Use and role of monoclonal antibodies and other biologics in preventive cardiology
DOI: https://doi.org/10.4414/smw.2015.14179
Baris
Gencer, Reijo
Laaksonen, Aliki
Buhayer, François
Mach
Summary
Biotechnological advances now enable the design of fully human antibodies to target specific antigens in a growing number of diseases. Monoclonal antibodies (mAbs) differ from traditional small chemical molecules in several ways: (1) biological production ‒ they are grown in and extracted from cell cultures; (2) specificity ‒ they demonstrate high target specificity, with a low risk of drug-drug interactions; (3) administration ‒ they are delivered parenterally (intravenously or subcutaneously); (4) dosage interval ‒ their extended half-lives generally allow for spaced dosing (from weekly to monthly). In cardiology, fully human mAbs directed against proprotein convertase subtilisin / kexin type 9 (PCSK9) have shown to be effective in reducing low-density lipoprotein cholesterol (LDL-C) in phase II clinical trials among patients with familial hypercholesterolaemia (FH). PCSK9 inhibitors have just received approval for the treatment of FH and clinical atherosclerotic disease, and patients not at target under maximally tolerated statin therapy or intolerant to statins. Large-scale phase III trials are currently assessing the role of PCSK9 inhibitors in the secondary prevention setting for patients with acute coronary syndromes (ACS) and poorly controlled LDL-C under evidence-based therapies. Another area currently under investigation for fully human mAbs in secondary prevention is their potential ability to inhibit inflammatory pathways. In this context, canakinumab, a specific mAb inhibiting interleukin-1β (IL-1β), has already received approval for the treatment of systemic juvenile idiopathic arthritis. The canakinumab anti-inflammatory thrombosis outcomes trial (CANTOS) is an ongoing trial assessing whether inhibition of IL-1β could reduce the occurrence of cardiovascular adverse events in 17,200 patients with ACS and with defined persisting inflammation.
Introduction
Biologics are complex products derived from natural sources and produced with the use of cutting-edge biotechnology [1]. Monoclonal antibodies (mAbs) constitute a class of biologics widely used for the treatment of immune disorders, but with limited sucess so far in the prevention or treatment of cardiovascular diseases (CVD) [2]. One of the few examples of a mAb that has proven successful in the field of cardiology is abciximab, a glycoprotein IIb/IIIa inhibitor used to prevent thrombosis during percutaneous coronary interventions [2]. This situation is expected to change soon since the recent approval of proprotein convertase subtilisin / kexin type 9 (PCSK9) inhibitors, a group of mAbs designed to target low-density lipoprotein cholesterol (LDL-C) and currently being investigated in large-scale clinical trials [3, 4]. Current focus is on the management of lipid disorders, such as familial hypercholesterolaemia (FH) and patients intolerant to statin. This article provides an overview of the role and use of biologics, and more precisely monoclonal antibodies in preventive cardiology.
What are biologics?
In contrast to traditional systemic drugs, biologics are complex proteins or oligopeptides derived from natural sources and manufactured using cutting-edge biotechnology processes [1]. Among biological products the World Health Organization (WHO) included vaccines, blood products for transfusion, allergenic extracts, human cells, tissues for transplantation, gene therapies and cellular therapies (cited from http://who.int/medicines/services/inn/BioRev2012.pdf). Biologics typically demonstrate high specificity for their target, bind notably at the extracellular level, and have a lower risk for drug-drug interactions as they are not metabolised via hepatic or renal pathways (table 1) [2]. Among the biological therapeutic products currently used are monoclonal antibodies, hormones (e.g., insulin, parathyroid hormone), interferons, interleukins, growth hormones, proteins, peptides and vaccines [2]. Monoclonal antibodies constitute a group of biologics designed to target specific components of cellular mechanisms or pathways and are widely used for the treatment of oncologic or immune diseases (table 1) [2]. Monoclonal antibodies are naturally produced by B cells and are also known as immunoglobulins (Igs), with their respective subtypes (IgA, IgD, IgE, IgG and IgM). The IgG subtype accounts for about 80% of all human antibodies and is the most commonly used class of Ig for the generation of therapeutic mAb hybridomas (B cells fused with an immortal cell line) [2]. Originally, the production of therapeutic mAbs involved the inoculation of mice with a given antigen, thus yielding mouse-based mAb treatments with high levels of immunogenicity and, subsequently, limited efficacy in patients. The invention of recombinant antigen engineering made it possible to move away from murine mAbs (0% human) to chimeric mAbs (65% human), and eventually to minimally immunogenic humanised mAb (>90% human). Today, XenoMouse Hybridoma Technology enables the production of fully human mAbs isolated from the spleen of transgenic mice that produce only human antibodies [2]. The "o", "xi/i", "zu" or "u" in the generic suffix of the International Nonpropriatary Name gives information on the origin of the antibody ("mab" is for "monoclonal antibody"): -omab for murine antibodies, -ximab for chimeric, -zumab for humanised and -umab for fully human antibodies (table 2) [2].
Therapeutic mAbs are administered parenterally (intravenously, subcutaneously or intramuscularly). Circulating mAbs typically have a half-life of 7–21 days and are eliminated via phagocytic and endothelial cells of the reticuloendothelial system. Safety considerations are mainly related to immune reactions (anti-antibody response) and adverse effects at the injection site. However, immune reactions have considerably diminished with the advent of fully human mAbs [2].
|
Table 1: Differences between monoclonal antibodies and small molecules. |
| |
Monoclonal antibodies
|
Small molecules
|
| Structure |
Immunoglobulin |
Small molecule |
| Production |
Cell culture media (hybridoma) |
Chemical synthesis |
| Target specificity |
High |
Low |
| Elimination |
Reticuloendothelial system |
Hepatic, renal, faeces |
| Administration |
Parenteral |
Oral, parenteral |
| Drug-drug interactions |
Low |
Potentially high |
| Dosing |
Every 1–8 weeks |
Daily, weekly |
| Costs |
High |
Low compared with monoclonal antbodies |
| Main indications |
Oncology, rheumatology, gastroenterology |
All |
| Contraindications |
Immunocompromised state; tuberculosis; specific to drug label |
Specific to drug label |
| Table adapted From Foltz et al. [2] |
|
Table 2: Examples of antibodies tested in cardiology. |
|
Generic suffix
|
Nature
|
Percentage human
|
Examples in cardiology
|
| Monoclonal antibodies against intrinsic targets: |
| -omab |
Murine |
0% |
|
| -ximab |
Chimeric |
65% |
Abciximab, basiliximab |
| -zumab |
Humanised |
>90% |
Bococizumab |
| -umab |
Fully human |
100% |
Alirocumab, evolocumab, canakinumab |
| Antibodies against extrinsic agents (antidote): |
| |
Ovine |
0% |
Digoxin-specific antibody (Digibind®) |
| Table adapted from Foltz et al. [2] |
Which antibody treatments are currently used in cardiology?
So far, mAbs have proved efficacious and safe mainly in oncology and immune disorders. Currently, mAbs approved for the treatment of cardiological indications are: (1) abciximab (ReoPro®), a glycoprotein IIb/IIIa inhibitor used as an adjunctive antiplatelet therapy during percutaneous coronary interventions in ACS patients; and (2) digoxin immune Fab (Digitalis-Antidote BM), a monovalent ovine Ig antibody-binding fragment used in the treatment of digoxin toxicity. Basiliximab (Simulect®) is a chimeric mouse-human monoclonal antibody to the CD25 antigen (surface IL-2 receptor α-chain), approved for the prophylaxis of acute organ rejection in patients receiving kidney transplantation. It is also used in the acute phase of operations, as some preliminary data point to its efficacy and safety after heart transplantation [5, 6].
Why target PCSK9?
The use of mAbs to target PCSK9 for the treatment of hypercholesterolaemia and secondary prevention of CVD, as well as for the reduction of inflammation associated with CVD, has previously been discussed in the Swiss Medical Weekly [4]. PCSK9 binds to the LDL-C receptor on the surface of hepatocytes, thereby preventing its recycling and enhancing its degradation in endosomes/lysosomes, resulting in reduced LDL-C clearance [7]. Mutations of PCSK9 have been identified as genetic markers of FH, and loss-of-function mutations of PCSK9 have been associated with reduced levels of LDL-c, as well as a lower risk of coronary heart disease (CHD) [8]. In addition, PCSK9 levels are increased by statin therapy, suggesting a role of PCSK9 in statin resistance [7]. These findings constitute the proof of concept for targeting PCSK9 in the treatment of hypercholesterolaemia.
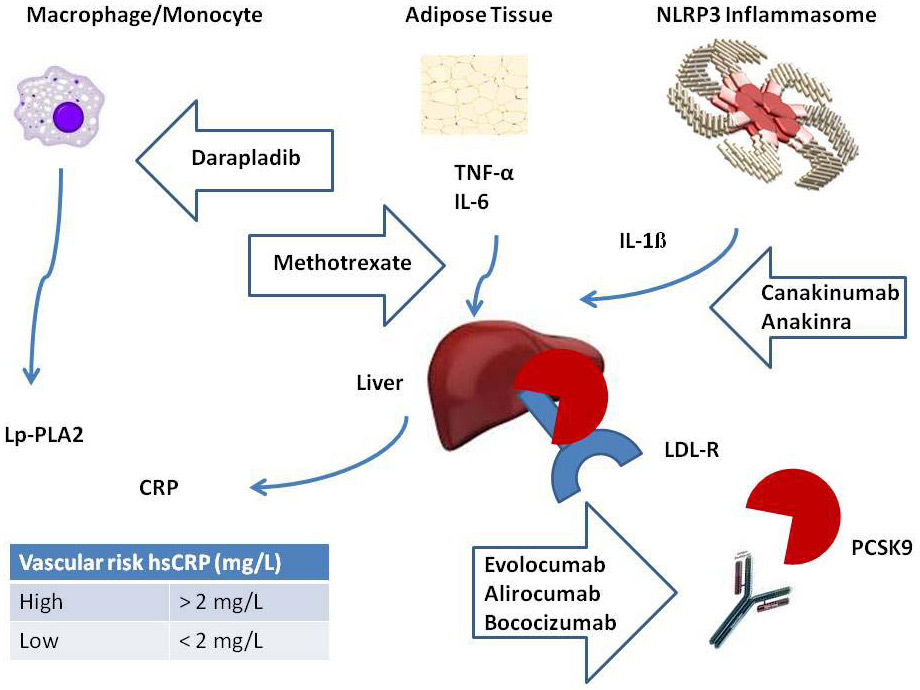
Figure 1
Targeting inflammatory and lipid pathways for the treatment of cardiovascular disease. Monoclonal antibodies against PCSK9 (evolocumab, alirocumab and bococizumab) decrease circulating LDL-C by an increase of LDL-R at the hepatocyte surface. Monoclonal antibodies against IL-1β (canakinumab) are currently being tested in the CANTOS trial [37]. Anakinra, an inhibitor of IL-1β, has been tested in the VCU-ART studies [46]. Methotrexate, an inhibitor of TNFα, IL-6 and hsCRP, is currently being tested in the CIRT trial [38]. Darapladib has been tested in the SOLID-TIMI 52 and STABILITY trials [44, 45].
CANTOS = Canakinumab Anti-inflammatory Thrombosis Outcomes Study; CIRT = Cardiovascular Inflammation Reduction Trial; hsCRP = high-sensitive C-reactive protein; IL = interleukin; LDL-C = low-density lipoprotein cholesterol; LDL-R = low-density lipoprotein receptor; PCSK9 = proprotein convertase subtilisin / kexin type 9; SOLID-TIMI 52 = stabilization of plaque using darapladib-thrombolysis in myocardial infarction 52; STABILITY = stabilization of atherosclerosis plaque by initiation of darapladib therapy. TNF = tumour necrosis factor; VCU-ART = Virginia Commonwealth University anakinra remodeling trial.
Figure adapted from Ridker [25].
Three PCSK9 inhibitors are currently being tested in double-blinded randomized controlled trials (RCTs): alirocumab, evolocumab and bococizumab. Alirocumab and evolocumab have already shown to be efficacious for the treatment of FH patients with poorly controlled LDL-C despite maximally tolerated statin therapy [4]. Trial results showed that both anti-PCSK9 mAbs reduced LDL-C levels systematically by 40%–70% compared with placebo, while also decreasing triglyceride and apolipoprotein B (ApoB) levels, and increasing HDL-C and apolipoprotein A1 (ApoA1) [9–15]. Recently, the ODYSSEY Combo trial showed alirocumab to be more effective than ezetimibe in reducing LDL-C levels, with a similar safety profile observed in both groups [16]. In the light of these findings, anti-PCSK9 mAbs seem to confirm the adage “lower is better” in their role as lipid lowering agents in FH patients, but also potentially for the reduction of cardiovascular events (CVE), as seen in exploratory analyses [17, 18]. The ODYSSEY LONG TERM trial randomised 2,341 patients with FH or coronary heart disease (CHD) to alirocumab 150 mg every 2 weeks vs placebo. At 52 weeks, alirocumab was associated with a 60% reduction in LDL-C (mean LDL-C 1.3 mmol/ for alirocumab vs LDL-C 3.1 mmol/L for placebo, p <0.001). In a post-hoc analysis, the rate of adjudicated major CVE requiring hospitalisation was 1.7% for alirocumab vs 3.3% for placebo (hazard ratio [HR] 0.52, 95% confidence interval [CI] 0.31‒090, p = 0.02) [17]. In the OSLER trial, where evolocumab was compared with standard therapy, evolocumab demonstrated a reduction of CVE events at 1 year (0.95% vs 2.18%, HR 0.47, 95% CI 0.28–0.27, p = 0.003) in an exploratory analysis [18]. Overall, mAb safety profiles seem to be comparable to placebo; however, in the anti-PCSK9 mAb arms more neurocognitive events have been reported (0.9% vs 0.3% for evolocumab and placebo, respectively, p-value not reported, and 1.2% vs 0.5% for alirocumab and placebo, respectively, p = 0.17) [17, 18]. A recent meta-analysis of 24 randomised controlled trials including 10,159 patients showed a significant reduction of all-cause mortality (odds ratios [OR] 0.45, 95% CI 0.23–0.86, p = 0.015), mortality (OR 0.50, 95% CI 0.23–1.10, p = 0.084) and myocardial infarction events (OR 0.49, 95% CI 0.26–0.93, p = 0.030) for anti-PCSK9 mAbs compared with standard therapy [19]. Serious adverse events were not increased by anti-PCSK9 mAbs, although specific neurocognitive events were not reported in this meta-analysis [19]. Another major concern could be demyelination in the central nervous system or leukoencephalopathy, the latter having led to a black box warning for rituximab. Administration of mAbs has, furthermore, been associated with serious reactions, such as acute anaphylaxis, serum sickness, the generation of antibodies directed towards the mAb treatment, and life threatening cytokine release syndrome [20, 21].
If PCSK9 therapies continue to demonstrate such impressive LDL-C lowering effects, physicians might look forward to having an effective add-on or alternative to statins as lipid lowering agents [22]. The approval of anti-PCSK9 mAbs for the treatment of FH and for patients intolerant to statins has just received approval in the US and Europe. Another area under investigation is the potential of anti-PCSK9 mAbs in the secondary setting, for the treatment of dyslipidaemia after ACS. Two large-scale phase III trials are currently recruiting patients with established CHD in order to assess whether PCSK9 inhibitors can reduce the occurrence of CVE events in patients at high risk, such as those hospitalised for ACS: FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibitors in subjects with Elevated Risk, NCT01764633) for evolocumab; and ODYSSEY OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab SAR236553/REGN727, NCT01663402) for alirocumab [23]. More than 20,000 patients are planned for enrolment in each study. Further large-scale studies are needed to assess the long-term safety and clinical use of anti-PCSK9 mAbs [24].
Why target inflammation?
Inflammation is a major component of atherosclerotic disease, including plaque rupture and the resulting acute CVE [25]. Innate and acquired immunity play a key role in the biology of atherogenesis, including cell adhesion, cell transmigration across the endothelium, fatty streak formation, smooth muscle cell migration, and plaque progression and rupture [26]. When activated, interleukin-1 (IL-1), tumour necrosis factor-α (TNF-α) or interleukin-6 (IL-6) pathways result in elevated levels of hepatic acute phase proteins, including C-reactive protein (CRP), fibrinogen and plasminogen activator inhibitor type-1 [25]. Epidemiological studies have shown inflammatory markers such as CRP, IL-6 and TNF-α to be associated with subsequent CVE events independently of hyperlipidaemia and other major cardiovascular risk factors [27, 28]. Considering the mechanistic actors of inflammation, targeting causal pathways of inflammation rather than a single marker is likely to be the most promising approach for emergent therapy developments [29]. For clinicians, CRP is a well-known marker used in daily practice to better identify patients at higher risk of CVD [30]. The reason inflammation is believed to play an independent role in the occurrence of CVD stems partially from the fact that half of all heart attacks and strokes occur in apparently healthy men and women with averagely low cholesterol levels or at low risk of CVD according to risk stratification recommendations (e.g., Framingham or SCORE) [31]. The JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial is a good example of integrating inflammation into the design of clinical trials. In JUPITER, 17,802 men and women with LDL-C below 130 mg/L (3.4 mmol/l) and high-sensitivity CRP >2 mg/dl were randomised to rosuvastatin 20 mg/day or placebo as primary prevention [32]. Rosuvastatin 20 mg/day was associated with a 50% LDL-C and 37% CRP reduction, respectively, as well as a reduced occurrence of the combined primary endpoint of myocardial infarction, stroke, revascularisation, hospitalisation for unstable angina and cardiovascular death (hazard ratio [HR] 0.56, 95% CI 0.46–0.69) after a mean follow-up of 1.9 years. The trial was prematurely stopped following an independent data board notification. The main outcome of the JUPITER trial was the additional benefit of rosuvastatin in subjects who had higher levels of CRP at baseline. The benefit of this study was to add inflammation to theinclusion criteria, as opposed to statin trials mostly designed on the basis of hypercholesterolaemia criteria only. The JUPITER trial was the first to demonstrate a greater clinical benefit of statin therapy among patients whose CRP and LDL-C levels were most reduced [29]. These findings have led to the new concept of CVD inflammation reduction trials. This approach aims at targeting inflammation independently from concomitant lipid-lowering or antiplatelet treatments. However, a treatment targeting a specific risk factor does not preclude a reduction of cardiovascular events. For instance, high levels of homocysteine are a known risk factor for CVD, but trials aiming to reduce homocysteine levels with B vitamins did not lower the risk of CVE events in secondary prevention [33].
Future perspectives for targeting inflammation in acute coronary syndromes: CANTOS and CIRT trials
NOD-like receptor pyrin domain 3 (NLRP-3) inflammasomes play a key role in the production of several proinflammatory cytokines such as IL-1β, following recognition of cholesterol crystal deposits within growing atheroma [34]. IL-1β is produced by monocytes and macrophages, which are key cells involved in atherosclerosis. Anakinra, an IL-1β receptor antagonist, has already shown to improve glycaemia and systemic inflammation in diabetic patients [35]. A pilot phase II trial in diabetic patients has also shown canakinumab, a human mAb targeting IL-1β, to be effective in reducing IL-6 and CRP (>50%) [36]. Canakinumab is approved for the treatment of systemic juvenile idiopathic arthritis, and is currently being investigated in ACS patients. The Canakinumab Anti-Inflammatory Thrombosis Outcomes Trial (CANTOS, NCT01327846) was designed to test whether a human mAb specifically inhibiting IL-1ß could reduce the occurrence of CVE in high risk patients [37]. The primary objective of the CANTOS trial is to assess whether long-term treatment with canakinumab (50, 150, or 300 mg every 3 months) compared with placebo will reduce the occurrence rates of CVE in patients who had myocardial infarction with coronary revascularisation and elevated inflammation (hsCRP >2 mg/L) despite intensive secondary prevention therapies. The primary outcomes will be a composite endpoint of major adverse CVE, defined as myocardial infarction, nonfatal stroke or cardiovascular death. Other exploratory endpoints will be the occurrence of heart failure events, atrial fibrillation, stent thrombosis and thromboembolic events. Immunocompromised patients and patients with heart failure, uncontrolled hypertension or diabetes, renal or hepatic failure and oncological disease will be excluded. A total of 17,200 patients randomized 1:1:1.5 to receive canakinumab 50 mg, 150 mg, 300 mg or placebo, respectively, will be needed to reach a number of 1,400 events for the primary endpoint and show a hazard reduction of 20% with canakinumab compared with placebo (all patients have the standard recommended therapies in secondary prevention). In terms of safety, specific attention will be given to the development of antibodies to canakinumab, as well as new cases of diabetes. CANTOS is the first major adverse cardiovascular events-driven trial to specifically address the inflammatory hypothesis of atherosclerosis, evaluating the impact of IL-1β inhibition compared with placebo in an event-driven protocol.
The second ongoing trial, using methotrexate, a non-biologic, is the Cardiovascular Inflammation Reduction Trial (CIRT, NCT01594333) among CVD patients (myocardial infarction specifically) [38]. In total, 7,000 subjects will be assigned to low-dose methotrexate, as used for the treatment of rheumatoid arthritis (15–20 mg weekly). Methotrexate is a dihydrofolate reductase inhibitor preventing the synthesis of both purine and pyrimidine nucleotides, which also constitutes the basis for its use in the treatment of malignancies. Methotrexate may reduce the production of cytokines (IL-6, TNF-α) and have several actions on cellular mechanisms of atheroma formation [39]. Data from epidemiological studies in patients with rheumatoid arthritis suggest that patients treated with methotrexate or other disease-modifying antirheumatic drugs (DMARDs) have fewer CVE [40]. Methotrexate at low dose inhibits atherosclerotic-driven inflammation and is known to have an acceptable safety profile in the treatment of rheumatoid arthritis [41, 42]. Low-dose methotrexate reduces several inflammatory markers, including CRP, IL-6 and TNF-α in patients with rheumatological disease [43]. The primary aim of CIRT is to test directly the inflammatory hypothesis of atherothrombosis by evaluating the effects of low-dose methotrexate on the rates of recurrent myocardial infarction, stroke, and cardiovascular death among stable coronary artery disease patients with type 2 diabetes or metabolic syndrome [29]. Both the CANTOS and CIRT trials will test the impact of reducing inflammation in patients at increased risk of CVD events and with persisting biological inflammation despite treatment with evidence-based therapies.
Other potential targets against inflammation
Recently, two nonbiological, potentially anti-inflammatory agents were investigated in patients with CHD. The first, darapladib, is a lipoprotein-associated phospholipase A2 inhibitor that has preventive effects on necrotic core expansion as assessed with intravascular ultrasound virtual histology. In the STABILITY (Stabilization of Atherosclerosis Plaque by Initiation of Darapladib Therapy) trial, darapladib did not significantly reduce the primary endpoint of cardiovascular death, myocardial infarction, or stroke in patients with stable CHD (HR 0.94, 95% CI 0.85–1.03, p = 0.20), but an effect was observed for major coronary events (9.3% vs 10.3%, HR 0.90, 95% CI 0.82–1.00, p = 0.045) and total coronary events (14.6% vs 16.1%, HR 0.91, 95% CI 0.84–0.98, p = 0.02) [44]. In the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52, NCT01000727) trial, 13,026 patients were randomised to darapladib (160 mg) or placebo within 30 days of hospitalisation for an ACS. No significant differences were observed between the two groups in terms of major coronary events (16.3% vs 15.6%, HR 1.00, 95% CI 0.91–1.09, p = 0.93) after 2.5 years of follow-up [45]. The second agent, anakinra, is an IL-1 receptor inhibitor that has been tested after acute myocardial infarction in the VCU-ART (Virginia Commonwealth University Anakinra Remodeling Trial) and VCU-ART2 pilot studies [46]. After 14 days of treatment, no difference was noted between the arms for the combined endpoint of death, cardiac death, recurrent acute myocardial infarction, stroke, unstable angina and symptomatic heart failure (HR 1.08, 95% CI 0.31–3.74, p = 0.90); however, the combined endpoint for death or heart failure was significantly reduced in the anakinra arm (HR 0.16, 95% CI 0.03–0.76, p = 0.008) [46].
Whereas inhibiting PCSK9 activity has a circumscribed effect on the LDL receptor, targeting inflammation via the IL-1 β-receptor with canakinumab or anakinra, or via lipoprotein-associated phospolipase A2 with darapladib is much less specific, as is methotrexate. Modulation of pathways for acute phase proteins may result in susceptibility to infections, as shown in trials with canakinumab for the treatment of juvenile arthritis [47]. So far, trials targeting inflammation with darapladip or anakinra have shown more or less neutral efficacy results, coupled with more side effects [44, 45]. There are no data on the consequences of long-term immunomodulatory therapy in patients with CVD.
Conclusion
Monoclonal antibodies appear to offer the most promising prospects for new therapeutic approaches for the treatment of dyslipidaemia. Anti-PCSK9 mAbs are a good example of effective treatment of hypercholesterolaemia. Other emergent monoclonal antibodies targeting inflammation in CVD, such as Il-1β receptor antagonists or canakinumab, are currently being studied in large-scale clinical trials. Compared with classical chemical substances, biologics have the advantages of providing high target specificity and lower risk of drug-drug interactions. Results from ongoing trials are expected to provide a more detailed understanding of the use and role of biologics in cardiology, especially regarding their long-term safety.
References
1 Morrow T, Felcone LH. Defining the difference: What Makes Biologics Unique. Biotechnology healthcare. Sep 2004;1(4):24–9.
2 Foltz IN, Karow M, Wasserman SM. Evolution and emergence of therapeutic monoclonal antibodies: what cardiologists need to know. Circulation. 2013;127(22):2222–30.
3 Gencer B, Mach F. Sweetless’n low LDL-C targets for PCSK9 treatment. Eur Heart J. 2015.
4 Gencer B, Lambert G, Mach F. PCSK9 inhibitors. Swiss Med Wkly. 2015;145:w14094.
5 Segovia J, Rodriguez-Lambert JL, Crespo-Leiro MG, et al. A randomized multicenter comparison of basiliximab and muromonab (OKT3) in heart transplantation: SIMCOR study. Transplantation. 2006;81(11):1542–8.
6 Delgado JF, Vaqueriza D, Sanchez V, et al. Induction treatment with monoclonal antibodies for heart transplantation. Transplant Rev (Orlando). 2011;25(1):21–6.
7 Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circulation research. 2014;114(6):1022–36.
8 Cohen JC, Boerwinkle E, Mosley TH, Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
9 Sullivan D, Olsson AG, Scott R, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–506.
10 Giugliano RP, Desai NR, Kohli P, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–17.
11 Koren MJ, Scott R, Kim JB, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995–2006.
12 Raal F, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408–17.
13 Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366(12):1108–18.
14 Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36.
15 Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891–900.
16 Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36(19):1186–94.
17 Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99.
18 Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–09.
19 Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of Proprotein Convertase Subtilisin/Kexin Type 9 Antibodies in Adults With Hypercholesterolemia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015.
20 Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nature reviews. Drug Discov. 2010;9(4):325–38.
21 Guan M, Zhou Y, Sun J, Chen S. Adverse Events of Monoclonal Antibodies Used for Cancer Therapy. Biomed Research International. 2015.
22 Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol. 2012;59(25):2354–5.
23 Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682–9.
24 Catapano AL, Papadopoulos N. The safety of therapeutic monoclonal antibodies: implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis. 2013;228(1):18–28.
25 Ridker PM. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur Heart J. 2014;35(9):540–3.
26 Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–13.
27 Rodondi N, Marques-Vidal P, Butler J, et al. Markers of atherosclerosis and inflammation for prediction of coronary heart disease in older adults. Am J Epidemiol. 2010;171(5):540–9.
28 Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–22.
29 Ridker PM. Testing the inflammatory hypothesis of atherothrombosis: scientific rationale for the cardiovascular inflammation reduction trial (CIRT). J Thromb Haemost. 2009;7(Suppl1):332–9.
30 Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73.
31 Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904.
32 Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207.
33 Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88.
34 Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–61.
35 Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–26.
36 Ridker PM, Howard CP, Walter V, et al. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126(23):2739–48.
37 Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am Heart J. 2011;162(4):597–605.
38 Everett BM, Pradhan AD, Solomon DH, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166(2):199–207 e115.
39 Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology (Oxford). 2003;42(10):1189–96.
40 Krishnan E, Lingala VB, Singh G. Declines in mortality from acute myocardial infarction in successive incidence and birth cohorts of patients with rheumatoid arthritis. Circulation. 2004;110(13):1774–9.
41 Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res. 2012;64(5):625–39.
42 Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108(9):1362–70.
43 Rho YH, Oeser A, Chung CP, Milne GL, Stein CM. Drugs Used in the Treatment of Rheumatoid Arthritis: Relationship between Current Use and Cardiovascular Risk Factors. Arch Drug Inf. 2009;2(2):34–40.
44 White HD, Held C, Stewart R, et al. Darapladib for preventing ischemic events in stable coronary heart disease. N Engl J Med. 2014;370(18):1702–11.
45 O’Donoghue ML, Braunwald E, White HD, et al. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID-TIMI 52 randomized clinical trial. JAMA. 2014;312(10):1006–15.
46 Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115(3):288–92.
47 Ruperto N, Brunner HI, Quartier P, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–406.
