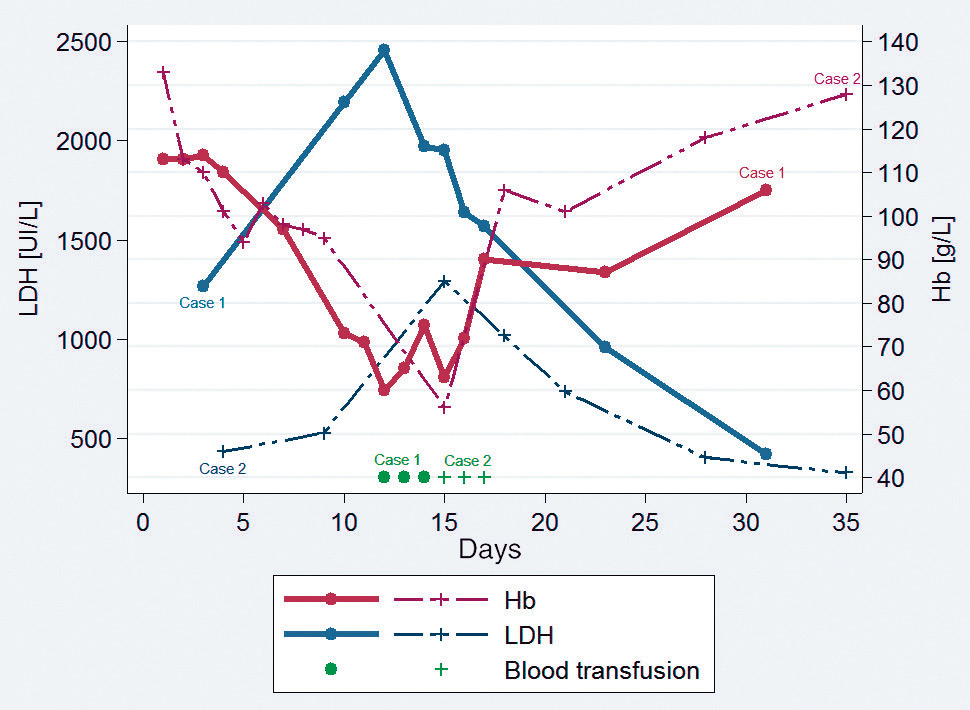
Figure 1
Time course of haemoglobin (Hb) levels and lactate dehydrogenase (LDH) levels in case 1 and case 2 (delayed pattern).
N.B.: Because of the low number of LDH data, case 3 is not shown.
DOI: https://doi.org/10.4414/smw.2015.14181
Abbreviations
CRP: C-reactive protein
F: female
G6PD: glucose-6-phosphate dehydrogenase
Hb: haemoglobin
HFR: high-fluorescence reticulocytes
LDH: lactate dehydrogenase
M: male
NA: not available
PADH: post-artesunate delayed haemolysis
SpO2: peripheral oxygen saturation
WHO: World Health Organization
Between 2000 and 2013, estimated malaria mortality rates fell by 47% [1]. Nevertheless, in 2013, an estimated 198 million cases of malaria were diagnosed worldwide, with more than 550 000 related fatalities. Annually, the World Health Organization (WHO) reports approximately 10 000 to 12 000 malaria cases imported to Europe [2] with a mortality ranging from 0.3% to 10.5% in severe cases [3, 4].
Intravenous artesunate has now replaced quinine as the first-line treatment for severe malaria in adults and children [5]. Its superiority was first demonstrated in endemic countries with a 34.7% mortality reduction in the Asian SEAQUAMAT trial and 22.5% in the African AQUAMAT trial [6, 7]. A recent meta-analysis confirmed these conclusions with a relative risk of mortality of 0.61 for adults and 0.76 for children treated with parenteral artesunate vs parenteral quinine [8]. This effect is probably related to a faster clearance of parasites. In addition, artesunate induces fewer adverse events such as cardiotoxicity, hypoglycaemia and hearing disturbances.
Since 2011, a number of cases of delayed haemolytic anaemia in travellers who had been treated with intravenous artesunate for severe Plasmodium falciparum malaria with hyperparasitaemia have been documented. Post-artesunate delayed haemolysis (PADH) appears after the end of treatment, following a first phase of clinical improvement without parasitaemia (explanations for the physiopathology are described in the discussion). This phenomenon, therefore, is not associated with a relapse of active malaria infection, and is different from the anaemia caused by P. falciparumduring the blood stages of the parasite. Indeed, as reported by Clark et al. [9] and Rolling et al. [10], anaemia during active malaria results from the destruction of erythrocytes (infected and noninfected) by the reticuloendothelial system and a low production of reticulocytes due to decreased erythropoiesis in the bone marrow secondary to a strong inflammatory response. The anaemia caused by the active infection lasts 1 week approximately. Thereby, parasite clearance is synchronous with the increase in reticulocyte production and rising haemoglobin level.
We hereby present four cases of PADH, diagnosed at Geneva University Hospitals since August 2012.
A 34-year-old Mexican female with no medical history and who had lived in Geneva for 5 years developed severe P. falciparum malaria with hyperparasitaemia (27.5%) and hyperbilirubinaemia (77 µmol/l) 15 days after returning from a 4-day professional trip to Malawi. She had not taken antimalaria chemoprophylaxis. She was admitted to the intermediate care unit and given three doses of intravenous artesunate (Malacef®, Artecef BV, The Netherlands) of 2.4 mg/kg each (cumulative dose of 7.2 mg/kg = 396 mg) at 12-hour intervals. Less than 24 hours later, the parasitaemia dropped to 0.02%. The treatment was switched to oral artemether/lumefantrine (Riamet®) for 3 days with a rapid clinical and laboratory improvement (haemoglobin 110 g/l, C-reactive protein [CRP] 24 mg/l, bilirubinaemia 31 µmol/l, and two negative blood smears 24 hours apart). The patient was then discharged on day 4. On day 7, she was seen for follow-up as an outpatient. Her temperature was 35.9 °C, haemoglobin 97 g/l and she had a negative peripheral blood smear. On day 10, the patient showed fatigue, exertional dyspnoea, fever of 39.4 °C and jaundice with dark urine. No clinical source of infection was noticed. She was re-admitted to a regular hospital ward. Blood analysis indicated a haemoglobin level of 77 g/l, CRP of 130 mg/l, bilirubinaemia of 50 µmol/l associated with haemolysis criteria: reticulocytes 531.73 G/l with 182.1‰, high fluorescence reticulocytes (HFR) 10.7%, lactate dehydrogenase (LDH) 2 195 UI/l, undetectable haptoglobin (<74 mg/l) and a free haemoglobin of 25.6 µmol/l. Blood smear was negative. Urinanalysis with a urine test strip and microscopy showed high levels of haemoglobinuria without erythrocyturia and positive nitrite. Ceftriaxone was introduced for 24 hours until urine culture became negative. Blood culture was also negative. No immunological tests (Coomb’s test) nor inherited haemolysis causes (glucose-6-phosphate dehydrogenase [G6PD] deficiency, sickle cell disease, spherocytosis) were performed. A diagnosis of PADH was made on the classical presentation and history. On day 12, the haemoglobin dropped to the lowest level of 60 g/l. The patient received three packed red blood cell transfusions. From day 14, the fever decreased spontaneously with concomitant normalisation of haemolytic markers. On day 15, the patient was discharged with a haemoglobin level of 106 g/l. Follow-up at days 18 and 28 did not show any relapse.
Two weeks after a 3-day professional trip to Burkina Faso, a 42-year-old Canadian female was admitted to the intensive care unit for severe P. falciparum malaria with 9% parasitaemia. She had not taken any chemoprophylaxis. She received 2.4 mg/kg of intravenous artesunate (Malacef®) on admission at 12-hour intervals for a total cumulative dose of 7.2 mg/kg (432 mg). Twenty-four hours after the first dose of intravenous artesunate, the parasitaemia dropped to 0.15%, and parasites were cleared on day 3. The treatment was switched to oral artemether/lumefantrine (Riamet®) and was subsequently interrupted 24 hours later because of side effects (headache). Then atovaquone/proguanil (Malarone®) was started for 2 days. Clinical improvement was slower than clearance of parasitaemia. Persistent fever as well as acute dyspnoea with hypoxaemia (SpO2 85%) was noted on day 5 and diagnosed as capillary leak syndrome. It resolved with diuretics. On day 9, the patient was discharged with a haemoglobin level of 95 g/l.
During follow-up on day 15, the patient presented asthenia, dyspnoea and pallor. She complained of fever for the past 2 days. Blood analysis indicated a haemoglobin level of 56 g/l, a CRP at 15.5 mg/l, bilirubinaemia at 34 µmol/l associated with haemolysis criteria: reticulocytes 219 G/l with 97.5‰, HFR 13.7%, LDH 1292 UI/l. Haptoglobin, free haemoglobin and Coomb’s test were not performed, but inherited haemolysis causes (G6PD deficiency, sickle cell disease, spherocytosis) were ruled out. Blood smear, and blood and urine cultures were negative. The patient was re-admitted and was transfused with a total of six units of packed red blood cells. From day 18, the fever decreased spontaneously with concomitant normalisation of haemolytic markers. The level of haemoglobin slowly increased. She was discharged on day 21. On day 35, the haemoglobin reached its prior level (128 g/l), with still a slight elevation of LDH (324 UI/l). The patient was asymptomatic at this time.
A 44-year-old patient from Venezuela presented at the hospital with a 3-day history of fever after traveling to Africa. He had spent 3 weeks in Ivory Coast and had not taken malaria chemoprophylaxis. According to the WHO 2010 criteria [5], severe P. falciparum malaria with 4% parasitaemia was diagnosed. The haemoglobin level was 159 g/l. He was admitted to the intermediate care unit and given four doses of intravenous artesunate (Malacef®) of 2.4 mg/kg at h0, h12, h24 and h48 (cumulative dose 9.6 mg/kg = 680 mg). After 48 hours, the patient was switched to oral artemether/lumefantrine (Riamet®). The patient’s condition improved and he was discharged on day 3 with a haemoglobin level of 143 g/l. On day 7, the haemoglobin was stable at 143 g/l, and the patient did not have any complaints.
On day 17, during a follow-up check, blood tests indicated a drop of 20 g/l in haemoglobin (124 g/l) and the presence of haemolysis markers such as increased reticulocytes (293.44 G/l with 69.7‰), HFR (8.8%), LDH (507 UI/l) and undetectable haptoglobin (<74 mg/l). The patient was asymptomatic and it was decided not to perform any laboratory examination to rule out relapse or other infections. Coomb’s test and a search for G6PD deficiency were not performed. A biological and clinical diagnosis of PADH was made. No transfusion was given. The level of haemoglobin slowly increased until it reached its prior level (140 g/l) on day 29.
A 39-year-old Colombian female was admitted with severe malaria. She had spent 8 weeks in West African countries (Guinea Bissau for 7 weeks, then Senegal) without taking any chemoprophylaxis. She had returned to Geneva 1 week previously and started complaining of fever. Importantly, a 7-week pregnancy was confirmed at that time. She had no other medical history. She presented with severe P. falciparum malaria (hyperparasitaemia 11%) with impaired consciousness, which led to endotracheal intubation. She was treated with intravenous artesunate on the admission day (h0), at h12 and then once a day for 3 more days. The total dose was 12 mg/kg (768 mg). Parasitaemia dropped to 0.06% less than 24 hours later but clinical improvement was slower. Extubation was possible only 40 hours later. Moreover, on day 2, she developed acute renal failure (anuria and serum creatinine 359 µmol/l) requiring renal replacement therapy on day 3. She had a rapid clinical and laboratory improvement, although renal replacement therapy had to be carried on for 4 weeks. On day 4, treatment was switched to an oral form (artemether/lumefantrine [Riamet®]) for 72 hours, and she was transferred to an internal medicine ward (with a haemoglobin level of 84 g/l, and two negative blood smears at 24 hours apart).
On day 8, the haemoglobin level was 66 g/l, LDH 435 UI/l and haptoglobin 227 mg/l. The patient was transfused with two units of packed red blood cells. On day 20, the haemoglobin level dropped again, to 67 g/l, with clearer haemolysis criteria (LDH 633 UI/l, undectable haptoglobin <74 mg/l). Reticulocytes were low (32.67 G/l) in the context of kidney failure. She received one more unit of packed red blood cells. On day 28, the patient was febrile, the haemoglobin level had dropped again to 66 g/l with LDH 724 UI/l, undectable haptoglobin (<74 mg/l) and free haemoglobin at 8.3 µmol/l. Urine culture, blood culture and a blood smear were negative. Reticulocytes were 107 G/l but difficult to interpret as she was at that time receiving erythropoietin in relation to the kidney failure. As liver enzymes were high, viral hepatitis was tested for and a diagnosis of Hepatitis A was confirmed. She was transfused again with packed red blood cells. A diagnosis of PADH (persistent pattern) was suspected but was not confirmed as she presented many other coexisting diseases (kidney failure with haemodialysis and erythropoietin substitution, acute hepatitis A) leading to possible misinterpretation.
These four cases show various presentations of PADH. The data are summarised in table 1. Two different patterns have been defined in the literature: delayed and persistent [11]. The first (fig. 1) is characterised by a haemoglobin drop and haemolysis (low haptoglobin, increased LDH) more than 7 days after the beginning of artesunate treatment. The second (fig. 2) is characterised by continuing haemolysis starting from and around day 7 after the beginning of artesunate treatment and persisting beyond day 14. According to case series, approximately 20% of adult travellers are affected by PADH [12, 13]. Two studies conducted in Ghana, Gabon and Democratic Republic of Congo on African children showed that prevalence was 7% to 11% [14, 15]. However, these studies were underpowered to assess the incidence of PADH accurately. At Geneva University Hospitals, intravenous artesunate has been available since August 2012. After 30 months, 18 patients were treated with it, following a diagnosis of severe malaria based on WHO 2010 criteria [5]. We here present the four patients (22%) in whom a likely or definitive diagnosis of PADH was made. Although clinical data mainly showed an association of PADH with the intravenous form, cases after intramuscular, intrarectal or oral artemisinin derivatives treatment have also been described [11].

Figure 1
Time course of haemoglobin (Hb) levels and lactate dehydrogenase (LDH) levels in case 1 and case 2 (delayed pattern).
N.B.: Because of the low number of LDH data, case 3 is not shown.
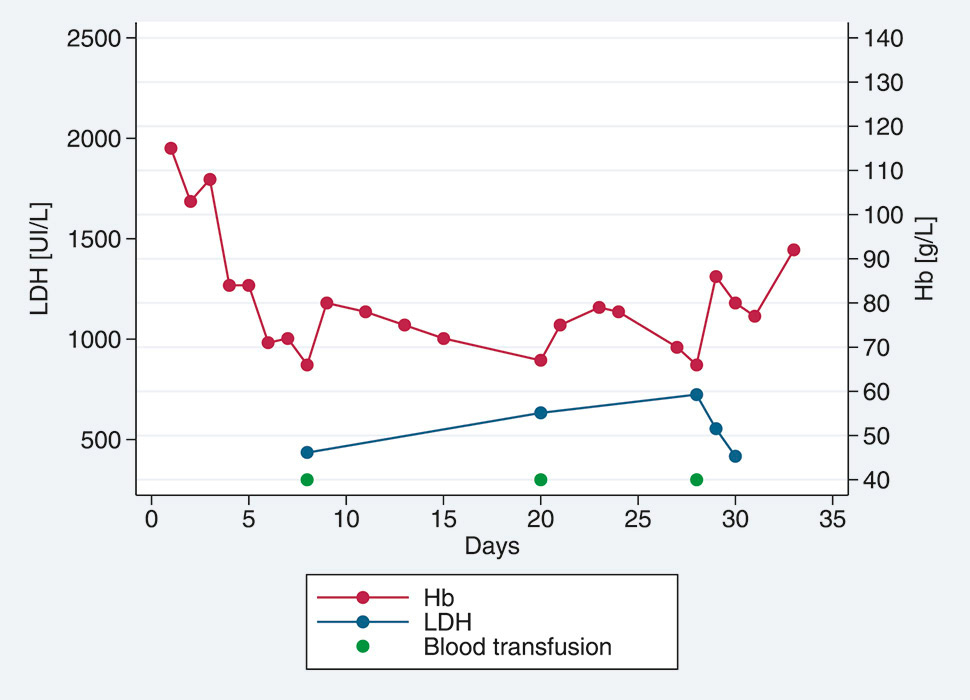
Figure 2
Time course of haemoglobin (Hb) levels and lactate dehydrogenase (LDH) levels in case 4 (persistent pattern).
Artesunate very quickly reduces parasitaemia by targeting the ring-stage malaria parasite inside the erythrocyte and stimulating the removal of dead parasites by the spleen without the lysis of the host erythrocytes. This process is known as “pitting”. These so-called “once-infected erythrocytes” (antigen positive, parasite negative) are consequently spared and they re-enter the circulation. However the pitting induces deformation and reduction in size of the once-infected erythrocytes which shortens their life-span. A prospective nonrandomised study showed a clear correlation between the occurrence of the post-artesunate haemolysis and the number of once-infected erythrocytes [13]. The simultaneous destruction of these once-infected erythrocytes during a short period of time produces the delayed haemolytic anaemia. The absence of delayed haemolytic anaemia in patients treated with quinine can be explained by the extremely low level of once-infected erythrocytes compared with artesunate treatment [16, 17]. Although the amount of once-infected erythrocytes is related to the level of parasitaemia, the pitting rate in the spleen is an individual variable, hence explaining the inconstant occurrence of PADH in a population of hyperparasitaemic patients [13]. The factors influencing the pitting rate remain unknown. Thus it is not possible to predict the occurrence of PADH solely on the basis of the level of parasitemia. Measurements by flow cytometry or conventional fluorescence mircroscopy might be a good predictive marker of delayed haemolysis [13] but are not yet available.
Other hypotheses have been proposed, but their aetiological role in PADH seems minor at the best. Toxicity caused by a manufacturing problem of the pharmaceutical agent has been recently rejected [18].
Direct artesunate toxicity has also been suggested. A temporary reduction in reticulocyte count after an injected dose of artesunate has been described in healthy volunteers and malaria patients [19]. However, late bone marrow suppression by artesunate is not supported by the fact that the reticulocyte count is increased during the delayed haemolytic anaemia [13]. Furthermore, no reports have linked the dose of artesunate with the occurrence of haemolysis. [10, 12, 20].
An autoimmune mechanism to explain PADH as the first cause seems unlikely. Most cases had a negative Coomb’s test and the haemolytic anaemia resolved without any immunosuppressive therapy [11]. However, in one recent published case report, a possible drug-induced immune delayed haemolytic anaemia was evoked in a semi-immune nonhyperparasitaemic patient treated with artesunate [21]. More research is needed to rule out an immune mechanism as cause of the delayed haemolysis.
Enzyme defect such as G6PD deficiency have been excluded in most published cases but haemoglobinopathies, membranopathies and other enzyme deficiencies have rarely been investigated.
Usually PADH is self-limited and the treatment is only supportive. No fatal outcome has been observed so far. However, blood transfusion has been required in approximately 70% of patients [11].
As in other case reports [21–23], we noticed relapsing fever in three of our four patients. Recurrence of malaria or other infectious diseases were ruled out. The fever and concomitant inflammatory process were self-limited and subsided coincidentally with the reduction in haemolysis. Even if the aetiology remains unknown, the fever is probably related to the severity of the haemolysis, as sometimes describe in other haemolytic anaemias. Even if the diagnosis of PADH is the most probable, it is recommended that other causes of fever are excluded.
In conclusion, for the last 5 years, artesunate has tremendously improved the management of severe malaria. It speeds up the clearance of parasites in an impressive way with a proven reduction in mortality of patients treated with artesunate compared with quinine. Furthermore, it reduces the length of stay in intermediate or intensive care units [24]. The physiopathology of the delayed haemolytic anaemia remains unclear, even if it might be linked to drug efficacy rather than to a conventional toxic effect. There has been no fatal outcome. However, treating physicians should be aware of this potentially late adverse reaction following the treatment of severe malaria with artemisinin derivatives. Consequently, in our opinion, the conventional follow-up recommended by the WHO (day 0, day 3, day 28) is not adequate anymore. We would like to emphasise the need for a closer follow-up (day 7, day 14, day 21 and day 28) for these patients, as a fifth of them will experience such haemolysis and the majority will need further blood transfusions.
| Table 1: Laboratory test results for the four patients with post artesunate delayed hemolysis. | |||||||||
| Age/sex | Blood smear | Cumulative dose of IV artesunate | Time for parasite clearance | Hb at the end of IV artesunate | Minimum Hb | Day of diagnosis of haemolysis | Maximum of LDH | Other laboratory examination | |
| Case 1 | 32/F | 27.5% | 7.2 mg/kg (396 mg) | 48 h | 110 g/l | 60 g/l | D12 | 2 456 UI/l | Free Hb: 25.6 µmol/L Haptoglobin <74 mg/l |
| Case 2 | 41/F | 9% | 7.2 mg/kg (432 mg) | 48 h | 110 g/l | 56 g/l | D15 | 1 292 UI/l | NA |
| Case 3 | 44/M | 4% | 9.6 mg/kg (680 mg) | 72 h | 143 g/l | 124 g/l | D17 | 507 UI/l | Haptoglobin <74 mg/l |
| Case 4 | 39/F | 11% | 12 mg/kg (768 mg) | 72h | 84 g/l | 66 g/l | D7 + D27 | 724 UI/l | Free Hb 8.3 µmol/l Haptoglobine <74 mg/l |
| F = female; IV =: Intravenous; Hb = haemoglobin; LDH = lactate dehydrogenase; M = male; NA = not available | |||||||||
Acknowledgement: We thank Dr C. Fumeaux for the help with data collection, and Dr M. Aldenkortt for having kindly revised the manuscript.
1 World Health Organization. Malaria report. Geneva, Switzerland: WHO; 2014.
2 World Health Organization: From malaria control to elimination in the WHO European region 2006–2015. Regional office for Europe, Copenhagen, Denmark; 2006.
3 Bruneel F, Tubach F, Corne P, Megarbane B, Mira J-P, Peytel E, et al. Severe imported falciparum malaria: a cohort study in 400 critically ill adults. PloS One 2010;5:e13236
4 Robert Koch Institut: Reiseassoziierte Infektionskrankheiten 2010. Epidemiologisches Bulletin. 2011;40:371–8. German.
5 World Health Organization: Guidelines for the treatment of malaria, second edition. Geneva, Switzerland; 2010.
6 Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial, group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366:717–25.
7 Dondorp AM, Fanello CI, Hendriksen ICE, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children: an open-label, randomised trial. Lancet. 2010;376:1647–57.
8 Sinclair D, Donegan S, Isba R, Lallo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database Syst Rev 2012; 6:CD005967.
9 Clark RL1 Hypothesized cause of delayed hemolysis associated with intravenous artesunate. Med Hypotheses. 2014;82(2):167–70.
10 Rolling T, Schmiedel S, Wichmann D, Wittkopf D, Burchard GD, Cramer JP. Post-treatment haemolysis in severe imported malaria after intravenous artesunate: case report of three patients with hyperparasitaemia. Malaria J. 2012;11:169.
11 Rehman K1, Lötsch F2, Kremsner PG3, Ramharter M4. Haemolysis associated with the treatment of malaria with artemisinin derivatives: a systematic review of current evidence. Int J Infect Dis. 2014;29C:268–73.
12 Centers for Disease Control and Prevention. Published reports of delayed HA after treatment with artesunate for severe malaria. Atlanta, USA: CDC; 2013.
13 Jauréguiberry S, Ndour PA, Roussel C, Ader F, Safeukui I, Nguyen M, et al. French Artesunate Working Group. Postartesunate delayed hemolysis is a predictable event related to the lifesaving effect of artemisinins. Blood. 2014;124(2):167–75.
14 Burri C, Ferrari G, Ntuku HM, Kitoto AT, Duparc S, Hugo P, Mitembo DK, Lengeler C.Delayed Anemia after Treatment with Injectable Artesunate in the Democratic Republic of the Congo: A Manageable Issue.Am J Trop Med Hyg. 2014;91(4):821–3.
15 Rolling T, Agbenyega T, Issifou S, Adegnika AA, Sylverken J, Spahlinger D, et al. Delayed hemolysis after treatment with parenteral artesunate in African children with severe malaria – a double-center prospective study. J Infect Dis. 2014;209(15):1921–8.
16 Chotivanich K, Udomsangpetch R, McGready R, Proux S, Newton P, Pukrittaya- kamee S, et al. Central role of the spleen in malaria parasite clearance. J Infect Dis. 2002;185(10):1538–41.
17 Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. In vivo removal of malaria parasites from red blood cells without their destruction in acute falciparum malaria. Blood. 1997;90:2037–40.
18 MMV. Experts Group Meeting on delayed anaemia following treatment with injectable artesunate. Wien, Austria; 2013. http://www.mmv.org/sites/default/files/uploads/docs/event/2013/InjectableArtesunateExpertGroupMeeting.pdf.
19 Robert L. Clark Effects of Artemisinins on Reticulocyte Count and Relationship to Possible Embryotoxicity in Confirmed and Unconfirmed Malarial Patients. Birth Defects Res A Clin Mol Teratol. 2012;94(2):61–75.
20 Zoller T, Junghanss T, Kapaun A, Gjorup I, Richter J, Hugo-Persson M, et al. Intravenous artesunate for severe malaria in travelers, Europe. Emerg Infect Dis. 2011;17:771–7.
21 Raffray L, Receveur MC, Beguet M, Lauroua P, Pistone T, Malvy D1. Severe delayed autoimmune haemolytic anaemia following artesunate administration in severe malaria: a case report. Malar J. 2014;13:398.
22 Caramello P, Balbiano R, De Blasi T, Chiriotto M, Deagostini M, Calleri G. Severe malaria, artesunate and haemolysis. J Antimicrob Chemother. 2012;67:2053–4.
23 Jarvis JN, Coltart CE, Pule M, Chiodini PL, Doherty T. Artemisinin therapy and severe delayed haemolysis. Lancet. 2013;382:180.
24 Kurth F, Kurth F, Develoux M, Mechain M, Clerinx J, Antinori S, et al. Intravenous Artesunate Reduces Parasite Clearance Time, Duration of Intensive Care, and Hospital Treatment in Patients With Severe Malaria in Europe: The TropNet Severe Malaria Study. Clin Infect Dis. 2015 Jul 17. pii: civ575. [Epub ahead of print]
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.