How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis
DOI: https://doi.org/10.4414/smw.2015.14190
Francesca
Oliviero, Paolo
Spinella, Ugo
Fiocco, Roberta
Ramonda, Paolo
Sfriso, Leonardo
Punzi
Summary
Arthritis encompasses a heterogeneous group of diseases characterised by inflammation that leads not only to joint damage, bone erosion, severe pain and disability, but also affects other organs of the body, resulting in increased morbidity and mortality. Although the mechanisms underlying the pathogenesis of joint diseases are for the most part unknown, a number of nutrient and non-nutrient components of food have been shown to affect the inflammatory process and, in particular, to influence clinical disease progression.
The Mediterranean diet model has already been linked to a number of beneficial health effects: both fat and non-fat components of the Mediterranean dietary pattern have been shown to exert important anti-inflammatory activities by affecting the arachidonic acid cascade, the expression of some proinflammatory genes, and the activity of immune cells. N-3 polyunsaturated fatty acids, in particular, have been shown to affect lymphocyte and monocyte functions, crucially involved in adaptive and innate immunity.
Although some aspects concerning the mechanisms of action through which the Mediterranean diet pattern exerts its beneficial effects remain to be elucidated, arthritis patients may potentially benefit from it in view of their increased cardiovascular risk and the treatment they require which may have side effects.
Introduction
Over the last 50 years growing attention has been focused on the role that certain foods play in the development and progression of chronic diseases. In this context the Mediterranean dietary pattern (MDP) has been shown to have a number of beneficial health effects not only with regard to cardiovascular diseases and cancer, but also to diabetes, metabolic syndrome, visceral obesity and arthritis [1, 2].
Arthritis encompasses a heterogeneous group of diseases characterised by inflammation that often leads not only to joint damage, bone erosion, severe pain and disability, but also affects other organs of the body, resulting in increased morbidity and mortality. Patients with chronic rheumatic diseases often require long-term costly medicines and present significantly higher levels of work productivity loss, not to mention a deterioration of quality of life [3]. Particular attention should, then, be devoted to all measures including prevention and nonpharmacological interventions to reduce this enormous burden. Some evidence indicates that lifestyle, and mainly diet, can improve the course of most of these diseases. The current article reviews the role of this particular diet pattern and of some of its components in the pathogenesis and progression of the most prevalent arthropathies. It examines, in particular, how specific components of the MDP modulate the inflammatory pathways in these chronic diseases.
Common inflammatory features and accelerated atherosclerosis in rheumatic diseases
Although with different expressions, rheumatic conditions share common inflammatory pathways mainly involving the joints. During the inflammatory process, joint tissue cells including synovial fibroblasts, synovial macrophages and chondrocytes, produce soluble immune mediators, such as cytokines and chemokines which contribute to the inflammatory response and to joint damage (fig. 1). These proinflammatory substances may lead to an extension of the inflammatory process to the body with a subsequent cascade of inflammatory reactions, the so-called acute phase response. Characteristic features of this at times powerful reaction are acute phase proteins, including C-reactive protein (CRP) produced by the liver following proinflammatory cytokine stimulation.
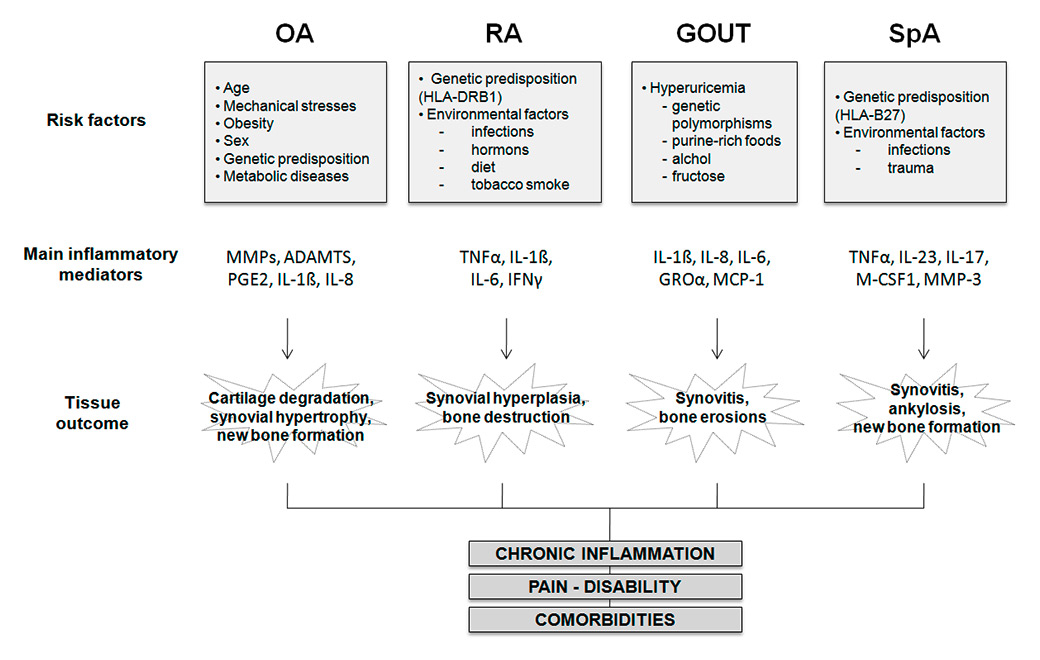
Figure 1
Schematic pathogenesis of most common arthropathies. Several risk factors are implicated in the development of osteoarthritis, rheumatoid arthritis, gout and spondyloarthritis. Each of these diseases is characterised by the release of specific inflammatory mediators which mainly lead to joint destruction, pain, disability, chronic inflammation and co-morbidities.
ADAMTS = a disintegrin and metalloproteinase with thrombospondin motifs; GRO = growth regulated oncogene; HLA = human leucocyte antigen; IFN = interferon; IL = interleukin; M-CSF = macrophage colony-stimulating factor; MMPs = metalloproteinases; OA = osteoarthritis; PGE = prostaglandin E; RA = rheumatoid arthritis; RANKL = Receptor activator of nuclear factor kappa-B ligand; SpA = spondyloarthritis; TNF = tumour necrosis factor.
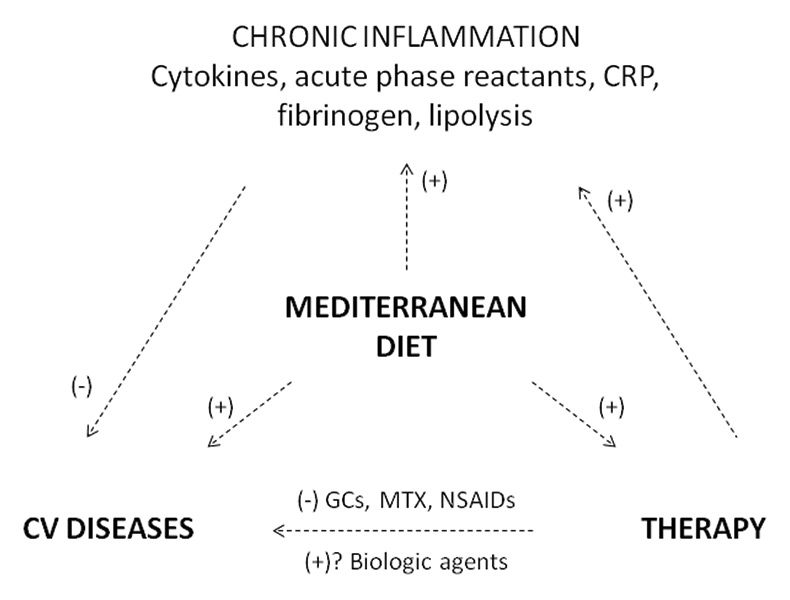
Figure 2
The relationship between chronic inflammation, the development of cardiovascular diseases, pharmacological therapy and the Mediterranean diet.
Cardiovascular diseases and, in particular, accelerated atherosclerosis, are comorbidities that are frequently observed in patients suffering from rheumatic conditions. They are caused not only by sustained inflammation, but also by the chronic use of pharmacological therapy. A healthy diet such as the Mediterranean one, with its anti-inflammatory and anti-oxidant components, could have beneficial effects by acting simultaneously on inflammation, the cardiovascular system and treatment.
CRP = C-reactive protein; CV = cardiovascular; GCs = glucocoticoids; MD = Mediterranean diet; MTX = methotrexate; NSAlDs = nonsteroidal antinflammatory drugs; (+) positive effect, (-) negative effect.
Comorbidities are frequently linked to arthritis, and these may be a result of both intrinsic factors, such as genetic predisposition, age and sex, and extrinsic ones, such as environmental variables, including diet and therapeutic drugs [4]. The inflammatory process is of crucial importance in this context and may be both affected by and responsible for the effects of these factors.
High levels of systemic inflammation seem, in particular, to contribute to accelerating atherosclerosis in patients with chronic inflammatory arthritis (fig. 2). Observational studies have, in fact, found a 50% higher risk of cardiovascular mortality in patients with rheumatoid arthritis compared with that in the general population [5]. A higher incidence of cardiovascular disease has likewise been noted in patients with gout [6] as well as in those with psoriatic disease [7].
Accelerated atherosclerosis in patients with inflammatory arthritis appears to be due to a complex interaction between inflammation, classical cardiovascular risk factors, co-morbidities and pharmacological treatment (fig. 2). Inflammation seems to contribute to accelerating atherosclerosis by promoting endothelial dysfunction, activating the coagulation cascade, inducing secondary dyslipidaemia and increasing atheromatous plaque vulnerability [8].
Pharmacological interventions such as nonsteroidal anti-inflammatory drugs, cyclooxygenase 2 (COX-2) inhibitors, disease-modifying drugs and corticosteroids used to treat inflammatory arthritis may affect classical risk factors for cardiovascular diseases (fig. 2) and induce or aggravate hypertension. The cardiovascular risk associated with other therapies, such as methotrexate or the new anti-tumour necrosis factor (TNF) biological agents, is not completely clear. While methotrexate may increase homocysteine levels, an independent risk factor for atherosclerotic disease, evidence from observational databases and registries indicates that biologics increase total cholesterol and high-density lipoproteins but not the overall atherogenic index [5].
The role of diet in the pathogenesis and progression of the most common forms of arthritis
The mechanisms underlying the pathogenesis of joint diseases are for the most part unknown. lt has, nevertheless, been demonstrated that diet and lifestyle can play a role in their pathogenesis as well as improve the course of most of these diseases [9, 10].
Rheumatoid arthritis
Both inherited genes and environmental factors have been implicated as causal factors in the pathogenesis of rheumatoid arthritis. Repeated inflammatory and immune insults may contribute, in genetically susceptible individuals, to a breakdown of self-tolerance and can lead to autoimmunity [11].
The incidence of rheumatoid arthritis appears to be lower in southern Europe than in north European and North American countries [12]. Some studies have, nevertheless, found that the radiological joint damage and extra-articular manifestations linked to rheumatoid arthritis are more severe in northern than in southern European populations [13]. lt has been hypothesised that, above and beyond genetics, environmental and lifestyle factors such as diet can contribute to the striking differences noted in disease expression and severity. While several studies have suggested that diet can influence clinical disease progression in patients with rheumatoid arthritis or other chronic joint diseases [14], there is no evidence that dietary factors play a specific role in their aetiology.
Other studies have, nevertheless, highlighted that the consumption of certain foods is associated with a higher or lower risk of developing rheumatoid arthritis, and that a diet rich in fish oil and some antioxidants is, in fact, associated with a lower one. Cooked vegetables and olive oil have been found to be inversely and independently associated with the risk of rheumatoid arthritis in a population sample living in southern Greece [15].
Using a multivariate model analysis, a large Danish prospective population-based cohort study demonstrated that an increase in intake of 30 g fatty fish (≥8 g fat / 100 g fish) per day was associated with a 49% reduction in the risk of rheumatoid arthritis, while the intake of medium fatty fish (3–7 g fat / 100 g fish) was associated with a significantly higher risk. The same study failed to find any associations between rheumatoid arthritis risk and the intake of citrus fruit, vegetables, retinol, beta carotene, vitamins A, E, C and D, zinc, selenium, iron and meat [16].
According to the data of the Norfolk Arthritis Register, individuals with lower intakes of fruit and vegetables (particularly rich in vitamin C) had an increased risk of developing inflammatory arthritis [17]. More evidence supporting the role of diet in rheumatoid arthritis was recently provided by the Nurses’ Health Study, which identified an association between sugar-sweetened soda and an increased risk of seropositive rheumatoid arthritis in women [18]; no association between protein or meat and the risk of rheumatoid arthritis was noted in the same large cohort [19].
The EIRA study (a population-based case-control study including more than 4 000 participants) showed that regular consumption of oily fish was associated with only a modestly decreased risk of developing rheumatoid arthritis [20].
As far as alcohol consumption and the risk of developing rheumatoid arthritis is concerned, some studies have reported a modest association between long-term moderate alcohol consumption and reduced risk of rheumatoid arthritis [21], whereas others have found no significant protective effect of alcohol consumption on disease development [22].
Spondyloarthropathies
Fewer data are available with regard to the connection between diet and spondyloarthropathies. The most frequent of these are ankylosing spondylitis and psoriatic arthritis, whose causes are thought to be multifactorial; various environmental factors seem to affect genetically predisposed subjects, and a strong association between ankylosing spondylitis and human leucocyte antigen (HLA) B27 has been noted. lt has been hypothesised, particularly in the light of the finding of a low prevalence of spondyloarthropathies in Alaskan Eskimos who have high intakes of omega-3 fatty acids despite the high prevalence of HLA-B27 (25–40%) in that population, that diet plays a role in spondyloarthropathy pathogenesis [23]. That disease is, moreover, often associated with bowel mucosa inflammation, and diet often appears to be correlated to gastrointestinal symptoms in these patients [24].
Some investigators have reported that the plasma phospholipid content of arachidonic acid is correlated with disease activity in ankylosing spondylitis patients and that the dietary habits characterising those patients living in the western hemisphere affect cardiovascular risks [25].
Some have postulated that a low starch diet can be beneficial in ankylosing spondylitis patients as it seems to lead to a reduction in the growth of microbes in the bowel flora which may be associated to higher immunoglobulin A (lgA) levels and to an increase in inflammation in patients with active disease [26].
Consistent with this hypothesis, eliminating dairy products from patients’ diets has been shown to have a beneficial effect on spondyloarthropathies as it modifies the content of the intestinal flora and consequently reduces the proliferation of pathogenic bacteria or it alters gut permeability linked to a chronic intestinal allergy to milk products [27].
An abnormal fatty acid pattern and low levels of serum selenium have been reported in patients with psoriatic arthritis; an association between total saturated fatty acids and disease duration, morning stiffness and erythrocyte sedimentation rate has, in particular, been observed [28].
Osteoarthritis
The most prevalent joint disease, osteoarthritis, is considered a multifactorial condition. Age and obesity, as well as mechanical stress, all seem to play a role in its development. Genome-wide association studies have uncovered no definitive, common highly penetrant allele that causes osteoarthritis. It has recently been hypothesised that epigenetic alterations (heritable modifications in gene function without changes in the DNA sequence) rather than genetic mutations contribute to the development of osteoarthritis and other chronic diseases [29].
A number of nutrient and non-nutrient dietary components have been shown to affect epigenetic signalling pathways, in particular DNA methylation at CpG sites (epimutations), histone deacetylation or chromatin remodelling of key inflammatory genes and noncoding RNAs [30]. There are some catechins among these which, as we have recently shown, can suppress the inflammatory response induced in vitro by pyrophosphate calcium crystals which, in osteoarthritis, are associated with a more severe disease form [31].
Since epigenetic changes, although heritable at the cellular level, are potentially reversible, epigenetics could be a new molecular target for osteoarthritis therapeutic interventions, especially at early stages of the disease, and diet could play a fundamental role in disease prevention.
Gout
Although some genetic polymorphisms have been associated with higher serum urate levels [32], it is widely accepted that diet plays a role in hyperuricaemia and in the development of the disease as the risk of recurrent attacks is increased after high intakes of purine-rich foods, especially from animal sources. Urate levels also rise markedly after high intakes of fructose-rich food, red meat and alcohol. Low-fat dairy products, whole grains, nuts and legumes, and low-sugar fruits and coffee are, instead, associated with a decreased risk of gout [33, 34]. While some investigators have reported that vitamin C supplements reduce hyperuricaemia [35], a recent Cochrane systematic review failed to uncover a clinically significant reduction in serum uric acid levels after vitamin C supplementation in comparison with the decrease linked to the use of standard urate-lowering agents [36].
Some have theorised that differences in food components present in different countries may play an important role not only in the prevalence of the disease but also in its severity and even in the mortality linked to it. Carried out on a large population sample, the ATTICA study [37] found that adherence to a Mediterranean diet was correlated to lower serum uric acid levels and fewer cases of hyperuricaemia.
The traditional Mediterranean dietary pattern
The traditional MDP is characterised by an abundance of plant foods, such as vegetables, legumes, fruits, grain, cereals and nuts, and fish. Although olive oil is the main source of fat, there is a moderate intake of poultry, dairy products and eggs. Variable amounts of wine are often consumed with meals. Culinary herbs and spices are another important component that seems to increase the health promoting characteristics of the diet and food palatability [38]. Many of the characteristic components of the traditional MDP are considered “functional foods”, which are known to have positive effects on health, capacity and well-being, and may be responsible for some of the advantages associated with the diet.
Unlike other fat-rich diets such as the Western one, most (about 85%) of the fat content of the MDP is furnished by a single food component which is, of course, olive oil. The MDP is, in fact, low in saturated fats and cholesterol and lacks trans fatty acids; it is instead high in monounsaturated fatty acids (MUFAs), and in particular oleic acid. In view of the particular gastronomic features of olive oil and a high content of micro components giving it its characteristic odour, colour and taste, its use facilitates the intake of some vegetables, legumes and cereals, all containing high proportions of low glycaemic index carbohydrates with a high health-promoting potential [39]. While the benefits of the MDP are not due exclusively to olive oil itself, a number of its properties depends on or are potentiated by it and the other health-promoting components of the diet [40].
The effects of the Mediterranean dietary pattern in modulating inflammation
Specific components of the MDP have received increasing attention because of a variety of health-promoting properties in chronic inflammatory and degenerative diseases [41].
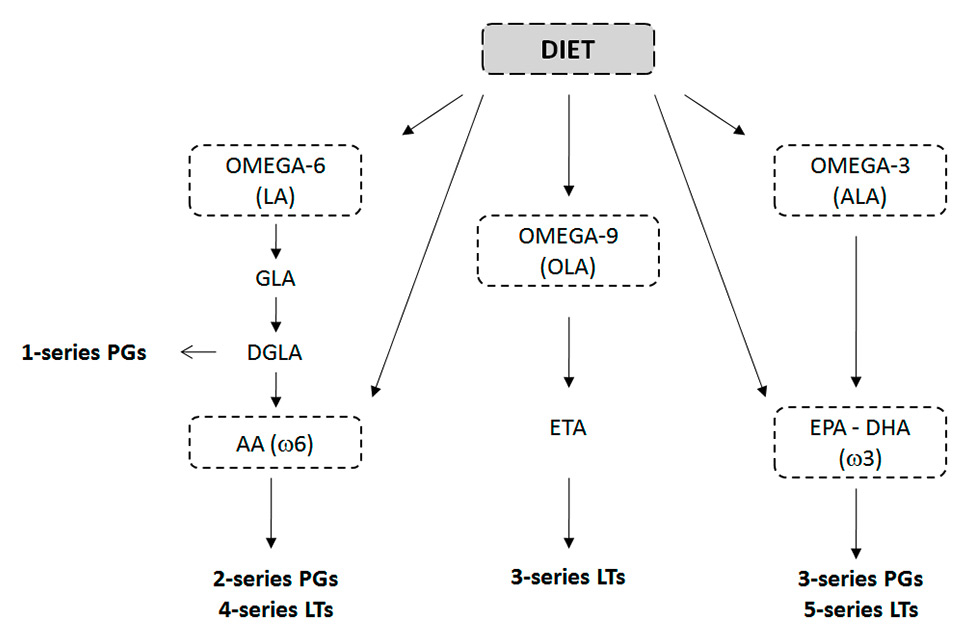
Figure 3
The influence of diet on prostaglandin and leukotriene production from polyunsaturated n-3 and n-6 fatty acids and monounsaturated n-9 fatty acids.
AA = arachidonic acid; ALA = α-linolenic acid; DGLA = dihomo-γ-linolenic acid; DHA = docosahexaenoic acid; DPA = docosapentaenoic acid; EPA = eicosapentaenoic acid; ETA = eicosatrienoic acid; GLA = γ-linolenic acid; LA = linoleic acid; LTs = leukotrienes; PGs = prostaglandins.
Both nutritive (fat) and non-nutritive (nonfat) components of the MDP have been shown to exert important anti-inflammatory activities bothin vitroand in vivo by modulating the arachidonic acid cascade, the expression of some proinflammatory genes, and the activity of immune cells [1].
Nutritive components
Arachidonic acid (20:4 n-6) is one of the most important polyunsaturated fatty acids, which modulates the inflammatory process. Associated with membrane phospholipids, it is metabolised by phospholipase C into a variety of eicosanoids. Among these, 2-series prostaglandins and 4-series leukotrienes exert proinflammatory, prothrombotic, and proatherogenic actions when chronically or excessively produced (fig. 3). It has been shown that dietary arachidonic acid can significantly affect tissue arachidonic acid content and, thus, eicosanoid production [42]. Conversely, dietary long chain n-3 polyunsaturated fatty acide (PUFAs) (found especially in salmon, walnuts and flaxseeds) can attenuate tissue arachidonic acid levels and eicosanoid production in vitro and in vivo by altering plasma phospholipid fatty acid composition and leading to the production of alternative 3- and 5-series prostaglandins and leukotrienes (fig. 3). lt has, nevertheless, been observed that when equivalent quantities of dietary arachidonic acid and eicosapentaenoic acid (EPA) are concomitantly assumed, the former is the more powerful of the two and is responsible for the effects that may be associated with the ingestion of n-3 PUFAs [43].
Another nutritive component of the MDP that has established anti-inflammatory properties is oleic acid (18:1 n-9), the main monounsaturated fatty acid contained in virgin olive oil. It has been observed that oleic acid is able to modulate the expression of pleiotropic genes involved in signal transduction pathways and cytokine production. Oral oleic acid administration in rats decreases the production of the inflammatory mediators IL-1ß, IL-6 and cytokine-induced neutrophil chemoattractants by resident macrophages and enhances neutrophil function [44]. Extra-virgin olive oil supplementation has also been shown to restore lubricin expression in cartilage after anterior cruciate ligament resection in rats [45].
The effect of prefeeding diets with different ratios of PUFAs, EPA and docosahexaenoic acid (DHA) on arthritis development and on disease severity has been evaluated in a rat model [46]. The type of diet and in particular the (n-6) to (n-3) fatty acid balance has been found to influence inflammation and oedema during the acute onset stage of arthritis and during the development of the chronic disease phase, thus highlighting the immunomodulatory effect of diet linked to the incorporation of EPA and DHA in cellular lipids and to the restricted production of arachidonic acid-derived mediators of inflammation [46].
Non-nutritive components
Non-nutritive components of the MDP, such as phenolic compounds from olives, virgin olive oil and red wine, have received a great deal of attention because of their important anti-inflammatory, antiangiogenic, cardioprotective and anticancer properties.
Oleuropein, hydroxytyrosol and resveratrol all exhibit a marked antioxidant activity in vitro, as they reduce the expression of endothelial adhesion molecules such as vascular cell adhesion molecule 1 (VCAM-1) and inhibit the activation of transcription factors NF-κB and AP-1 [47]. The inhibitory effects of phenolic compounds have been noted with regard to cyclooxygenase-2 (COX-2) protein expression and prostanoid and metalloproteinase production [48] known to play a crucial role in angiogenesis, a key pathogenic process associated not only with atherosclerotic vascular diseases and cancer, but also with inflammatory chronic joint diseases.
As far as joint inflammation is concerned, hydroxytyrosol supplementation has been shown to significantly improve disease severity in the collagen-induced model in rats, down-regulating COX-2 and inducible nitric oxide synthase (iNOS) expression [49]. Another bioactive phenol present in virgin olive oil, secoiridoid oleocanthal, has been found to possess a strong anti-inflammatory potential by down-regulating some cytokines such as IL-6, IL-1, TNFα, granulocyte-macrophage colony stimulating factor (GM-CSF) and macrophage inflammatory protein-1α (MIP-1α) in activated macrophages and chondrocytes [50]. A member of the same family, oleuropein aglycone has been shown to modulate the inflammatory response in collagen-induced arthritic mice and to ameliorate the tissue damage linked to it [51]. A joint protective effect by polyphenol extract from extra virgin olive oil has recently been observed in the same model of arthritic mice. Oral administration of this extract was found, in fact, to reduce joint oedema, cell migration, cartilage degradation and bone erosion, affecting the c-Jun NH(2)-terminal kinase (JNK), p38, STAT3 and NF-κB signalling pathways [52].
Carnosol, another nonfat component that is extracted from Mediterranean herbs such as rosemary and sage has been found to have promising anti-cancer and anti-inflammatory properties. It deregulates inflammatory signalling including nitric oxide and leukotrienes, antagonises the intracellular Ca++ mobilisation, and inhibits the secretion of leucocyte elastase [53].
The effect of Mediterranean dietary components on immune cell functions
It is well established that diet can modulate immune functions and, in particular, the level of fat and the types of fatty acids [54]. By altering membrane composition and their interaction with membrane-bound enzymes and receptors, fatty acids are able to modulate lymphocyte and monocyte functions, which are crucially involved in adaptive and innate immunity [55].
A number of studies have investigated the effects of both n-3 (EPA, DHA) and n-6 (glinolenic acid [GLA], dihomo-g-linolenic acid [DGLA], arachidonic acid) fatty acids on the immune system and in particular on lymphocyte proliferation, cytokine secretion, natural killer (NK) cell activity and cytotoxic T lymphocyte activity. GLA, EPA, DHA are the fatty acids that appear to have the most potent immunological effects.
It has been observed, for example, that a DHA-enriched diet reduces regulatory (Treg) cell functions in mice. DHA diminishes the capacity of Treg cells to inhibit effector T cell proliferation, reduces the migration of Treg cells toward chemokines, and downregulates the expression of chemokine receptors in these cells [56]. On the other hand, EPA down regulates the T-helper 1-type response which is associated with chronic inflammatory diseases [52]. A lower lymphocyte proliferation and NK cell activity have also been observed ex vivo in connection to oleic acid [57].
A recent study investigating the contribution of Th cells and monocytes to the immunomodulatory effect of pure EPA or DHA in vitro found a pro-resolving rather than inhibitory effect of EPA and DHA on immune cell functions [58]. EPA, and to a lesser extent DHA, were found to decrease the intracellular concentrations of IL-2, TNFα and IL-4 in Th cells acting at least in part through PPAR gamma. More interestingly, neither EPA nor DHA reduced TNFα or IL-6 in monocytes but they stimulated the expression of the immunoregulatory IL-10 highlighting a cell type-specific fatty acid effect.
N-3 PUFAs have also been shown to suppress dendritic cell activation [59] and the adaptive immune response affecting B lymphocyte function, in particular through modifications of lipid raft organisation [60].
With regard to non-fat factors, an interesting action on immune cell functions has been noted with regard to the phenolic compounds isolated from olive oil, which increase the release of Ca2+ from intracellular stores, an important signal regulating fundamental biological processes [61].
Although the proportion of dietary PUFAs is important to obtain functional effects in vitro, it is possible that variations in the intake of specific fatty acids or some nonnutritive components of the diet may affect immune function and the progression of diseases that have an immunological component such as rheumatoid arthritis.
Mediterranean diet and gut microbiota
Adopting an anti-inflammatory dietary pattern, such as the MDP, contributes to maintaining “good” gut microbiota and leading to healthy outcomes. Gut microbiota exert, in fact, an important influence on local and systemic processes, such as immunity and metabolism and their composition is strictly dependent on diet [62].
The MDP, which is rich in complex carbohydrates and fibres and low in animal proteins and fats, is able to promote saccharolitic microbiota favouring short-chain fatty acid production. These metabolites have been shown to possess positive immune-modulating activity by modifying the cytokine production profile of TH cells, promoting intestinal epithelial barrier integrity, resolving intestinal inflammation, and regulating the acetylation of lysine residues, a covalent modification that affects proteins involved in a variety of signalling and metabolic processes [63].
Increasing experimental evidence has highlighted the connection between microbial dysbiosis and many inflammatory rheumatic diseases including spondyloarthropathies, lupus, gout, and rheumatoid arthritis [64].
Although the mechanism by which intestinal microbiota influence autoimmunity or trigger innate immunity responses is not completely understood, the beneficial effects of the MDP might be implicated also in view of its effect on the gut microbiota.
The influence of Mediterranean dietary components on epigenetic modifications
Several studies have demonstrated that both the induction and prevention of different diseases are linked with epigenetic modifications, which are stable and heritable changes in gene expression not due to alterations in the DNA sequence.
In recent years, a number of dietary compounds have been shown to interact with the genome, to modify gene expression, and to alter protein and metabolite composition within the cell by affecting epigenetic states [65].
As far as the components of the Mediterranean diet (MD) are concerned, only a few studies have examined their role in epigenetic modifications. Extra virgin olive oil has, in fact, been shown to selectively regulate CNR1 gene expression in human colon cancer cells and in the rat colon, via a significant reduction in the methylation levels of CNR1 promoter. This effect was attributed to the phenolic fraction since use of olive oil deprived of the phenolic fraction produced no effects on CNR1 methylation and messenger RNA levels [66].
A recent cohort study assessing and comparing MD and control groups showed that subjects showing greater adherence to MD had diminished changes in the global DNA methylation pattern and gene-specific methylation such as the stearoyl Coenzyme A desaturase (SCD1) gene involved in the synthesis of MUFAs from saturated fatty acids [67].
|
Table 1: Clinical studies focusing on the Mediterranean diet in rheumatoid arthritis. |
|
Authors
|
Type of study
|
Patients
|
Study period
|
Main results
|
| Skoldstam et al., 2003 [58] |
Single centre, randomised, parallel |
51 RA,
25 CD + 26 MD |
3 months |
Significant improvement of DAS28, HAQ, VAS pain, SF36, CRP and cholesterol. |
| Michalsen et al., 2005 [59] |
Single centre, parallel |
16 RA,
9 FT + 7 MD |
13 days |
Non-significant improvement of DAS28. |
| Mc Kellar et al., 2007 [61] |
Single centre, parallel |
130 RA,
55 CD + 75 MD |
6 months |
Non-significant improvement of EMS, HAQ, patient global VAS, VAS pain and sBP. |
| Abendroth et al., 2010 [60] |
Single centre, parallel |
50 RA,
22 FT + 28 MD |
13 days |
Significant improvement of DAS28.
Non-significant improvement of VAS pain and SF36. |
| CD = control diet; DAS28 = disease activity composite score (swollen and tender joints, erythrocyte sedimentation rate, general health); EMS = early morning stiffness; FT = fasting therapy; HAQ = health assessment questionnaire; MD = Mediterranean diet; RA = rheumatoid arthritis; SF36 = short-form health survey; VAS = visual analogue scale. |
Clinical studies evaluating the effect of the Mediterranean diet in arthritis
Studies investigating the efficacy of the MDP on disease activity in patients suffering with chronic joint diseases all concern rheumatoid arthritis and are outlined in table 1.
The first single-centre, randomised, parallel study on patients following the Mediterranean diet (MD) was carried out by Sköldstam and colleagues over a 3-month period [68]. The clinical outcome of 26 rheumatoid arthritis patients prescribed a MD was compared with that of 25 rheumatoid arthritis patients all following a control diet after a 3-week outpatient-based rehabilitation programme. At the end of the study, the disease activity score (DAS) 28, the health assessment questionnaire (HAQ), and the Short Form-36 Health Survey (SF-36), which were used as the study’s primary efficacy variables, were all significantly improved with respect to baseline values in the MD group. The secondary efficacy variables, and in particular CRP and the visual analogue scale (VAS) pain score, were also improved in the MDP group, but there was no change in any of the efficacy variables in the control one.
An observational study on the effect of the MD with respect to fasting therapy (300 kcal/day) on disease outcome was carried out by Michalsen and colleagues in rheumatoid arthritis patients [69]. During the 2-week study period, the DAS28 disease activity index improved in both groups of patients although to a slightly greater degree in the fasting patients (table 1).
Another study comparing the effect of MD with that of fasting therapy in rheumatoid arthritis patients was carried out by Abendroth and colleagues [70]. For 2 weeks, 28 rheumatoid arthritis patients followed a MD while 22 followed a fasting diet (300 kcal/day). In contrast to Michalsen's study, improvement in disease activity was comparable in the two groups. The VAS pain score improved in both groups and did so significantly on day 7 in the fasting group. At the same time, health and quality of life, as measured by the SF-36 scores, were slightly improved in both groups; no differences were found in the HAQ score.
Finally, a large number of participants were enrolled in McKellar and colleagues' pilot study during which 75 rheumatoid arthritis patients followed a MD and 55 a control one [71]. After 3 months, the VAS pain and HAQ scores were significantly improved in the MD compared with the control group; after 6 months the former also showed a significant clinical improvement in the global assessment and in early morning stiffness. The effect of the diet on some cardiovascular risk factors was also evaluated; there was a significant decline in systolic blood pressure in the Mediterranean diet group, while cholesterol, HDL and glutathione levels were unaltered.
Conclusions
Many of the anti-inflammatory effects of the MDP are linked to the intake of foods that are rich in PUFAs with a lower n-6 to n-3 fatty acid ratio and extra-virgin olive oil which, with its high content of MUFAs and non-fat micro-components such as phenolic compounds, has been found to have important anti-inflammatory effects both in vitro and in vivo.
Most interventional and epidemiological studies focusing on the influence of the MDP on disease development have been concerned with cardiovascular diseases and various forms of cancer. Clinical research on the effect of the Mediterranean diet on arthritis is limited to only a few studies, all carried out in rheumatoid arthritis patients. These studies have for the most part produced contrasting results. It is, moreover, difficult to compare directly the results of these trials as they are characterised by differences in the components making up the diet, the length of the study periods, patients’ characteristics, and, in particular, disease duration and disease activity. In addition, fasting therapy led to a better clinical response in some cases, and, although the study periods were perhaps too short to produce reliable results, disease activity improvement may be linked to alterations in the intestinal flora produced by fasting itself.
Some aspects underlying the mechanisms of action by which the MDP exerts its beneficial effects and if, in effect, it objectively modifies disease progression, remain to be elucidated.
Since it includes all the food groups, the Mediterranean diet is considered nutritionally adequate and complete and, in any case safe, heart-healthy, easy to follow, and associated with long-term weight control and the maintenance of “good” gut microbiota. The benefits to arthritis patients may be even greater in view of their increased risk of cardiovascular disease whose treatment has been linked to adverse side effects. Although further controlled clinical studies focusing on the role of the MDP in different forms of arthritis are warranted, the regimen can in any case be considered a valuable part of an arthritis patient’s global health management plan.
References
1 Preedy VR, Watson RR, eds. The Mediterranean Diet: An Evidence-Based Approach. Academic Press; 2015.
2 Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health. Biofactors. 2013;39:335–42.
3 Wolf AD, Pfleger B. Burden of Major Musculoskeletal Conditions. Policy and Practice. Special Theme-Bone and Joint Decade 2000-2010. Bulletin of the World Health Organization. 2003;81:646–56.
4 Ingegnoli F, Gualtierotti R, Artusi C, Lubrano E. Focus on the potential effects of treatments for spondylarthritides on cardiovascular risk. Expert Rev Clin Immunol. 2014;10:307–15.
5 John H, Kitas G. Inflammatory arthritis as a novel risk factor for cardiovascular disease. Eur J Intern Med 2012;23:575-579.
6 Bhole V, Krishnan E. Gout and the heart. Rheum Dis Clin North Am. 2014;40:125–43.
7 Husted JA, Thavaneswaran A, Chandran V, Eder L, Rosen CF, Cook RJ, et al. Cardiovascular and other comorbidities in patients with psoriatic arthritis: a comparison with patients with psoriasis. Arthritis Care Res (Hoboken). 2011;63:1729–3.
8 Ramonda R, Lo Nigro A, Modesti V, Nalotto L, Musacchio E, Iaccarino L, et al. Atherosclerosis in psoriatic arthritis. Autoimmun Rev. 2011;10:773–8.
9 Pattison DJ, Symmons DP, Young A. Does diet have a role in the aetiology of rheumatoid arthritis? Proc Nutr Soc. 2004;63:137–43.
10 Choi HK. Dietary risk factors for rheumatic diseases. Curr Opin Rheumatol. 2005;17:141–6.
11 Firestein GS. The T cell cometh: interplay between adaptive immunity and cytokine networks in rheumatoid arthritis. J Clin Invest. 2004;114:471–4.
12 Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36:182–8.
13 Drosos AA, Lanchbury JS, Panayi GS, Moutsopoulos HM. Rheumatoid arthritis in Greek and British patients. A comparative clinical, radiologic, and serologic study. Arthritis Rheum. 1992;35:745–8.
14 González Cernadas L, Rodríguez-Romero B, Carballo-Costa L. Importance of nutritional treatment in the inflammatory process of rheumatoid arthritis patients; a review. Nutr Hosp. 2014;29:237–45.
15 Linos A, Kaklamani VG, Kaklamani E, Koumantaki Y, Giziaki E, Papazoglou S, et al. Dietary factors in relation to rheumatoid arthritis: a role for olive oil and cooked vegetables? Am J Clin Nutr. 1999;70:1077–8.
16 Pedersen M, Stripp C, Klarlund M, Olsen SF, Tjønneland AM, Frisch M. Diet and risk of rheumatoid arthritis in a prospective cohort. J Rheumatol. 2005;32:1249–52.
17 Symmons D, Harrison B. Early inflammatory polyarthritis: results from the norfolk arthritis register with a review of the literature. I. Risk factors for the development of inflammatory polyarthritis and rheumatoid arthritis. Rheumatology (Oxford). 2000;39:835–43.
18 Hu Y, Costenbader KH, Gao X, Al-Daabil M, Sparks JA, Solomon DH, et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr. 2014;100:959–67.
19 Benito-Garcia E, Feskanich D, Hu FB, Mandl LA, Karlson EW. Protein, iron, and meat consumption and risk for rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther. 2007;9:R16.
20 Rosell M, Wesley AM, Rydin K, Klareskog L, Alfredsson L; EIRA study group. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology. 2009;20:896–901.
21 Jin Z, Xiang C, Cai Q, Wei X, He J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose response meta-analysis of prospective studies. Ann Rheum Dis. 2014;73:1962–7.
22 Sundström B, Johansson I, Rantapää-Dahlqvist S. Diet and alcohol as risk factors for rheumatoid arthritis: a nested case-control study. Rheumatol Int. 2015;35(3):533–9.
23 Boyer GS, Templin DW, Cornoni-Huntley JC, Everett DF. Prevalence of spondyloarthropathies in Alaskan Eskimos. J Rheumatol. 1994;21:2292–7.
24 Sundström B, Wallberg-Jonsson S, Johansson G. Diet, disease activity, and gastrointestinal symptoms in patients with ankylosing spondylitis. Clin Rheumatol. 2011;30:71–6.
25 Sundström B, Johansson G, Kokkonen H, Cederholm T, Wallberg-Jonsson S. Plasma phospholipid fatty acid content is related to disease activity in ankylosing spondylitis. J Rheumatol. 2012;39:327–33.
26 Ebringer A, Wilson C. The use of a low starch diet in the treatment of patients suffering from ankylosing spondylitis. Clin Rheumatol. 1996;15(Suppl1):62–6.
27 Appelboom T, Durez P. Effect of milk product deprivation on spondyloarthropathy. Ann Rheum Dis 1994;53:481–2.
28 Azzini M, Girelli D, Olivieri O, Guarini P, Stanzial AM, Frigo A, et al. Fatty acids and antioxidant micronutrients in psoriatic arthritis. J Rheumatol. 1995;22:103–8.
29 Barter MJ, Bui C, Young DA. Epigenetic mechanisms in cartilage and osteoarthritis: DNA methylation, histone modifications and microRNAs. Osteoarthritis Cartilage. 2012;20:339–49.
30 McKay JA, Mathers JC. Diet induced epigenetic changes and their implications for health. Acta Physiol (Oxf). 2011;202:103–18.
31 Oliviero F, Sfriso P, Scanu A, Fiocco U, Spinella P, Punzi L. Epigallocatechin-3-gallate reduces inflammation induced by calcium pyrophosphate crystals in vitro. Front Pharmacol. 2013;4:51.
32 Prasad P, Krishnan E. Filipino gout: a review. Arthritis Care Res (Hoboken). 2014;66:337–43.
33 Torralba KD, De Jesus E, Rachabattula S. The interplay between diet, urate transporters and the risk for gout and hyperuricemia: current and future directions. Int J Rheum Dis. 2012;15:499–506.
34 Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–16.
35 Juraschek SP, Miller ER 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken). 2011;63:1295–306
36 Andrés M, Sivera F, Falzon L, Buchbinder R, Carmona L. Dietary supplements for chronic gout. Cochrane Database Syst Rev. 2014;10:CD010156
37 Kontogianni MD, Chrysohoou C, Panagiotakos DB, Tsetsekou E, Zeimbekis A, Pitsavos C, et al. Adherence to the Mediterranean diet and serum uric acid: the ATTICA Study. Scand J Rheumatol. 2012;41:442–9.
38 Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Rinaldi Alvarenga JF, Leal LN, Lamuela-Raventos RM. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014;154:299–307.
39 Serra-Majem L, Ngo de la Cruz J, Ribas L, Tur JA. Olive oil and the Mediterranean diet: beyond the rhetoric. Eur J Clin Nutr. 2003;57(Suppl 1):S2–7.
40 Pérez-Jiménez F, Ruano J, Perez-Martinez P, Lopez-Segura F, Lopez-Miranda J. The influence of olive oil on human health: Not a question of fat alone. Mol Nutr Food Res. 2007;51:1199–208.
41 Oliviero F, Punzi L, Spinella P. Mediterranean Food Pattern in Rheumatoid Arthritis. Current Rheumatology Rev. 2009;5:233–40.
42 Ferretti A, Nelson GJ, Schmidt PC, Kelley DS, Bartolini G, Flanagan VP. Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids. 1997;32:435–9.
43 Li B, Birdwell C, Whelan J. Antithetic relationship of dietary arachidonic acid and eicosapentaenoic acid on eicosanoid production in vivo. J Lipid Res. 1994;35:1869–77.
44 Magdalon J, Vinolo MA, Rodrigues HG, Paschoal VA, Torres RP, Mancini-Filho J, et al. Oral administration of oleic or linoleic acids modulates the production of inflammatory mediators by rat macrophages. Lipids. 2012;47:803–12.
45 Musumeci G, Trovato FM, Pichler K, Weinberg AM, Loreto C, Castrogiovanni P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: an in vivo and in vitro study on lubricin expression. J Nutr Biochem. 2013;24:2064–75.
46 Volker DH, Fitzgerald PE, Garg ML. The eicosapentaenoic to docosahexaenoic acid ratio of diets affects the pathogenesis of arthritis in Lew/SSN Rats. J Nutr. 2000;130:559–65.
47 Carluccio MA, Siculella L, Ancora MA, Massaro M, Scoditti E, Storelli C, et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–9.
48 Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–9.
49 Silva S, Sepodes B, Rocha J, Direito R, Fernandes A, Brites D, et al. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J Nutr Biochem. 2015;26:360–8.
50 Scotece M, Gómez R, Conde J, Lopez V, Gómez-Reino JJ, Lago F, et al. Further evidence for the anti-inflammatory activity of oleocanthal: inhibition of MIP-1α and IL-6 in J774 macrophages and in ATDC5 chondrocytes. Life Sci. 2012;91:1229–35.
51 Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Morittu VM, et al. Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J Pharmacol Exp Ther. 2011;339:859–69.
52 Rosillo MA, Alcaraz MJ, Sánchez-Hidalgo M, Fernández-Bolaños JG, Alarcón-de-la-Lastra C, Ferrándiz ML. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. J Nutr Biochem. 2014;25:1275–81.
53 Johnson JJ. Carnosol: a promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011;305:1–7.
54 Calder PC, Yaqoob P, Thies F, Wallace FA, Miles EA. Fatty acids and lymphocyte functions. Br J Nutr. 2002;87Suppl1:S31–48.
55 Jaudszus A, Gruen M, Watzl B, Ness C, Roth A, Lochner A, et al. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. J Lipid Res. 2013;54:923–35.
56 Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexainoic acid HA reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009;50:2377–88.
57 Kremer JM, Lawrence DA, Jubiz W, DiGiacomo R, Rynes R, Bartholomew LE, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 1990;33:810–20.
58 Jaudszus A, Gruen M, Watzl B, Ness C, Roth A, Lochner A, et al. Evaluation of suppressive and pro-resolving effects of EPA and DHA in human primary monocytes and T-helper cells. J Lipid Res. 2013;54:923–35.
59 Teague H, Rockett BD, Harris M, Brown DA, Shaikh SR. Dendritic cell activation, phagocytosis and CD69 expression on cognate T cells are suppressed by n-3 long-chain polyunsaturated fatty acids. Immunology. 2013;139:386–9.
60 Rockett BD, Melton M, Harris M, Bridges LC, Shaikh SR. Fish oil disrupts MHC class II lateral organization on the B-cell side of the immunological synapse independent of B-T cell adhesion. J Nutr Biochem. 2013;24:1810–6.
61 Palmerini CA, Carlini E, Saccardi C, Servili M, Montedoro G, Arienti G. Activity of olive oil phenols on lymphomonocyte cytosolic calcium. J Nutr Biochem. 2005,16:109–13.
62 Montemurno E, Cosola C, Dalfino G, Daidone G, De Angelis M, Gobbetti M, et al. What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press Res. 2014;39:114–23.
63 Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36.
64 Rogier R, Koenders MI, Abdollahi-Roodsaz S. Toll-like receptor mediated modulation of T cell response by commensal intestinal microbiota as a trigger for autoimmune arthritis. J Immunol Res. 2015;2015:527696
65 Lima SCS, Ribeiro Pinto LF, Herceg Z. The Effects of Diet on Epigenetic Processes. Handbook of Epigenetics. The New Molecular and Medical Genetics. Edited by: Trygve Tollefsbol
66 Di Francesco A, Falconi A, Di Germanio C, Micioni Di Bonaventura MV, Costa A, Caramuta S, et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J Nutr Biochem. 2015;26:250–58.
67 Martín-Núñez GM, Cabrera-Mulero R, Rubio-Martín E, Rojo-Martínez G, Olveira G, Valdés S, et al. Methylation levels of the SCD1 gene promoter and LINE-1 repeat region are associated with weight change: an intervention study. Mol Nutr Food Res. 2014;58:1528–36.
68 Sköldstam L, Hagfors L, Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:208–14.
69 Michalsen A, Riegert M, Lüdtke R, Bäcker M, Langhorst J, Schwickert M, et al. Mediterranean diet or extended fasting’s influence on changing the intestinal microflora, immunoglobulin A secretion and clinical outcome in patients with rheumatoid arthritis and fibromyalgia: an observational study. BMC Complement Altern Med. 2005;5:22.
70 Abendroth A, Michalsen A, Lüdtke R, Rüffer A, Musial F, Dobos GJ, et al. Changes of Intestinal Microflora in Patients with Rheumatoid Arthritis during Fasting or a Mediterranean Diet. Forsch Komplementmed. 2010;17:307–13.
71 McKellar G, Morrison E, McEntegart A, Hampson R, Tierney A, Mackle G, et al. A pilot study of a Mediterranean-type diet intervention in female patients with rheumatoid arthritis living in areas of social deprivation in Glasgow. Ann Rheum Dis. 2007:66;1239–43.


