Gestational age-adapted oxygen saturation targeting and outcome of extremely low gestational age neonates (ELGANs)
DOI: https://doi.org/10.4414/smw.2015.14197
Anja
Hergenhan, Martina
Steurer, Thomas M.
Berger
Summary
QUESTIONS UNDER STUDY: Optimal oxygen saturation (SpO2) targets for extremely low gestational age neonates (ELGANs, gestational age [GA] <28 weeks) are unknown. Conflicting results from five recently published multicentre trials, which randomised ELGANs to high (91 to 95%) or low (85 to 89%) SpO2 targets from birth up to a corrected GA of 36 weeks, prompted us to examine our experience with two different SpO2policies.
METHODS: We retrospectively compared outcomes of two cohorts of ELGANs which were exposed to two different SpO2 target policies adapted to the infants’ corrected GA. Between 1 January 2000 and 30 June 2007, SpO2 targets were 85 to 95% at <30 weeks and 88 to 97% at ≥30 weeks (high SpO2 target cohort, n = 157). Between 1 July 2007 and 31 December 2011, SpO2 targets were lowered to 80 to 90% at <30 weeks, 85 to 95% between 30 and 34 weeks and finally 88 to 97% at ≥34 weeks (low SpO2 target cohort, n = 84).
RESULTS: There were no statistically significant differences between the high and low SpO2 target cohorts in mortality rates (15.9 vs 17.9%, risk ratio [RR] 0.89; 95% confidence interval [CI] 0.50–1.60), incidences of severe retinopathy of prematurity (2.3 vs 0%, RR 3.68; 95% CI 0.19–70.3), or moderate/severe bronchopulmonary dysplasia (14.4 vs 21.1%, RR 0.68; 95% CI 0.37–1.26).
CONCLUSIONS:Adapting SpO2 targets to the advancing corrected GA seems safe and is associated with low incidences of short-term complications. Mortality rates did not vary with the two different SpO2 target policies utilised and were comparable to those reported from recently published randomised controlled SpO2 target trials.
A Swiss single centre experience over 12 years
Abbreviations
ANC antenatal corticosteroids
BOOST benefits of oxygen saturation targeting
BPD bronchopulmonary dysplasia
cPVL cystic periventricular leukomalacia
ELGANs extremely low gestational age neonates
GA gestational age
NEC necrotising enterocolitis
NICU neonatal intensive care unit
PIVH periventricular/intraventricular haemorrhage
ROP retinopathy of prematurity
SUPPORT surfactant, positive pressure, and pulse oximetry randomised trial
Introduction
Large-scale use of supplemental oxygen in neonatology started more than 75 years ago, to alleviate symptoms of neonatal respiratory distress, the leading cause of death among preterm infants at the time [1]. When Wilson and colleagues published their observation that periodic breathing and apnoea spells in preterm infants decreased if they were nursed in 70% oxygen [2], and the link between perinatal lack of oxygen and neurological damage was more fully appreciated, liberal use of oxygen became the standard of care for a variety of neonatal conditions [3].
By 1950, retrolental fibroplasia (now known as retinopathy of prematurity, ROP) was recognised as the principal cause of blindness in infants and oxygen toxicity was suspected to be an important factor in its pathogenesis [4]. Oxygen use in premature infants was generally restricted to an FiO2 of 40% [5]. Only a few years later, Avery and Oppenheimer concluded from an autopsy study of two cohorts with different use of oxygen (1944 to 1948: FiO2 60–80%, 1954–1958: FiO2 rarely exceeding 40%) that a restricted oxygen supplementation policy might be associated with increased mortality rates from hyaline membrane disease [6].
In the 1970s and 1980s, technologies became available to monitor transcutaneous partial pressure of oxygen and transcutaneous oxygen saturation (SpO2). Although the administration of supplemental oxygen had become more reliable, ROP and bronchopulmonary dysplasia (BPD) remained two of the most feared complications of prematurity for many years to come. In 2001, Tin and colleagues reported that the incidence of threshold ROP in neonatal intensive care units (NICUs) with low SpO2 targets (i.e., 70 to 90%) were almost two thirds lower than in NICUs with high SpO2 targets (i.e., 88 to 98%). Importantly, survival rates and rates of neurodevelopmental impairment at one year of age did not differ between the groups [7]. Additional support for lower SpO2 targets came from the STOP-ROP [8] and the Benefits of Oxygen Saturation Targeting (BOOST) trials [9].
In recent years, the results of five large prospective randomised controlled multicentre trials (RCTs) (SUPPORT: Surfactant Positive Pressure and Pulse Oximetry Randomised Trial, n = 1 316; BOOST II [UK, Australia, New Zealand]: Benefits of Oxygen Saturation Targeting Trial II, n = 2 448; and COT: Canadian Oxygen Trial, n = 1 201), which explored the effects of low (i.e., 85 to 89%) versus high (i.e., 91 to 95%) SpO2 targets initiated immediately after birth and maintained up to a corrected age of 36 weeks of gestation in extremely low gestational age neonates (ELGANs), have been published [10–14]. Despite the fact that the study designs of the five RCTs were almost identical, the results differed. While SUPPORT and BOOST II UK and Australia (subgroup with revised pulse oximeter-calibration algorithm only, n = 1 187) reported significantly higher mortality rates before discharge among infants in the low SpO2 target groups [11, 12], BOOST II New Zealand [10] and COT [14] found no difference in death independent of the pulse oximeter-calibration algorithm used. Patients randomised to the low SpO2 target group had significantly lower rates of severe ROP in SUPPORT and BOOST II, but not in COT [11, 12, 14].
As editorials accompanying the publications of these RCTs highlighted, it remains challenging to know how to incorporate these conflicting results into clinical practice [15, 16]. The study oximeter masking algorithms may have had unanticipated effects on how caregivers dealt with SpO2 deviations [17, 18], and, in fact, the optimal SpO2 target ranges for ELGANs remain elusive. This, coupled with the fact that we have historically used two different SpO2 target policies that included adaptation to the patient’s corrected gestational age (GA), prompted us to analyse and compare the outcome of ELGANs cared for in our NICU over a 12-year-period.
Patients and methods
Patient population
We retrospectively compared the outcome of two cohorts of ELGANs (gestational age <28 weeks) treated at the Children’s Hospital of Lucerne, who were exposed to two different SpO2 target policies. Between 1 January 2000 and 30 June 2007, SpO2 targets were 85 to 95% for ELGANs at a corrected GA of <30 weeks and 88 to 97% at a corrected GA of ≥30 weeks (high SpO2 target cohort). Between 1 July 2007 and 31 December 2011, SpO2 targets were lowered to 80 to 90% for ELGANs at a corrected GA of <30 weeks, 85 to 95% between 30 and 34 weeks and finally 88 to 97% at more than 34 weeks (low SpO2 target cohort) (fig. 1). The decision to lower the SpO2 targets was based on data from literature that had accumulated at the time, which showed lower rates of ROP and BPD without affecting survival and neurodevelopmental impairment rates [7–9]. Other policies that might have had an impact on the outcomes studied, such as the use of oxygen in the delivery room, surfactant administration, diagnosis and treatment of haemodynamically significant persistent ductus arteriosus, and nutrition strategies remained unchanged. Infants were allocated to their gestational-age specific SpO2 targets on NICU admission. Over the entire study period, the same pulse oximetry system was used (Nellcor® sensors in combination with Philips FAST®-SpO2 algorithm). SpO2 alarms were set at the upper and lower values of the SpO2 targets when supplemental oxygen was required.The well-trained nurses were instructed to respond to both low and high SpO2 alarms promptly; it was their responsibility to assess the reason for the alarm and to take appropriate action. They were allowed to adjust the FiO2 autonomously and would only call for physician support if they could not rapidly resolve the problem on their own. Infants with major congenital abnormalities and infants who had died in the delivery room following primary nonintervention were excluded. The following perinatal data were recorded: GA, birth weight (BW), sex, singleton/multiple births, antenatal corticosteroid (ANC) and surfactant administration.
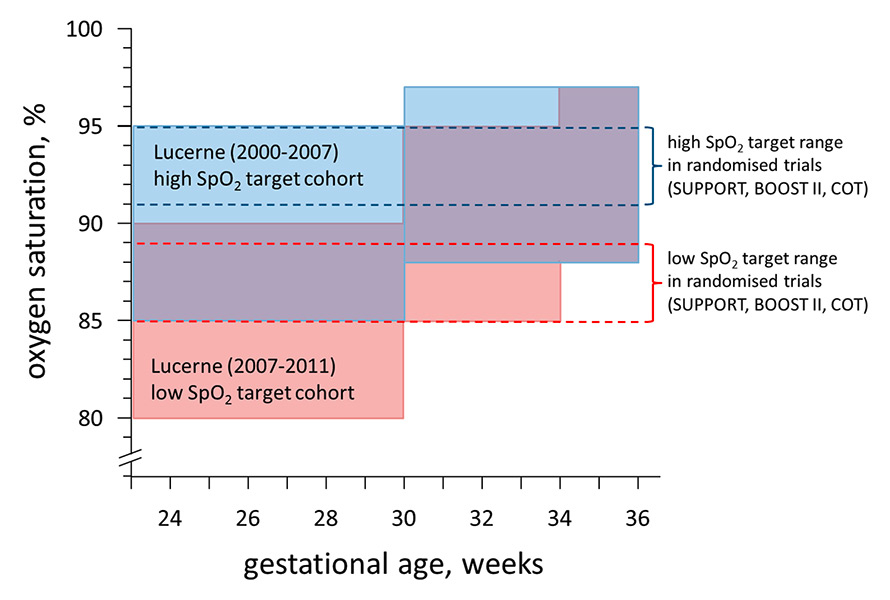
Figure 1
Oxygen saturation (SpO2) target policies at the Children’s Hospital of Lucerne: (A) between 1 January 2000 and 30 June 2007, SpO2 targets were 85 to 95% at <30 weeks and 88 to 97% at ≥30 weeks (high SpO2 target cohort); (B) between 1 July 2007 and 31 December 2011, SpO2 targets were lowered to 80 to 90% at <30 weeks, 85 to 95% between 30 and 34 weeks and finally 88 to 97% at ≥34 weeks (low SpO2 target cohort).
Mortality rates, timing and cause of death
Mortality rates before discharge and at the neurodevelopmental follow-up (12–24 months) were determined. In addition, age at death and cause of death were analysed. Based on a detailed chart review by two of the authors (AH, TMB), the main cause of death was classified into six categories: respiratory failure, cardiovascular failure, central nervous system complication, gastrointestinal failure, sepsis or multiorgan system failure.
Rates of neonatal morbidities and neurodevelopmental impairment
To allow comparison with outcomes from the recently published RCTs on SpO2 targeting in ELGANs, the following neonatal morbidities were analysed: rates of severe ROP (defined as stage ≥III) [19], moderate/severe BPD defined as a requirement for supplemental oxygen and/or mechanical respiratory support at 36 weeks postmenstrual age [20], severe (grade 3 or 4) periventricular/intraventricular haemorrhage (PIVH) [21], cystic periventricular leukomalacia (cPVL) [22], as well as necrotising enterocolitis (NEC) (Bell’s stage ≥2) [23].
Developmental evaluation was performed using either the Bayley Scales of Infant Development, second edition (BSID-II), or the Griffiths Mental Developmental Scales (GMDS). The BSID-II included determination of the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI). Disability was defined as either moderate neurodevelopmental impairment (i.e., PDI and/or MDI between 55 and 69, GMDS DQ between 55 and 69, or bilateral visual or hearing impairment) or severe (i.e., cerebral palsy resulting in severely impaired mobility [PDI <55], severe cognitive impairment [MDI <55], GMDS DQ <55, bilateral blindness or deafness). Finally, the rates of the following combined outcomes were determined: death before discharge or severe ROP, death or moderate/severe BPD by 36 weeks, and death or disability by 12–24 months.
Statistical analysis
Baseline characteristics are presented as mean and standard deviation (SD) for continuous variables and in percentages for binary variables. Chi square test was used to compare proportions. Associations between the the high and low SpO2 target cohorts and the outcomes are presented as risk ratios with corresponding 95% confidence intervals (CIs). All statistical analyses were performed with the statistical software Stata (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).
|
Table 1:Mortality and morbidity rates. |
|
|
High SpO2 cohort
(n = 157)
|
Low SpO2 cohort
(n = 84)
|
Relative risk
(95% confidence interval)
|
p-value
|
|
no. / total no. (%)
|
|
|
|
Death before discharge
|
25/157 |
(15.9) |
15/84 |
(17.9) |
0.89 (0.50–1.60) |
0.70 |
|
Individual morbidities
|
|
|
|
|
|
|
| Severe ROP* |
3/132 |
(2.3) |
0/69 |
(0) |
3.68 (0.19–70.3) |
0.39 |
| Moderate/severe BPD* |
19/132 |
(14.4) |
15/71 |
(21.1) |
0.68 (0.37–1.26) |
0.22 |
| NEC |
10/157 |
(6.4) |
2/84 |
(2.4) |
2.68 (0.60–11.9) |
0.20 |
| PIVH grade 3 or 4 |
12/157 |
(7.6) |
8/84 |
(9.5) |
0.80 (0.34–1.89) |
0.61 |
| cPVL |
4/157 |
(2.5) |
2/84 |
(2.4) |
1.07 (0.20–5.72) |
0.94 |
| Disability†
|
14/121 |
(11.6) |
5/61 |
(8.2) |
1.41 (0.53–3.74) |
0.49 |
|
Combined outcomes
|
|
|
|
|
|
|
| Death before discharge or severe ROP |
28/157 |
(17.8) |
15/84 |
(17.9) |
1.00 (0.56–1.76) |
1.00 |
| Death or moderate/severe BPD by 36 weeks |
44/157 |
(28.0) |
28/84 |
(33.3) |
0.84 (0.57–1.25) |
0.39 |
| Death or disability†
|
39/146 |
(26.7) |
20/76 |
(26.3) |
1.02 (0.64–1.61) |
0.95 |
| BPD = bronchopulmonary dysplasia; cPVL = cystic periventricular leukomalacia; NEC = necrotising enterocolitis; PIVH = periventricular/intraventricular haemorrhage; ROP = retinopathy of prematurity
* rates among survivors only
† data on disability missing for 11 and 8 patients in the high and low SaO2 cohorts, respectively |
Results
Patient characteristics
Overall, 241 ELGANs were included in our analysis; of these, 157 infants belonged to the high and 84 infants to the low SpO2 target cohorts. Baseline characteristics among the newborns in both cohorts were comparable with a mean GA of 26.3 ± 1.1 and 26.1 ± 1.1 weeks and a mean BW of 860 ± 198 g and 811 ± 205 g in the high and low SpO2 cohorts, respectively. In both time periods, there were slightly more males than females. The rates of any ANC administration were ≥85% in both time periods, and the vast majority of ELGANs were treated with exogenous surfactant (96% and 94% in the high and low SpO2 cohorts, respectively).
Mortality rates, age at death and cause of death
There was no statistically significant difference between the high and low SpO2 target cohorts in rates of death before discharge (15.9 vs 17.9%; RR 0.89, 95% CI 0.50–1.60) (table 1). The age at death did not differ between the two cohorts with the majority of patients dying within the first week of life. In the high SpO2 cohort, respiratory failure was the leading cause of death (40%), whereas cardiovascular failure predominated among infants in the low SpO2 cohort (40%).
Neonatal morbidities and neurodevelopmental impairment
Although there was a trend towards higher incidence of severe ROP (2.3 vs 0%; RR 3.68, 95% CI 0.19–70.3) and NEC (6.4 vs 2.4%; RR 2.68, 95% CI 0.60–11.9) in the high SpO2 target cohort, the difference did not reach statistical significance. Also, there was a statistically nonsignificant decreased rate of moderate/severe BPD in the high compared to the low SpO2 target cohort (14.4 vs 21.1%; RR 0.68, 95% CI 0.37–1.26). Severe PIVH (7.6 vs 9.5%; RR 0.80, 95% CI 0.34–1.89) and cPVL (2.5 vs 2.4%; RR 1.07, 95% CI 0.20–5.72) occurred at similar rates in both SpO2 cohorts (table 1).
Disability, defined as moderate or severe neurodevelopmental impairment, was observed among 11.6% of survivors in the high SpO2 cohort compared with 8.2% in the low SpO2 cohort (RR 1.41, 95% CI 0.53–3.74). Data were missing or incomplete for 9% of patients in both cohorts (table 1).
Finally, the combined outcomes of death before discharge or severe ROP (17.8 vs 17.9%; RR 1.00, 95% CI 0.57–1.76), death or moderate/severe BPD by 36 weeks (28.0 vs 33.3%; RR 0.84, 95% CI 0.57–1.25) and death or disability at 12–24 months (26.7 vs 26.3%; RR 1.02, 95% CI 0.64–1.61) were also comparable (table 1).
|
Table 2:Comparison of baseline population characteristics from current study and randomised controlled SpO2 target trials. |
| |
Lucerne
|
SUPPORT
|
BOOST II
|
COT
|
|
High SpO2 cohort
(n = 157)
|
Low SpO2 cohort
(n = 84)
|
High SpO2 cohort
(n = 662)
|
Low SpO2 cohort
(n = 654)
|
High SpO2 cohort
(n = 1 224)
|
Low SpO2 cohort
(n = 1 224)
|
High SpO2 cohort
(n = 599)
|
Low SpO2 cohort
(n = 602)
|
| Gestational age, weeks (mean ± SD) |
26.3 ± 1.1 |
26.1 ± 1.1 |
26.0 ± 1.0 |
26.0 ± 1.0 |
26.0 ± 1.2 |
26.0 ± 1.2 |
25.6 ± 1.2 |
25.6 ± 1.2 |
| Birth weight, grams (mean ± SD) |
860 ± 198 |
811 ± 205 |
825 ± 193 |
836 ± 193 |
837 ± 189 |
826 ± 184 |
845 ± 197 |
829 ± 188 |
| Male sex, % |
54.1 |
51.2 |
56.0 |
52.1 |
52.4 |
56.0 |
54.4 |
54.7 |
| Multiple births, % |
34.4 |
9.5 |
26.6 |
24.6 |
25.9 |
26.3 |
30.4 |
34.2 |
| ANC, any, % |
84.7 |
88.1 |
95.6 |
96.8 |
90.7 |
89.6 |
89.8 |
88.4 |
| ANC, complete course, % |
63.9 |
70.2 |
70.2 |
73.3 |
59.6 |
57.6 |
NA |
NA |
| Surfactant administration, % |
96.2 |
94.0 |
84.5 |
81.3 |
NA |
NA |
84.8 89.5 |
|
| ANC = antenatal corticosteroids; SD = standard deviation |
|
Table 3:Comparison of major outcomes from the current study and randomized controlled SpO2 target trials. |
| |
Lucerne
|
|
SUPPORT
|
|
BOOST II
|
|
COT
|
|
|
High SpO2 cohort
(n = 157)
|
Low SpO2 cohort
(n = 84)
|
RR
|
High SpO2 cohort
(n = 662)
|
Low SpO2 cohort
(n = 654)
|
RR
|
High SpO2 cohort
(n = 1224)
|
Low SpO2 cohort
(n = 1224)
|
RR
|
High SpO2 cohort
(n = 599)
|
Low SpO2 cohort
(n = 602)
|
RR
|
| Death before discharge, % |
15.9 |
17.9 |
0.9 |
16.2 |
19.9 |
0.8 |
16.6/15.9* |
19.2/23.1* |
0.9/O.7*,§
|
NA |
NA |
NA |
| Death at 12‒24 months, % |
15.9 |
17.9 |
0.9 |
18.2 |
22.1 |
0.8 |
15.9†/18.7‡
|
14.7†/27.2‡
|
1.1†/0.7‡,** |
15.3 |
16.6 |
0.9 |
| Severe ROP, % |
2.3 |
|
NA |
17.9 |
8.6 |
2.1 |
13.5 |
10.6 |
1.3 |
13.1 |
12.8 |
1.0 |
| Moderate/severe BPD¶, % |
14.4 |
21.1 |
0.7 |
41.7 |
38.8 |
1.1 |
45.7 |
45.3 |
1.0 |
33.14 |
31.84 |
1.0 |
| NEC, % |
6.4 |
2.4 |
2.7 |
10.8 |
11.9 |
0.9 |
8.0 |
10.4 |
0.8 |
9.3 |
12.3 |
0.8 |
| Death or disability, % |
26.7 |
26.3 |
1.0 |
27.5 |
30.2 |
0.9 |
45.5†/46.3‡
|
38.9†/51.1‡
|
1.2†/0.9‡
|
49.7 |
51.6 |
1.0 |
| BPD = bronchopulmonary dysplasia; NEC = necrotising enterocolitis; ROP = retinopathy of prematurity; RA = risk ratio
* Pooled cohort / revised oximeter calibration algorithm only.
† BOOST II New Zealand (n = 340)
‡ BOOST II United Kingdom (n = 722) preliminary data only
¶ Only severe BPD in COT
§ 95% confidence interval 0.6–0.9
** 95% confidence interval 0.6–1.0 |
Discussion
In our retrospective cohort study, comparing two cohorts exposed to two different SpO2 target policies (both of which included adjustments with advancing corrected GA), we found no statistically significant differences in rates of death before discharge or rates of significant neonatal complications, such as severe ROP and moderate/severe BPD. Importantly, identical oximeters and oximeter-calibration algorithms were used in both cohorts.
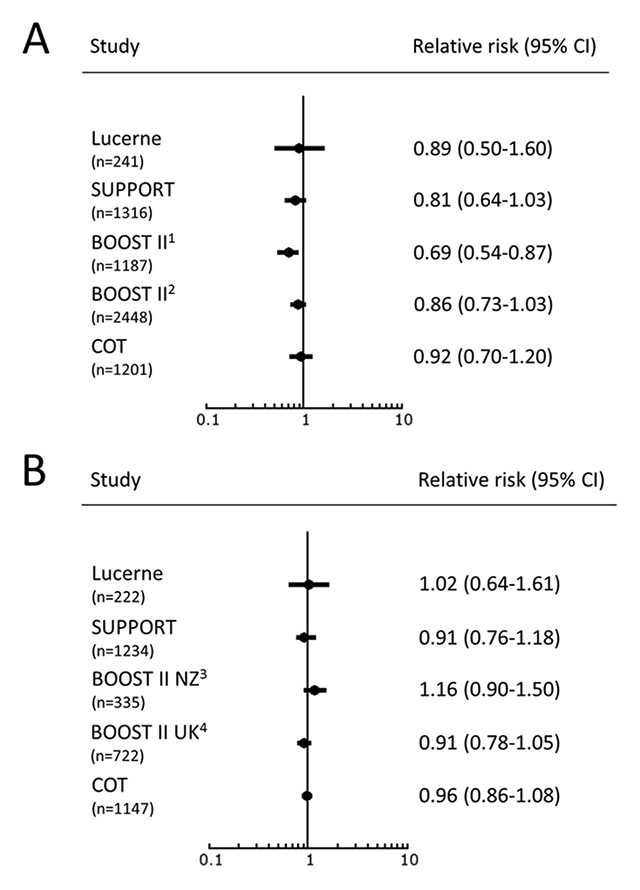
Figure 2
Forest plots of mortality (A) and death or disability (B) in ELGANs in high vs low SpO2 target cohorts.
(RR below 1 favours high SpO2 target)
1 revised oximeter-calibration algorithm cohort only
2 pooled oximeter-calibration algorithm cohort
3 New Zealand cohort only
4 UK revised oximeter-calibration algorithm cohort only, preliminary data only
The patient population characteristics of our two cohorts are very similar to those described in the recently published SpO2 target RCTs (table 2), suggesting that comparison of the results is possible. Mortality rates in the two cohorts of the present study (15.9 and 17.9% in the high and low SpO2 cohorts, respectively) are comparable to those reported from the recent RCTs (table 3). Both SUPPORT and BOOST II (subgroup with revised pulse oximeter-calibration algorithm only) observed significantly lower rates of death before discharge in their high SpO2 target groups (16.2 and 15.9%, respectively) compared with their low SpO2 target groups (19.9 and 23.1%, respectively) [11, 12]. This was also the case for the mortality rates before 18–24 months in BOOST II United Kingdom (18.7 vs 27.2%) [24], but not in BOOST II New Zealand (15.9 vs 14.7%) [10] and COT (15.3 vs 16.6%) [14] in the high and low SpO2 target groups, respectively (table 3, fig. 2A).
Age at death and cause of death did not differ between the two cohorts in our study. This is also true for SUPPORT and BOOST II. In these RCTs, the difference in the proportions of ELGANs dying in the two SpO2 groups accumulated gradually after the first week of life [11, 12]. No particular cause of death occurred more commonly in the low SpO2 target groups. Therefore, neither SUPPORT nor BOOST II could explain what might have caused excess deaths in their low SpO2 target groups [25]. Di Fiore et al. have shown in a subcohort of SUPPORT that intermittent hypoxaemia events (i.e., SpO2 ≤80% for ≥10 seconds and ≤3 minutes) occurred more frequently among ELGANs in the low SpO2 target group [26]. It has been speculated that these episodes might trigger some long-term effects that predispose for death in vulnerable infants at a later time point [25]. On the other hand, since oxygen delivery depends on other factors than SpO2 (e.g., haemoglobin concentration and cardiac output), a wide range of SpO2 values may or may not be associated with tissue hypoxia [27].
The rates of severe neonatal complications in our study were well below those reported from SUPPORT, BOOST II and COT. In our two cohorts, severe ROP occurred in fewer than 3% of patients; whereas, it was observed in 13.1 to 17.9% in the high SpO2 target groups and in 8.6 to 12.8% in the low SpO2 target groups in the RCTs (table 3). Similarly, the rates of moderate/severe BPD were less than half of those observed in SUPPORT and BOOST II; severe BPD was diagnosed in ≤4% of our patients, whereas more than 30% of the patients in the COT trial required more than 30% oxygen or were still on noninvasive or invasive respiratory support at a corrected GA of 36 weeks (table 3).
These differences in morbidity rates are remarkable and difficult to explain. While our GA-adapted SpO2 target policies are likely to decrease the risk of oxygen toxicity in the most immature infants early in life, it is unclear why the rates of ROP and BPD are so much lower than those reported for the low SpO2 target groups in the RCTs. Obviously, other factors may play a role. Differences in baseline population characteristics, nutritional factors affecting insulin-like growth factor 1 (IGF-1) concentrations and conditions associated with pre- and postnatal inflammation are known to modify the risk of severe ROP [28]. Oxygen toxicity is only one factor in the pathogenesis of BPD, with volutrauma and infections being additional important contributors. We therefore suggest that, apart from SpO2 targeting, other aspects of care (e.g., liberal use of early prophylactic surfactant administration in the delivery room, strict adherence to lung protective ventilation strategies, including elective use of high frequency oscillatory ventilation in the smallest patients, nosocomial infection prevention bundles) could be responsible for the low rates of neonatal complications in our cohort.
Finally, the rate of the combined outcome of death or disability is comparable to the rate reported in SUPPORT [11], but lower than those observed in BOOST II (New Zealand) [10], BOOST II (United Kingdom, preliminary data only) [24] and COT [14] (table 3). None of the studies observed a statistically significant difference between the high and low SpO2 target cohorts (fig. 2B). More information from the five SpO2 target RCTs with more than 5000 ELGANs enrolled will be reported by the Neonatal Oxygenation Prospective Meta-analysis (NeOProM) Collaboration in the near future [29].
Limitations
Our study has several limitations. First, the most significant limitation is the lack of power to detect small but potentially significant clinical differences between the two groups: The current sample is powered to detect a difference of 15% between groups. In order to detect a difference of 5% with a power of 80%, a sample size of about 1 500 infants would be necessary. Second, the SpO2 target policies of the two time periods analysed showed some significant overlap, limiting the ability to detect any beneficial or harmful effects; in addition, the policies differed in the way SpO2 targets were adapted to the corrected GA. Third, because of the retrospective design, we cannot determine how stringently the predefined SpO2 targets were reached in our patients. Other studies have shown that high SpO2 alarms are tolerated for longer periods of time than low SpO2 alarms (i.e., periods of hyperoxia are considered to be more acceptable than periods of hypoxia) [30]. Finally, no data on neurodevelopmental impairment were available for 9% of survivors and assessment was not uniform since both BSID-II and GMDS were used.
Conclusion
In our retrospective observational cohort study, different SpO2 target policies for ELGANs were not associated with statistically significant differences in mortality rates before discharge, rates of neonatal morbidities or disability at 12–24 months of life. When compared with the results of international RCTs on high versus low SpO2 targets, we found comparable mortality rates but lower rates of ROP and moderate/severe BPD. It is tempting to speculate that our policy of adapting SpO2 targets to the infants’ corrected GA, which differs from the strategies used in the randomised controlled SpO2 target trials, might be responsible for this observation [31].
In addition, the somewhat wider SpO2 target ranges used in our cohorts may have decreased periods of both hypoxia and hyperoxia [32]. The undisputed goal of oxygen therapy is to deliver sufficient oxygen to the tissues, while minimising oxygen toxicity and oxidative stress. However, at this point, it remains uncertain how this balance can be safely achieved in the most immature infants who are especially vulnerable to the harmful effects of oxygen [14, 32].
The results of our observational study add to the on-going discussion about the optimal SpO2 target for preterm infants. Given the study design and the small sample size, the results cannot be used to change any existing policies, but they are hypothesis generating and may be helpful when designing any future randomised controlled trials.
Disclosure statement: No financial support and no other potential conflict of interest relevant to this article was reported.
References
1 Philip AGS. The evolution of neonatology. Pediatr Res. 2005;58:799–815.
2 Wilson J, Long S, Howard P. Respiration of premature infants: response to variations of oxygen and to increased carbon dioxide in inspired air. Am J Dis Child. 1942;63:1080–5.
3 Anonymous. The early history of oxygen use for premature infants. Pediatrics. 1976;57:592–8.
4 Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia: a clinical approach. Med J Austral. 1951;2:48–50.
5 Robertson AF. Reflections on errors in neonatology: I. The “Hands-Off” years, 1920 to 1950. J Perinatol. 2003;23:48–55.
6 Avery M, Oppenheimer E. Recent increase in mortality from hyaline membrane disease. J Pediatr. 1960;57:553–9.
7 Tin W, Milligan DW, Pennefather P, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:F106–10.
8 The STOP-ROP Multicenter Study Group. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy Of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310.
9 Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. N Engl J Med. 2003;349:959–67.
10 Darlow BA, Marschner SL, Donoghoe M, Battin MR, Broadbent RS, Elder MJ, et al. Randomized controlled trial of oxygen saturation targets in very preterm infants: two year outcomes. J Pediatr. 2014;165:30–5.
11 Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69.
12 Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–104.
13 Vaucher YE, Peralta-Carcelen M, Finer NN, Carlo WA, Gantz MG, Walsh MC, et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N Engl J Med. 2012;367:2495–504.
14 Schmidt B, Whyte RK, Asztalos EV, Moddemann D, Poets C, Rabi Y, et al. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: a randomized clinical trial. JAMA. 2013;309:2111–20.
15 Bancalari E, Claure N. Oxygenation targets and outcomes in premature infants. JAMA. 2013;309:2161–2.
16 Polin RA, Bateman D. Oxygen-saturation targets in preterm infants. N Engl J Med. 2013;368:2141–2.
17 Schmidt B, Roberts RS, Whyte RK, Asztalos EV, Poets C, Rabi Y, et al. Impact of study oximeter masking algorithm on titration of oxygen therapy in the canadian oxygen trial. J Pediatr. 2014;165:666–71.
18 Samiee-Zafarghandy S, Saugstad OD, Fusch C. Do we have an answer when it comes to providing extremely preterm infants with optimal target oxygen saturation? Acta Paediatr. 2015;104:e130–3
19 The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
20 Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
21 Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34.
22 De Vries LS, Eken P, Dubowitz LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res. 1992;49:1–6.
23 Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
24 Stenson B, Brocklehurst B, Cairns S, Deshpande S, Fleck BW, Halliday HL, et al. Abstracts of the Perinatal Medicine 2014 Congress, 9-11 June 2014, Harrogate, UK. Arch Dis Child Fetal Neonatal Ed. 2014;99(Suppl 1):A23.
25 Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2014;105:55–63.
26 Di Fiore JM, Walsh M, Wrage L, Rich W, Finer N, Carlo WA, et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J Pediatr. 2012;161:1047–52.
27 Lakshminrusimha S, Manja V, Mathew B, Suresh GK. Oxygen targeting in preterm infants: a physiological interpretation. J Perinatol. 2015;35:8–15.
28 Hellstrom A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013;382:1445–57.
29 Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr. 2011;11:6.
30 Claure N, Bancalari E, D’Ugard C, Nelin L, Stein M, Ramanathan R, et al. Multicenter crossover study of automated control of inspired oxygen in ventilated preterm infants. Pediatrics. 2011;127:e76–83.
31 Chen ML, Guo L, Smith LEH, Dammann CEL, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity: a meta-analysis. Pediatrics. 2010;125:e1483–92.
32 Sola A, Golombek SG, Montes Bueno MT, Lemus-Varela L, Zuluaga C, Dominguez F, et al. Safe oxygen saturation targeting and monitoring in preterm infants: can we avoid hypoxia and hyperoxia? Acta Paediatr. 2014;103:1009–18.

