Figure 1
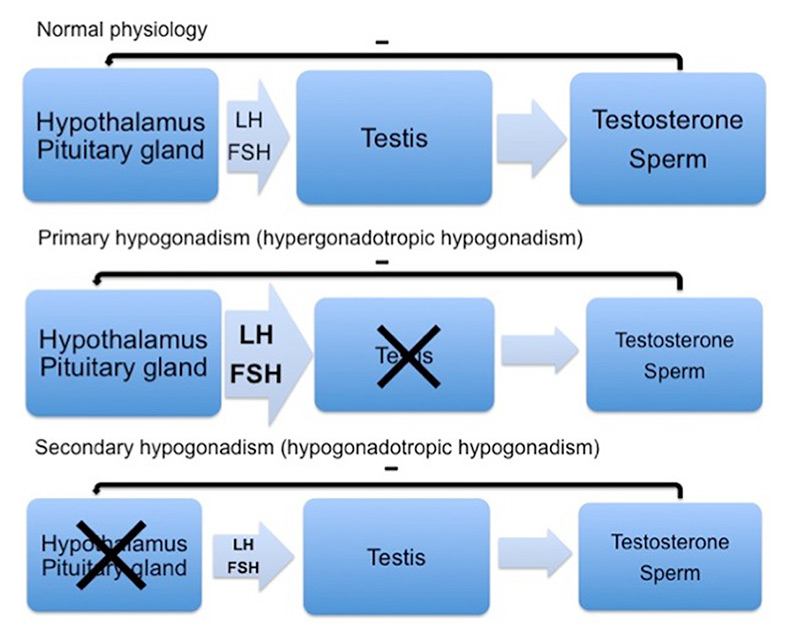
Hypothalamic-pituitary-gonadal axis and forms of hypogonadism.
FSH = follicle stimulating hormone; LH = luteinising hormone
DOI: https://doi.org/10.4414/smw.2015.14216
Male hypogonadism is defined as the failure to maintain physiological concentrations of testosterone, a physiological quantity of sperm or the combination of both and a wide variety of disorders can be the cause [1]. The Leydig cells of the testes produce most of the circulating androgens, while a small amount is derived from the adrenal glands. Testosterone is the most important androgen and is in males responsible for the development of primary and secondary sexual characteristics as well as the preservation of the male phenotype and reproductive function.

Figure 1
Hypothalamic-pituitary-gonadal axis and forms of hypogonadism.
FSH = follicle stimulating hormone; LH = luteinising hormone
Androgen deficiency can originate either from the testes (primary hypogonadism), resulting in low testosterone levels, impairment of spermatogenesis and elevated levels of luteinising hormone (LH) and follicle stimulating hormone (FSH), or from the hypothalamic and pituitary regulation of the testicular function (secondary hypogonadism), resulting in low testosterone levels, impaired spermatogenesis and low or inadequately normal LH and FSH levels (fig. 1) [1, 2].
Table 1 shows a compilation of the diverse aetiologies of hypogonadism [1–4]; it should be noted, that some conditions, such as haemochromatosis, cause central as well as peripheral gonadal insufficiency. The classification of hypogonadism into central (“secondary”) and peripheral (“primary”) is important, because it implies different therapeutic strategies, for example with regard to the restoration of fertility. While true hypogonadism, aetiologically linked to an underlying disorder (such as Klinefelter’s syndrome) [3], is unequivocally an indication for testosterone replacement therapy, this is much less clear for the aging male with low testosterone levels.
Testosterone has multiple actions in the body, such as sexual differentiation and reproduction, effects on muscle, bone, haematopoiesis and behaviour. Its effects (and the symptoms when lacking) mirror the multiple sites of action. The time of onset of testosterone deficiency is relevant. When occurring prenatally or before puberty, it may lead to delayed or incomplete formation of primary and secondary sexual characteristics. In contrast, testosterone deficiency in the postpubertal state may lead to decreased pubic and axillary hair, diminished shaving frequency, reduced spontaneous erections and libido, and many other nonspecific symptoms (see table 2) [1, 2, 4–6]. The biological effect of testosterone is distributed via testosterone itself by binding to the androgen receptor, via conversion to dihydrotestosterone by the enzyme 5-alpha-reductase or via oestradiol after being converted by the enzyme aromatase [1, 2].
| Table 1: Causes of primary and secondary hypogonadism. | ||
| Primary hypogonadism | Secondary hypogonadism | |
| Congenital | Klinefelter’s syndrome | Isolated hypogonadotropic hypogonadism |
| Mutations in LH and FSH receptors | Prader-Willi syndrome | |
| Myotonic dystrophy | Mutations in β-subunit of LH and FSH | |
| Cryptorchidism | ||
| Y-chromosome microdeletions | ||
| Acquired | Irradiation of the testis | Pituitary damage of any cause |
| Orchitis (mumps) | Tumours | |
| Chemotherapy | Apoplexy | |
| Haemochromatosis | Infections | |
| Sickle-cell disease | Infiltrative disease | |
| Cirrhosis | Haemochromatosis | |
| Testicular trauma or torsion | Head trauma | |
| Autoimmune testicular failure | Medications (opioids, glucocorticoids, GnRH agonists) | |
| Toxic (alcohol, etc.) | Morbid obesity or diabetes | |
| Eating disorder | ||
| Excessive exercise | ||
| Idiopathic hypogonadotropic hypogonadism | ||
| Hyperprolactinaemia | ||
| FSH = follicle stimulating hormone; LH= luteinising hormone | ||
| Table 2: Effects of testosterone, physiological and/or pharmacological, and clinical signs and symptoms of testosterone deficiency. | ||
| Effect (physiological and/or pharmacological) | Deficit | |
| Sexual organs | Male phenotype Development of primary and secondary sexual characteristics Maintenance of reproductive function | Libido↓ Sexual activity↓ |
| Body fat | Abdominal fat↑ | |
| Muscles | Muscle mass↑ | Muscle mass↓ |
| Bones | Bone mass↑ | Bone mass↓ Risk for osteoporosis↑ |
| Blood | Haemoglobin↑ Haematocrit↑ | Haemoglobin↓ Haematocrit↓ |
| Lipid profile | Total cholesterol↓ LDL cholesterol↓ HDL cholesterol↓ | Total cholestero↑ LDL cholesterol↑ |
| Psyche | Risk-taking behaviour↑ | Depressive disorders Well-being↓ |
| HDL = high-density lipoprotein; LDL = low-density lipoprotein | ||
The diagnosis of hypogonadism is a composite of (i) an illness known to cause hypogonadism (i.e. indicating a high a prioriprobability for true hypogonadism to be present),(ii) clinical symptoms and signs (table 2) [1, 2, 4, 5], as well as (iii) a low total testosterone concentration measured in serum [2].
Since free testosterone levels are delicate to determine and their reference values are not well established, total serum testosterone should be measured in most cases where hypogonadism is clinically suspected. In addition, total testosterone levels correlate better with the clinical symptoms of androgen deficiency than bioavailable testosterone estimates [7]. Importantly, testosterone levels must be measured fasting in the morning because of the underlying circadian rhythm. There is evidence that this rhythm is altered in the aging male [8, 9], but the testing of testosterone in the aging male should nevertheless take place in the morning, because Brambilla et al. have shown that, similarly to younger subjects, some of their older patients had low testosterone levels in the afternoon, but not in the morning [10]. The testosterone measurement should always be repeated at least once [5].
In cases where the measured total testosterone level is considered low, the aetiology of the hypogonadism needs to be determined (see table 1) [1–4]; this also applies to elderly males, since a bona fide pathology of the hypothalamic-pituitary region needs to be ruled out. The first step is to quantify LH and FSH [5]; if elevated, the cause of the hypogonadism is testicular and further evaluation depends on the age and clinical situation, but should include a careful personal history as well as the exclusion of haemochromatosis. In patients where the gonadotropin levels are low or (inadequately) normal, a central cause is suspected. After exclusion of obvious causes in the medical history (such as anorexia, opiates, glucocorticoid therapy), the further evaluation should include the measurement of prolactin and computed tomography or magnetic resonance imaging of the hypothalamic-pituitary region to exclude a mass lesion [2, 5, 11]. Christ-Crain et al. have shown in their study that additional gonadotropin releasing hormone (GnRH) testing, as compared with measuring LH and FSH only, could reduce the need for cranial imaging. In their study elderly men with a testosterone level below 11.7 nmol/l and an increase of LH upon GnRH-stimulation of more than 15 mU/l had a 100% sensitivity to exclude a hypothalamic-pituitary origin of the hypogonadism [12].
The most challenging question is the definition of the lower threshold of the reference range for testosterone. This value is not only age-dependent, but is different among subjects: testosterone declines with age [13–19] and there is a significant interindividual variability in the testosterone levels leading to symptoms of androgen deficiency [10, 20]; moreover, the threshold for the various distinct symptoms of low testosterone is also different [10, 20–22]. Although no good substantiating evidence exists, the current guidelines recommend a threshold value of 10.4 nmol/l for the lower normal range of total serum testosterone [5], corresponding to the limit in young men [5, 20]. However, applying this value to men over the age of 60 years leads to the classification of 20% of these subjects as being androgen deficient [15].
Besides the question regarding the definition of “normal” testosterone serum levels, another challenging issue is whether the age-related decline in testosterone levels is a variation of normality or has a true disease value requiring therapy [13–19]. This problem has been very controversial, discussed over the past 10–20 years, and the only meaningful answers would come from large, long-term randomized and placebo-controlled trials, which will be discussed in detail below. To further confound matters, several names have been attached to the age-related decline in testosterone levels, such as andropause, late onset hypogonadism, partial androgen deficiency of the aging male or “low T syndrome” [5, 11, 23].
Morley et al. developed a screening questionnaire for androgen deficiency in aging males (ADAM-questionnaire) [24]. This questionnaire includes the topics of libido, erectile function, mood, physical activity and muscle strength, and the authors reported a sensitivity of 88% and a specificity of 60% for predicting a low testosterone level [24]. However, Christ-Crain et al. showed that there was no correlation between a positive ADAM-questionnaire and the measured testosterone levels in 51 men [25]. In another study, Wu et al. [21] reported from a population survey of 3 369 men (40 to 79 years of age), that the existence of at least three sexual symptoms (decreased frequency of morning erection, erectile dysfunction, decreased frequency of sexual thoughts) is associated with a decreased total testosterone level. No correlation was, however, found with psychological symptoms (fatigue, loss of energy, sadness). They therefore suggest a combination of the named three sexual symptoms and a total testosterone level of less than 11 nmol/l as a definition for the diagnosis of late-onset hypogonadism [21]. In the EMAS study population [26], use of this definition (i.e. a combination of three sexual symptoms and a total testosterone less than 11 nmol/l), gave a prevalence of 2.1% in the entire study population (63 out of 2966 men), which increased with age from 0.1% in men 40 to 49 years of age, to 5.1% for those 70 to 79 years of age [21].
However, the answer regarding the risks and benefits of treating the age-related decline in testosterone levels must come from adequately powered long-term randomised trials. Although such trials provide only very limited support for testosterone supplementation in the aging male, as described below, testosterone prescriptions have increased dramatically in the United States over the past two decades, from around $18 million until 1988 to around $400 million at the end of 2002 [27].
The debate is still ongoing over who could benefit from testosterone replacement therapy and this is mostly due to the absence of adequate trial data. Based on physiological considerations and data from hypogonadal men, we would expect positive actions of testosterone on bone, muscle, body-fat, libido, reproductive function, psyche and lipid profile (see table 2) [1, 2, 4, 5].
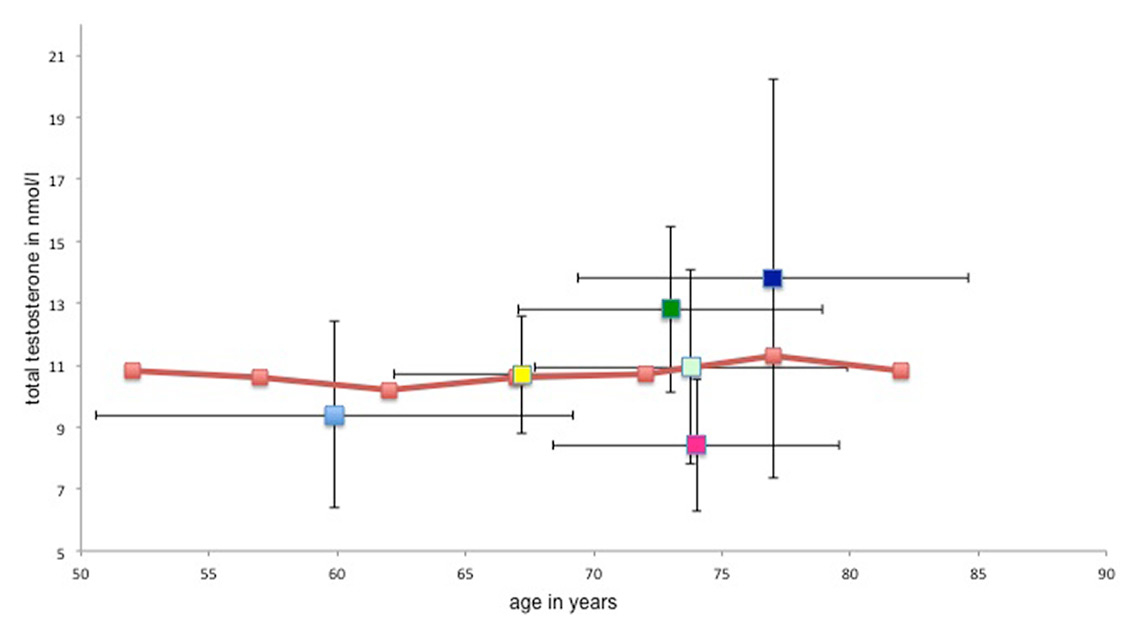
Figure 2
The mean age and mean testosterone levels at baseline from six study populations in comparison with adopted data from the Rancho-Bernardo study.
Light blue = Jones et al. [30]; yellow = Emmelot-Vonk et al. [28, 29]; light green = Srinivas-Shankar et al. [34]; dark green = Snyder et al. [32, 33]; pink = Basaria et al. [27]; purple = Kenny et al. [31]; orange line = mean age-dependent total serum testosterone levels in the Rancho-Bernardo study [13]
We searched the literature for randomised, controlled trials of testosterone administration in aging males, a size of at least 100 patients and a follow-up of more than 3 years – unfortunately, not a single trial fulfils these criteria (the longest follow-up was 36 months in two studies) and we decreased the minimal requirement for follow-up to 6 months. With these eligibility criteria we identified eight studies (see table 3) [28–35], which include two studies of Snyder et al. [33, 34] and two studies of Emmelot-Vonk et al. [29, 30], who each use the same study population in both of their studies. The identified studies are heterogeneous; total patient number varies between 108 and 262, the mean age between 60 years and 77 years, as well as the formulation (transdermal, oral, scrotal patch) and dosage of testosterone (transdermal between 5 mg to 100 mg daily, oral 160 mg daily). In addition, the mean baseline testosterone levels vary widely and some of the studies (e.g. Snyder et al., Kenny et al., Srinivas-Shankar et al., Emmelot-Vonk et al. [29, 30, 32–35]) do not fulfil the current criteria of low testosterone discussed above [5].
Snyder et al. [33, 34] dealt in their two studies with the questions whether testosterone treatment would lead to an increase in bone density, lean body mass and muscle strength, and decrease in body fat. They randomised 108 men over 65 years of age (mean age 73 years) to either placebo or testosterone scrotal patch (approximately 6 mg testosterone daily). The inclusion criteria was a testosterone at least 1 standard deviation below the mean for healthy young men (16.5 nmol/l) and the participants did not have to complain of any symptoms of low testosterone for inclusion [33, 34]. The mean total testosterone level for the men included in the study was 12.8 nmol/l. A strength of these studies is that the follow-up period was 36 months. Nevertheless, neither an increase in overall bone density, nor an increase in muscle strength measured by knee extension and flexion could be noticed [33, 34].
Similar findings were reported in the study of Kenny et al. [32]: they investigated the effects of transdermal testosterone treatment (5 mg daily for at least 12 months) on bone density, body composition, muscle and physical function in 131 men (mean age 77 years); the mean total testosterone level at baseline was 13.8 nmol/l [32]. The effect of testosterone therapy on bone density was site-dependent, as there was a small effect on bone mineral density in the spine (3.25% increase, p = 0.005), but no significant effect on the hip and even a decrease in wrist bone density [32]. As in the studies of Snyder et al. [33, 34], an increase in lean body mass and decrease in body fat was noted, although these changes were not associated with differences in strength or physical performance [32].
The results of the studies of Emmelot-Vonk et al. [29, 30] are similar to the trials discussed above [32–34]. No significant differences between testosterone treatment (testosterone twice daily orally, 160 mg/d for 6 months) and placebo in 223 men (mean age 67 years, mean total testosterone 10.7 nmol/l) were reported concerning bone density, functional mobility, muscle strength or cognitive function. In addition, these authors analysed the effects of testosterone treatment on the sexual function and did not find a difference during the follow-up period [29, 30].
Exploring similar endpoints (muscle mass and strength, physical function) Srinivas-Shankar et al. [35] found positive results, although of debatable clinical relevance. The 262 men included in the study (mean age 74 years, mean total testosterone 10.9 nmol/l) received either transdermal testosterone gel (50 mg/d) or placebo gel. After 6 months of treatment, the isovolumetric knee extension improved in the testosterone group by 8.6 Newton-metres (95% confidence interval [CI] 1.3–16.0; p = 0.02). The authors claim that even these modest changes are associated with improvement in overall physical function, although they fail to document this by examining measures relevant for daily activities [35].
A different approach was used in the TIMES2 Study [31], where 220 hypogonadal men (mean age 60 years, mean total testosterone 9.4 nmol/l) with type 2 diabetes and/or metabolic syndrome received transdermal testosterone (60 mg daily) or placebo over 12 months. The main goal was to evaluate the effect of testosterone treatment on insulin resistance. Taken together, no relevant differences could be shown in this study [31].
The last study of the eight intervention studies qualifying for this review is the study of Basaria et al. [28], also known as the “Testosterone in Older Men with Mobility Limitations (TOM) – Trial” which was designed to evaluate the effect of testosterone therapy on lower-extremity strength and physical function over a period of 6 months in 209 men (mean age 74 years, mean total testosterone 8.4 nmol/l). The participants received a placebo or 100 mg of testosterone dermal gel daily. The trial was stopped before enrolment had been completed because of a higher incidence of adverse cardiovascular events in the testosterone group (acute coronary syndrome, stroke, etc; see discussion below). The analysis of all the participants who underwent at least one outcome assessment showed a significant increase in the testosterone group regarding leg-press strength, chest-press strength and stair climbing power while carrying a load [28].
In figure 2 we show the distribution of age and mean serum testosterone in the eight trials discussed above with regard to the natural age-related decline in serum testosterone as documented in the “Rancho Bernardo study” [14, 28–35]. In this study, Ferrini and Barrett-Connor [14] examined the age-associated variations in endogenous sex hormones in 810 community-dwelling men in Rancho Bernardo, California, in the years 1984–1987. They showed that there was a small, but age-related decline of testosterone levels in their population (age between 24–90 years, mean age 69.6 years). Figure 2 demonstrates that the mean total testosterone level in most of the relevant intervention studies discussed above [28–35] was higher at baseline than the testosterone in the men in the Rancho Bernardo study of similar age [14]. Hence, only the studies of Basaria and Jones [28–35] included patients with mean testosterone levels below the age-dependent mean of the Rancho Bernardo population – and one of these studies had to be terminated early because of serious cardiovascular adverse events [28].
This emphasises the need for further studies [36] to define the treatment threshold for testosterone levels in the aging male, as well as with regards to the long-term (3–10 year) risks and (clinically relevant!) benefits of testosterone therapy in this population.
| Table 3: Summary of findings of randomised controlled trials administering testosterone. | ||
| Outcome | Results (eight studies) | |
| Inclusion criteria for trials: minimum of 100 patients, follow-up at least 6 months | ||
| Significant | Not significant | |
| Strength and physical function | Basaria et al. [28]: increase in leg-press (in Newtons, difference between groups: mean 129.8 (43.9 to 215.6, p = 0.003) and chest-press strength (in Newtons, difference between groups: mean 34.5 (13.2 to 55.8, p = 0.002), stair climbing power with a load (in Watts, difference between groups: mean 30.2 (0.3–60.1, p = 0.05) | Basaria et al. [28]: no change in grip-strength, 50-m walking speed, lift and lower test |
| Srinivas-Shankar et al. [35]: improvement in isovolumetric knee extension in testosterone group by 8.6 Newton-meters (95% confidence interval, 1.3–16.0; p = 0.02). | Srinivas-Shankar et al. [35]: no significant difference in grip strength or physical function test | |
| Emmelot-Vonk et al. [29]: no significant changes in both groups in score on the Hamilton Assessment Questionnaire, isometric grip strength, isometric leg extensor strength and timed get-up-and-go test | ||
| Kenny et al. [32]: no significant difference in strength or physical performance | ||
| Snyder et al. [34]: no change in knee strength or physical function | ||
| Bone mineral density | Kenny et al. [32]: increase on bone mineral density in the spine (3.25% increase, p = 0.005) | Kenny et al. [34]: no significant effect on hip and even a decrease in wrist bone density (1.2% decrease, p = 0.008) |
| Snyder et al. [33]: increase in mean bone density in lumbar spine (p <0.001) in both groups (testosterone-group [4.2 ± 0.8%] and placebo-group [2.5 ± 0.6%]) | ||
| Emmelot-Vonk et al. [29]: no change in bone mineral density (total hip and lumbar spine) in both groups | ||
| Body composition | Emmelot-Vonk et al. [29]: significant decrease in total fat mass (in kg, change difference: −1.3 (−1.8 to – 0.8, p <0.001) and increase in lean mass (in kg, change difference: 1.2 (−0.7 to 1.7, p <0.001) in testosterone group, no difference in placebo group | |
| Srinivas-Shankar et al. [35]: significant increase in lean body mass in testosterone group vs placebo (mean difference in kg: 1.1 (0.6–1.5; p = <0.001), decrease in fat mass (mean difference in kg: 0.6 (−1.1 to −0.1; p = 0.01) | ||
| Snyder et al. [34]: decrease in fat mass (−3.0 ± 0.5 kg, p = 0.001), increase in lean mass (1.9 ± 0.3 kg, p = <0.001) in testosterone group | ||
| Change in glucose, plasma lipids and insulin resistance | Jones et al. [31]: significant reduction in testosterone group in HOMA-IR at 6 (15.2%, p = 0.018) and 12 months (16.4%, p = 0.006) | Jones et al. [31]: no significant changes in BMI, waist circumference, abdominal obesity, percentage body fat, triglycerides |
| Emmelot-Vonk et al. [29]: significant decrease in total cholesterol (change difference −0.2 [−0.4 to 0], 0.03) and HDL (change difference −0.1 [−0.2 to −0.1, p <0.001]) | ||
| Cognition | Emmelot-Vonk et al. [29]: both groups increased in cognitive function scores, but no significant differences between groups | |
| Sexual function | Emmelot-Vonk et al. [30]: no difference in scores on sexual function between the groups | |
| BMI = body mass index; HDL = high-density lipoprotein: HOMA-IR = homeostasis model assessment of insulin resistance | ||
| Table 4: Testosterone formulations approved in Switzerland. | |||
| Registered trade name | Application(recommended starting dose) | Preparation | Costs(approximately) |
| Intramuscular | |||
| Nebido® | 1000 mg, every 12 weeks | Testosterone undecanoate | 670 CHF/y |
| Testoviron® | 250 mg, every 2–3 weeks | Testosterone enanthate | 320–490 CHF/y |
| Transdermal | |||
| Testogel® | 50 mg in 5 g gel, once daily | Testosterone | 1 150 CHF/y |
| Oral | |||
| Andriol® | 40 mg capsules, 3–4 capsules daily in the first 2–3 weeks, followed by 1–3 capsules daily | Testosterone undecanoate | 290–860 CHF/y |
Testosterone can be administered by several routes – transdermally, intramuscularly and orally – and each route is accompanied by different pharmacokinetics (see table 4) [5, 37]. The replacement therapy is usually in the form of testosterone, which can be converted to oestradiol and dihydrotestosterone [38]. Finkelstein et al. [39] showed in a study with two cohorts of men (aged 20–50, with testosterone levels in the normal range) that the amount of testosterone required to maintain lean mass, fat mass, strength and sexual function varied widely in the study population. Moreover, Finkelstein has shown that oestrogen deficiency was associated with an increase in body fat, whereas both androgen and oestrogen deficiency accounted for a decline in sexual function [39]. This study emphasises the importance of the administration of natural testosterone as well as the challenge of interindividual thresholds for the action of testosterone.
The main adverse actions of testosterone are related to the haematological, cardiovascular and urogenital systems.
Testosterone replacement therapy leads to an increase in haematocrit and this is the most common adverse event of androgen therapy. It was observed that the increase in haematocrit was more distinct in older men compared with younger hypogonadal men [40–42]. Coviello et al. have shown that haemoglobin and haematocrit increase in a linear, dose-dependent manner [41]. The rise in haematocrit starts 1 week after the introduction of testosterone therapy and continues in some individuals until 12 weeks [41]. There is some evidence that the application of testosterone intramuscularly is associated with a higher incidence of erythrocytosis [43].
The mechanisms by which testosterone leads to erythroctyosis remain unclear. In most studies there was no evidence of an increase in erythropoietin levels under testosterone replacement therapy [41, 44]. Bachman et al. demonstrated that hepcidin, which is an iron regulatory peptide, is suppressed under testosterone administration [45], where a lowering in hepcidin levels leads to an increase in bioavailable iron, hence predisposing to the development of erythrocytosis [46]. Using the data from the TOM trial [28], Bachman et al. could show that the increase of haemoglobin and haematocrit under testosterone replacement therapy is accompanied by a transient increase in erythropoietin levels at 1 and 3 months after the initiation of treatment, as well as a decrease in hepcidin and ferritin levels [47]. At 6 months, the erythropoietin levels returned to a (nonsuppressed) baseline under continued testosterone application, while haemoglobin and haematocrit remained elevated. Therefore, the authors suggest that there must be a new “set-point” of erythropoietin in relation to the haemoglobin level, thereby continuously promoting erythrocytosis [47].
Because of this common side effect, haematocrit values must be checked at baseline, at 3 to 6 months and then annually under testosterone replacement-therapy [5]. If the haematocrit rises above 54%, testosterone application should be stopped and can be reinitiated with a lower dose of testosterone after the haematocrit has fallen below 50% [5].
There is an ongoing debate whether testosterone replacement therapy is associated with an increase or decrease in cardiovascular events.
Low testosterone is associated with an increase in cardiovascular risk factors such as a rise of cholesterol levels, insulin resistance and a gain in abdominal fat [48]; hence, it is tempting to speculate that low testosterone might lead to a higher risk for cardiovascular events and mortality. There are various prospective community-based studies that indeed found a correlation between low testosterone levels and cardiovascular events and mortality [49–52]. On the other hand, two meta-analyses [42, 53] have not shown an association between testosterone therapy and an increase in cardiovascular events and/or mortality in randomised controlled trials. The most recent meta-analysis concerning this topic showed an increased risk of cardiovascular events [54], as can also be suspected from the effects of androgens on erythropoiesis and the coagulation system. This meta-analysis, together with the studies of Basaria et al. [28] and Finkle et al. [55], raised the concern about the cardiovascular safety of testosterone replacement therapy.
The TOM trial [28] mentioned above was originally designed to evaluate the effect of testosterone therapy on lower-extremity strength and physical function. The trial was stopped before enrolment had been completed because of a higher incidence of adverse cardiovascular events in the testosterone group, although the study was not powered to assess this aspect. The reported events were diverse and included acute coronary syndrome, syncope, peripheral oedema, stroke, cardiac arrhythmias, etc. [28].
Another cohort-study [55] was an attempt to evaluate the risk of myocardial infarction following the beginning of testosterone therapy. From a large healthcare database (n = 55 593) the incidence rate of myocardial infarction in the 90 days following the initial testosterone prescription (postprescription interval) was compared with the rate 1 year prior to the initial prescription (preprescription interval). The post-/preprescription rate ratio (RR) for testosterone treatment prescription was 1.36 (95% CI 1.03–1.81) in all subjects and it was shown that there is an increase in RR for testosterone treatment prescription in older men (men <55 years 0.95 [95% CI 0.54–1.67], men ≥75 years 3.43 [95% CI 1.54–7.56]). The authors suggest that there is an increase in the risk of experiencing a myocardial infarction after initiation of testosterone therapy in older men [55].
Taken together, large-scale, prospective, randomised controlled trials are needed to evaluate the benefits and risks of testosterone therapy in the aging male [36].
Prostate tissue is well known to be an androgen-sensitive tissue [1]. Therefore, the concern that testosterone therapy will lead to an increase in prostate-related events is justified. Benign prostatic hyperplasia is a common finding in the aging male, as is prostate cancer. Testosterone therapy can be administered in patients known to have benign prostatic hypertrophy as long as they have mild to moderate lower urinary tract symptoms [5, 27]. The symptoms can be evaluated by the IPSS (International Prostate Symptom Score). If a patient has severe symptoms of the lower urinary tract (IPSS >19) testosterone therapy should be closely monitored because an increase in prostate volume under therapy could exaggerate the obstructive symptoms [5, 27].
The current literature shows no clear evidence that testosterone therapy increases the risk for prostate cancer [5, 11, 56]. Men with metastatic prostate cancer or breast cancer are at high risk for adverse events under testosterone replacement therapy and testosterone use is not recommended in such patients [5]. In various meta-analyses [40, 42], no significant difference was shown in the incidence of prostate-related events. Kaufman and Vermeulen [57] showed in their review that, in the few available intervention studies of testosterone therapy in the aging male, some cases of prostate cancer were diagnosed, although this does not prove causality [57]. The definitive answer to the question whether testosterone replacement is safe in relation to the development of prostate cancer in the aging male will depend on studies with a follow-up duration of 5–10 years or more.
Under testosterone therapy close monitoring for adverse effects is mandatory: testosterone levels should be obtained 3 to 6 months after therapy initiation; the goal is to raise total testosterone levels into the lower-normal range for elderly patients, around 10 nmol/l [5]. Furthermore, current recommendations include the measurement of haematocrit at baseline and 3 and 6 months, and then annually, as well as performing a digital rectal examination in men over 40 years with a baseline prostate-specific antigen over 0.6 ng/ml; other aspects also require attention, such as urinary symptoms, as detailed in reference [5].
Testosterone declines in the aging male [13–19] and the decision at what point this decline is clinically relevant remains uncertain. There have been different attempts to define the state of low testosterone in the aging male. Wu et al. [21] suggest that a combination of at least three sexual symptoms and a serum total testosterone level below 11 nmol/l (fasting in the morning) may be a possible definition of late-onset hypogonadism. However, a wide interindividual range in the decline of testosterone levels exists [10, 20] as well as with regard to the sensitivity of target tissues.
A critical evaluation of each patient and his complaints must precede the measurement of testosterone (establish a high clinical a priori probability for clinically relevant hypogonadism to be present), since an abnormal serum level invariably leads to the sometimes nearly irresistible temptation to replace the missing hormone. The usually recommended threshold value is a total testosterone level of 10 nmol/l [5] and we suggest that this cut-off should be used in subjects with a high a priori likelihood for testosterone deficiency (risk factors for hypogonadism, history, physical examination); however, in patients with a lower probability, a cut-off of 7 nmol/l should be considered in order to avoid exposing nonhypogonadal men to the risks of testosterone replacement therapy. Testosterone is normally administered intramuscularly and regular monitoring of the potential haematological, cardiovascular and urogenital side effects is mandatory.
However, before testosterone medication is begun, the aetiology of the hypogonadism should be established in order to exclude a systemic or local disease, which must be treated specifically.
In summary, after all the past decades of testosterone replacement therapy and the rise in its use in aging males, we still do not know the threshold value of testosterone in the aging male and whether the benefits might be bigger than the risks. This mostly due to the lack of adequate randomised controlled trials, but also hampered by the presence of distinct interindividual differences in testosterone levels and sensitivity. Until we have more solid data on these important issues, testosterone replacement therapy should be reserved for patients with bona fide hypogonadism, i.e., risk factors and clinical findings supporting the clinical relevance of a low serum testosterone level.
1 Bhasin S. Testicular Disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polanski KS, editors. Williams Textbook of Endocrinology. 11th Edition, Philadelphia: Elsevier; 2008. p. 645–99.
2 Basaria S. Male hypogonadism. Lancet. 2014;383(9924):1250–63.
3 Cheng Xu LM, Nelly Pitteloud. Hypogonadismus beim Mann. Schweiz Med Forum. 2015;15(10):218–24.
4 Isidori AM, Buvat J, Corona G, Goldstein I, Jannini EA, Lenzi A, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol. 2014;65(1):99–112.
5 Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–59.
6 Coates JM, Herbert J. Endogenous steroids and financial risk taking on a London trading floor. Proc Natl Acad Sci U S A. 2008;105(16):6167–72.
7 Christ-Crain M, Meier C, Huber P, Zimmerli L, Trummler M, Muller B. Comparison of different methods for the measurement of serum testosterone in the aging male. Swiss Med Wkly. 2004;134(13-14):193–7.
8 Bremner WJ, Vitiello MV, Prinz PN. Loss of Circadian Rhythmicity in Blood Testosterone Levels with Aging in Normal Men. J Clin Endocrinol Metab. 1983;56(6):1278–81.
9 Diver MJ, Imtiaz Ke, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf). 2003;58(6):710–7.
10 Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf). 2007;67(6):853–62.
11 Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol. 2008;159(5):507–14.
12 Christ-Crain M, Meier C, Huber PR, Zimmerli L, Mueller B. Value of Gonadotropin-Releasing Hormone Testing in the Differential Diagnosis of Androgen Deficiency in Elderly Men. J Clin Endocrinol Metab. 2005;90(3):1280–6.
13 Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age Trends in the Level of Serum Testosterone and Other Hormones in Middle-Aged Men: Longitudinal Results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87(2):589–98.
14 Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147(8):750–4.
15 Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. J Clin Endocrinol Metab. 2001;86(2):724–31.
16 Lapauw B, Goemaere S, Zmierczak H, Van Pottelbergh I, Mahmoud A, Taes Y, et al. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. 2008;159(4):459–68.
17 Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–3.
18 Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, et al. Hypothalamic-Pituitary-Testicular Axis Disruptions in Older Men Are Differentially Linked to Age and Modifiable Risk Factors: The European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–45.
19 Zmuda JM, Cauley JA, Kriska A, Glynn NW, Gutai JP, Kuller LH. Longitudinal Relation between Endogenous Testosterone and Cardiovascular Disease Risk Factors in Middle-aged Men: A 13-Year Follow-up of Former Multiple Risk Factor Intervention Trial Participants. Am J Epidemiol.1997;146(8):609–17.
20 Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab. 2004;89(8):3813–7.
21 Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, et al. Identification of Late-Onset Hypogonadism in Middle-Aged and Elderly Men. N Engl J Med. 2010;363(2):123–35.
22 Zitzmann M, Faber S, Nieschlag E. Association of Specific Symptoms and Metabolic Risks with Serum Testosterone in Older Men. J Clin Endocrinol Metab. 2006;91(11):4335–43.
23 Morales A, Heaton JPW, Carson CC, Andropause: a misnomer for a true clinical entity. J Urol. 2000;163(3):705–12.
24 Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239–42.
25 Christ-Crain M, Mueller B, Gasser TC, Kraenzlin M, Trummler M, Huber P, et al. Is there a clinical relevance of partial androgen deficiency of the aging male. J Urol. 2004;172(2):624–7.
26 Tajar A, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, et al. Characteristics of Androgen Deficiency in Late-Onset Hypogonadism: Results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508–16.
27 Bhasin S, Singh AB, Mac RP, Carter B, Lee MI, Cunningham GR. Managing the Risks of Prostate Disease During Testosterone Replacement Therapy in Older Men: Recommendations for a Standardized Monitoring Plan. J Androl. 2003;24(3):299–311.
28 Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse Events Associated with Testosterone Administration. N Engl J Med. 2010;363(2):109–22.
29 Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299(1):39–52.
30 Emmelot-Vonk MH, Verhaar HJ, Nakhai-Pour HR, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on sexual functioning in aging men: a 6-month randomized controlled trial. Int J Impot Res. 2009;21(2):129–38.
31 Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, et al. Testosterone Replacement in Hypogonadal Men With Type 2 Diabetes and/or Metabolic Syndrome (the TIMES2 Study). Diabetes Care. 2011;34(4):828–37.
32 Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, et al. Effects of Transdermal Testosterone on Bone and Muscle in Older Men with Low Bioavailable Testosterone Levels, Low Bone Mass and Physical Frailty. J Am Geriatr Soc. 2010;58(6):1134–43.
33 Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, et al. Effect of Testosterone Treatment on Bone Mineral Density in Men Over 65 Years of Age. J Clin Endocrinol Metab. 1999;84(6):1966–72.
34 Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of Testosterone Treatment on Body Composition and Muscle Strength in Men Over 65 Years of Age. J Clin Endocrinol Metab. 1999;84(8):2647–53.
35 Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MDL, Adams JE, Oldham JA, et al. Effects of Testosterone on Muscle Strength, Physical Function, Body Composition, and Quality of Life in Intermediate-Frail and Frail Elderly Men: A Randomized, Double-Blind, Placebo-Controlled Study. J Clin Endocrinol Metab. 2010;95(2):639–50.
36 Endocrine Society. The risk of cardiovascular Events in Men Receiving Testosterone Therapy [statement]. 2014 Feb; Available from: https://www.endocrine.org/~/media/endosociety/Files/Advocacy%20and%20Outreach/Position%20Statements/Other%20Statements/The%20Risk%20of%20Cardiovascular%20Events%20in%20Men%20Receiving%20Testosterone%20Therapy.pdf
37 swissmedic, Schweizerisches Heilmittelinstitut [internet]. [updated may 2015, cited may 2015]. Available from: https://www.swissmedic.ch
38 Nieschlag E. Current topics in testosterone replacement of hypogonadal men. Best Pract Res Clin Endocrinol Metab. 2015;29(2015)77–90.
39 Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–22.
40 Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical Review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(6):2560–75.
41 Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of Graded Doses of Testosterone on Erythropoiesis in Healthy Young and Older Men. J Clin Endocrinol Metab. 2008;93(3):914–9.
42 Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, et al. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci. 2005;60(11):1451–7.
43 Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, Efficacy, and Safety of a Permeation-Enhanced Testosterone Transdermal System in Comparison with Bi-Weekly Injections of Testosterone Enanthate for the Treatment of Hypogonadal Men. J Clin Endocrinol Metab. 1999;84(10):3469–78.
44 Maggio M, Snyder PJ, Ceda GP, Milaneschi Y, Luci M, Cattabiani C, et al. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology. 2013;1(1):24–8.
45 Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, et al. Testosterone Suppresses Hepcidin in Men: A Potential Mechanism for Testosterone-Induced Erythrocytosis. J Clin Endocrinol Metab. 2010;95(10):4743–7.
46 Fleming MD. The Regulation of Hepcidin and Its Effects on Systemic and Cellular Iron Metabolism. Hematology Am Soc Hematol Educ Program. 2008:151–8.
47 Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, et al. Testosterone Induces Erythrocytosis via Increased Erythropoietin and Suppressed Hepcidin: Evidence for a New Erythropoietin/Hemoglobin Set Point. J Gerontol A Biol Sci Med Sci. 2014;69(6):725–35.
48 J Jones TH. Testosterone deficiency: a risk factor for cardiovascular disease?. Trends Endocrinol Metab. 21(8):496–503.
49 Khaw K-T, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, et al. Endogenous Testosterone and Mortality Due to All Causes, Cardiovascular Disease, and Cancer in Men: European Prospective Investigation Into Cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–701.
50 Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75.
51 Tivesten Å, Vandenput L, Labrie F, Karlsson MK, Ljunggren Ö, Mellström D, et al. Low Serum Testosterone and Estradiol Predict Mortality in Elderly Men. J Clin Endocrinol Metab. 2009;94(7):2482–8.
52 Haring R, Völzke H, Steveling A, Krebs A, Felix SB, Schöfl C, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J. 2010 Jun;31(12):1494–501.
53 Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, et al. Testosterone and Cardiovascular Risk in Men: A Systematic Review and Meta-analysis of Randomized Placebo-Controlled Trials. Mayo Clin Proc. 2007;82(1):29–39.
54 Xu L, Freeman G, Cowling BJ, Schooling CM. Testosterone therapy and cardiovascular events among men: a systematic review and meta-analysis of placebo-controlled randomized trials. BMC Med. 2013;11:108.
55 Finkle WD, Greenland S, Ridgeway GK, Adams JL, Frasco MA, Cook MB, et al. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PloS one. 2014;9(1):e85805.
56 Endogenous Hormones and Prostate Cancer Collaborative Group, Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous Sex Hormones and Prostate Cancer: A Collaborative Analysis of 18 Prospective Studies. J Natl Cancer Inst. 2008;100(3):170-83.
57 Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–76.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.