the discharges from all Swiss university care providers and ran a third calculation.
The analysis was based on the dataset of all inpatient burn cases discharged during the study period from any of the five university hospitals of Switzerland, which included 370 966 coded cases (fig. 1). The data were provided by the committee coordinating the efforts of all five Swiss university hospitals relating to reimbursement tariffs (UNIFIN), which consists of representatives from the finance departments of the five centres. The Diagnosis Related Group (DRG) as well as the calculated effective case weight under the catalogue version of the year 2014 was obtained by grouping the cases by the SwissDRG catalogue version 3.0. At all five hospitals, cases were coded with the International Classification of Diseases, 10th revision, German modification (ICD-10 GM) and the Swiss medical procedure catalogues of 2012 and 2013, respectively. The same national coding guidelines of 2012/2013 applied to all five centres.
Costs were defined as total direct costs of inpatient care, allocated to each case under the REKOLE® full cost accounting method [15]. Revenue was defined as the case weight of each case grouped under SwissDRG catalogue version 3.0 (2014), multiplied by a base rate of 11 100 Swiss Francs (CHF) (10 230 Euros [EUR]). In the preliminary analysis, additional supplemental payments (“Zusatzentgelte”) were included in the analysis. However, as these supplemental payments constituted less than 1% of the total revenue and the amount of these payments was not available from other university hospitals, these payments were not considered for the validation of the regression model. The case earnings were calculated by subtracting the case costs from the calculated case revenue under SwissDRG 3.0. The accuracy of coding and cost data in Switzerland is continuously subject to external and independent audits.
A severe burn was defined as being a case grouped into the DRG Y01Z or into a Iong-term mechanical ventilation DRG. To classify as Y01Z, the case needed a burns injury principal diagnosis code and at least one of the following: mechanical ventilation >95 h, thirddegree burns >20% total body surface area (TBSA), burns of at least grade 2a of body sites such as head or torso and third degree burns, complicated operating room procedures and complicating secondary diagnosis such as sepsis. A long-term mechanical ventilation DRG is a DRG with a Major Diagnostic Category (MDC) being a pre-MDC, excluding transplantation, apheresis, multimodal pain management or radiotherapy DRGs. Cases classify into a long-term mechanical ventilation DRG if the duration of mechanical ventilation exceeded 95 hours or the cumulated NEMS (nine equivalents of nursing manpower use score) and SAPS (simplified acute physiology score) II score for adults exceeds 552 [16] (fig. 2).
In the first phase, all discharged patients with ICD-10 GM version 2012 codes T20.*, T21.*, T22.*, T23.*, T24.*, T25.*, T29.*, T30.*, T31.*, T32.* at the University Hospital of Zurich during 2012-2013 were included, the * indicating the variable last position of the code. In four patients, the code T31.*! was corrected manually. In all of these four cases, the percentage of the total body surface area (%TBSA) with third degree burns was documented in the medical records, but was coded as “unknown”. Computerised medical records of patients meeting the inclusion criteria were reviewed for a minimal clinical dataset including gender, age, referral from or to our centre, insurance coverage, length of stay (LOS), LOS in the intensive care unit (ICU), duration of mechanical ventilation, number of transfused red blood cell concentrates, thrombocyte concentrates or fresh frozen plasma concentrates, percent of body surface area affected, percent of body surface area affected by thirddegree burns, systemic inflammatory response syndrome (SIRS), psychiatric illness, the presence or absence of electrical injury and mortality.
The cases were divided into two groups, severe burns and nonsevere burns. Total costs of cases were obtained from the dataset submitted to SwissDRG.
In the second phase, our regression model was validated with cases from the other four university hospitals in Switzerland. The study population for validation included all discharged patients with ICD-10 GM version 2012 codes T20.*, T21.*, T22.*, T23.*, T24.*, T25.*, T29.*, T30.*, T31.*, T32.* from CHUV Lausanne, Hopital Universitaire de Geneve, Inselspital Bern and University Hospital of Basel grouped according to SwissDRG 3.0.
In the third phase, the regression model was run on the dataset including all cases from the five university hospitals in Switzerland between 2012 and 2013.
The UNIFIN data and the discharges from our hospital were grouped with the online grouping software provided by SwissDRG AG [17]. The data were transferred into the business intelligence software QlikView® for further preparation, before being exported as a dataset file (Microsoft Excel 2010). All statistical analysis was done using IBM SPSS Statistics version 22 [18].
As the distribution of patient costs is right-skewed, a log-normal distribution can be assumed [19]. Hence, we used the decadic logarithm of total costs per case as the dependent variable. Univariate analysis was performed with the Spearman rank correlation for continuous variables determining total cost, the Mann-Whitney U-test for binary nominal variables and the Kruskal-Wallis test for nominal variables with more than two categories. We decided to model the prediction of cost with a linear regression. With an α-level deemed acceptable at 0.05, regression coefficients were considered significant at a Bonferroni-corrected significance level of p <0.025 (two coefficients).
The exchange rate on 25 February 2015 of approximately 1 CHF to 0.93 EUR was used to convert the main financial results into Euros and an exchange rate of approximately 1 US-dollar (USD) to 0.88 EUR was used to convert financial results to EUR.
Approval of the study from the cantonal ethics board of Zurich was obtained prior to the analysis (KEK-ZH-Nr. 2014-0231). All involved hospitals approved the study. All patient-based data from our centre was anonymised before the analysis. The UNIFIN data had already been anonymised before it was obtained by the research group.
| Table 1: Descriptive statistics of the study population: all discharges with coded burns diagnoses from any of the five Swiss university hospitals (n = 828). | ||||
| All cases (n = 828) | Nonsevere burns (n = 719) | Severe burns (n = 109) | p-value | |
| Total cost per case (CHF) | 14 292 (5 527–41 215) | 11 312 (4 874–27 783) | 179 949 (96 782–328 618) | <0.001 |
| DRG revenue per case (CHF) | 17 396 (8 647– 8 647) | 11 455 (8 647–22 389) | 139 871 (139 871–214 508) | <0.001 |
| Earnings per case (CHF) | 343 (–10 312 – 5 654) | 588 (–6 720 – 5 354) | –33 178 (–95 533 – 23 662) | <0.001 |
| Age, years (range) | 35 (14–55) | 32 (8–53) | 49 (28–62) | <0.001 |
| Sex (female %) | 320 (38.6) | 277 (38.5) | 43 (39.4) | 0.86 |
| Referral from other hospitals | 85 (10.3%) | 60 (8.3%) | 25 (22.9%) | <0.001 |
| Referral to other Hospitals | 21 (2.5%) | 12 (1.7%) | 9 (8.3%) | <0.001 |
| Hospital mortalities | 23 (2.8%) | 12 (1.7%) | 11 (10.1%) | <0.001 |
| LOS, days (range) | 7.0 (2.0–16.0) | 6.0 (2.0–12.0) | 35.0 (24.0–59.0) | <0.001 |
| Duration of mechanical ventilation, hours (range) | 0 (0–0) | 0 (0–0) | 168 (94–516) | <0.001 |
| SIRS diagnosis | 41 (5.0%) | 10 (1.4%) | 31 (28.4%) | <0.001 |
| Psychiatric diagnosis | 210 (25.4%) | 129 (17.9%) | 81 (74.3%) | <0.001 |
| DRG = diagnosis-related group; LOS = length of stay; SIRS = systemic inflammatory response syndrome Continuous variables are presented with median and interquartile range, categorical variables with n (%); p-values were determined with the Mann-Whitney U-test for continuous variables and categorical variables with the chi squared test. | ||||
The population characteristics of the UNIFIN cases of all five university care centres are summarised in table 1. In short, the population was comprised of 828 cases, 109 (13.2%) cases of severe burns and 719 (86.8%) cases of nonsevere burns. The cohorts were comparable in terms of gender, but in the severe burns cohort, the patients were older, stayed longer in the hospital and were mechanically ventilated for longer.
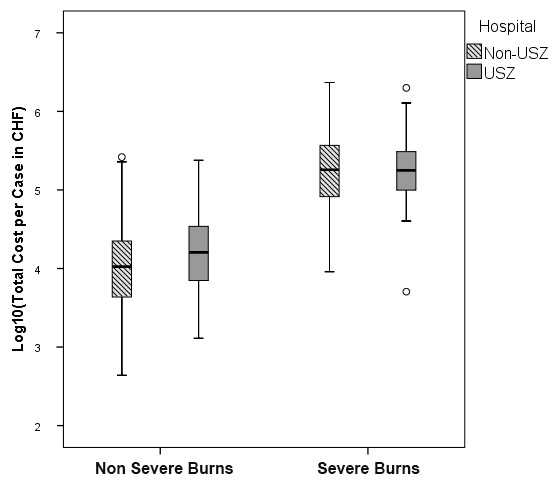
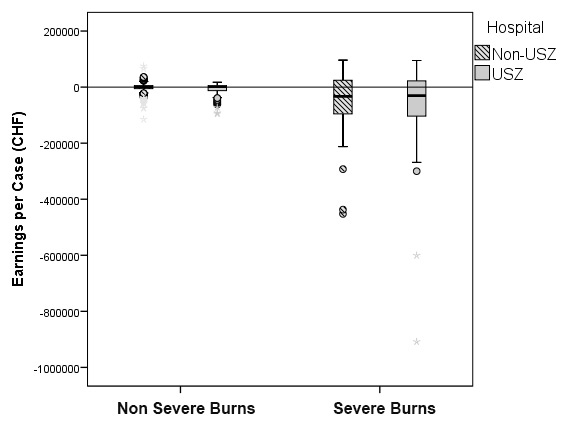
Figure 3
Distribution of total costs per case (fig. 3A) and total earnings per case (fig. 3B) for severe and nonsevere burns. A decadic logarithmic transformation of the cost function was performed to allow for easier analysis. The y-axis was limited to –1 000 000 CHF, hence one severe burn case (earnings of –1 324 732 CHF) is not visible. Study population: discharges from our hospital (n = 268) and all other Swiss university hospitals (n = 560). Severe burns (n = 109, of which n = 56 from our hospital and n = 53 from the four other university hospitals) and nonsevere burns (n = 719, n = 212 from our hospital and n = 507 from the four other university hospitals). Our hospital = USZ, the four other university hospitals =: Non-USZ. The upper and lower bounds of the boxes denote the upper and lower quartile respectively, with the broad line in the box denoting the median. Whiskers were defined by 1.5x interquartile range (IQR). Outliers were defined as either <1.5 x IQR of the first quartile or >1.5 x IQR of the third quartile. Outliers are represented as circles or asterisks, where asterisks denote extreme values (<3 x IQR of the first quartile or >3 x IQR of the third quartile).
The %TBSA was only available for patients discharged from our centre (University of Zurich Hospital) (table 2). To sum up, only 9.4% of the nonsevere burn cohort had involved TBSA of ≥20%, whereas in cases with severe burns, 67.9% had a reported TBSA ≥20%. Deep, third or fourth degree burns of TBSA >20% were recorded in 2.8% in the nonsevere burn cohort and the corresponding figure was 32.1% in the severe burn cohort.
For the calculation of financial results, all burns cases from all five university hospitals were considered. The total costs per case were more than tenfold higher in the severe burns cohort (median 179 949 CHF [167 353 EUR] vs 11 312 CHF [10 520 EUR] per case, interquartile range (IQR) 96 782 to 328 618 CHF per case, p <0.001). Revenues were also higher, with a median of 139 871 CHF [130 080 EUR] vs 11 455 CHF [10 653 EUR] per case (IQR 139 871 to 214 508 vs 8 647 to 22 389 CHF per case, p <0.001). Bottom-line earnings were a median deficit of –33 178 CHF (30 856 EUR) per case for severe burns and a median profit of 588 CHF (547 EUR) for nonsevere burns (IQR –95 533 to 23 662 vs –6 720 to 5 354 CHF per case, p <0.001) (table 1).
The distribution of the decadic logarithm of total costs per case for severe and nonsevere burns is illustrated in figure 3A. The earnings for the nonsevere burns appeared tightly grouped around 0 CHF (fig. 3B). SwissDRG 3.0 is capable of assuring cost-coverage at a base rate of 11 100 CHF (10 323 EUR) for nonsevere burns. However, this is not the case for the severe burns. We therefore decided to focus on the subgroup of severe burns for further analysis.
We formed two subgroups of the 109 severe burns; one cohort comprising the cases from our centre (University Hospital of Zurich [USZ]) to establish the model (n = 56), and the second cohort comprising the cases from the four other centres for validation (n = 53). The two subgroups had a similar distribution of costs and earnings.
We analysed the correlation strength of the continuous variables with total costs per case by Spearman’s rank correlation coefficient ρ(supplemental table S1). LOS (ρ= 0.89), LOS in the ICU (ρ= 0.95), duration of mechanical ventilation (ρ= 0.67) and number of transfused red blood cell units (ρ= 0.78) correlated significantly with total costs (p <0.001). The SIRS diagnosis (Mann-Whitney p = 0.002) and DRG LOS status, length of hospital stay below the low trim-point, between the high trim-point and the low trim-point or above the high trim point, (Kruskal-Wallis p <0.001) were significant predictors of higher total costs (supplemental table S2).
We designed a model with LOS as the independent variable, (cohort of severe burns, discharges from the University Hospital of Zurich, n = 56). The model had an adjusted R2 of 0.0.64, p <0.001. LOS had a standardised coefficient of β = 0.803 and was significant at p <0.025 (p <0.001) (supplemental table S3).
Subsequently, we tested the model with the data from all discharges, n = 53, of the four Swiss university hospitals excluding the cases from our hospital. We obtained a model with an adjusted R2 of 0.71, p <0.001. LOS had a standardised coefficient of β = 0.846 and was significant at p <0.025 (p <0.001) (supplemental table S4).
In a last step, we ran the model with the data of all discharges from the five Swiss university hospitals. In the study population of severe burns discharged from all university centres (n = 109), our model had an adjusted R2 of 0.67, p <0.001. LOS had a standardised coefficient of β = 0. 821 and was significant at p <0.025 (LOS: p <0.001) (table 3). If the outlier with a deficit of –1.3 million CHF and case costs totalling more than 23 million CHF was excluded from the analysis, the regression model had an adjusted R2 of 0.66, the standardised coefficient of LOS being β = 0.814, significant at p <0.025 (p <0.001) (supplemental table S5).
| Table 2: Descriptive statistics, severity of burns. Study population: all burns cases discharged from the University Hospital of Zurich (n = 268). | ||||
| Total (n = 268) | Nonsevere burns (n = 212) | Severe burns (n = 56) | ||
| Burns % body surface area | Data not available | 20 (7.5%) | 18 (8.5%) | 2 (3.6%) |
| 0–9 | 139 (51.9%) | 136 (64.2%) | 3 (5.4%) | |
| 10–19 | 51 (19.0%) | 38 (17.9%) | 13 (23.5%) | |
| 20–29 | 22 (8.2%) | 11 (5.2%) | 11 (19.6%) | |
| 30–39 | 20 (7.5%) | 2 (0.9%) | 18 (32.1%) | |
| 40–49 | 5 (1.9%) | 2 (0.9%) | 3 (5.4%) | |
| 50–59 | 4 (1.5%) | 1 (0.5%) | 3 (5.4%) | |
| 60–69 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 70–79 | 4 (1.5%) | 1 (0.5%) | 3 (5.4%) | |
| 80–89 | 1 (0.4%) | 1 (0.5%) | 0 (0%) | |
| 90–100 | 2 (0.7%) | 2 (0.9%) | 0 (0%) | |
| Burns % body surface area third–fourth degree | Data not available | 20 (7.5%) | 18 (8.5%) | 2 (3.6%) |
| 0–9 | 209 (78.0%) | 182 (85.8%) | 27 (48.2%) | |
| 10–19 | 15 (5.6%) | 6 (2.8%) | 9 (16.1%) | |
| 20–29 | 6 (2.2%) | 0 (0%) | 6 (10.7%) | |
| 30–39 | 8 (3.0%) | 1 (0.5%) | 7 (12.5%) | |
| 40–49 | 3 (1.1%) | 1 (0.5%) | 2 (3.6%) | |
| 50–51 | 2 (0.7%) | 0 (0%) | 2 (3.6%) | |
| 60–69 | 1 (0.4%) | 1 (0.5%) | 0 (0%) | |
| 70–79 | 2 (0.7%) | 1 (0.5%) | 1 (1.8%) | |
| 80–89 | 1 (0.4%) | 1 (0.5%) | 0 (0%) | |
| 90–100 | 1 (0.4%) | 1 (0.5%) | 0 (0%) | |
| Values given in absolute numbers (% of column total) | ||||
| Table 3: Summary of regression coefficients – linear regression model predicting the decadic logarithm of total costs per case. Study population: severe burns discharges from all Swiss university care centres (n = 109). | ||||
| Model | Unstandardised coefficients B (SE) | Standardised coefficients β | p-value | 95% confidence interval for unstandardised coefficients |
| Constant | 4.802 (0.038) | <0.001 | 4.726–4.878 | |
| LOS (days) | 0.009 (0.001) | 0.821 | <0.001 | 0.008–0.010 |
| LOS = length of stay; SE = standard error | ||||
Herein, we completed a new estimation tool for predicting the inpatient treatment costs from the healthcare provider perspective in patients with severe burns. Based on the cost and medical records of every discharged patient with severe burn injury in the university hospitals in Switzerland during 2012–2013, we determined a cost of treatment per day of 5 998 CHF. Benchmarking our results, we found ouur calculated costs per day of treatment for severe burns to be considerably higher than the cost per day for burns treatment in the ICU reported by Berger et al. for Swiss patients in 2010, which amounted to 1 991 EUR [20]. Further, our cost exceeds the costs for sepsis treatment in the ICU by nearly two-fold [21]. Regarding international studies on burns, our cost ranks to the second highest per day [6]. The comparatively high costs in this study are explained by the burn severity and the Swiss high price level of 155% for gross domestic product and 181% for health expenditure in comparison with the OECD average [13].
Treatment of severe burns in specialised centres is resource intensive [2, 3, 6, 7]. The infrastructure of specialised care includes labour costs of specialised professionals, operative treatment, wound dressings, medications, laboratory and radiological tests [22]; the longer a patient requires these resources, the more expensive the treatment. It is therefore plausible that LOS is our strongest predictor of total costs: more severe injuries or patients with complications require longer treatment periods [23–25]. Hop et al. reported average burn centre costs of 2 380 EUR per day and 2 784 EUR per day for ICU care. They also calculated the total treatment costs per 1% TBSA burnt which amounted to 3 660 EUR [6]. DRG-based reimbursement systems aim at mirroring resource utilisation, assuring cost coverage on average within a specific DRG [12]. However, this does not seem to be the case for severe burns, as insufficient cost coverage is a reported issue from within different DRG-based reimbursement systems [26–29].
Severe burns are grouped into Y01Z under SwissDRG version 3.0. Cases of this DRG were allocated a case weight of 12.601 in 2014 [30]. Hence, assuming a base rate of 11 100 CHF (10 323 EUR) per case weight point, this leads to a fee of 139 871 CHF (130 080 EUR) billed to third party payers for an inlier, excluding supplemental payments. This is less than the reported amount of on average 208 699 EUR for DRG 504 and only slightly more than the average of 107 028 EUR for DRG 506, two US-American DRGs for the most severe burns reported by the National Burn Repository 10-year review of the United States in 2006 [31]. Severe burns seem to be better funded in the United States, especially if purchasing power parity is taken into account [13]. However, in 2015 funding for severe burns is reduced by approximately 2.3%, as the DRG catalogue now allocates 12.305 case weight points to Y01Z, a reduction of nearly .3 points (approximately 3 000 CHF or 2 790 EUR). To our knowledge, no systematic changes to the classification algorithm of severe burns are planned for the near future.
We compared our results with the reimbursement of severe burns within the DRG systems of four other European DRG systems – Germany, Austria, Italy and Spain – published by Lotter et al. [32]. The reimbursement systems of all four countries have specific burns DRGs. As of 2011, Austria had only three specific burns DRGs, whereas Spain had eight and Germany as well as Italy had 10. Our cohort of cases had an average LOS of 46.6 days (standard deviation of 41.4 days) and an average effective case weight of 18.551 points (standard deviation 15.915 points) and the care provided would therefore, by indicators of case severity, not be sufficiently funded in the reimbursement systems of Austria, Italy or Spain. However, since introduction of G-DRG in 2003, the German DRG system reimburses severe burns on a per diem basis (Y01Z) [33]. This approach would be adaptable to reimburse sufficiently the care for severe burns in Switzerland. Nonetheless, the per diem premium would have to be set at about 6 000 CHF per day to assure cost coverage.
One limitation of our study is the limited number of cases included in our regression model. Indeed, a population of 109 cases is realtively small for estimating a statistical model. In a larger population, a model including other predictors such as LOS may even have increased the precision of the model.
In this current study, LOS showed the highest correlation with the decadic logarithm of total costs per case of all quantitative scaled independent variables. Indeed, although there is controversy regarding the reduction of costs through reduction in LOS [34, 35], as most of hospital costs are fixed (salaries and infrastructure), an additional day of hospitalisation means that these resources generating fixed costs are employed to treat that particular patient for one day longer. Patients with a longer length of stay absorb more of the hospital costs and therefore LOS is a favoured predictor for estimating inpatient costs [23, 36]. It is therefore plausible that LOS is one of the strongest predictors for total costs in our model. The only qualitative independent variable with a significant predictive value at the 0.001 level in the univariate analysis was SIRS. The lack of significance of SIRS in the subpopulation used for data validation is most probably because there were only 6 cases of coded SIRS in comparison with 25 from our centre. Literature suggests that SIRS affects over 80% of surgical ICU patients [37, 38]. Even though SIRS seemed a promising variable in the univariate analysis, we saw SIRS as unfit to be a discriminatory attribute and therefore did not include it as a dependent variable in our analysis.
Changes in the reimbursement system can be used to incentivise desired behaviour of healthcare providers [39]. DRGs were introduced to increase transparency, efficiency and quality of care [10]. One risk of the introduction of a per diem reimbursement model for severe burns is the financial incentivisation of longer lengths of stay. Indeed, of the 56 severe burns treated at our centre during the time period, nearly half were discharged to rehabilitation centres (26 cases, 46%), three more (5%) discharged to psychiatric care and nine (16%) to other acute care hospitals. Eleven patients (20%) deceased. Therefore the introduction of a per diem reimbursement premium could encourage continuation of care at tertiary acute care providers and discourage discharges to other care providers with less high levels of complex care infrastructure, thus unnecessarily reducing efficiency of care provided. Furthermore, a per-diem reimbursement of 6 000 CHF per day would have increased the cost of care of the 109 severe burns cases by roughly 7.6 million CHF, an additional financial burden which would have had to be borne by society as a whole. Another model of reimbursement is bloc contract funding, as seen in some parts of the UK National Health Services: a lump sum is paid for the total provision of specific services, irrespective of the volume of care [39]. However, as this is not an activity-based healthcare reimbursement system either, it is questionable if this form of funding would increase efficiency. A further difficulty would most certainly be the Swiss federal system, with a multitude of third party payers, as the negotiations on who is to be the contract partner with the financial responsibility of funding the services on a national level could prove to be very difficult indeed. We therefore believe that a per diem based payment system, although certainly not perfect, is the best option to date for funding acute care for severe burns. Indeed, our regression model with LOS as the independent variable had a high predictive value for total cost, with an adjusted R2of 0.67. LOS seems to be the parameter by which an accurate calculation of the costs incurred by the treatment of severe burns is possible. The statistical results are also clinically explainable. Patients with a higher %TBSA affected by burns have a longer average length of stay: the average LOS more than doubles between the cohort of patients with an affected %TBSA of 9–10% to the cohort of patients with an affected %TBSA of 10–19% (supplemental table S6). Moreover, due to the small number of patients, high proportion of outliers and the high variance in treatment costs, a calculation of average costs per DRG becomes very difficult. In view of the strength of our regression model and the statistical difficulties of calculating precise averages with low variances in small populations, we suggest a modified reimbursement system for severe burns similar to the German DRG system. This could be, optionally, differentiated for diagnosis of SIRS. Practically speaking, the modifications necessary under SwissDRG to implement the suggested reimbursement model are quite simple: the grouper logic could be modified to include all cases with a burns diagnosis, which until now are assigned to a long-term ventilation DRG or the DRG Y01Z.
Switzerland has the advantage of widespread use of electronic medical records and national standards for medical data submission [40]. Therefore, Switzerland provides a good study population for the modelling of a reimbursement system for burn cases in industrialised countries. Further advantages of Swiss cost studies are the determined, accurate accounting method for hospitals [15] and the regular external auditing of cost, coding and revenue data. The burns study population analysed here is the largest reported from a European centre within the last 10 years. That said, this study is limited by its retrospective nature and possible coding inaccuracies [41], and the exclusion of burns treated at nonuniversity care facilities. Similar to the German DRG system, the Swiss DRG reimbursement system reimburses certain expensive medications or procedures, such as dialysis and certain transfusions of blood products, via supplemental payments («Zusatzentgelte»). These services are bracketed out of the DRG premiums as their reimbursement cannot be adequately calculated in the flat-rate reimbursement system. As the amount of supplemental payments («Zusatzentgelte») per case were not available for cases discharged from university centres other than ours and as the volume of these supplemental payments accounted for less than 1% of the revenue for burns cases from our centre, supplemental payments in general were not taken into account when calculating earnings. Although the accounting methods in Swiss hospital warrant relative accuracy for cost allocation, there is still room for improvement.
We conclude that inpatient care for severe burns treatment is underfunded in Switzerland under the present reimbursement system. We suggest a simple model with LOS as the independent variable predicting total cost to be used in the redesign of the reimbursement system for severe burns. We conclude that a per diem remuneration would be able to reflect actual treatment costs with higher accuracy than the present DRGs.
| Table S1: Univariate analysis of continuous variables predicting total cost per case for severe burns. Study population: discharges from the University Hospital of Zurich (n = 56). Bivariate correlation determined with Spearman correlation. | ||
| ρ | p value | |
| Age (years) | –0.27 | 0.044 |
| LOS (days) | 0.89 | <0.001 |
| LOS ICU (days) | 0.95 | <0.001 |
| Duration of mechanical ventilation (h) | 0.67 | <0.001 |
| Red blood cell units | 0.78 | <0.001 |
| ICU = intensive care unit; LOS = length of stay. | ||
| Table S2: Univariate analysis of categorical variables predicting total cost per case for severe burns. Study population: discharges from the University Hospital of Zurich (n = 56). Influence of predictive value determined with the Mann-Whitney-U test (binomial variables) or the Kruskal-Wallis test (more than two categories per variable). | ||||
| n | Total cost per case (CHF) | p-value | ||
| Sex (1 = female, 2 = male) | 1 | 20 | 196 366 (88 277–420 242) | 0.87 |
| 2 | 36 | 171 524 (102 387–283 105) | ||
| Referral from another hospital | No | 31 | 190 281 (99 487–291 374) | 0.99 |
| Yes | 25 | 176 850 (93 132–329 968) | ||
| Referral to another hospital | No | 47 | 166 198 (99 487‒291 374) | 0.99 |
| Yes | 9 | 230 285 (93 132‒329 968) | ||
| Hospital mortality | No | 54 | 190 281 (111 892‒323 557) | 0.12 |
| Yes | 11 | 99 487 (75 743‒253 403) | ||
| SIRS diagnosis | No | 31 | 127 415 (84 124‒212 411) | 0.002 |
| Yes | 25 | 291 374 (166 198‒445 015) | ||
| Psychiatric diagnosis | No | 13 | 99 487 (79 771‒230 285) | 0.12 |
| Yes | 43 | 190 281 (111 892‒323 711) | ||
| Electric burn | No | 51 | 176 850 (99 487‒292 638 | 0.97 |
| Yes | 5 | 200 905 (73 340‒445 015) | ||
| Insurance (mandatory = 1, half-private = 2, private = 3) | 1 | 41 | 178 386 (96 782‒292 638) | 0.60 |
| 2 | 5 | 217 873 (153 815‒274 836) | ||
| 3 | 10 | 153 238 (105 287‒443 072) | ||
| DRG LOS status 3.0 (1 = low outlier, 2 = inlier, 3 = high outlier) | 1 | 7 | 75 743 (40 267‒99 487) | <0.001 |
| 2 | 33 | 153 815 (105 287‒217 873) | ||
| 3 | 16 | 383 391 (269 172‒532 306) | ||
| Burns % TBSA | 0–9 | 3 | 190 281 (105 287‒291 374) | 0.11 |
| 10–19 | 13 | 121 424 (84 124‒292 638) | ||
| 20–29 | 11 | 166 198 (93 132‒263 507) | ||
| 30–39 | 18 | 155 866 (99 322‒329 968) | ||
| 40–49 | 3 | 178 386 (79 771‒212 411) | ||
| 50–59 | 3 | 443 072 (153 815‒1 991 344) | ||
| 60–69 | – | |||
| 70–79 | 3 | 562 452 (514 363‒1 277 343) | ||
| 80–89 | – | |||
| 90–100 | – | |||
| DRG = drug-related group; LOS = length of stay; SIRS = systemic inflammatory response syndrome; TBSA = total body surface area Total costs are presented as median with interquartile range. | ||||
| Table S3: Summary of regression coefficients – linear regression model predicting the decadic logarithm of total costs per case. Study population: discharges from the University Hospital of Zurich (n = 56). | ||||
| Model | Unstandardised coefficients B (standard error) | Standardised coefficients β | p-value | 95.0% confidence interval for unstandardised coefficients |
| Constant | 4.869 (0.50) | <0.001 | 4.769–4.969 | |
| Length of stay (days) | 0.009 (.001) | 0.803 | <.0001 | 0.007–0.11 |
| Table S4: Summary of regression coefficients – linear regression model predicting decadic logarithm of total costs per case. Study population: discharges from CHUV Lausanne, University Hospitals of Geneva, Bern and Basel (n = 53). | ||||
| Model | Unstandardised coefficients B (standard error) | Standardised Coefficients β | p-value | 95.0% confidence interval for unstandardised coefficients |
| Constant | 4.725 (0.057) | <0.001 | 4.610–4.841 | |
| Length of stay (days) | 0.010 (0.001) | 0.846 | <0.001 | 0.008–0.011 |
| Table S5: Summary of regression coefficients – linear regression model predicting the decadic logarithm of total costs per case, excluding the outlier with a final balance of –1’3 million CHF. Study population: severe burns discharges from all Swiss university care centres (n = 108). | ||||
| Model | Unstandardised coefficients B (standard error) | Standardised Coefficients β | p-value | 95.0% confidence interval for unstandardised coefficients |
| Constant | 4.787 (0.039) | <0.001 | 4.710–4.864 | |
| Length of stay (days) | 0.010 (0.001) | 0.814 | <0.001 | 0.008–0.011 |
| Table S6:Length of stay and category of %TBSA affected by burns. All burns discharges from the University Hospital of Zurich with an available %TBSA category (n = 248). | ||
| %TBSA | Number of cases | Length of stay |
| 0–9 | 139 | 9.4 (±12.0) |
| 10–19 | 51 | 20.8 (±18.8) |
| 20–29 | 22 | 24.6 (±22.2) |
| 30–39 | 20 | 33.0 (±24.8) |
| 40–49 | 5 | 15.6 (±17.8) |
| 50–59 | 4 | 75.5 (±96.9) |
| 70–79 | 4 | 74.5 (±63.1) |
| 80–89 | 1 | 1.0 (± NA) |
| 90–100 | 2 | 1.0 (±0) |
| %TBSA = percentage of total body surface area affected | ||
1 Al-Mousawi AM, Mecott-Rivera GA, Jeschke MG, Herndon DN. Burn teams and burn centers: the importance of a comprehensive team approach to burn care. Clin Plast Surg. 2009;36:547–54.
2 Sanchez JL, Pereperez SB, Bastida JL, Martinez MM. Cost-utility analysis applied to the treatment of burn patients in a specialized center. Arch Surg. 2007;142:50–7; discussion 7.
3 Stavrou D, Weissman O, Winkler E, Millet E, Nardini G, Tessone A, et al. Managing the relationship between quality and cost-effective burn care. Burns: journal of the International Society for Burn Injuries. 2011;37:367–76.
4 Peck MD. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns. 2011;37:1087–100.
5 Brusselaers N, Monstrey S, Vogelaers D, Hoste E, Blot S. Severe burn injury in Europe: a systematic review of the incidence, etiology, morbidity, and mortality. Critical Care. 2010;14:R188.
6 Hop MJ, Polinder S, van der Vlies CH, Middelkoop E, van Baar ME. Costs of burn care: A systematic review. Wound Repair Regen. 2014;22:436–50.
7 Wheeler JRC, Vanharrison R, Wolfe RA, Payne BC. The Effects of Burn Severity and Institutional Differences on the Costs of Care. Med Care. 1983;21:1192–203.
8 Fetter RB, Shin Y, Freeman JL, Averill RF, Thompson JD. Case mix definition by diagnosis-related groups. Medical Care. 1980;18:iii:1–53.
9 Fetter RB. DRGs: Their Design and Development. Ann Arbor, MI: Health Administration Press; 1991.
10 Busse R, Geissler R, Quentin W, Wiley M. Diagnosis-Related Groups in Europe: Moving towards transparency, efficiency and quality in hospitals. 1st Edition ed. Maidenhead, England: McGraw-Hill, Open University Press; 2011.
11 Fallpauschalen in Schweizer Spitälern – Basisinformationen für Gesundheitsfachleute.
12 Jencks SF, Dobson A. Refining Case-Mix Adjustment – the Research Evidence. N Engl J Med. 1987;317:679–86.
13 OECD. 2011 PPP Benchmark results. 2011.
14 OECD. Population and Vital Statistics (ALFS). 2015.
15 REKOLE® Handbuch – Betriebliches Rechnungswesen im Spital. 3rd Edition ed. Bern, Switzerland2014.
16 SwissDRG 3.0 Abrechnungsversion (2014/2014) Definitionshandbuch. Bern, Switzerland: SwissDRG AG; 2013.
17 AG S. Batchgrouper, V3.0 Abrechnungsversion 2014/2014. 2014.
18 IBM C. IBM SPSS Statistics for Windows. Version 22.0 ed. Armonk, NY: IBM Corp.; 2013.
19 LK L. Hospital information systems for casemix management. New York: Wiley; 1986.
20 Berger MM, Davadant M, Marin C, Wasserfallen JB, Pinget C, Maravic P, et al. Impact of a pain protocol including hypnosis in major burns. Burns: journal of the International Society for Burn Injuries. 2010;36:639–46.
21 Schmid A, Pugin J, Chevrolet JC, Marsch S, Ludwig S, Stocker R, et al. Burden of illness imposed by severe sepsis in Switzerland. Swiss Med Wkly. 2004;134:97–102.
22 Patil V, Dulhunty JM, Udy A, Thomas P, Kucharski G, Lipman J. Do burn patients cost more? The intensive care unit costs of burn patients compared with controls matched for length of stay and acuity. Journal of burn care & research: official publication of the American Burn Association. 2010;31:598–602.
23 Polverejan E, Gardiner JC, Bradley CJ, Holmes-Rovner M, Rovner D. Estimating mean hospital cost as a function of length of stay and patient characteristics. Health Economics. 2003;12:935–47.
24 Ishak KJ, Stolar M, Hu MY, Alvarez P, Wang Y, Getsios D, et al. Accounting for the relationship between per diem cost and LOS when estimating hospitalization costs. BMC Health Serv Res. 2012;12:439.
25 Fine MJ, Pratt HM, Obrosky DS, Lave JR, McIntosh LJ, Singer DE, et al. Relation between length of hospital stay and costs of care for patients with community-acquired pneumonia. Am J Med. 2000;109:378–85.
26 Holmes JHt. Critical issues in burn care. Journal of burn care & research: official publication of the American Burn Association. 2008;29:S180–7.
27 Kagan RJ, Gamelli R, Saffle JR. DRG 504: the effect of 96 hours of mechanical ventilation on resource utilization. Journal of burn care & research: official publication of the American Burn Association. 2007;28:664–8.
28 Kagan RJ, Edelman L, Solem L, Saffle JR, Gamelli R. DRG 272: does it provide adequate burn center reimbursement for the care of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis? Journal of burn care & research: official publication of the American Burn Association. 2007;28:669–74.
29 Oestreich K, Jester A, Ohlbauer M, Schroter B, Germann G, Pelzer M. Survival strategy of burn centers in the context of the German DRG system: reimbursement. Der Unfallchirurg. 2006;109:505–10.
30 Fallpauschalenkatalog SwissDRG 3.0 2013.
31 Latenser BA, Miller SF, Bessey PQ, Browning SM, Caruso DM, Gomez M, et al. National Burn Repository 2006: a ten-year review. Journal of burn care & research: official publication of the American Burn Association. 2007;28:635–58.
32 Lotter O, Jaminet P, Amr A, Chiarello P, Schaller HE, Rahmanian-Schwarz A. Reimbursement of burns by DRG in four European countries: an analysis. Burns. 2011;37:1109–16.
33 Wappler F, Spilker G. Verbrennungsmedizin. Stuttgart: Georg Thieme Verlag; 2008.
34 Kahn JM, Rubenfeld GD, Rohrbach J, Fuchs BD. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46:1226–33.
35 Taheri PA, Butz DA, Greenfield LJ. Length of stay has minimal impact on the cost of hospital admission. J Am Coll Surg. 2000;191:123–30.
36 Jansen LA, Hynes SL, Macadam SA, Papp A. Reduced length of stay in hospital for burn patients following a change in practice guidelines: financial implications. Journal of burn care & research: official publication of the American Burn Association. 2012;33:e275–9.
37 Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive care medicine. 2000;26(Suppl 1):S64–74.
38 Pittet D, Rangel-Frausto S, Li N, Tarara D, Costigan M, Rempe L, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21:302–9.
39 Marshall L, Charlesworth A, Hurst J. The NHS payment system: evolving policy and emerging evidence. Nuffield Trust; 2014.
40 Statistik Bf. Medizinische Statistik der Krankenhäuser Neuchâtel, Switzerland 2015.
41 Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, et al. Systematic review of discharge coding accuracy. Journal of public health (Oxford, England). 2012;34:138–48.
Authors’ contribution: JP and RMM contributed equally.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.